Chapter 70 Surgical Management of Cerebellar Stroke—Hemorrhage and Infarction
Relevant Anatomy
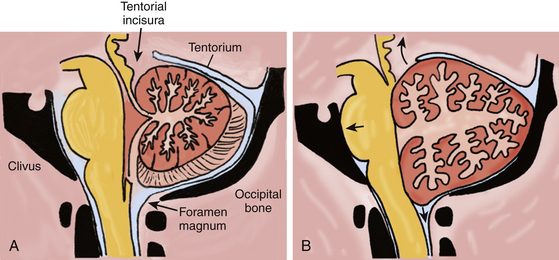
Composed of two hemispheres and a midline vermis, the cerebellum sits in the posterior fossa dorsal to the brain stem, in a space constrained by three surfaces: (1) the tentorium superiorly, (2) the skull base formed by the petrous bone and clivus ventrally, and (3) the suboccipital skull convexity surface dorsally and inferiorly (Fig. 70-1A). The posterior fossa communicates with the supratentorial space via the tentorial incisura, through which passes the upper brain stem (midbrain); and it communicates with the spinal canal via the foramen magnum, through which passes the lower brain stem (medulla) and upper cervical spinal cord.
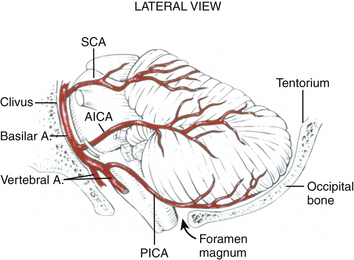
Blood is supplied to the cerebellum via three pairs of arteries from the vertebrobasilar system, each of which originates ventrally in the posterior fossa, and must encircle (and supply) the appropriate brain-stem region to reach the cerebellum (Fig. 70-2). These three trunks include: (1) the posterior inferior cerebellar artery (PICA), which originates from the vertebral artery 1 to 3 cm proximal to the vertebrobasilar junction and supplies the lateral medulla, inferior vermis, and posterior-inferior cerebellar surface, (2) the anterior inferior cerebellar artery (AICA), which usually originates from the inferior third of the basilar artery, and supplies the caudal pons and the petrosal surface of the cerebellum, and (3) the superior cerebellar artery (SCA), which supplies the caudal midbrain/rostral pons, the superior cerebellar peduncle and dentate nucleus, the superior vermis and the tentorial surface of the cerebellum.
Pathogenesis of Cerebellar Hemorrhage and Infarction
The infratentorial compartment, or posterior fossa, is approximately one-eighth of the entire intracranial space.1 Based on MRI volumetric analysis, the volume of the posterior fossa is approximately 200 cc in men and slightly less in women.2,3 The three components of that volume are brain parenchyma (80%), circulating blood (10%), and CSF (10%).4 An expanded cerebellum associated with a clot or edema can directly compress the brain stem against the clivus, obliterating the subarachnoid cisterns. A mass lesion in the cerebellum, such as a hematoma or edematous infarct, can also cause effacement of either the fourth ventricle or the aqueduct, and lead to obstructive hydrocephalus.
If we estimate 18-20 cc of CSF in the posterior fossa, an infarct or hematoma adding 18 cc of mass to the posterior fossa is sufficient to displace the entire volume of CSF in this compartment. After the CSF reserve space is obliterated, any further enlargement of the hematoma or edema of the surrounding brain and/or infarction reduces the volume of circulating blood, and begins to force brain parenchyma out of the posterior fossa through the tentorial incisura or the foramen magnum (Fig. 70-1B). Given that the volume of a sphere is 4/3×πr3 (where r is the radius), an 18-cc spherical hematoma with a diameter of 3.2 cm (radius 1.6 cm) correlates with the popular assertion that a 3-cm cerebellar hematoma usually warrants urgent surgical evacuation. The causes of cerebellar infarction and hemorrhage are outlined in Table 70-1.
| Cerebellar Hemorrhage | Cerebellar Infarction |
|---|---|
| Hypertension | Vertebral artery atherosclerosis |
| Coagulopathy | Cardioembolism |
| Amyloid angiopathy | Artery to artery embolism |
| Hemorrhagic transformation of an infarct | Patent foramen ovale |
| Tumor | Vertebral artery dissection |
| Arteriovenous malformation | Hypercoagulable states |
| Cavernous malformation | Vasculitis |
| Supratentorial surgery with cerebral spinal fluid drainage | Venous sinus thrombosis |
| Spinal surgery with durotomy | Cocaine use |
Hemorrhage
Hypertension is the most common identifiable risk factor for cerebellar hemorrhage, occurring in 36% to 89% of cases in various series.5 Hematomas occur in the cerebellar hemisphere and vermis with roughly equal frequency, and may extend into the ventricular system. The SCA and AICA territories are more often involved, particularly in the region of the dentate nucleus, which is supplied by perforating arteries from the SCA.6 A few cases of microaneurysms have been reported in histopathologic studies of perihemorrhagic tissue.7
Less frequent causes of cerebellar hemorrhage are coagulopathy (typically warfarin use), amyloid angiopathy, hemorrhagic transformation of an infarct, tumors, and vascular malformations.8–10 Remote cerebellar hemorrhage related to the release of large volumes of CSF intra- or peri-operatively has been well described in the literature as an unexpected outcome of supratentorial or spinal surgery.11–14
Infarction
The most common cause of cerebellar infarction in older patients is vertebral artery atherosclerosis, which may be intracranial, extracranial, or both. The PICA distribution is the most likely territory involved clinically. Cardiac and artery-to-artery embolization is also an important cause, and may explain “nonterritorial” infarcts that involve smaller regions of the cerebellum.15 In patients under age 40, patent foramen ovale is an important consideration.16 Vertebral artery dissection is another important cause, with or without a history of trauma, chiropractic manipulation, or prolonged neck hyperextension (“beauty parlor stroke”). Most infarcts caused by vertebral artery dissection also occur in the PICA territory.17
Less common disorders associated with cerebellar infarction include hypercoagulable states, vasculitis, venous sinus thrombosis, and cocaine use.16 Such infarcts may involve the typical PICA, SCA, or AICA territories, watershed regions, or smaller, nonterritorial areas.15
Clinical Manifestations
While cerebellar stroke is the most worrisome cause of dizziness, nausea, and gait instability, the same constellation of symptoms may result from a peripheral disorder, such as vestibular neuritis or labyrinthitis. Furthermore, the clinical presentation of cerebellar hemorrhage is not reliably distinct from that of cerebellar infarction. In Raco’s series comparing 70 patients with cerebellar hemorrhage and 52 patients with cerebellar infarction, both entities commonly presented with the acute onset of nausea, vomiting, dizziness, and unsteady gait. Headache was more common as an initial symptom in cerebellar hemorrhage.6 Dizziness may occur with or without vertigo.16 On physical exam, dysarthria, ataxia, and nystagmus are common. Ataxia may be appendicular, axial or both, depending on the relative involvement of the cerebellar hemispheres and vermis. Nystagmus and/or hearing loss may be a sign of cerebellar stroke or of a peripheral vestibular disorder.16
Posterior circulation strokes, particularly infarction, often involve both the cerebellum and the brain stem. In such situations, various brain-stem stroke syndromes are evident in combination with the manifestations of cerebellar dysfunction. Depending on the level affected, these syndromes may include ipsilateral hemifacial analgesia, contralateral hemibody analgesia, Horner’s syndrome, motor deficits, and cranial neuropathies (Table 70-2). The neurologic deficit often indicates the arterial territory affected by the infarct, but anatomic variations are common between AICA and PICA, and the territory affected can vary considerably.
| Infarct Territory | Clinical Features |
|---|---|
| Posterior inferior cerebellar artery (lateral medullary syndrome or Wallenberg’s syndrome) |
Distinguishing the signs of primary brain-stem stroke from those of secondary brain-stem compression is extremely important. Direct brain-stem compression can manifest either as gaze restrictions, lower cranial nerve dysfunction, or altered sensorium from suppression of the reticular activating system. After the early symptoms and signs of the infarction are defined, delayed neurologic deterioration 1 to 7 days following symptom onset is often indicative of cerebellar swelling and impending upward (transtentorial) or downward (tonsilar) herniation.18–23 Hydrocephalus may further contribute to the decline. Certainly, any change in sensorium warrants urgent surgical intervention, as the patient can quickly proceed through the later stages of brain-stem compression, which include coma, abnormal respirations, posturing, and death.
Radiologic Evaluation
Several CT criteria have been correlated with neurologic deterioration and the need for surgical intervention. The concept of a “tight posterior fossa” was described by Weisberg in a series of 14 patients with cerebellar hemorrhage, all of whom had effacement of the basal cisterns and obstructive hydrocephalus. Nonsurgical management resulted in 100% mortality; of the eight patients who underwent craniectomy and hematoma evacuation, all survived and six became ambulatory.24 Taneda and colleagues correlated the degree of quadrigeminal cistern effacement with patient outcomes.25 Many surgeons consider a hematoma diameter of greater than 3 cm to be an indication for surgery.26–28 Another treatment algorithm stresses the importance of fourth ventricular compression over hematoma size.5
Initial Management
An acute cerebellar hemorrhage or infarct requires neurosurgical evaluation. A patient with an acute cerebellar stroke without hydrocephalus or mass effect should be admitted for observation and continued evaluation, generally in a neurologic ICU. Serial neurologic exams and brain imaging are complementary. For ischemic stroke, antiplatelet and thrombolytic therapy may be given in accordance with American Heart Association guidelines, but should be avoided if surgical intervention is likely.30
In cerebellar hemorrhage, follow-up CT scans should be done daily (or more often in unstable patients) for the first several days following the ictal event, to evaluate for edema progression and/or rehemorrhage. Cerebellar infarction can be expected to increase in volume, with the first radiographic signs of mass effect developing 1 to 6 days after onset. Patients with progressive neurologic deterioration typically have characteristic findings over time on CT scanning, including fourth ventricular shift, basal cistern compression, brain-stem deformity, and hydrocephalus.29
Indications and Timing for Surgical Intervention
Hemorrhage
There is now general consensus that in cases of cerebellar hemorrhage with a tight posterior fossa, early surgery reduces mortality and can have good outcomes.5,24,25,31–34 This group of patients has a high mortality when the hematoma is not evacuated, whether or not a ventriculostomy is performed.31,35 While a hematoma diameter of 3 cm or larger is a popular indication for surgery, any cerebellar hemorrhage causing obstructive hydrocephalus, compression of the brain stem, or effacement of the cisterns should be a potential candidate for emergent surgical decompression and clot evacuation.
Infarction
The surgical management of cerebellar infarction has been the subject of debate. Cerebellar infarction, or “softening” as it was called, was first recognized as a neurosurgical emergency by Fairburn and Oliver in 1956.36 It is now commonly agreed that surgical decompression can be lifesaving. Nevertheless, some surgeons favor a “gradual” approach to the treatment of massive infarction, beginning with ventriculostomy. If neurologic deficits progress despite this measure, these surgeons would proceed with craniectomy.6,37 Several series describe a subgroup of patients with cerebellar infarcts who were treated with ventriculostomy alone.38–40 Of those initially treated with ventriculostomy, 30% to 40% ultimately required a suboccipital craniectomy.
With cerebellar infarction causing brain-stem compression, it is well-established that suboccipital craniectomy reduces mortality.19,41 In a series of 13 patients with cerebellar infarction and progressive deterioration of consciousness, all 7 who underwent craniectomy lived; the one patient treated with ventriculostomy alone died, and 4 of the 5 patients treated medically died.31 With timely decompression, good functional outcomes are common.18,42–44 One series reports that even comatose patients have a 38% chance of good recovery with decompressive surgery.38
When cerebellar infarction is massive enough to cause hydrocephalus, there are intuitive reasons that suboccipital surgery (often with ventriculostomy) is preferable to ventriculostomy alone. Hydrocephalus in this setting is caused by effacement of the fourth ventricle or the aqueduct, and it implies some degree of acute brain-stem compression, which would not be relieved by CSF drainage alone. Waiting to see if the brain-stem compression increases and causes progressive neurologic deterioration puts the patient at undue risk of acute decline and often catastrophic outcome. Other disadvantages of ventriculostomy without suboccipital craniectomy include the risk of upward transtentorial herniation and the prolonged need for catheter drainage, with associated rates of infection and shunt requirement.18,45
Surgical Intervention
A midline incision is preferable for the craniectomy, although occasionally a paramedian incision may be utilized. Bone removal is wide enough to decompress cerebellar swelling as well as to provide access to remove the offending pathology. The bone removal must extend inferiorly to access the cisterna magna and widely decompress the foramen magnum, and generally includes removal of the posterior arch of C1. The dura is opened to expose the midline and lateral cerebellar surface affected by the hematoma or infarct, and the incision is carried inferiorly below the foramen magnum so that the tips of the cerebellar tonsils are visualized and that adequate decompression of the brain stem has been accomplished.
Cases
Case 1
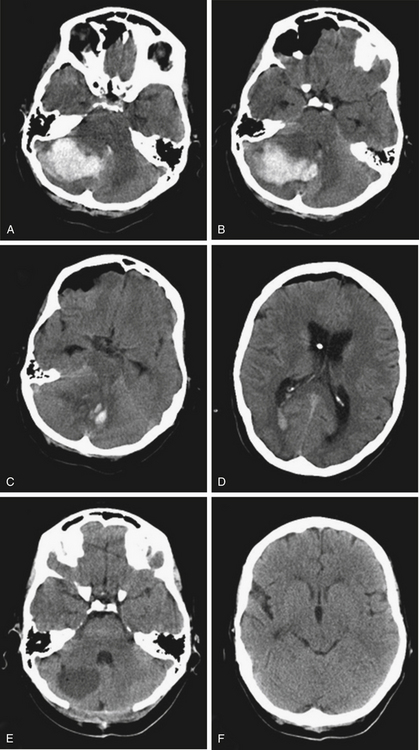
A 51-year-old woman with a history of hypertension presented to the emergency room of another facility after the acute onset of headache, nausea, vomiting, and gait instability. She became comatose in the ER and was intubated. After a CT showed a large right cerebellar hemorrhage with hydrocephalus, a right frontal ventriculostomy was placed, and the patient was transferred to our institution. Her CT showed a 5×3-cm hematoma in the right cerebellar hemisphere and vermis, causing deformity of the brain stem, effacement of the basal cisterns, compression of the fourth ventricle, and hydrocephalus (Fig. 70-3). She underwent an emergent suboccipital craniectomy and evacuation of the hematoma, and made a dramatic recovery postoperatively. At follow-up 4 months later, she was neurologically intact except for mild gait instability.
Case 2
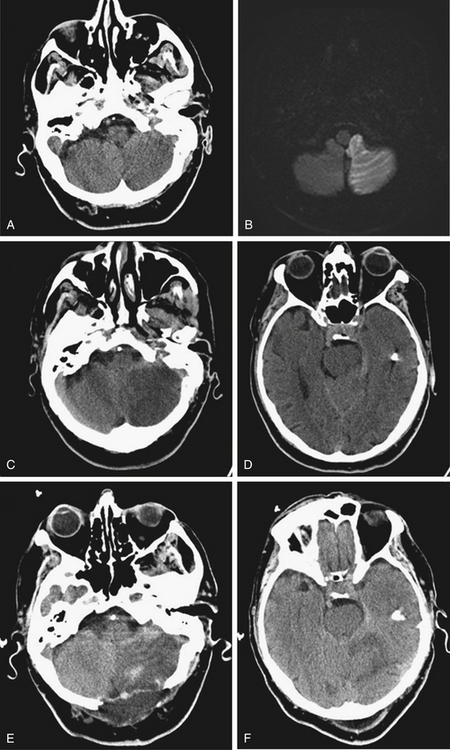
An 81-year-old man with a history of hypertension and atrial fibrillation presented after the acute onset of nausea and gait instability. His neurologic examination showed left dysmetria and gaze-paretic nystagmus. His initial CT was normal, and MRI showed diffusion restriction in the left PICA distribution (Fig. 70-4A and B). He was admitted to the neurologic intensive care unit, and found to have dysarthria 40 hours after the onset of symptoms. A CT showed the left cerebellar infarction with mass effect, causing transtentorial compression of the brain stem (Fig. 70-4C and D). He underwent an emergent suboccipital decompression, and recovered well after the surgery.
Conclusion
While the pathophysiology of cerebellar hemorrhage is distinct from that of cerebellar infarction, it is appropriate to consider these two entities together, because they have similar neurologic sequelae. Both conditions typically present acutely with symptoms and signs of cerebellar dysfunction, and may progress rapidly to coma from mass effect in the posterior fossa. When brain-stem compression and obstructive hydrocephalus occurs, urgent suboccipital decompression is warranted. Timely surgery can be life-saving, and patients often have a good functional outcome.
Adams H.P., del Zoppo G., Alberts M.J., et al. Guidelines for the early management of adults with ischemic stroke. Stroke. 2007;38:1655-1711.
Amarenco P., Levy C., Cohen A., et al. Causes and mechanisms of territorial and nonterritorial cerebellar infarcts in 115 consecutive patients. Stroke. 1994;25:105-112.
Auer L.M., Auer T., Sayama I. Indications for surgical treatment of cerebellar hemorrhage and infarction. Acta Neurochir (Wien). 1986;79:74-79.
Barinagarrementeria F., Amaya L.E., Cantu C. Causes and mechanisms of cerebellar infarction in young patients. Stroke. 1997;28:2400-2404.
Chen H.J., Lee T.C., Wei C.P. Treatment of cerebellar infarction by decompressive suboccipital craniectomy. Stroke. 1992;23:957-961.
De Oliveira J.G., Rassi-Neto A., Ferraz F.A.P., et al. Neurosurgical management of cerebellar cavernous malformations. Neurosurg Focus. 2006;21:1-8.
Duncan G.W., Parker S.W., Fisher C.M. Acute cerebellar infarction in the PICA territory. Arch Neurol. 1975;32:364-368.
Edlow J.A., Newman-Toker D.E., Savitz S.I. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008;7:951-964.
Fairburn B., Oliver L.C. Cerebellar softening: a surgical emergency. BMJ. 1956;1:1335-1336.
Friedman J.A., Piepgras D.G., Duke D.A., et al. Remote cerebellar hemorrhage after supratentorial surgery. Neurosurg. 2001;49:1327-1340.
Heros R.C. Surgical treatment of cerebellar infarction. Stroke. 1992;23:937-938.
Hornig C.R., Rust D.S., Busse O., et al. Space-occupying cerebellar infarction: clinical course and prognosis. Stroke. 1994;25:372-373.
Juttler E., Schweickert S., Ringleb P.A., et al. Long-term outcome after surgical treatment for space-occupying cerebellar infarction: experience in 56 patients. Stroke. 2009;40:3060-3066.
Kirollos R.W., Tyagi A.K., Ross S.A., et al. Management of spontaneous cerebellar hematomas: a prospective treatment protocol. Neurosurg. 2001;49:1378-1387.
Kobayashi S., Sato A., Kageyama Y., et al. Treatment of hypertensive cerebellar hemorrhage: surgical or conservative management. Neurosurgery. 1994;34:246-251.
Koh M.G., Phan T.G., Atkinson J.L.D., et al. Neuroimaging in deteriorating patients with cerebellar infarcts and mass effect. Stroke. 2000;31:2062-2067.
Kudo H., Kawaguchi T., Minami H., et al. Controversy of surgical treatment for severe cerebellar infarction. J Stroke Cerebrovasc Dis. 16, 2007. 259-262
Park J.S., Hwang J.H., Park J., et al. Remote cerebellar hemorrhage complicated after supratentorial surgery: retrospective study with review of articles. J Korean Neurosurg Soc. 2009;46:136-143.
Pfefferkorn T., Eppinger U., Linn J., et al. Long-term outcome after suboccipital decompressive craniectomy for malignant cerebellar infarction. Stroke. 2009;40:3045-3050.
Raco A., Caroli E., Isidori A., et al. Management of acute cerebellar infarction: one institution’s experience. Neurosurgery. 2003;53:1061-1066.
Rhoton A.L. Cerebellum and fourth ventricle. Neurosurgery. 2000;47:S7-27.
Salvati M., Cervoni L., Raco A., et al. Spontaneous cerebellar hemorrhage: clinical remarks on 50 cases. Surg Neurol. 2001;55:156-161.
Wakai S., Nagai M. Histological verification of microaneurysms as a cause of cerebral haemorrhage in surgical specimens. J Neurol Neurosurg Psychiatry. 1989;52:595-599.
Weisberg L.A. Acute cerebellar hemorrhage and CT evidence of tight posterior fossa. Neurology. 1986;36:858-860.
Yanaka K., Meguro K., Fujita K., et al. Immediate surgery reduces mortality in deeply comatose patients with spontaneous cerebellar hemorrhage. Neurol Med Chir (Tokyo). 2000;40:295-300.
1. Rhoton A.L. Cerebellum and fourth ventricle. Neurosurgery. 2000;47:S7-27.
2. Horinek D., Brezova V., Nimsky C., et al. The MRI volumetry of the posterior fossa and its substructures in trigeminal neuralgia: a validated study. Acta Neurochir. 2009;151:669-675.
3. Badie B., Mendoza D., Batzdorf U. Posterior fossa volume and response to suboccipital decompression in patients with Chiari I malformation. Neurosurgery. 1995;37:214-218.
4. Rengachary S.S., Duke D.A. Increased intracranial pressure, cerebral edema, and brain herniation. In: Rengachary S.S., Wilkins R.H. Principles of Neurosurgery. London: Mosby-Wolfe, 1994. pp. 2.2-2.14
5. Kirollos R.W., Tyagi A.K., Ross S.A., et al. Management of spontaneous cerebellar hematomas: a prospective treatment protocol. Neurosurg. 2001;49:1378-1387.
6. Raco A.. Surgical management of cerebellar hemorrhage and cerebellar infarction, Schmidek H.H., Roberts D.W. Schmidek and Sweet Operative Neurosurgical Techniques, 5th ed, Philadelphia: Saunders Elsevier, 2006. pp. 859-872
7. Wakai S., Nagai M. Histological verification of microaneurysms as a cause of cerebral haemorrhage in surgical specimens. J Neurol Neurosurg Psychiatry. 1989;52:595-599.
8. McCormick W.F., Nofzinger J.D. Cryptic vascular malformations of the central nervous system. J Neurosurg. 1966;24:865-875.
9. Arnaout O.M., Gross B.A., Eddleman C.S., et al. Posterior fossa arteriovenous malformations. Neurosurg Focus. 2009;26:1-6.
10. De Oliveira J.G., Rassi-Neto A., Ferraz F.A.P., et al. Neurosurgical management of cerebellar cavernous malformations. Neurosurg Focus. 2006;21:1-8.
11. Friedman J.A., Piepgras D.G., Duke D.A., et al. Remote cerebellar hemorrhage after supratentorial surgery. Neurosurg. 2001;49:1327-1340.
12. Konya D., Ozgen S., Pamir M.N. Cerebellar hemorrhage after spinal surgery: case report and review of the literature. Eur J Spine. 2006;15:95-99.
13. Brockmann M.A., Groden C. Remote cerebellar hemorrhage: a review. Cerebellum. 2006;5:64-68.
14. Park J.S., Hwang J.H., Park J., et al. Remote cerebellar hemorrhage complicated after supratentorial surgery: retrospective study with review of articles. J Korean Neurosurg Soc. 2009;46:136-143.
15. Amarenco P., Levy C., Cohen A., et al. Causes and mechanisms of territorial and nonterritorial cerebellar infarcts in 115 consecutive patients. Stroke. 1994;25:105-112.
16. Edlow J.A., Newman-Toker D.E., Savitz S.I. Diagnosis and initial management of cerebellar infarction. Lancet Neurol. 2008;7:951-964.
17. Barinagarrementeria F., Amaya L.E., Cantu C. Causes and mechanisms of cerebellar infarction in young patients. Stroke. 1997;28:2400-2404.
18. Chen H.J., Lee T.C., Wei C.P. Treatment of cerebellar infarction by decompressive suboccipital craniectomy. Stroke. 1992;23:957-961.
19. Heros R.C. Surgical treatment of cerebellar infarction. Stroke. 1992;23:937-938.
20. Duncan G.W., Parker S.W., Fisher C.M. Acute cerebellar infarction in the PICA territory. Arch Neurol. 1975;32:364-368.
21. Sypert G.W., Alvord E.C. Cerebellar infarction: a clinicopathologic study. Arch Neurol. 1975;32:357-363.
22. Norris J.W., Eisen A.A., Branch C.L. Problems in cerebellar hemorrhage and infarction. Neurology. 1969;19:1043-1050.
23. Lehrich J.R., Winkler G.F., Ojemann R.G. Cerebellar infarction and brain stem compression: diagnosis and surgical treatment. Arch Neurol. 1970;22:490-498.
24. Weisberg L.A. Acute cerebellar hemorrhage and CT evidence of tight posterior fossa. Neurology. 1986;36:858-860.
25. Taneda M., Hayakawa T., Mogami H. Primary cerebellar hemorrhage: quadrigeminal cistern obliteration on CT scans as a predictor of outcome. J Neurosurg. 1987;67:545-552.
26. Kobayashi S., Sato A., Kageyama Y., et al. Treatment of hypertensive cerebellar hemorrhage: surgical or conservative management. Neurosurgery. 1994;34:246-251.
27. Koziarski A., Frankiewicz E. Medical and surgical treatment of intracerebellar hematomas. Acta Neurochir (Wien). 1991;110:24-28.
28. Mezzadri J.J., Otero J.M., Ottino C.A. Management of 50 spontaneous cerebellar haemorrhages: importance of obstructive hydrocephalus. Acta Neurochir (Wien). 1993;122:39-44.
29. Koh M.G., Phan T.G., Atkinson J.L.D., et al. Neuroimaging in deteriorating patients with cerebellar infarcts and mass effect. Stroke. 2000;31:2062-2067.
30. Adams H.P., del Zoppo G., Alberts M.J., et al. Guidelines for the early management of adults with ischemic stroke. Stroke. 2007;38:1655-1711.
31. Auer L.M., Auer T., Sayama I. Indications for surgical treatment of cerebellar hemorrhage and infarction. Acta Neurochir (Wien). 1986;79:74-79.
32. Da Pian R., Bazzan A. Pasqualin A: Surgical versus medical treatment of spontaneous posterior fossa hematomas: a cooperative study on 205 cases. Neurol Res. 1984;6:145-151.
33. Salvati M., Cervoni L., Raco A., Delfini R. Spontaneous cerebellar hemorrhage: clinical remarks on 50 cases. Surg Neurol. 2001;55:156-161.
34. Yanaka K., Meguro K., Fujita K., et al. Immediate surgery reduces mortality in deeply comatose patients with spontaneous cerebellar hemorrhage. Neurol Med Chir (Tokyo). 2000;40:295-300.
35. Firsching R., Huber M., Frowein R. Cerebellar haemorrhage: management and prognosis. Neurosurg Rev. 1991;14:191-194.
36. Fairburn B., Oliver L.C. Cerebellar softening: a surgical emergency. BMJ. 1956;1:1335-1336.
37. Cioffi F.A., Bernini F.P., Punzo A., D’Avanzo R. Surgical management of acute cerebellar infarction. Acta Neurochir (Wien). 1985;74:105-112.
38. Hornig C.R., Rust D.S., Busse O., et al. Space-occupying cerebellar infarction: clinical course and prognosis. Stroke. 1994;25:372-373.
39. Jauss M., Krieger D., Hornig, et al. Surgical and medical management of patients with massive cerebellar infarctions: results of the German-Austrian cerebellar infarction study. J Neurol. 1999;246:257-264.
40. Raco A., Caroli E., Isidori A., et al. Management of acute cerebellar infarction: one institution’s experience. Neurosurgery. 2003;53:1061-1066.
41. Feely M.P. Cerebellar infarction. Neurosurgery. 1979;4:7-11.
42. Kudo H., Kawaguchi T., Minami H., et al. Controversy of surgical treatment for severe cerebellar infarction. J Stroke Cerebrovasc Dis. 16, 2007. 259-262
43. Juttler E., Schweickert S., Ringleb P.A., et al. Long-term outcome after surgical treatment for space-occupying cerebellar infarction: experience in 56 patients. Stroke. 2009;40:3060-3066.
44. Pfefferkorn T., Eppinger U., Linn J., et al. Long-term outcome after suboccipital decompressive craniectomy for malignant cerebellar infarction. Stroke. 2009;40:3045-3050.
45. Cunes R.A., Caronna J.J., Pitts L., et al. Upward transtentorial herniation: seven cases and a literature review. Arch Neurol. 1979;36:618-623.