Chapter 66 Surgical Management of Extracranial Carotid Artery Disease
Since the first description of a carotid endarterectomy (CEA) for the prevention of stroke,1 the operation has been widely debated and often criticized; yet the numbers of endarterectomy procedures performed annually have steadily increased.2 Early studies suggested that medical management was superior to surgical intervention.3,4 This is clearly no longer the case. Gratifying and unimpeachable results from recent multicenter trials have advocated surgical therapy over medical management in specific cases5–7 of both asymptomatic and symptomatic carotid stenosis. The North American Symptomatic Carotid Endarterectomy Trial data indicate that CEA has benefit for all symptomatic patients with lesions of more than 70% linear stenosis and for specific subgroups of symptomatic patients with more than 50% stenosis. The Asymptomatic Carotid Atherosclerosis Study indicates that asymptomatic patients with more than 60% stenosis have a better outcome with CEA than with medical management.8
Surgical Technique
Surgical Magnification
We perform the operation with 3.5× loupe-magnified technique. Microscopic repair of the internal carotid artery (ICA), which we have also tried, allows a primary repair that is unquestionably finer than a loupe-magnified technique,3,9–11 but which in our experience did not alter the overall patient outcome or incidence of restenosis or acute occlusion. In the ongoing effort to reduce morbidity, we have instead adopted universal patch grafting with collagen-impregnated Dacron (Hemashield graft), which has essentially eliminated the problem of acute postoperative thrombosis or rapid restenosis. In our opinion, the graft procedure is more easily and expeditiously accomplished with 3.5× magnification rather than the microscope. There is no doubt that the suture lines are not as fine with this method, but the added lumen diameter with patch angioplasty renders the microscopic technique unnecessary in our routine practice.
Anesthetic Technique
General anesthesia and local anesthesia are both in common use for CEA. We routinely use general anesthesia with both full-channel electroencephalographic and concurrent somatosensory-evoked potential (SSEP) monitoring. Proponents of local anesthesia cite the advantages of patient response to questioning as a superior method of assessing the need for intraoperative shunting while minimizing anesthetic risks, reducing postoperative morbidity, and shortening length of stay. The patient has local anesthesia with light sedation, which allows the patient to perform a simple task with the contralateral hand during cross-clamping. The disadvantages include risk of contamination and patient movement during the procedure, along with the increased psychological stress of remaining awake. A recent review comparing our technique with that of an institutional vascular surgeon using local anesthesia showed a decreased incidence of electroencephalographic changes and intraoperative shunting with local anesthesia. However, there was no difference in stroke rate, complications, length of stay, or overall outcome.12,13
We prefer general anesthesia for a number of reasons, not the least of which is the controlled environment. Additionally, all commonly used inhalational anesthetic agents and intravenous barbiturates significantly reduce the cerebral metabolic rate of oxygen consumption,14 giving a theoretical protective effect in the setting of cerebral ischemia. We keep our patients normocapnic. Although there has been much interest in arterial levels of carbon dioxide, nonphysiologic hypercapnia and hypocapnia provide no cerebral protection.15–18 Gross and colleagues19 found that there was a 40% decrease in electroencephalographic changes with cross-clamping in those patients receiving either one or two units of 6% hetastarch (500 to 1000 ml). They had acceptable outcomes with a postoperative stroke and mortality rate of 1.3%. We ourselves have not adopted this technique because of our policy of shunting quickly without hesitation for any hint of an EEG or SSEP change. Finally, blood pressure is maintained at normotensive levels with a tolerance of as high as a 20% increase in systolic pressure.11 Although some surgeons prefer to induce hypertension at cross-clamping if there are electroencephalographic changes and then shunt if no improvement is seen in the electro-encephalographic recordings, we are not trying to avoid shunt use, and as mentioned we have a policy to shunt immediately if any monitoring changes are evident.
Monitoring Techniques
Monitoring techniques can be divided into two categories: (1) tests of vascular integrity, such as stump pressure measurements, xenon regional cerebral blood flow studies, transcranial Doppler and, to a lesser extent, intraoperative oculoplethysmography (OPG), Doppler/duplex scanning, and angiography; and (2) tests of cerebral function, such as electroencephalography, electroencephalographic derivatives, and/or SSEP monitoring. The newly described near-infrared spectroscopy technique bridges both categories. We use a full-channel electroencephalography interpreted by a neurologist. After completion of arteriotomy closure, an intraoperative Doppler examination is performed of the common carotid artery (CCA), the ICA, and the external carotid artery (ECA).20,21
Intraoperative Shunting
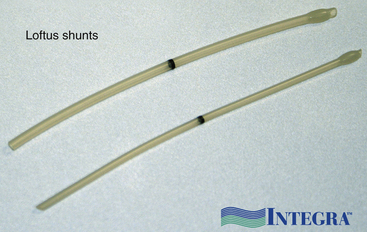
Generally speaking, there are three schools of thought about intraoperative shunting.22 Carotid surgeons shunt in every case, shunt when indicated by some form of intraoperative monitoring, or never place a shunt.23 In our institution, we perform monitor-dependent shunting based on electroencephalographic criteria. We use a custom commercial shunt of our own design (Loftus shunt, Integra Neurocare, Plainfield, NJ).24 In our experience, we shunt approximately 15% of CEAs. This increases to approximately 25% if the contralateral carotid is occluded. After the shunt is placed, the monitoring should return to baseline. If this does not occur, the shunt must be inspected for possible kinking, thrombosis, or misplacement. We always auscultate the shunt with a Doppler probe that confirms patency and shunt flow.
Proponents of universal shunting tout the benefits of the maximal degree of cerebral protection in every case while eliminating dependence on specialized intraoperative monitoring techniques. They assert that shunt placement is benign and allows extra time to ensure meticulous intimal dissection and arteriotomy repair.25–30
Proponents of nonshunting believe that shunt placement is not benign. In one series, there was a higher stroke rate with shunting compared with nonshunting,31 indicating that embolization from shunt placement, especially by surgeons inexperienced in the procedure, is a real risk. Another documented concern is distal intimal damage leading to embolization or carotid artery dissection.32
Many surgeons, in part because of the concerns previously discussed, choose not to shunt. There have been multiple series that have good surgical results with no shunts being used.33–40 These authors do not deny the existence of postoperative stroke, but they strongly believe that neurologic deficits from carotid artery surgery are invariably embolic rather than hemodynamic in nature and that intraoperative monitoring and/or shunt placement will not further reduce the already low morbidity in their series.39
As discussed previously, we prefer to shunt when there are changes in the EEG and/or SSEP with cross-clamping. This policy has been well supported by several reports of large series of patients.41,42 Of note, there are also several authors who normally practice selective shunting but who advocate shunting all patients who have had recent strokes or reversible neurologic events (due to their belief that intraoperative monitoring is unreliable in the face of recent ischemic events).43–45 Whereas we understand their concerns, this has not been our practice.
Patch Graft Angioplasty
Almost all carotid surgeons perform patch angioplasty for recurrent carotid stenosis. Many will also use selective patching in cases in which the internal carotid is small, where the arteriotomy may have extended far up the ICA, or in any similar case in which compromise of the lumen and a high risk of thrombosis is anticipated. We have taken this policy one step further, and for a number of years have used a Hemashield patch graft primarily in all our patients. We have not encountered any restenosis or acute occlusions since using the patch universally. There are several other synthetic grafts available depending on surgeon preference, and of course autologous sapenous vein grafts can be used, but we prefer Hemashield, mindful of the real concern of central patch rupture with autologous vein, especially in women and patients with diabetes. Rupture risk can be reduced by harvesting the graft from a high femoral site rather than at the ankle.46
Heparinization
A single dose of intravenous heparin is given to the patient at some point before cross-clamping. This dose is between 2500 and 10,000 U of heparin, depending on the surgeon’s preference. There are no published reports to support one dose versus another; however, Poisik and colleagues38 recently reported their results using weight-based dosing of heparin at 85 U/kg. Although they did not see any statistically significant differences between fixed-dose heparinization (5000 U) and weight-based dosing, there were trends of decreased complications of hematoma formation and neuropsychometric testing differences. Some individuals reverse the heparinization with protamine after the operation.39,40 We have not found any benefit in this practice. For those patients who come to the operating room on a continuous heparin drip, we continue the infusion until the arteriotomy closure is finished. With meticulous technique, bleeding in these cases has not been a problem.
Tacking Sutures
Tandem sutures to secure the distal intima in the ICA after plaque removal are considered a great advance by some26 and are deemed unnecessary by others27,39,40 The concern with tacking sutures is that they may narrow the lumen, but to us this risk seems small compared with the concern of intimal dissection from an unsecured intimal flap. Several authors28,39,40 state that, if the arteriotomy is carried far enough to see normal intima distal to the plaque, the tacking sutures are unnecessary. We strongly agree with an arteriotomy that extends past the plaque, but we are not always satisfied with how the intimal plaque tapers at the distal endpoint. In recent years, because of negative experiences with plaque that does not feather cleanly when pulled down from the distal ICA, we have adopted the use of fine scissors to “trim” the plaque cleanly in the ICA as it is removed. When this is done, tacking sutures are rarely necessary. We estimate that we now selectively place tacking sutures in the distal ICA in approximately 10% of cases.
Surgery
We think that the meticulous anatomic dissection and identification of vital cervical structures needed to minimize postoperative complications can be achieved only with a bloodless field. Accordingly, we do not consider elapsed time to be a factor in the performance of carotid artery surgery. In our institution, CEA requires from 2 to 2.5 hours of operating time and the average cross-clamp time is between 30 and 40 minutes. No untoward effects from the length of the procedure have been observed in any patient, and we are convinced that the risk of cervical nerve injury or postoperative complications related to hurried closure of the suture line is significantly reduced by meticulous attention to detail.47
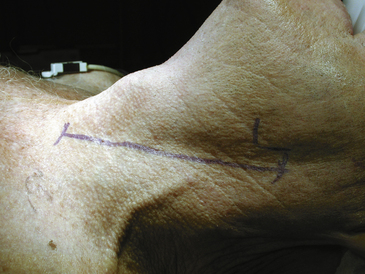
The position of the carotid bifurcation has been likewise determined before surgery from the angiogram and the skin incision is planned accordingly. We always use a linear incision along the anterior portion of the sternocleidomastoid muscle (Fig. 66-1). This may go as low as the suprasternal notch and as high as the retroaural region depending on the level of the bifurcation. The skin and subcutaneous tissues are divided sharply to the level of the platysma, which is always identified and divided sharply as well. Hemostasis often requires the generous use of bipolar electrocautery. If careful attention is paid to all bleeding points during the opening, there will be little or no bleeding when heparin is administered and the closure will be much simpler.
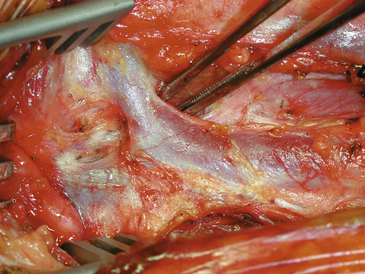
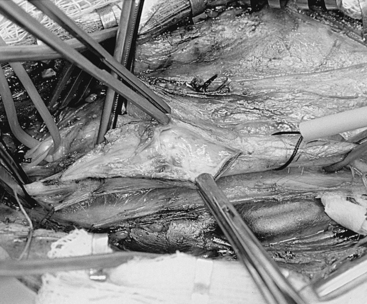
It is to be emphasized that the jugular vein is the key landmark in this exposure and complete dissection of the medial jugular border should always be carried out before proceeding to the deeper structures. In some corpulent individuals, the vein is not readily apparent and a layer of fat between it and the sternomastoid must be entered to locate the jugular itself. If this is not done, it is possible to fall into an incorrect plane lateral and deep to the jugular vein. As soon as the jugular is identified, dissection is shifted to come along the medial jugular border and the vein is held back with blunt retractors.48 The importance of the blunt retractor in preventing vascular injury at this point cannot be overemphasized. In this process, several small veins and one large common facial vein are customarily crossing the field and need to be doubly ligated and divided (Fig. 66-2). The underlying carotid artery is soon identified once the jugular is retracted. Most often we come upon the CCA first, and at the point of first visualization, the anesthesiologist is instructed to give 5000 U of intravenous heparin, which, as discussed previously, is never reversed. Dissection of the carotid complex is then straightforward, and the CCA, ECA, and ICA are isolated with the gentlest possible dissection and encircled with 00 silk ties (or vessel loops, if preferred) passed with a right-angle clamp. We no longer routinely inject the carotid sinus; however, the anesthesiologist is notified when the bifurcation is being dissected, and if any changes in vital signs ensue, the sinus can be injected with 2 to 3 ml of 1% plain Xylocaine through a short 25-gauge needle (this has not been necessary for the past several years). Although the carotid complex is completely exposed, the CCA and ECA are not routinely dissected free from their underlying beds to prevent postoperative kinking and coiling of these vessels. These arteries are dissected circumferentially only in those areas where silk ties or clamps are placed around them. Posterior dissection is more extensive in the region of the ICA, where in an occasional case posterior tacking sutures may later be placed and tied.
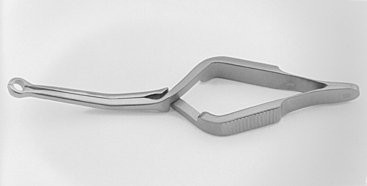
It is vital to have adequate exposure of the ICA and control distal to the plaque before opening the vessel. The extent of the plaque can be readily palpated with some experience by a moistened finger. There is also a visual cue when the vessel becomes pinker (instead of hard and yellow) and more normal appearing distal to the extent of the plaque. If high exposure is needed, the posterior belly of the digastric muscle can be cut with impunity, although this is necessary only in a small percentage of cases. When complete exposure is achieved, the final step in preparation for cross-clamping is to ensure that the shunt clamp can be fitted around the ICA to secure the shunt if one is used. We designed and use a custom commercial spring-loaded pinch clamp (Loftus carotid shunt clamp, Scanlan Instruments, St. Paul, MN), which is available in several angles and has a special head exactly sized to grasp the ICA and indwelling shunt without leakage from back-bleeding. The Loftus shunt clamp is illustrated in Fig. 66-3.
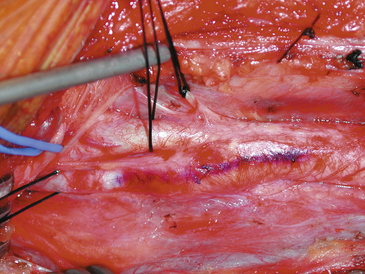
We also use a sterile marking pen to draw the proposed arteriotomy line along the vessel, which is helpful in preventing a jagged or curving suture line (Fig. 66-4). The arteriotomy is made on the anterior surface of the ICA to facilitate the subsequent repair.
The monitoring systems (we use concurrently both EEG and SSEP now) are then rechecked, and the technicians are notified of impending cross-clamping. Once a suitable period of baseline has been recorded, the CCA is occluded with a large DeBakey vascular clamp and small, straight bulldog clamps or Yasargil aneurysm clips are used to occlude the ICA and ECA. We always occlude the ICA first in the belief that this approach has the lowest risk of embolization associated with clamping. A no. 11 blade is then used to begin the arteriotomy in the CCA and when the lumen is identified, a Potts scissors is used to cut straight up along the marked line into the region of the bifurcation and then up into the internal until normal ICA is entered (Fig. 66-5). In severely stenotic vessels with friable plaque, the lumen is not always easily discerned and false planes within the lesion are often encountered; great care must be taken to ensure that the back wall of the carotid is not lacerated and that the true lumen is identified before attempted shunt insertion.
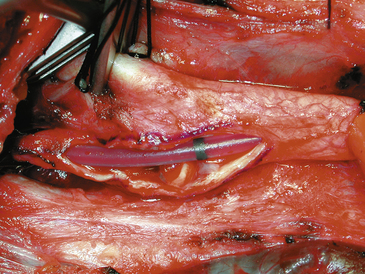
Changes in the electroencephalogram mandate a rapid trial of induced hypertension for some surgeons, but for an intraluminal shunt is placed for any change on any monitor. The wisdom of shunt use is discussed elsewhere in the text. Numerous shunt types are available. We now use and recommend a customized indwelling shunt, the Loftus carotid endarterectomy shunt (Integra Neurocare, Plainsboro, NJ) (Fig. 66-6). This is a 15-cm straight silicone tube, singly packed in two diameters (10 Fr and 12 Fr), with tapered ends for easy insertion and a bulb at the proximal end to facilitate anchoring by the Rummel tourniquet. This shunt has a black marker band directly in the center of the shunt, so that cephalad shunt migration can be readily discerned and corrected. The shunt is first inserted into the CCA and secured by pulling up on the silk ties; a mosquito clamp then holds the rubber sleeve in place to snug the silk around both the vessel and the intraluminal shunt. The shunt tubing is held closed at its midportion with a heavy vascular forceps and then briefly opened to confirm blood flow and evacuate any debris in the shunt tubing. Suction is then used by the assistant to elucidate the lumen of the ICA, and the distal end of the shunt tubing is placed therein. After the shunt is again bled, flushing any debris from the ICA, the bulldog clamp is removed and the shunt is advanced up the ICA until the black dot lies in the center of the arteriotomy. The shunt, if properly placed, should slide easily into the ICA, and no undue force should be employed to prevent intimal damage and possible dissection. The Loftus shunt clamp is then used to secure the shunt distally in the ICA. Visualization of the dot in the center of the arteriotomy confirms constant correct positioning of the shunt (Fig. 66-7). A handheld Doppler probe can be applied to the shunt tubing to audibly confirm flow.
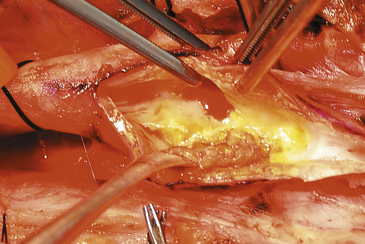
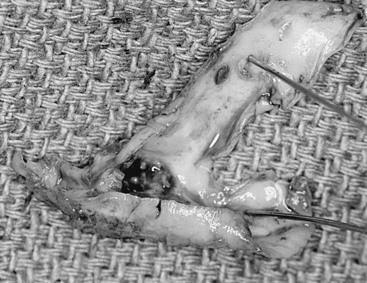
With or without the shunt, the plaque is next dissected from the arterial wall with a Freer elevator. A vascular pickup is used to hold the wall, and the Freer elevator is moved from side to side developing a plane first in the lateral wall of the arteriotomy (Fig. 66-8). The plaque is usually readily separated in a primary case, and we go approximately half way around the wall before proceeding to the other side. The plaque is then dissected on the medial side of the CCA and transected proximally with a Potts or Church scissors. A clean feathering away of the plaque is almost never possible in the CCA, and the goal here is to transect the plaque sharply, leaving a smooth transition zone. We like to pass a right-angle clamp between the plaque and the normal vessel and cut sharply along the clamp blade with the no. 15 knife (Fig. 66-9). It is important to note that, despite the direction of flow, the proximal end point can create a flap and the surgeon should ensure that the CCA end point is adherent. Attention is then directed to the ICA where likewise the plaque is dissected first laterally and then medially and then an attempt is made to feather the plaque down smoothly from the ICA (Fig. 66-10). However, we find that, in some cases no matter how far up the ICA we go, a shelf of normal intima remains and tacking sutures are required. Attention is finally directed to the final point of plaque attachment at the orifice of the ECA. The vascular pickup is used to grip across the entire plaque at the ECA opening, and, with some traction on the plaque, the ECA can be everted such that the plaque can be dissected quite far up into that vessel (Fig. 66-11). The eversion of the external and thus optimal plaque removal can be facilitated by “pushing” the distal external artery proximally with the clamp or forceps. The plaque is often tethered in the ECA by the clamp and as long as the lumen is held closed with the heavy forceps, this clamp can be removed without untoward bleeding, allowing avulsion of the distal plaque. The clamp must be quickly reapplied to stem back-bleeding that occurs when the plaque is removed from the ECA. It should be stressed that if plaque removal is inadequate in the ECA, thrombosis may ensue, which can occlude the entire carotid tree with disastrous results. If there is any question of incomplete removal of the external plaque, we do not hesitate to extend the arteriotomy up the ECA itself and close it via a separate suture line.

FIGURE 66-10 The dissection of the plaque is continued with the Freer elevator along the medial edge.
After gross plaque removal, a careful search is made for any remaining fragments adherent to the arterial wall (Fig. 66-12). Suspect areas are gently stroked with a peanut sponge, and every attempt is made to remove all loose fragments in a circumferential fashion, elevating them the complete width of the vessel until they break free at the arteriotomy edge. (The fine Scanlan Loftus micro-ring-tip forceps are ideal for this, as the fragments are often too fine to be grasped with conventional DeBakey forceps.) Although it is important to remove all loose fragments, no attempt is made to elevate firmly attached fragments, which pose no danger of elevating or breaking off.
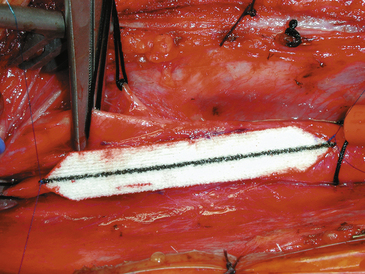
Several special aspects of plaque removal need to be considered. The simplest plaque to remove is the soft, friable plaque with intraplaque hemorrhage and thrombus, which dissect quite readily and from which fragments are easily removed (Fig. 66-13). The more difficult is the severely stenotic, stony hard plaque in which a plane of dissection at the lateral border of the carotid may not be readily apparent. This situation is analogous to the gross appearance in a case of recurrent carotid stenosis. In these instances, even the most gentle plaque removal results in areas of thinning, where only an adventitial layer is left in the posterior wall of the carotid. We have occasionally treated these thin spots by primary plication with one or two double-armed interrupted stitches of 6-0 Prolene placed in the same fashion as the tacking sutures, and no untoward consequences have ensued. Likewise, we have occasionally encountered an intraluminal thrombus emanating from a congenital web or shelf in the lumen of the vessel, and the shelf has been successfully tacked down with a posteriorly placed stitch of double-armed 6-0 Prolene. In all cases, the goal is to leave as smooth an arteriotomy bed as is possible with minimal areas of denudation or roughness available as sites of thrombus formation. Finally, at the completion of the repair, when the clamps are off, we sometimes reinforce thin back wall areas with an encircling “diaper” of Hemashield graft, loosely held together at the top by 6-0 sutures (Fig. 66-14).49
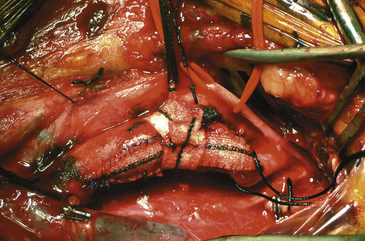
FIGURE 66-14 A Hemashield is wrapped around the carotid reconstruction to reinforce a tenuous back wall.
Attention is then directed to the arterial repair. If desired, the operating microscope can be brought into the field at this point or in some cases sooner to allow for removal of the small fragments under high magnification.9–1150 Our personal preference is to continue with 3.5× loupe magnification. If tacking sutures are required, double-armed sutures of 6-0 Prolene are placed vertically from the inside of the vessel out such that they traverse the intimal edge and are tied outside the adventitial layer. Most often two such sutures are used, placed at the 4- and 8-o’clock positions.
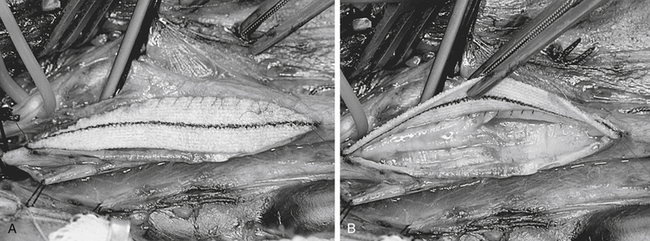
The patch, whatever material is employed, is then fashioned, if the surgeon has chosen this option. The patch material (Hemashield for the purposes of this description and illustrations) is placed over the arteriotomy and cut to the exact length of the opening. After removal from the field, the ends are trimmed and tapered to a point with fine Metzenbaum scissors. Each end of the patch is then anchored to the arteriotomy with double-armed 6-0 Prolene sutures, and the needles are left on and secured with rubber-shod clamps (Fig. 66-15). The medial wall suture line is closed first, and a running, nonlocking stitch is brought from the ICA anchor to the CCA anchor where it is tied to a free end of the CCA anchor Prolene (Fig. 66-16). The lateral wall is then closed (with the remaining limb of the ICA anchor stitch) from the ICA to just below the level of the carotid bulb. At this point, the second arm of the CCA anchor stitch is used to run up the CCA lateral wall to meet the ICA limb. Small bites are taken just at the arterial edge throughout (being certain, however, that all layers are included), and sutures are placed relatively close together to prevent leaks. Care is also taken so that no stray adventitial tags or suture ends are sewn into the lumen where they might induce thrombosis. Several millimeters of unsewn vessel are left on the lateral wall ensuring room to remove the shunt, if one has been used. After the EEG/SSEP teams are again notified, the shunt is double-clamped with two parallel straight mosquitoes and then cut between them and removed in two sections, one from each end. A common error at this point is to mistakenly entangle the suture material in the shunt clamps and thereby hamper smooth shunt removal. With or without shunt, the arteriotomy is completely closed as follows: all three vessels are first opened and closed sequentially to ensure that back-bleeding is present from the ICA, ECA, and CCA. The ICA is back-bled last to ensure that it is free of debris. The two stitches are then held taut by the surgeon while the assistant introduces a heparinized saline syringe with a blunt needle into the arterial lumen. The vessel is filled with heparinized saline, and in this process, all air is evacuated from the lumen. As the stitches are drawn up and a surgeon’s knot is thrown, the blunt needle is withdrawn, allowing no air to enter. Ten more knots are then placed in this most crucial stitch (Fig. 66-17). The declamping sequence is first from the ECA, then from the CCA, and, finally, some 10 seconds later, from the ICA. In this fashion, all loose debris and remaining microbubbles of air are flushed into the ECA circulation. Meticulous attention is paid to evacuation of all debris and air before opening the ICA in every case. However, in the rare case in which there is a known ECA occlusion (although most of these can be reopened at surgery with an ECA endarterectomy), this technique is extremely crucial as there is no ECA safety valve and all intraluminal contents will be shunted directly into the intracranial circulation.
An alternative method for completing the repair involves removal of the ligature or clip from the superior thyroid artery or ECA before final closure, allowing back-bleeding from that vessel to fill the lumen and eliminate the air and debris while the final stitches are placed and tied. We do not use this because we find the bleeding to be annoying.
When the clamps have been removed, the suture lines are inspected for leaks, which are customarily controlled with pressure, patience, and Surgicel gauze. In occasional cases, a single throw of 6-0 Prolene is necessary to close a persistent arterial hemorrhage. Suture repairs of bleeding points are more likely if a patch graft has been placed. It is almost never necessary to reapply clamps to the artery if the repair has been properly performed. The repair is then lined with Surgicel, and the three vessels are tested with a handheld Doppler to ensure patency. The retractors are removed and hemostasis is confirmed both along the jugular vein and from the surrounding soft tissues. Persistent oozing is often encountered in these patients who have often received large doses of antiplatelet agents in addition to their intraoperative heparin. A final Doppler check is made, and the wound is closed in layers. The carotid sheath is first closed to provide a barrier against infection, and the platysma is then closed as a separate layer to ensure a good cosmetic result. Either running or interrupted subcuticular stitches may be used to close the skin edges. A Hemovac drain is left inside the carotid sheath. It is removed on the first postoperative day. Patients are continued on aspirin after surgery and are discharged in 1 to 2 days.51–57
We manage any postoperative neurologic deficit, including a transient ischemic attack alone, with immediate assessment of the technical adequacy of repair. If high-quality color duplex ultrasonography is available, this may allow quick documentation of patency and identify any partially obstructing defects. Computed tomography angiography is another excellent and rapid strategy, and these techniques have essentially replaced the rush to catheter angiography. Any occluded carotid artery postoperatively is re-explored and repatched immediately, although since adopting the primary Hemashield patch repair, the incidence of postoperative occlusion has been zero.58
Special Situations
Complete Occlusion
Surgery is generally indicated for cases of acute carotid occlusion if the patient is not so debilitated as to make recovery untenable. Surgery is essentially always indicated for known acute postoperative occlusion, reflecting technical error in most cases. Surgery is sometimes indicated for cases of subacute carotid occlusion and in cases of chronic occlusion if the possibility of a “string sign” minimally patent vessel (which can usually be reopened) exists, justifying exploration. Our surgical technique for complete CCA/ICA occlusion involves opening (or reopening) of the CCA and ICA once the vessels have been controlled. The thrombus is usually seen at the carotid bulb and extending into the distal ICA; in our experience, the ECA is usually patent. Removal of thrombus and associated ICA plaque may establish back-bleeding; if not, the ICA can be explored with a no. 8 feeding tube cut to a 15-cm length and attached to a 10-ml syringe. The tube is advanced into the ICA, and the syringe is drawn back to establish suction, which often will pull down the distal thrombus as the tubing is withdrawn. If this fails, Fogarty catheters are passed into the ICA, but the risk of establishing a carotid-cavernous fistula with these must be considered. If back-bleeding cannot be established, we cleanly ligate the distal and proximal ICA stumps and perform a CCA/ECA endarterectomy and repair. If 6 hours or more have passed since occlusion, the likelihood of successful neurologic salvage is diminished, and the risk of intracerebral hemorrhage appears to increase.59
Stump Syndrome
The term “stump syndrome” describes the continuation of ipsilateral ischemic symptoms after ICA occlusion; the mechanism for this is the retrograde passage of emboli from the ICA intraluminal thrombus that gain entry to the intracranial circulation via the ECA and its collateral blood flow. After strict indications criteria are met,60 surgical correction is undertaken via a standard common to external carotid CEA. After removal of the thrombus from the ICA stump, we attempt to reopen the ICA and establish back-bleeding. If this is not possible (it usually is not), the stump is obliterated with inside-out sutures or with external application of large Weck clips. We stress that the ICA lumen must be obliterated. A standard external CEA is then performed (we place a CCA to ECA patch graft), and the arteriotomy is closed in the usual fashion.
Bilateral Carotid Endarterectomy
Bilateral CEA runs the risk of extreme swings in blood pressure from concurrent denervation of both carotid sinuses61 and from the risk of bilateral cranial nerve injury. For those patients who require bilateral endarterectomy, we recommend a staged procedure, with at least a 6-week window between the procedures. We customarily have laryngoscopy performed by an otolaryngologist to rule out recurrent laryngeal nerve palsy before the second procedure is undertaken. Unilateral nerve dysfunction in the cervical region is troublesome, but a bilateral one can be disabling. We have on occasion deferred the second surgery due to an occult vocal cord paralysis. When this happens, the patient is maintained on medical management until such time as cord function returns (as it usually does), after which the second side CEA is performed.
Plaque Morphology
The correlation of plaque ulceration with ischemic neurologic symptoms and the need for surgery is difficult for several reasons. First, studies have shown poor interobserver variability, either on ultrasound or arteriographic examinations, and poor correlation between pathologic specimens and radiographically demonstrated ulceration. Second, in symptomatic patients, deep ulceration is most commonly found in conjunction with significant degrees of carotid stenosis, and it becomes difficult to separate clinical symptomatology between these two findings.62,63 The most recent data from the North American Symptomatic Carotid Endarterectomy Trial7 show that in medically treated patients with 50% to 99% stenosis (now proven to be unequivocal surgical candidates), the presence of plaque ulceration in conjunction with stenosis significantly increases the risk of stroke.64 Whether the presence of ulceration in plaques of less than 50% linear stenosis significantly increases the risk of stroke remains an unanswered question that has not been addressed by cooperative trial data.
The significance of intraplaque hemorrhage as a predictor of ischemic symptoms is also unclear. Heterogeneous plaque morphology on duplex scanning (consistent with intraplaque hemorrhage) was a significant risk factor for subsequent neurologic events in one study if the underlying stenosis was more than 50%.65 Although one recent review also suggested that intraplaque hemorrhage was found more commonly in patients with symptomatic carotid artery disease,62 other studies suggest that there is a low correlation between ischemic symptoms and plaque hematoma in patients who undergo CEA.66
Concurrent Coronary Artery Disease
Surgical intervention is the most effective treatment option for patients with carotid artery stenosis.67 Alternate treatment modalities have arisen—the most notable being carotid artery stenting (CAS)—offering novel therapeutic options for extracranial carotid stenosis; but the preponderance of data continues to favor CEA as the therapeutic of choice.68–72 Even so, there is a subset of high-risk patients with carotid artery stenosis and significant comorbidities such as poor cardiac function who, in the past, have justified consideration of alternative therapies and/or more measured approaches to CEA. For the most part, these patients still benefit greatly from CEA; but the timing of surgery and the spectrum of attendant risks can vary depending on the patient’s comorbidities.73
Cardiac disease is probably the most common comorbidity that we deal with in planning strategy for carotid patients.74,75 The basic dictum is—fix the offending lesion first.76 In general, patients with symptomatic coronary artery disease (CAD) severe enough to warrant coronary artery bypass grafting (CABG), and surgical but asymptomatic carotid stenosis, should have their CEA delayed until they have had their CABG performed and been allowed adequate time to recover. While true that there appears to be some increase in stroke risk among CABG patients with synchronous carotid disease, the bulk of post-CABG cerebral infarctions (85%) occur in patients with little to no (<50% stenosis bilaterally) carotid disease.77 Likewise, reviews of ischemic patterns in post-CABG patients with carotid artery disease and CVAs have found that 60% to 80% of their infarctions lie in either the posterior circulation or bilateral territories, making their ischemia difficult to attribute to unilateral carotid disease.77,78 Particularly for those patients with unilateral, asymptomatic disease, the risk of post-CABG stroke appears to be exceedingly low (0%–2.9%).79–81 Within this patient population, the risk of myocardial infarction in the perioperative period post-CEA appears to outweigh the potential risk of stroke post-CABG. It has therefore been recommended that surgeons consider delaying CEA for asymptomatic disease in patients with significant cardiac disease until after their cardiac issues have been adequately addressed.
Patients with symptomatic carotid disease are more aggressively managed even in the face of significant CAD. Naylor, et al. noted in a series of 4638 patients undergoing isolated CABG procedures that those with less than 50% carotid stenosis bilaterally had a 1.8% rate of CVAs, those with more than 50% unilateral stenoses had a rate of 3.2%, and those with unilateral carotid occlusions had a rate of 11.6%.77 These data do not distinguish between symptomatic or asymptomatic patients, but imply that the risk of postprocedural cerebral infarction after a CABG becomes unacceptably high (>1%–2%) when carotid disease is compounded by tandem lesions or complete occlusion.82,83 Similarly, some small studies have noted that coronary artery revascularization patients with pre-CABG complaints of strokes or TIAs ipsilateral to known carotid artery disease suffered a roughly sixfold increase in post-CABG strokes compared to patients with carotid artery disease who were asymptomatic or had discordant neurologic symptoms.79 Taken together, these data argue for preemptive CEAs among cardiopaths with preoperative symptomatic carotid disease or marked bilateral stenoses/occlusions.84
Advanced Age
Age over 70 years, is considered by many to be an independent risk factor for carotid reconstruction, and suggestions are made that elderly patients are better treated with CAS-P. Current data do not support this for two reasons: the risk of CEA is probably not significantly higher in the elderly; and the risk of CAS-P clearly is much higher with advancing age, because of the tortuosity of the great vessels and the difficulty of access for endovascular treatment. A review of 53 patients over 70 years old at the University of Iowa Hospital found no differences in CEA outcomes when compared to historic, younger controls.85 Likewise, several prospective and retrospective studies have compared CEA and CAS in the elderly and found that age does not significantly alter CEA results. A meta-analysis of 8 CAS study groups and 33 CEA study groups that selected for patients over 80 years of age identified increased rates of postprocedure stroke among CAS patients, similar to those rates noted in past randomized controlled trials (RCTs). No other differences were seen in periprocedural or long term mortality and myocardial events in these studies.86 Finally, the lead-in data from the credentialing phase of the CREST trial confirm that the risk of CAS-P in patients in their 70s and 80s is unacceptably high compared to routine CEA (stroke/death rate 5.3% for ages 70 to 79 and 12.1% for ages over 80).87 As long as the predicted life expectancy of the patient exceeds 5 years, CEA should continue to be considered as the treatment of choice for extracranial carotid disease exceeding 50% to 60% among the elderly.88,89
Complicated Neck Anatomy
Patients with previous neck surgeries, high carotid bifurcations, irradiated cervical fields, and inextensible necks may be considered by some more efficaciously approached via an endovascular route. Although such anatomic challenges may make for a more difficult dissection, an experienced surgeon offers long-term benefits with CEA for such patients with significant carotid stenosis. A case-controlled trial in 2009 looking at 154 carotid artery stenosis patients prospectively followed and paired CEA and CAS patients. In the final analysis, no difference in periprocedural ischemic events or mortality was noted. A higher rate of postprocedural cranial nerve injuries (5 vs. 1, p = 0.03) did appear in the CEA group, although long-term outcomes did not vary.90 These initial data would give credence to the thought that the “hostile neck” has little bearing on CEA outcomes when a skilled surgeon performs the procedure. In addition, the application of a NIMS endotrachael tube for vocal cord monitoring during the procedure was not reviewed in the above series and may resolve or at least minimize the issue of increased cranial nerve injuries among CEAs performed in “hostile neck” patients. We now use this method in every case.
Intraluminal Thrombi
The problem of surgical timing in patients with angiographically demonstrated propagating intraluminal thrombus remains an open question among cerebrovascular experts.74,75,91–98 In patients who present with a transient ischemic attack (which, in our experience, has always resolved with anticoagulation) and a long intraluminal thrombus (which goes too far up the internal carotid to be exposed and controlled), we have opted for delayed surgery (at 6 weeks after repeat angiography) in every case and have never seen a negative outcome from intercurrent embolization once heparin is instituted. The small “bullet-type” thrombus at the bifurcation, which can be isolated with cross-clamps, is operated without delay.
Likewise, there is a small subset of patients with postoperative neurologic events (most often transient ischemic attacks) after CEA who are found to have a fresh thrombus adherent to the suture line (by angiography), partially occluding the artery, and which is presumably the source of embolic phenomena. If there is no other angiographic evidence of technical inadequacy, we have chosen to manage these patients conservatively as well, with full anticoagulation and 6-week follow-up angiography. In every case, the thrombus has resolved, and there have been no negative neurologic outcomes in our series with this plan of management.93 Despite the surgeon’s natural inclination to fix a problem with bold action, we have found that a measured conservative approach yields good results in cases of fresh or propagating thrombus and in our experience is superior to undertaking a high-risk surgical procedure.
Tandem Lesions of the Carotid Siphon
In the North American Symptomatic Carotid Endarterectomy Trial, symptomatic patients were excluded if the degree of siphon stenosis exceeded that at the carotid bifurcation.7 The presence of stenotic disease at the carotid siphon has been proposed as a contraindication to CEA because of both the inability to pinpoint the symptomatic source and the reputed increased possibilities of postoperative occlusion from decreased carotid flow velocity. This has not been our experience, and we do not hesitate to operate on patients with tandem lesions if we are convinced that an active plaque at the carotid bifurcation is the source of their embolic phenomena.
Intracranial Aneurysm (Silent)
There is always a concern that cervical carotid revascularization (for either symptomatic or asymptomatic carotid stenosis, especially high grade) will lead to rupture of a known intracranial aneurysm when both lesions are present. Although this is no doubt a small risk, several articles have shown that it is safe to proceed with a CEA with a silent intracranial aneurysm discovered on angiography.94,95 Obviously, the symptomatic lesion should be treated first. We do not hesitate to operate in the light of an asymptomatic intracranial aneurysm, but we do customarily recommend subsequent craniotomy and aneurysm clipping as well.
Recurrent Stenosis
There is a quantifiable incidence of recurrent carotid stenosis after primary CEA. We have seen a decrease in our restenosis rate (to zero) after adopting patch graft angioplasty in all our cases. Piepgras and colleagues96 show a symptomatic restenosis rate of 1% with an asymptomatic restenosis rate of 4% to 5% at 2-year follow-up using a patch graft. Aside from technical inadequacies, it has been difficult to identify risk factors associated with recurrent carotid stenosis, although continuation of smoking habits after endarterectomy has proved to be a significant risk factor in several studies,97,98 whereas hypertension, diabetes mellitus, family history, lipid studies, aspirin use, and coronary disease may not be as important.
Carotid Endarterectomy versus Carotid Artery Stenting
There have been, to date, over 11 RCTs comparing CEA and CAS.99–110 The results have roundly failed to validate CAS as a noninferior treatment for carotid artery stenosis. More to the point, several have found CAS to bear a much higher risk of subacute CVAs than carotid surgery. In 1998, the Leicester trial, although limited by a small sample size, found a 45% increase in periprocedure CVAs among CAS patients compared to CEA patients; and, in 2001, the WALLSTENT multicenter RCT demonstrated an alarming rate of ipsilateral strokes and deaths among CAS patients (CAS 12.1% vs. CEA 3.6%).106,108 These results have been cause for much concern, but supporters of CAS have often only responded by citing the nascent understanding of CAS and lack of experience with the procedure during these early studies.
As such, a number of RCTs since WALLSTENT have attempted to reverse the stigma of 30-day and 1-year infarcts from carotid stenting. Largely, they failed. The SAPPHIRE trial, released in 2004, stands as the lone exception. It showed in a group of 334 randomized patients a 24.6% and 30.3% stroke rate between CAS and CEA. The difference was not statistically significant. We note, however, that there were an enormous number of patients (415 of the original 741 patients), nearly all stented, who were treated outside of the trial, which always introduces the specter of selection bias against surgery. In addition, the SAPPHIRE results claiming equipoise, unfortunately, have not been reproducible in subsequent, larger RCTs.109,110
In the final analysis, the authors of CREST noted no difference in primary endpoints between the two treatment arms (7.2% CAS versus 6.8% CEA; 95% CI, 0.81 to 1.51; p = 0.51). Though these results have been lifted as evidence of equipoise between CAS and CEA, the data underpinning these conglomerate endpoint data leads to a different interpretation. Looking at stroke and myocardial infarction independently, the two largest endpoints comprising CREST’s general endpoint, the study found an elevated periprocedural stroke rate among CAS patients (4.1% CAS versus 2.3%, p = 0.012), on par with previous studies, and an elevated myocardial infarction rate among CEA patients (1.1 CAS versus 2.3%, p = 0.032). Looking at quality of life measures recorded over the study’s 4-year follow-up (Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36)), myocardial infarctions had no demonstrable effect on patients’ quality of life, while periprocedural strokes did. Further, the rate of major ipsilateral stokes among CAS-treated patients, despite the use of an embolic protection device, ran nearly double that of the CEA patients (15.2% CAS versus 8.0% CEA).111
Summary
Multicenter trials have advocated surgical therapy over medical management in the cases of symptomatic patients with more than 50% stenosis, and asymptomatic patients with more than 60% stenosis. We perform the operation using a 3.5× loupe-magnified technique. Universal patch grafting has essentially eliminated the problem of acute postoperative thrombosis or rapid restenosis. We prefer general anesthesia with full-channel EEG and concurrent SSEP monitoring. We perform selective shunting based on monitoring criteria. This has resulted in an approximately 15% shunt placement rate in our experience. We do not reverse heparinization with protamine after the operation. We only place tacking sutures in approximately 10% of cases due to the use of fine scissors to trim the plaque cleanly in the ICA as it is removed. We emphasize meticulous hemostasis in our technique.
Alberts M.J., McCann R., Smith T.P., et al. A randomized trial of carotid stenting versus endarterectomy in patients with symptomatic carotid stenosis: study design. J Neurovasc Dis. 1997;2:228-234.
Bailes J., Spetzler R.F. Microsurgical Carotid Endarterectomy. New York: Lippincott-Raven; 1996.
Brooks W.H., McClure R.R., Jones M.R., et al. Carotid angioplasty and stenting versus carotid endarterectomy: randomized trial in a community hospital. J Am Coll Cardiol. 2001;38:1589-1595.
Eliasziw M., Streifler J.W., Fox A.J., et al. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. Stroke. 1994;25:304-308.
Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid stenosis. JAMA. 1995;273:1421-1428.
Fields W.S., Maslenikov V., Meyer J.S., et al. Joint study of extracranial arterial occlusion. V: progress report of prognosis following surgery or nonsurgical treatment for transient cerebral ischemic attacks and cervical carotid artery lesions. JAMA. 1970;211:1993-2003.
Heros R.C. Carotid endarterectomy in patients with intraluminal thrombus. Stroke. 1990;19:667-668.
Honeycutt J.H., Loftus C.M. Carotid stump syndromes and external revascularization. In: Loftus C.M., Kresowik T.F. Textbook of Carotid Artery Surgery. New York: Thieme; 2000:315-320.
Loftus C.M. Carotid Artery Surgery: Principles and Technique, 2nd ed. New York: Informa Publishing; 2006.
Loftus C.M. Carotid endarterectomy: the asymptomatic carotid. In: Batjer H.H., editor. Cerebrovascular Disease. New York: Lippincott-Raven; 1996:409-420.
Loftus C.M. Carotid Endarterectomy: Principles and Technique. St. Louis: Quality Medical Publishing; 1995.
Loftus C.M. Concomitant carotid and coronary disease. Patient Care. 1992;15:49-66.
Loftus C.M. Propagating intraluminal carotid thrombus: surgery or anticoagulation? In: Loftus C.M., Kresowik T.F. Carotid Artery Surgery. New York: Thieme; 2000:321-327.
Loftus C.M. Surgical management options to prevent ischemic stroke. Neurosurg Q. 1994;4:1-38.
Loftus C.M. Technical aspects of carotid endarterectomy with hemashield patch graft. Neurol Med Chir (Tokyo). 1997;37:805-818.
Loftus C.M. Technical fundamentals, monitoring, and shunt use during carotid endarterectomy. Tech Neurosurg. 1997;3:16-24.
Loftus C.M., Kresowik T.F. Anatomic basis and technique of carotid endarterectomy. In: Loftus C.M., Kresowik T.F. Carotid Artery Surgery. New York: Thieme Medical Publishers; 2000:245-256.
Naylor A.R., Bolia A., Abbott R.J., et al. Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: a stopped trial. J Vasc Surg. 1998;28:326-334.
Naylor A.R., Mehta Z., Rothwell P.M., Bell P.R.F. Stroke during coronary artery bypass surgery: a critical review of the role of carotid artery disease. Eur J Vasc Endovasc Surg. 2002;23:283-294.
North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445-453.
Schiro J., Mertz G.H., Cannon J.A., et al. Routine use of a shunt for carotid endarterectomy. Am J Surg. 1981;142:735-738.
Solomon R.A., Loftus C.M., Quest D.O., et al. Incidence and etiology of intracerebral hemorrhage following carotid endarterectomy. J Neurosurgery. 1986;64:29-34.
Sundt T.M. The ischemic tolerance of neural tissue and the need for monitoring and selective shunting during carotid endarterectomy. Stroke. 1983;14:93-98.
Yadav J.S., Wholey M.H., Kuntz R.E., et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493-1501.
1. Eastcott H.H.G., Pickering G.W., Rob C.G. Reconstruction of internal carotid artery in a patient with intermittent attacks of hemiplegia. Lancet. 1954;2:994-996.
2. Loftus C.M. Surgical management options to prevent ischemic stroke. Neurosurg Q. 1994;4:1-38.
3. Fields W.S., Maslenikov V., Meyer J.S., et al. Joint study of extracranial arterial occlusion. V: progress report of prognosis following surgery or nonsurgical treatment for transient cerebral ischemic attacks and cervical carotid artery lesions. JAMA. 1970;211:1993-2003.
4. Shaw D.A., Venables G.S., Cartlidge N.E.F., et al. Carotid endarterectomy in patients with transient cerebral ischaemia. J Neurol Sci. 1984;64:45-53.
5. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. Endarterectomy for asymptomatic carotid stenosis. JAMA. 1995;273:1421-1428.
6. MRC European Carotid Surgery Trial. Interim results for symptomatic patients with severe (70-99%) or with mild (0-29%) carotid stenosis. Lancet. 1991;337:1235-1243.
7. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high grade stenosis. N Engl J Med. 1991;325:445-453.
8. Loftus C.M. Carotid endarterectomy: the asymptomatic carotid. In: Batjer H.H., editor. Cerebrovascular Disease. New York: Lippincott-Raven; 1996:409-420.
9. Bailes J., Spetzler R.F. Microsurgical Carotid Endarterectomy. New York: Lippincott-Raven; 1996.
10. Spetzler R.F., Martin N., Hadley M.N., et al. Microsurgical endarterectomy under barbiturate protection: a prospective study. J Neurosurg. 1986;65:63-73.
11. Steiger H.J., Schaffler L., Liechti S. Results of microsurgical carotid endarterectomy. Acta Neurochir (Wien). 1989;100:31-38.
12. Wellman B.J., Loftus C.M., Kresowik T., et al. The differences in electroencephalographic changes in awake versus anesthetized carotid endarterectomy patients. Paper presented at the AANS/CNS Joint Section on Cerebrovascular Surgery. 2nd Annual Meeting. February 4-6, 1997. Anaheim, CA
13. Loftus C.M. Anesthesia for carotid endarterectomy: general vs. local? In: Bederson J.B., Tuhrim S. Treatment of Carotid Disease: a Practitioner’s Manual. Park Ridge, IL: AANS Publications; 1998:181-190.
14. Gelb A.W. Anesthetic considerations for carotid endarterectomy. Int Anesthesiol Clin. 1984;22:153-164.
15. Baker W.H., Rodman J.A., Barnes R.W., et al. An evaluation of hypocarbia and hypercarbia during carotid endarterectomy. Stroke. 1976;7:451-454.
16. Boysen G., Ladegaard-Pedersen H.G., Henriksen H., et al. The effect of PaCO2 on regional cerebral blood flow and internal carotid arterial pressure during carotid clamping. Anesthesiology. 1971;35:286-300.
17. Fourcade H.E., Larson C.P., Ehrenfeld W.K., et al. The effects of CO2 and systemic hypertension on cerebral perfusion pressure during carotid endarterectomy. Anesthesiology. 1970;33:383-390.
18. Pistolese G.R., Citone G., Faragilia V., et al. Effects of hypercapnia on cerebral blood flow during the clamping of the carotid arteries in surgical management of cerebrovascular insufficiency. Neurology. 1971;21:95-100.
19. Gross C.E., Bednar M.M., Lew S.M., et al. Preoperative volume expansion improves tolerance to carotid artery cross-clamping during endarterectomy. Neurosurgery. 1998;43:222-228.
20. Loftus C.M. Monitoring during extracranial carotid reconstruction. In: Loftus C.M., Traynelis V.C. Intraoperative Monitoring Techniques in Neurosurgery. New York: McGraw-Hill; 1994:3-8.
21. Loftus C.M. Technical fundamentals, monitoring, and shunt use during carotid endarterectomy. Tech Neurosurg. 1997;3:16-24.
22. Loftus C.M. Overview of shunt controversy. In: Loftus C.M., Kresowik T.F. Carotid Artery Surgery. New York: Thieme; 2000:409-419.
23. Loftus C.M. Overview of shunt controversy. In: Loftus C.M., Kresowik T.F. Carotid Artery Surgery. New York: Thieme Medical Publishers; 2000:409-420.
24. Loftus C.M. Design characteristics and clinical of a newly designed carotid artery shunt. Neurol Res. 2000;22:443-448.
25. Benoit B.G., Navavi N.L. The “routine” use of intraluminal shunting in carotid endarterectomy. Can J Neurol Sci. 1978;5:339.
26. Gianotta S.L., Dicks R.E., Kindt G.W. Carotid endarterectomy: technical improvements. Neurosurgery. 1980;7:309-312.
27. Javid H., Julian O.C., Dye W.S., et al. Seventeen-year experience with routine shunting in carotid artery surgery. World J Surg. 1979;3:167-177.
28. Patterson R.H. Technique of carotid endarterectomy. In: Smith R.R., editor. Stroke and the Extracranial Vessels. New York: Raven Press; 1984:177-185.
29. Schiro J., Mertz G.H., Cannon J.A., et al. Routine use of a shunt for carotid endarterectomy. Am J Surg. 1981;142:735-738.
30. Thompson J.E. Protection of the brain during carotid endarterectomy. Int Anesthesiol Clin. 1984;22:123-128.
31. Prioleau W.H., Aiken A.F., Hairston P. Carotid endarterectomy: neurologic complications as related to surgical techniques. Ann Surg. 1977;185:678-683.
32. Loftus C.M., Dyste G.N., Reinarz S.J., et al. Cervical carotid dissection following carotid endarterectomy: a complication of indwelling shunt? Neurosurgery. 1986;19:441-445.
33. Allen G.S., Preziosi T.J. Carotid endarterectomy: a prospective study of its efficacy and safety. Medicine (Baltimore). 1981;60:298-309.
34. Baker W.H., Dorner D.B., Barnes R.W. Carotid endarterectomy: is an indwelling shunt necessary? Surgery. 1977;82:321-326.
35. Bland J.E., Lazar M.L. Carotid endarterectomy without shunt. Neurosurgery. 1981;8:153-157.
36. Ferguson G.G. Carotid endarterectomy: indications and surgical technique. Int Anesthesiol Clin. 1984;22:113-121.
37. Ferguson G.G. Carotid endarterectomy: to shunt or not to shunt? Arch Neurol. 1986;43:615-617.
38. Poisik A., Heyer E.J., Solomon R.A., et al. Safety and efficacy of fixed-dose heparin in carotid endarterectomy. Neurosurgery. 1999;45:434.
39. Ferguson G.G. Extracranial carotid artery surgery. Clin Neurosurg. 1982;29:543-574.
40. Ferguson G.G. Intra-operative monitoring and internal shunt: are they necessary in carotid endarterectomy? Stroke. 1982;13:287-289.
41. Ferguson G.G. Shunt almost never. Int Anesthesiol Clin. 1984;22:147-152.
42. Messick J.M., Sharbrough F., Sundt T. Selective shunting on the basis of EEG and regional CBF monitoring during carotid endarterectomy. Int Anesthesiol Clin. 1984;22:137-145.
43. Sundt T.M. The ischemic tolerance of neural tissue and the need for monitoring and selective shunting during carotid endarterectomy. Stroke. 1983;14:93-98.
44. Moore W.S., Yee J.M., Hall A.D. Collateral cerebral blood pressure: an index to tolerance to temporary carotid occlusion. Arch Surg. 1973;106:520-523.
45. Rosenthal D., Stanton P.E., Lamis P.A. Carotid endarterectomy: the unreliability of intraoperative monitoring in patients having had a stroke or reversible ischemic neurological deficit. Arch Surg. 1981;116:1569-1575.
46. Loftus C.M. Technical aspects of carotid endarterectomy with hemashield patch graft. Neurol Med Chir (Tokyo). 1997;37:805-818.
47. Honeycutt J.H., Loftus C.M. Carotid endarterectomy: general principles and surgical technique. Neurosurg Clin North Am. 2000;11:279-297.
48. Loftus C.M., Kresowik T.F. Anatomic basis and technique of carotid endarterectomy. In: Loftus C.M., Kresowik T.F. Carotid Artery Surgery. New York: Thieme Medical Publishers; 2000:245-256.
49. Loftus C.M., Robertson S.C. Technique of carotid endarterectomy with special emphasis on Hemashield patch graft technique. In: Proceedings of the 11th International Congress of Neurological Surgery. Bologna, Italy: Monduzzi Editore; 1997:1049-1057.
50. Findlay J.M. Carotid microendarterectomy. Neurosurgery. 1993;32:792-798.
51. Baron E.M., Baty D.E., Loftus C.M. Carotid endarterectomy. In: Connolly E.S., McKhann G.M., Huang J., Choudri R.F., Mocco J. Fundamentals of Operative Techniques in Neurosurgery. New York: Thieme Medical Publishing, 2007.
52. Loftus C.M. Carotid Artery Surgery: Principles and Technique, 2nd ed. New York: Informa Publishing; 2006.
53. Loftus C.M. Carotid Endarterectomy. Tech Neurosurg. 1997;3:1-84.
54. Loftus C.M. Carotid endarterectomy: how the operation is done. Clin Neurosurg. 1997;44:243-265.
55. Loftus C.M. Technique of carotid endarterectomy. Contemp Neurosurg. 1988;10:1-6.
56. Loftus C.M., Quest D.O. Technical issues in carotid surgery 1995. Neurosurgery. 1995;36:629-647.
57. Honeycutt J.H., Loftus C.M. Carotid endarterectomy: general principles and surgical technique. Neurosurg Clin North Am. 2000;11:279-297.
58. Loftus C.M. Carotid Endarterectomy: Principles and Technique. St. Louis: Quality Medical Publishing; 1995.
59. Solomon R.A., Loftus C.M., Quest D.O., et al. Incidence and etiology of intracerebral hemorrhage following carotid endarterectomy. J Neurosurg. 1986;64:29-34.
60. Honeycutt J.H., Loftus C.M. Carotid stump syndromes and external revascularization. In: Loftus C.M., Kresowik T.F. Textbook of Carotid Artery Surgery. New York: Thieme; 2000:315-320.
61. Wade J.G., Larson C.P., Hickey R.F., et al. Effect of carotid endarterectomy on carotid chemoreceptor and baroreceptor function in man. N Engl J Med. 1970;282:F823-F829.
62. Gomez C.R. Carotid plaque morphology and risk for stroke. Stroke. 1990;21:148-151.
63. Wechsler L.R. Ulceration and carotid artery disease. Stroke. 1998;19:650-653.
64. Eliasziw M., Streifler J.W., Fox A.J., et al. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. Stroke. 1994;25:304-308.
65. Sterpetti A.V., Schultz R.D., Feldhaus R.J., et al. Ultrasonographic features of carotid plaque and the risk of subsequent neurologic deficits. Surgery. 1988;104:652-660.
66. Lennihan L., Kupsky W.J., Mohr J.P., et al. Lack of association between carotid plaque hematoma and ischemic cerebral symptoms. Stroke. 1987;18:879-881.
67. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325:445-453.
68. Naylor A.R., Bolia A., Abbott R.J., et al. Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: a stopped trial. J Vasc Surg. 1998;28:326-334.
69. Brooks W.H., McClure R.R., Jones M.R., et al. Carotid angioplasty and stenting versus carotid endarterectomy: randomized trial in a community hospital. J Am Coll Cardiol. 2001;38:1589-1595.
70. Alberts M.J., McCann R., Smith T.P., et al. A randomized trial of carotid stenting versus endarterectomy in patients with symptomatic carotid stenosis: study design. J Neurovasc Dis. 1997;2:228-234.
71. Yadav J.S., Wholey M.H., Kuntz R.E., et al. Protected carotid-artery stenting versus endarterectomy in high risk patients. N Engl J Med. 2004;351:1493-1501.
72. Gurm H.S., Yadav J.S., Fayad P., et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572-1579.
73. Snell B.E., Loftus C.M. Carotid endarterectomy. Part II: surgical technique and management of concurrent vascular diseases. Contemp Neurosurg. 2003;25:1-6.
74. Gerraty R.P., Gates P.C., Doyle J.C. Carotid stenosis and perioperative stroke risk in symptomatic and asymptomatic patients undergoing vascular or coronary surgery. Stroke. 1993;24:1115-1118.
75. Newman D.C., Hicks R.G. Combined carotid and coronary artery surgery: a review of the literature. Ann Thorac Surg. 1988;45:574-581.
76. Loftus C.M. Concomitant carotid and coronary disease. Patient Care. 1992;15:49-66.
77. Naylor A.R., Mehta Z., Rothwell P.M., Bell P.R.F. Stroke during coronary artery bypass surgery: a critical review of the role of carotid artery disease. Eur J Vasc Endovasc Surg. 2002;23:283-294.
78. Barbut D., Grassineau D., Lis E., et al. Posterior distribution of infarcts in strokes related to cardiac operations. Ann Thorac Surg. 1998;65:1656-1659.
79. D’Agostino R.S., Svenssson L.G., Neumann D.J., et al. Screening carotid ultrasonography and risk factors for stroke in coronary artery surgery patients. Ann Thorac Surg. 1996;62:1714-1723.
80. Nakamura M., Okamoto F., Nakanishi K., et al. Does intensive management of cerebral haemodyamics and atheromatous aorta reduce stroke after coronary artery surgery? Ann Thorac Surg. 2008;85:513-519.
81. Gosh J., Murray D., Khwaja N., et al. The influence of asymptomatic significant carotid disease on mortality and morbidity in patients undergoing coronary artery bypass surgery. Eur J Vasc Endovasc Surg. 2005;23:88-90.
82. Schwartz L.B., Bridgman A.H., Kieffer R.W. Asymptomatic carotid artery stenosis and stroke in patients undergoing cardiopulmonary bypass. J Vasc Surg. 1995;21:146-153.
83. Salasidis G.C., Latter D.A., Steinmetz O.K., et al. Carotid duplex scanning in pre-operative assessment for coronary artery revascularisation: the association between peripheral vascular disease, carotid artery stenosis and stroke. J Vasc Surg. 1995;21:154-162.
84. Jones R.H., Loftus C.M., Sheldon W.C., et al. Concomitant carotid and coronary disease. Patient Care. 1992;15:49-66.
85. Loftus C.M., Biller J., Godersky J.C., et al. Carotid endarterectomy in symptomatic elderly patients. Neurosurgery. 1988;22:676-680.
86. Usman A.A., Tang G.L., Eskandari M.K. Metaanalysis of procedural stroke and death among octogenarians: carotid stenting versus carotid endarterectomy. J Am Coll Surg. 2009;208(6):1124-1131.
87. Hobson R.W., Howard V.J., Roubin G.S., et al. Carotid artery stenting is associated with increased complications in octogenarians: 30-day stroke and death rates in the CREST lead-in phase. J Vasc Surg. 2004;40:1106-1111.
88. Loftus C.M. High risk carotid endarterectomy and high-risk carotid surgery: is surgery or stenting the best choice? In: Watanabe K., editor. Developments in Neuroscience. Excerpta Medica International Congress Series 1247. Amsterdam: Elsevier; 2002:319-326.
89. Loftus C.M. Occlusive cerebrovascular disease in geriatric patients. In: Selman W.R., editor. Geriatric Neurosurgery. Park Ridge, IL: AANS Publications; 1999:103-111.
90. Frego M., Bridda A., Ruffolo C., et al. The hostile neck does not increase the risk of carotid endarterectomy. J Vasc Surg. 2009;50:40-47.
91. Biller J., Adams H.P., Boarini D., et al. Intraluminal clot of the carotid artery. Surg Neurol. 1986;25:467-477.
92. Heros R.C. Carotid endarterectomy in patients with intraluminal thrombus. Stroke. 1990;19:667-668.
93. Loftus C.M. Propagating intraluminal carotid thrombus: surgery or anticoagulation? In: Loftus C.M., Kresowik T.F. Carotid Artery Surgery. New York: Thieme; 2000:321-327.
94. Ladowski J.S., Webster M.W., Yonas H.O., et al. Carotid endarterectomy in patients with asymptomatic intracranial aneurysms. Ann Surg. 1984;200:70-73.
95. Stern J., Whelan M., Brisman R., et al. Management of extracranial carotid stenosis and intracranial aneurysms. J Neurosurg. 1979;51:147-150.
96. Piepgras D.G., Sundt T.M., Marsh W.R., et al. Recurrent carotid stenosis: results and complications of 57 operations. In: Sundt T.M., editor. Occlusive Cerebrovascular Disease: Diagnosis and Surgical Management. Philadelphia: WB Saunders; 1987:286-297.
97. Clagett G., Rich N., McDonald P., et al. Etiologic factors for recurrent carotid artery stenosis. Surgery. 1983;2:313-318.
98. Dempsey R.J., Moore R., Cordero S. Factors leading to early reoccurrence of carotid plaque after carotid endarterectomy. Surg Neurol. 1995;43:278-283.
99. Halliday A., Mansfield A., Marro J., et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2005;363:1491-1502.
100. Halliday A.W., Thomas D., Mansfield A. The Asymptomatic Carotid Surgery Trial (ACST). Rationale and design. Steering Committee. Eur J Vasc Surg. 1994;8:703-710.
101. Mas J.L., Chatellier G., Beyssen B., et al. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660-1671.
102. Ringleb P.A., Allenberg J., Bruckmann H., et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239-1247.
103. CAVATAS Investigators. Endovascular versus surgical treatment in patients with carotid stenosis in the Carotid and Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomised trial. Lancet. 2001;357:1729-1737.
104. CARESS Steering Committee. Carotid Revascularization Using Endarterectomy or Stenting Systems (CARESS): phase I clinical trial. J Endovasc Ther. 2003;10:1021-1030.
105. SPACE Collaborative Group, Ringleb P.A., Allenberg J., et al. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomized non-inferiority trial. Lancet. 2006;368:1239-1247.
106. Naylor A.R., Bolia A., Abbott R.J., et al. Randomized study of carotid angioplasty and stenting versus carotid endarterectomy: a stopped trial. J Vasc Surg. 1998;28:326-334.
107. Brooks W.H., McClure R.R., Jones M.R., et al. Carotid angioplasty and stenting versus carotid endarterectomy: randomized trial in a community hospital. J Am Coll Cardiol. 2001;38:1589-1595.
108. Alberts M.J., McCann R., Smith T.P., et al. A randomized trial of carotid stenting versus endarterectomy in patients with symptomatic carotid stenosis: study design. J Neurovasc Dis. 1997;2:228-234.
109. Yadav J.S., Wholey M.H., Kuntz R.E., et al. Protected carotid-artery stenting versus endarterectomy in high risk patients. N Engl J Med. 2004;351:1493-1501.
110. Gurm H.S., Yadav J.S., Faya P., et al. Long-term results of carotid stenting versus endarterectomy in high-risk patients. N Engl J Med. 2008;358:1572-1579.
111. Mantese V.A., Timaran C.H., Chiu D., et al. The carotid revascularization endarterectomy versus stenting trial (CREST). Stroke. 2010;41:S31-S34.
112. Mattos M.A., Shamma A.R., Rossi N., et al. Recurrent cartoid stenosis: is duplex follow-up cost effective in the first postoperative year? Am J Surg. 1988;156:91-95.