Chapter 74 Surgical Management of Anterior Communicating and Anterior Cerebral Artery Aneurysms
The anterior communicating artery (ACoA) aneurysm is the most common aneurysm encountered in neurosurgical practice, accounting for one quarter to one third of all microsurgically treated aneurysms in published experiences1–3 and 21% of the senior author’s experience with 2320 aneurysms. This aneurysm has a propensity to hemorrhage, often at or below size limits considered safe for conservative management and often in younger patients for whom microsurgical clipping might be favored over endovascular coiling.2,4 Consequently, neurosurgeons must be prepared to deal with this lesion. This particular aneurysm, more than any other, has an unusually wide variety of complexity and technical difficulty that depends on variations in parent artery anatomy, aneurysm projection, and clinical presentation. In addition, the ACoA complex is adjacent to the hypothalamus, optic apparatus, and cognitive/emotional centers in the basal frontal lobes, while arteries emanating from the ACoA complex affect the basal ganglia, internal capsule, and motor/sensory cortex. Therefore, surgery for ACoA aneurysms is associated with elevated risks. This chapter discusses the factors and techniques that facilitate surgical management of ACoA aneurysms and help improve patient outcome.
Anatomy
Nomenclature
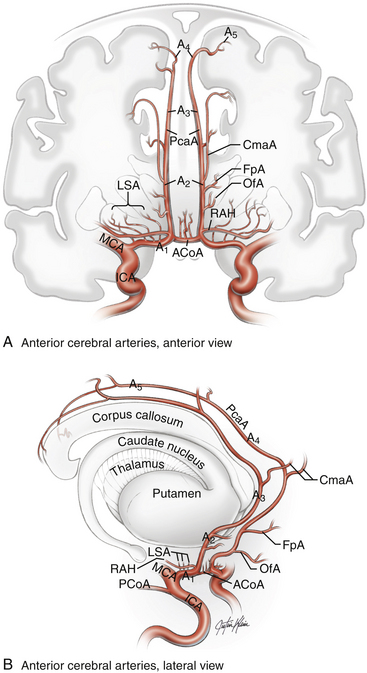
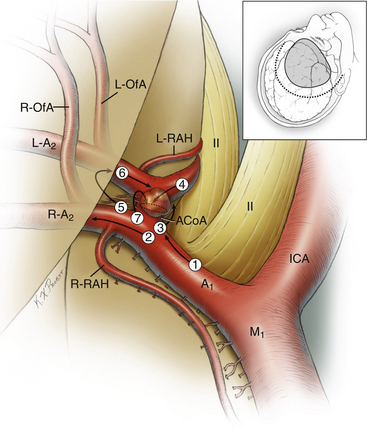
The anterior cerebral artery (ACA) is divided into five segments (Fig. 74-1). The A1 segment, also known as the precommunicating or horizontal segment of the ACA, originates with the ACA at the internal carotid artery (ICA) bifurcation and extends to the junction with the ACoA. The A2 segment, also known as the postcommunicating segment, originates at the ACoA and extends to the genu of the corpus callosum, following the contour of the rostrum. The A3 segment curves around the genu and extends to the body of the corpus callosum, where it assumes a posterior course. The A4 and A5 segments continue over the body of the corpus callosum, the division between them being located at the plane of the coronal suture. Contrary to popular opinion, this nomenclature is not defined by the bifurcation into pericallosal and callosomarginal arteries. This bifurcation is usually located along the A3 segment (approximately 60% of patients), but it can be more proximal on the A2 segment (10%), it can be more distal on the A4 segment (12%), or the pericallosal artery can be absent (18%).5
Normal Anatomy
The A1 segment courses medially and anteriorly over the optic tract and chiasm to the ACoA complex. Numerous penetrating branches (average 8, range 2-15)5 originate from this segment and course superiorly to supply the anterior perforated substance, subfrontal area, dorsal surface of the optic apparatus, hypothalamus, anterior commissure, septum pellucidum, and paraolfactory structures. Collectively, these perforators are also known as the medial lenticulostriate arteries, as opposed to the lateral lenticulostriate arteries that originate from the M1 segment of the middle cerebral artery (MCA). The most important of these perforators is the recurrent artery of Heubner, which originates from the proximal A2 segment on its lateral wall, just distal to the ACoA. This artery can arise from the distal A1 segment, just proximal to the ACoA, in 14% of patients or at the level of the ACoA in 8% of patients but is within 4 mm of the ACoA in 95% of patients.5 The artery is almost always present (98%) and can be duplicated (2%).5 The artery follows a course parallel to the A1 segment, either superior (60%) or anterior (40%) to it.5 The recurrent artery is typically seen before the A1 segment when the frontal lobe is retracted, making it a useful landmark to identify the A1 segment and the ACoA. The recurrent artery of Heubner supplies the head of the caudate nucleus, putamen, outer segment of the globus pallidus, and anterior limb of the internal capsule. Therefore, arterial injury can produce weakness involving the contralateral face and arm and expressive aphasia in the dominant hemisphere.6
The ACoA joins the two ACAs as they arrive in the interhemispheric fissure, completing the anterior part of the circle of Willis. Normally, the ACoA diameter is about half that of the A1 segments, but there is a direct correlation between asymmetry in the A1 segments and ACoA diameter. In other words, the ACoA diameter increases as the caliber of a hypoplastic A1 segment decreases, thereby compensating for the asymmetry in the A1 inflow.7 The ACoA can be duplicated in one third of patients, and triplicated in 10% of patients, but is always present.5 An ACoA that is not visualized angiographically is usually explained by an absence of cross-filling rather than an absence of the ACoA itself, and it can be visualized by a carotid cross-compression maneuver during angiography. The ACoA gives rise to important perforating arteries, which originate from its superior (54%) and posterior (36%) surfaces.5 These perforators course to the hypothalamus, median paraolfactory nuclei, genu, columns of the fornix, septum pellucidum, and anterior perforated substance. There can be perforators originating from the anterior and inferior aspects of the ACoA, supplying the dorsal optic chiasm, but these are few in number.
After making a right-angle turn from the horizontal A1 ACA, the A2 ACA runs superiorly in the interhemispheric fissure, coursing in front of the lamina terminalis and tracing the curvature of the genu. The branches arising from this segment are important more for correctly deciphering the anatomy of the ACoA complex than for their vascular supply. The orbitofrontal artery is the first cortical ACA branch, arising from the A2 segment approximately 5 mm distal to the ACoA from the anterolateral surface and coursing perpendicularly over the gyrus rectus and olfactory tract.6 This artery supplies the gyrus rectus, orbital gyri (anterior, posterior, medial, and lateral), and olfactory bulb/tract. It is important to avoid mistaking the recurrent artery of Heubner for this orbitofrontal artery. Both can be seen on the medial aspect of the gyrus rectus, but their origins from the A2 segment are separated and their courses are different, with the recurrent artery of Heubner eventually rejoining the A1 segment, even if it meanders under the gyrus rectus along its more proximal course. Correctly identifying the recurrent artery is especially important when resecting gyrus rectus. The recurrent artery is also typically small in caliber, with a diameter of approximately 1 mm.5 The orbitofrontal artery is often encountered draped over or adherent to the dome of a superiorly projecting aneurysm, which can tether the dome and make it more difficult to mobilize. The course of the orbitofrontal artery can also lead it across the neck of an ACoA aneurysm, requiring additional dissection before clipping.
The last relevant branch artery is the frontopolar artery, which originates from the A2 segment 14 mm, on average, from the ACoA near the genu.5 This artery courses anteriorly in the interhemispheric fissure and supplies the ventromedial frontal lobes. Rarely, it can originate from a common trunk with the orbitofrontal artery. Like the orbitofrontal artery, the frontopolar artery can be draped over the dome of a superiorly projecting aneurysm and should not be misinterpreted.
Variant Anatomy
Asymmetry in the caliber of the A1 segments is seen in approximately 10% of patients, with a hypoplastic segment defined arbitrarily as having a diameter of 1.5 mm.5 Smaller diameters are observed less frequently, with approximately 2% of patients having an A1 segment diameter of 1 mm.5 Aplastic A1 segments are rare, despite their suggestion on angiography with a contralateral A1 ACA that fills both distal ACAs. At surgery, a small A1 segment is typically found. The dominance of an A1 segment is relevant to aneurysm formation, projection of the dome, site of hematoma, and choice of side. Dominant A1 segments are frequently seen in association with ACoA aneurysms, which typically project in the direction of blood flow in that segment.7 As discussed later, it is usually advantageous to surgically approach these aneurysms from the side of the dominant A1 ACA, both for easier proximal control of the aneurysm and for better clipping of the neck. One final variant, the duplicated A1 segment, is rare (2%) and almost always unilateral.5
Variations in ACoA anatomy include duplications, triplications, and fenestrations.8,9 Accessory ACoAs are typically small and without significant perforators, making it important to recognize the primary ACoA and preserve both it and its perforators. Fenestrations can be the site of aneurysm formation and require special attention to differentiate the normal artery from the pathology. More important than these structural variations in the ACoA is its orientation. In only 18% of patients is the ACoA oriented in the transverse plane, as illustrated in anatomic textbooks.5 Instead, the ACoA complex is usually rotated or tilted, causing the A2 ACAs to course obliquely to one another in the interhemispheric fissure. This variation in orientation can direct an A2 segment more posteriorly, making its visualization more difficult. Rotation of the ACoA can shift the location of perforators from posterior to lateral, again making visualization more difficult, particularly when the aneurysm lies between the neurosurgeon and the perforators. These subtle changes in angles can be misleading, particularly when visualization is compromised by factors such as a swollen brain, a large aneurysm, and thick hematoma.
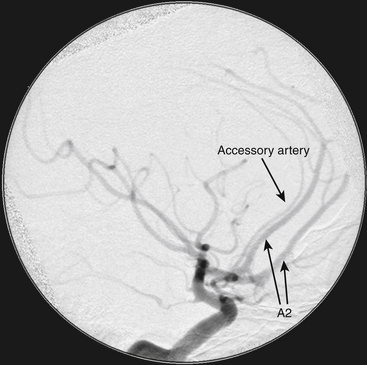
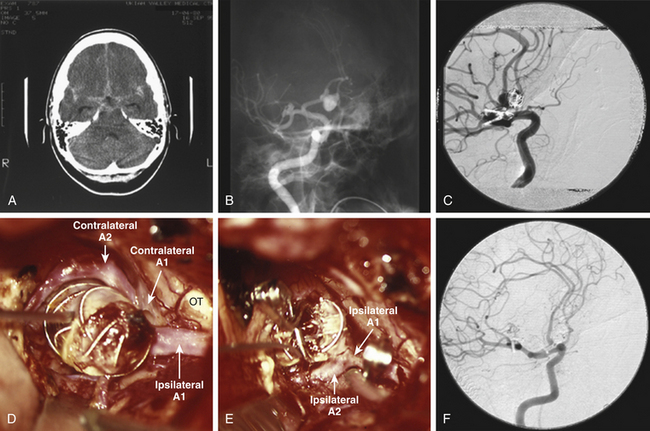
Variant anatomy in the efferent arteries can be the most difficult to deal with and is best understood in terms of the classification by Baptista.10 After analyzing 381 brain specimens, he defined three types of efferent artery anatomy. The type I anomaly, referred to as the azygos or “unpaired” ACA, is a single midline vessel arising from the confluence of the A1 segments (Fig. 74-2). Distally, the azygos ACA divides into pericallosal and callosomarginal arteries, with bifurcations, trifurcations, and quadrifurcations having been reported. This variant occurs in 0.3% to 2% of patients. The type II anomaly, referred to as the bihemispheric ACA, is an A2 ACA that transmits branches across the midline to supply both hemispheres, usually in the presence of a contralateral A2 segment that is hypoplastic or terminates early in its course toward the genu. This anomaly can be seen in as many as 12% of patients. The type III anomaly, referred to as an accessory ACA, is defined as a third artery originating from ACoA, in addition to the paired A2 segments and usually between them (Fig. 74-3). This accessory ACA variant is the most difficult one surgically because it results in some of the most unusual anatomic puzzles. If this variation is not appreciated, then a critical A2 ACA could be missed or, worse, sacrificed inadvertently during the aneurysm clipping (Fig. 74-4). The accessory ACA varies in caliber from a small remnant of the median artery of the corpus callosum (MACC) to a hyperplastic ACA that can resemble an azygos ACA when the two A2 segments are small in caliber and terminate early. A careful analysis of the angiogram preoperatively can alert the neurosurgeon to this challenging variant.
The MACC just mentioned originates during embryogenesis when elongating ACAs coalesce in the midline to form plexiform anastomoses at 44 days.11 Normally, the MACC regresses and disappears as the A2 segments mature, but vestigial remnants can account for the accessory ACA.
Aneurysm Anatomy
The true ACoA aneurysm arises from ACoA and is defined further by the projection of its dome in an anterior, superior, posterior, or inferior direction. In Yasargil’s experience with ACoA aneurysms, the superiorly and anteriorly projecting aneurysms were the most common (34% and 23%, respectively), while posteriorly and inferiorly projecting aneurysms were the least common (14% and 13%, respectively).12 Some aneurysms (approximately 16%) have mixed projection or multiple lobes.
The superiorly projecting aneurysm is less favorable than the anterior projecting aneurysm, mainly because of the contralateral A2 ACA and the perforators (Fig. 74-5). Proximal control is straightforward, as the aneurysm is away from the A1 segments and the view to the contralateral side is unobstructed. However, the aneurysm is interposed between the neurosurgeon and the contralateral A2 ACA, requiring some manipulation of the aneurysm to locate this artery. Furthermore, larger aneurysms can displace perforators laterally or posteriorly, and their adherence to the aneurysm necessitates some delicate dissection. In addition, the A2 segments can be adherent, requiring aggressive dissection along the plane between this efferent artery and the fundus to fully expose the neck. Unlike the anteriorly projecting aneurysms, where the view behind the aneurysm is panoramic, the view behind a superiorly projecting aneurysm requires more extensive dissection. The ipsilateral A2 ACA must be mobilized anteriorly and can require dissection of its branches (i.e., the orbitofrontal and frontopolar arteries). The clip application is often more complex with these aneurysms, sometimes requiring fenestrated clips that encircle the ipsilateral A2 ACA.
Posteriorly projecting ACoA aneurysms are arguably the most challenging to clip. With these lesions, the A1 and A2 segments can be identified readily, but the perforators are markedly more difficult to visualize and preserve. They are often displaced laterally, where they become obstacles to the clip blades during clip application, and/or they are displaced posteriorly, where they can easily elude detection. In addition, the parent arteries of the ACoA complex are interposed between the surgeon and the aneurysm neck, making it more difficult to dissect and apply the clips to the neck. Fortunately, these aneurysms are uncommon.12
The inferiorly projecting aneurysm is another favorable aneurysm, with the one caveat: its dome often adheres to the optic apparatus, making it susceptible to avulsion and rupture early in the dissection with frontal lobe retraction. From that standpoint, it can be treacherous, because at that point in the dissection, proximal control is inadequate and the aneurysm anatomy has not been analyzed or even exposed. However, care in retracting the frontal lobe can avert this complication, and the advantages of an inferior projection make it a relatively straightforward aneurysm. Like the anteriorly projecting aneurysm, the ipsilateral A1 and bilateral A2 segments are easily visualized. The contralateral A1 segment can be obscured, which could compromise proximal control of the aneurysm, but the other critical anatomy is accessible. Perforators are rarely a problem, and the necks of these aneurysms are easily closed. These aneurysms can be adherent to the optic nerves and/or chiasm, and often it is preferable to leave the dome undissected or amputate it after clipping the neck, rather than manipulating the optic nerves with unnecessary dissection.
Variant aneurysms include aneurysms that arise from a fenestration, duplicated or accessory ACoA, accessory A2 ACA, or azygos ACA. Accessory anatomy can pose additional risks, because it can mislead the surgeon and result in inadvertent arterial occlusions if not carefully protected. For example, the neurosurgeon might erroneously clip an important accessory A2 segment if an ipsilateral and contralateral A2 segment have already been identified. Therefore, thorough preoperative review of the anatomy and intraoperative dissection is critical before the final clipping is performed. Infundibuli arising from the ACoA have been observed, particularly with other vascular lesions such as arteriovenous malformations. These infundibuli, like those at the posterior communicating artery and elsewhere, can appear on angiography like aneurysms but transmit normal arteries and must be preserved. Dissection along the course of these arteries distinguishes them from aneurysms. ACoA aneurysms can be giant, atherosclerotic, calcified, or thrombotic. Aneurysms at this location can also be nonsaccular and due to other causes, including infection, trauma, and dissection.
Clinical Presentation
Aneurysm rupture is the most common presentation of patients with ACoA aneurysms, with the classic headache characterized by its sudden onset and severity.1 Patients can present in much worse neurologic condition, with obtundation or coma depending on the extent of hemorrhage and presence or absence of hydrocephalus. ACoA aneurysms are notoriously small, often rupturing at sizes smaller than those that would be considered a threshold for treatment. Therefore, advance symptoms are uncommon. When large or giant, ACoA aneurysms can produce symptoms from mass effect on the optic apparatus (visual field deficits), hypothalamus (endocrine dysfunction), hydrocephalus (obstruction of the foramen of Monro), or frontal lobes (cognitive dysfunction, memory impairment, and seizure).13,14
Diagnostic Imaging
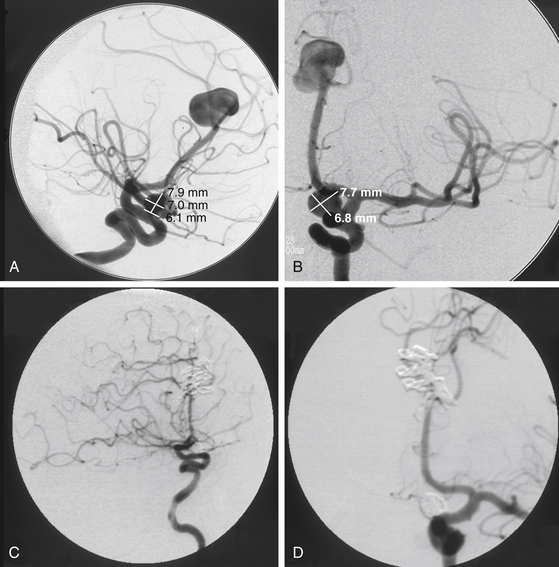
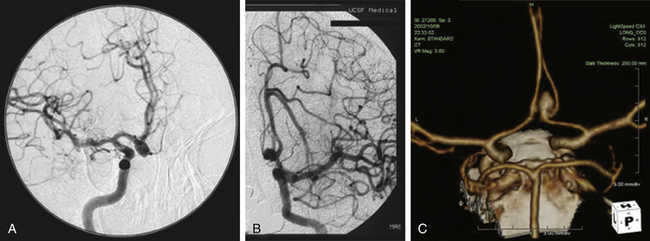
The diagnosis of subarachnoid hemorrhage (SAH) requires the confirmation of blood in the subarachnoid space, which can be accomplished best with a computed tomography (CT) scan. Blood is easily seen on a noncontrast CT scan, with a sensitivity of greater than 95%.15 CT can also pinpoint an aneurysm location based on SAH distribution, in addition to revealing the presence of intraparenchymal or intraventricular blood. Subarachnoid blood from a ruptured ACoA aneurysm tends to localize in the interhemispheric fissure.16 The direction of aneurysm projection influences this pattern of blood distribution, with superior and posterior projecting aneurysms filling the interhemispheric fissure and anterior and inferior projecting aneurysms bleeding more diffusely. While interhemispheric SAH is the most common finding with ruptured ACoA aneurysms, intracerebral hemorrhage can be observed in the gyrus rectus with laterally projecting aneurysms and intraventricular hemorrhage can be observed with aneurysms that rupture through the lamina terminalis. Pericallosal artery aneurysms tend to have blood more distally located, such as over the genu or body of the corpus callosum. CT scanning is quick and definitive, can diagnose associated conditions such as hydrocephalus, and with an additional bolus of intravenous contrast, can generate a CT angiogram (Fig. 74-5C).
While catheter angiography remains the gold standard for the diagnosis of aneurysms, it has disadvantages: it is invasive, time consuming, costly, and associated with some risk of dissection, embolization, and groin hematoma. Angiograms generated with CT or magnetic resonance data (computed tomography angiography, or CTA, and magnetic resonance angiography, respectively) are superb and, with computerized, three-dimensional reconstruction, can generate images with startlingly high resolution that are adequate for preoperative and intraoperative planning.17,18 CTA in particular is noninvasive, easy to obtain, and fast, offering an alternative to catheter angiography in unstable patients with ruptured aneurysms that need to get to the operating room emergently.
Lumbar puncture is the only laboratory study that needs to be considered in the evaluation of an aneurysm patient and is used only when the patient’s history is strongly suggestive of SAH but the CT scan is normal.19 There are two explanations for this inconsistency. The first explanation is a sentinel hemorrhage has leaked so little blood that it is not radiographically apparent, in which case cerebrospinal fluid (CSF) may be blood tinged and will not clear in successive tubes. The second explanation is a delayed CT scan was performed days after the SAH. A delay in seeking medical attention or in ordering the CT scan allows subarachnoid blood to disperse, making it difficult to detect on the imaging study. CSF is thus xanthochromic. CSF from a lumbar puncture that is positive for new or old blood indicates further evaluation with an angiogram.
ACoA aneurysms have a rate of false-negative angiography that is higher than that observed with other aneurysms.16,20 Patients with a characteristic aneurysmal SAH on CT scan and a negative angiogram should undergo repeat angiography within 1 week with careful attention focused on the ACoA region. This region is notorious for hiding small aneurysms that might elude detection due to intraluminal thrombus in the aneurysm, extraluminal thrombus compressing the aneurysm, or vasospasm in the afferent arteries.
Preoperative Management
After a ruptured aneurysm is diagnosed, the aneurysm is secured as quickly as possible, typically within 48 to 72 hours of the hemorrhage. In the meantime, efforts are made to stabilize the patient and minimize the risk of rerupture. There is a 4% risk of rehemorrhage in the first 24 hours after hemorrhage, with an associated mortality rate of 27% to 43%.1 Most important is blood pressure control. Rebleeding is caused by absolute elevations and rapid variations in blood pressure. Blood pressure should be carefully monitored with invasive arterial lines in an intensive care unit (ICU) setting where intravenous agents or drips can be administered to keep the systolic blood pressure under 140 mm Hg. Hydrocephalus is present in approximately one fourth of SAH patients21 and resolves with the insertion of a ventriculostomy. External ventricular drainage can improve the clinical status of patients dramatically and is recommended in all obtunded or comatose patients. In addition, ventriculostomy allows intracranial pressure (ICP) to be transduced and can guide preoperative management of increased pressures. In patients with a poor Hunt-Hess grade, intubation and mechanical ventilation are usually needed to protect the airway and sometimes to hyperventilate the patient for ICP management. Other comfort measures such as bed rest, sedation, and analgesics help minimize agitation that might precipitate rerupture. Seizures can precipitate rerupture, and anticonvulsants are given in the immediate post-SAH period when there is intraparenchymal hematoma.
Microsurgical Management
Exposure
The first decision in the surgical plan for an ACoA aneurysm is the choice of approach. For most aneurysms, a standard pterional craniotomy is sufficient. The alternative approach is the orbitozygomatic approach, which increases the exposure for large, giant, or complex ACoA aneurysms. The orbitozygomatic approach is, in essence, an application of skull base surgery principles, removing the orbital rim, roof, and inferior frontal skull to maximize the operating space under the brain and minimize retraction. The difference in exposure between the standard pterional and the orbitozygomatic approaches is well documented in cadaveric and clinical studies,22,23 but the decision to remove the orbitozygomatic unit should be made judiciously. In the senior author’s experience with 485 ACoA aneurysms, the orbitozygomatic approach was used in just 16% of patients. As with most maneuvers to expose ACoA aneurysms, the orbitozygomatic approach should be used only when necessary, since additional surgery adds to the patient’s cumulative risk and impact from surgery.
The orbitozygomatic approach differs only in the dissection of the temporalis muscle and in the osteotomies of the orbit and zygoma.24 We prefer the subfascial dissection to expose the zygoma and lateral orbit, but an interfascial dissection can also be used.25 The osteotomies are performed after the craniotomy, yielding a separate bone flap and orbitozygomatic unit that reassemble with excellent cosmetics postoperatively. The plates that replace the orbitozygomatic unit are applied and screw holes are predrilled before the unit is detached to ensure proper realignment. In addition, we prefer a reciprocating saw over a high-speed drill to make the osteotomies, and we prefer stepped cuts rather than linear cuts because they interlock the orbitozygomatic unit to the skull, enhancing the reconstruction.
Clipping
Successful clipping depends on thorough dissection and interpretation of the anatomy, which can be challenging at the ACoA complex (Fig. 74-6). In all, 11 arteries need to be identified: bilateral A1 ACAs, bilateral A2 ACAs, bilateral recurrent arteries of Heubner, ACoA, bilateral orbitofrontal arteries, and bilateral frontopolar arteries. The last two arteries are often outside the surgical field and are relevant only in rare cases where they course inferiorly into the surgical field. The first two arteries are the most important, because they provide the proximal control. Identification of the contralateral A1 ACA requires dissection across the region of the aneurysm, so its projection must be appreciated and the dissection should steer clear of the dome. Gyrus rectus resection or interhemispheric fissure dissection identifies the ipsilateral A2 ACA. The contralateral A2 ACA is the most difficult of the arteries to identify because it is the deepest artery in the surgical field and its visualization is usually obscured to some degree by the aneurysm. Therefore, the contralateral A2 ACA is typically the last of the five major arteries (two A1 ACAs, two A2 ACAs, and ACoA) to be found, often requiring some manipulation of the aneurysm. Alternatively, it can sometimes be seen in the interhemispheric fissure distal to the aneurysm and traced back to the ACoA complex. The orbitofrontal arteries are identified at their origin just distal to the recurrent artery and often course over the aneurysm or region of the dissection. Anatomic variations at the ACoA complex may add to or subtract from the 11 arteries.
After visualizing the parent arteries, attention is directed to the aneurysm neck and perforator dissection. ACoA aneurysms are notoriously adherent to surrounding structures. Aneurysms projecting superiorly typically adhere to the A2 ACAs, while those projecting inferiorly adhere to the optic apparatus. Posteriorly projecting aneurysms typically adhere to perforators, while anteriorly projecting aneurysms adhere to the frontal lobes. Therefore, the final steps in the dissection are often completed with the aneurysm softened with temporary clips on the A1 segments, mobilizing it from its surrounding adhesions and preparing it for clip application. For this, cerebroprotection with barbiturates is used, in conjunction with neurophysiologic monitoring.26 Electroencephalography and somatosensory evoked potentials are monitored during administration of barbiturates, with the anesthetic dose titrated to achieve electroencephalogram burst suppression. Mild hypothermia is also used routinely for cerebroprotection. Temporary clips can then be applied, with neurophysiologic monitoring indicating the patient’s tolerance to parent artery occlusion during the dissection. If changes in neurophysiologic monitoring are observed, temporary clips can be removed to reperfuse the ischemic territory, or the blood pressure can be elevated with vasopressor agents. Typically, only a few minutes are needed to complete the dissection and apply the clips. Minimizing temporary clip times requires maximizing the dissection that can be done beforehand, preselecting permanent clips, and having an experienced scrub nurse. Surgeon speed is of utmost importance during temporary clipping to reduce risk of ischemic injury. Temporary clipping relaxes the aneurysm to give the neurosurgeon the opportunity to perform the final, risky maneuvers, whether mobilizing the aneurysm to identify the remaining anatomy, clearing the perforators from the path of the clip blades, and/or establishing the cleavage planes between branch arteries and the aneurysm neck.
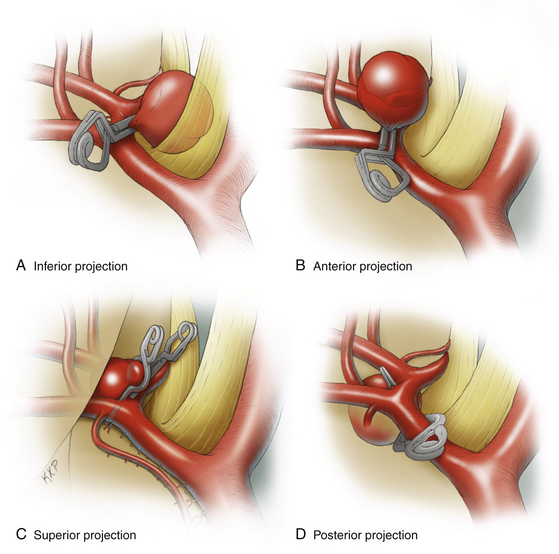
Permanent clipping depends on the aneurysm projection, size, and anatomy of the A1–A2 junction (Fig. 74-7). Aneurysms that project anteriorly and inferiorly can usually be clipped with a straight clip, since the aneurysm and the afferent/efferent arteries are on opposite sides of the ACoA. With these aneurysms, the viewing angle is along the length of the neck, parallel to the ACoA, with good visualization of posterior perforators and parent arteries. Aneurysms that project superiorly can be difficult because of the intimate relationship between the neck and the A2 segments. A clip that parallels the ACoA must be positioned so that its tips do not compromise the contralateral A2 ACA, with the tips either stopping just short of this segment or passing anterior or posterior to it. On the ipsilateral side, a fenestrated clip is often needed to close the proximal neck without compromising the ipsilateral A2 ACA. These requirements demand precise clip application, but fortunately the perforators are not as difficult to dissect as with posteriorly projecting aneurysms. Alternatively, a more posteriorly directed clip can sometimes be applied perpendicular to the ACoA. This clip application can produce a dog-ear remnant below the clip, which might require additional clips below the original one. Aneurysms that project posteriorly can be difficult because not only are the A2 segments intimately associated with the neck on its anterior aspect but so are the perforators on its posterior aspect. These aneurysms often require fenestrated clips such as the superiorly projecting aneurysms, because the clip blades pass through or around the ACA vessels. Most importantly, the back blade must course safely through a complex array of treacherous perforators.
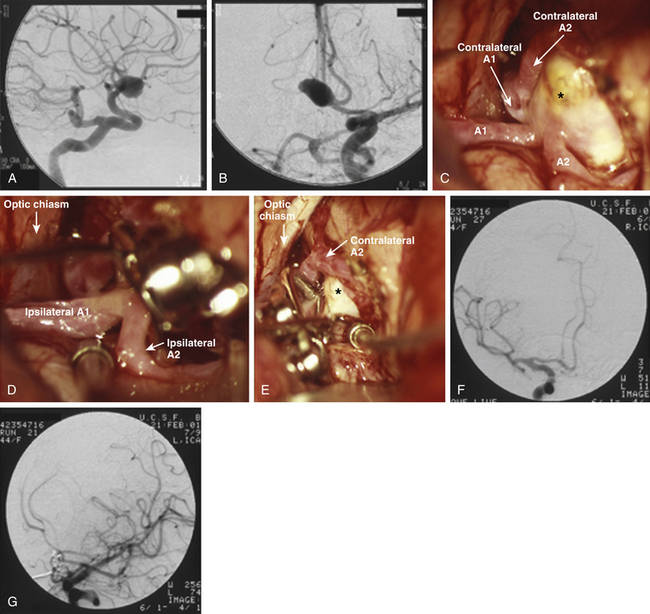
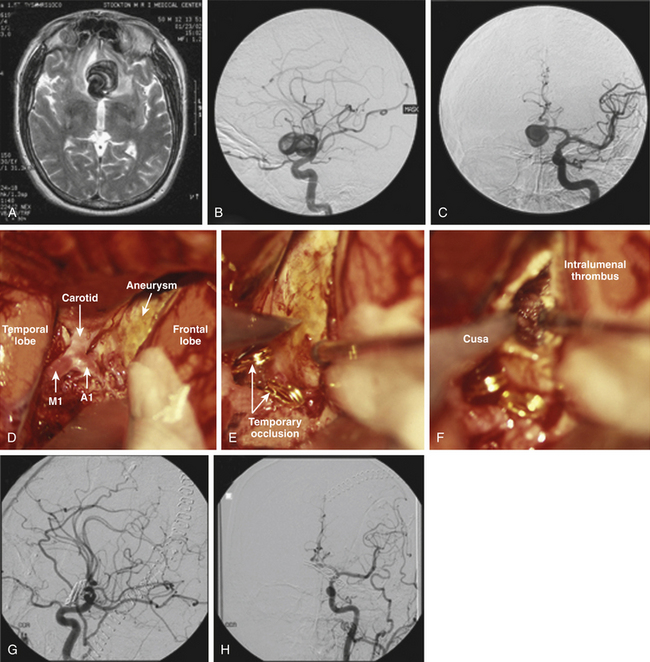
The large and giant ACoA aneurysms have challenges associated with their size, as they do at other locations. Wide necks, fusiform morphology, or an atherosclerotic artery contribute to the complexity for endovascular and microsurgical treatment. These aneurysms often necessitate closure with multiple clips. Single straight clips on these aneurysms tend to fail, because tissue in the proximal blades prevents the distal tip from closing. Fenestrated clips address this problem, with the fenestration encircling only the proximal neck of the aneurysm rather than an A2 segment. Then, a second clip can close the proximal neck encircled by the first clip, thereby completing the repair and contouring the reconstruction. Some of the most challenging aneurysms are those with A2 ACAs originating from the base of the aneurysm (Fig. 74-8), with acute angles between the A1 and the A2 segments or, even worse, with A2 segments that leave the aneurysm parallel to the A1 segments. Clip reconstruction for this unusual anatomy is associated with a significant risk of efferent artery compromise and often requires intraoperative angiography.
Alternative Techniques
Giant aneurysms require techniques not unlike the ones already discussed. The additional room provided by the orbitozygomatic approach makes it routine for these aneurysms.22 Temporary clipping is particularly valuable, because a giant aneurysm that no longer has antegrade filling is deflated and mobile, thereby opening the surgical field and facilitating the dissection and clipping.
With thrombotic aneurysms, the aneurysm does not respond to temporary clipping because thrombus transforms the aneurysm into a solid mass. Therefore, thrombectomy is needed to convert this mass into a deformable sac (Fig. 74-9). After trapping the aneurysm with temporary clips on both A1 and both A2 ACAs, it is entered by incising the wall. With some giant thrombotic aneurysms, complete control is not possible at the outset because the bulk of the aneurysm prevents visualization of contralateral anatomy. In these cases, bleeding can be controlled from inside the aneurysm with a Cottonoid over the orifice of the artery until the involved artery is isolated and temporarily clipped. A Cavitron ultrasonic surgical aspirator (CUSA) is typically used to break up thrombus and clean out the aneurysm lumen, with attention focused at the neck where the clip needs to be placed. Transecting the aneurysm can expedite thrombectomy by freeing the essential tissue needed to reconstruct a neck, rather than gutting the entire lumen and mobilizing the sac. This transaction maneuver can be important, because it minimizes ischemia time and risk of neurologic complication.
Previously coiled aneurysms that have residual filling or that recur are often managed like thrombotic aneurysms (Fig. 74-10). They too behave like a solid mass, and their intraluminal content is largely thrombus. Ideally, coils that have compacted create a neck beneath the coils that will accept a clip, thereby simplifying the management. In other cases, thrombectomy is required to mobilize the neck for clip reconstruction. The CUSA can be used as with purely thrombotic aneurysms, leaving the coil mass in place and clipping around the coils. Only rarely is it advisable to attempt to extract coils from the aneurysm.
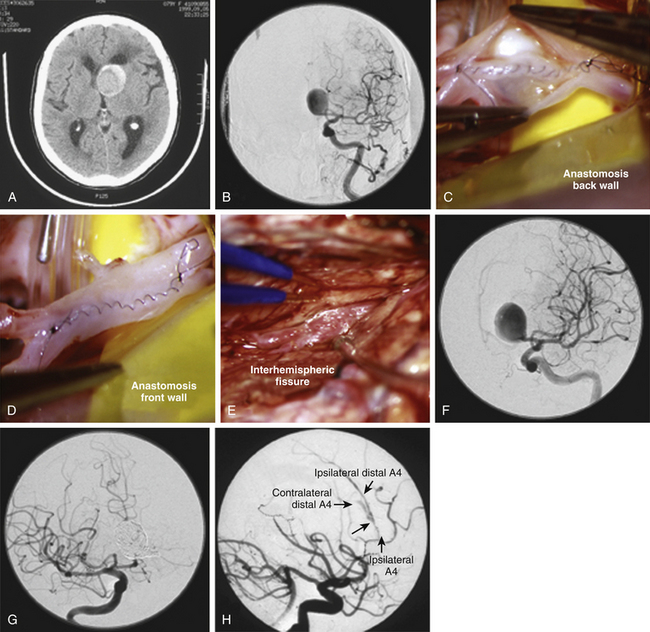
Bypass techniques allow complex aneurysms to be proximally occluded or trapped with minimal risk of ischemic complications. However, revascularization of the distal ACA territory for ACoA aneurysms is more difficult than with other vascular territories and aneurysms. The superficial temporal artery (STA) and occipital artery, the two most commonly used donor arteries for other extracranial-to-intracranial bypasses, are not long enough for a distal ACA bypass. Interposition grafts, such as the radial artery or saphenous vein, bridge from the carotid artery or STA to the ACA. They are long, require extensive exposure, and are prone to late occlusions. In situ bypasses that connect the A3 or A4 ACAs in the interhemispheric fissure with a side-to-side anastomosis can be used when blood flow in one of the efferent A2 arteries can be preserved during the aneurysm occlusion (Fig. 74-11). Most of these bypass procedures require an interhemispheric approach, separate from the pterional approach used for the ACoA aneurysm. A multimodality approach that creates a surgical bypass first and then occludes the aneurysm endovascularly in a separate stage has appeal. It minimizes the extent of surgery, limiting the craniotomy to just an interhemispheric approach rather than a combined pterional–bifrontal approach. It also confirms bypass patency before arterial sacrifice and allows the patient’s medical status and hemodynamics to be optimized for the second-stage procedure. This multimodality strategy has proved effective with atherosclerotic, fusiform, mycotic/infectious, and traumatic aneurysms involving the ACoA complex.27
Postoperative Management
Postoperatively, patients are assessed neurologically and radiographically with CT scan and digital subtraction angiogram. Angiography is essential in documenting complete aneurysm obliteration, patency of parent and branch arteries, and the presence of vasospasm. Patients at risk of developing vasospasm are monitored closely in an ICU. All patients are given nimodipine, starting within 4 days of SAH and continuing for 21 days, regardless of admission grade, at a dosage of 60 mg every 4 hours.28,29 Vasospasm is detected with angiography, transcranial Doppler (TCD) ultrasonography, or changes in neurologic examination. Angiography is the optimal diagnostic study, but it is limited by its invasiveness. TCD ultrasonography is noninvasive and can be done repeatedly to detect trends. Rising TCD velocities guide the timing of more aggressive measures, such as hypertensive therapy and angioplasty.
The mainstays of medical management are hypervolemia, hypertension, and hemodilution, collectively known as HHH therapy.30 Volume expansion is achieved with packed red blood cells, albumin solution, or hypertonic saline solution. Invasive monitoring with either a central venous pressure line or a pulmonary artery catheter is required to guide fluid management. Volume expansion to central venous pressures greater than 8 mm Hg or diastolic pulmonary artery pressures greater than 14 mm Hg is usually enough to dilute the hematocrit to less than 35%. In addition, volume expansion may increase systolic blood pressure to desired end points. As the patient’s clinical condition demands, the blood pressure is elevated further with pressor agents to systolic values between 180 and 220 mm Hg.31
Endovascular therapies for vasospasm are used when aggressive medical management fails, when TCD velocities rise, or when there are multiple risk factors for severe vasospasm. Transluminal balloon angioplasty (TBA) mechanically dilates segments of large cerebral arteries that are in spasm, restoring the normal caliber of the lumen, improving blood flow to ischemic brain, and resulting in clinical improvement.32 Furthermore, the effects of angioplasty appear to last up to a week, which corresponds to the duration of vasospasm.33 The success of this intervention has largely to do with timing. Early angioplasty before or immediately after neurologic deterioration enhances its efficacy. TBA is limited to large cerebral arteries such as the ICA, MCA, and ACA. Smaller distal arteries, notably the A2 ACAs, are not amenable to angioplasty and instead are treated with an intra-arterial papaverine or verapamil infusion. Superselective infusion of a vasodilator can improve the caliber of vasospastic arteries, but the effects are short lived (less than 12 hours). Repeated treatments may be needed for severe distal vasospasm, which limits its utility.
Hydrocephalus is a common complication of SAH, especially when there is significant intraventricular hemorrhage. Up to 65% of all patients requiring an external ventricular drain for hydrocephalus or intraventricular hemorrhage also require permanent ventriculoperitoneal shunting.34 Fenestration of the lamina terminalis during surgery appears to reduce the need for ventriculoperitoneal shunting.
Patients with ACoA aneurysms are susceptible to two postoperative entities: electrolyte abnormalities and the ACoA syndrome. Hyponatremia is frequently seen in SAH patients, with an incidence of around 18%, and lasts from 1 to 5 days.35 This electrolyte abnormality is the result of cerebral salt wasting, a natriuresis and secondary water loss due to the kidneys’ inability to retain sodium. Clinically, cerebral salt wasting can mimic the ischemic neurologic deterioration observed in patients with vasospasm. The laboratory picture of cerebral salt wasting is similar to that of the syndrome of inappropriate antidiuretic hormone secretion (SIADH), except that extracellular fluid volumes are low with cerebral salt wasting and elevated with SIADH. The routine treatment of SIADH, namely, fluid restriction, can be dangerous in patients with cerebral salt wasting, since further dehydration can exacerbate the diminished cerebral blood flow and ischemia in patients with vasospasm. Instead, cerebral salt wasting is treated with normal saline infusion, supplemental salt intake, and sometimes hypertonic saline infusion. The ACoA syndrome after SAH is characterized by impaired memory, personality changes, and confabulation.36
Avci E., Fossett D., et al. Branches of the anterior cerebral artery near the anterior communicating artery complex: an anatomic study and surgical perspective. Neurol Med Chir (Tokyo). 2003;43(7):329-333. discussion 333
Gonzalez L.F., Crawford N.R., et al. Working area and angle of attack in three cranial base approaches: pterional, orbitozygomatic, and maxillary extension of the orbitozygomatic approach. Neurosurgery. 2002;50(3):550-555. discussion 555–557
Iwanaga H., Wakai S., et al. Ruptured cerebral aneurysms missed by initial angiographic study. Neurosurgery. 1990;27(1):45-51.
Kassell N.F., Torner J.C., et al. The international cooperative study on the timing of aneurysm surgery. Part 1: Overall management results. J Neurosurg. 1990;73(1):18-36.
Kerber C.W., Imbesi S.G., et al. Flow dynamics in a lethal anterior communicating artery aneurysm. AJNR Am J Neuroradiol. 1999;20(10):2000-2003.
Molyneux A., Kerr R., et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267-1274.
Perlmutter D., Rhoton A.L.Jr. Microsurgical anatomy of the anterior cerebral-anterior communicating-recurrent artery complex. J Neurosurg. 1976;45(3):259-272.
Yasargil M.G. Microneurosurgery. New York: Georg Thieme Verlag/Thieme Stratton; 1984.
1. Kassell N.F., Torner J.C., et al. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg. 1990;73(1):18-36.
2. Molyneux A., Kerr R., et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360(9342):1267-1274.
3. Rhoton A.L.Jr. Aneurysms. Neurosurgery. 2002;51(Suppl 4):S121-S158.
4. Barker F.G.2nd, Amin-Hanjani S., et al. Age-dependent differences in short-term outcome after surgical or endovascular treatment of unruptured intracranial aneurysms in the United States, 1996-2000. Neurosurgery. 2004;54(1):18-28. discussion 28–30
5. Perlmutter D., Rhoton A.L.Jr. Microsurgical anatomy of the anterior cerebral-anterior communicating-recurrent artery complex. J Neurosurg. 1976;45(3):259-272.
6. Avci E., Fossett D., et al. Branches of the anterior cerebral artery near the anterior communicating artery complex: an anatomic study and surgical perspective. Neurol Med Chir (Tokyo). 2003;43(7):329-333. discussion 333
7. Kerber C.W., Imbesi S.G., et al. Flow dynamics in a lethal anterior communicating artery aneurysm. AJNR Am J Neuroradiol. 1999;20(10):2000-2003.
8. Namiki J., Doumoto Y. Microsurgically critical anomaly of the anterior communicating artery complex during the pterional approach to a ruptured aneurysm: double fenestration of the proximal A2 segments. Neurol Med Chir (Tokyo). 2003;43(6):304-307.
9. Stefani M.A., Schneider F.L., et al. Anatomic variations of anterior cerebral artery cortical branches. Clin Anat. 2000;13(4):231-236.
10. Baptista A.G. Studies on the arteries of the brain. II. The anterior cerebral artery: some anatomic features and their clinical implications. Neurology. 1963;13:825-835.
11. Serizawa T., Saeki N., et al. Microsurgical anatomy and clinical significance of the anterior communicating artery and its perforating branches. Neurosurgery. 1997;40(6):1211-1216. discussion 1216–1218
12. Yasargil M.G. Microneurosurgery. New York: Georg Thieme Verlag/Thieme Stratton; 1984.
13. Lownie S.P., Drake C.G., et al. Clinical presentation and management of giant anterior communicating artery region aneurysms. J Neurosurg. 2000;92(2):267-277.
14. Chan J.W., Hoyt W.F., et al. Pathogenesis of acute monocular blindness from leaking anterior communicating artery aneurysms: report of six cases. Neurology. 1997;48(3):680-683.
15. Sames T.A., Storrow A.B., et al. Sensitivity of new-generation computed tomography in subarachnoid hemorrhage. Acad Emerg Med. 1996;3(1):16-20.
16. Iwanaga H., Wakai S., et al. Ruptured cerebral aneurysms missed by initial angiographic study. Neurosurgery. 1990;27(1):45-51.
17. Dehdashti A.R., Rufenacht D.A., et al. Therapeutic decision and management of aneurysmal subarachnoid haemorrhage based on computed tomographic angiography. Br J Neurosurg. 2003;17(1):46-53.
18. Villablanca J.P., Jahan R., et al. Detection and characterization of very small cerebral aneurysms by using 2D and 3D helical CT angiography. AJNR Am J Neuroradiol. 2002;23(7):1187-1198.
19. Vermeulen M. Subarachnoid haemorrhage: diagnosis and treatment. J Neurol. 1996;243(7):496-501.
20. Bradac G.B., Bergui M., et al. False-negative angiograms in subarachnoid haemorrhage due to intracranial aneurysms. Neuroradiology. 1997;39(11):772-776.
21. Dorai Z., Hynan L.S., et al. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003;52(4):763-769. discussion 769–771
22. Gonzalez L.F., Crawford N.R., et al. Working area and angle of attack in three cranial base approaches: pterional, orbitozygomatic, and maxillary extension of the orbitozygomatic approach. Neurosurgery. 2002;50(3):550-555. discussion 555–557
23. Schwartz M.S., Anderson G.J., et al. Quantification of increased exposure resulting from orbital rim and orbitozygomatic osteotomy via the frontotemporal transsylvian approach. J Neurosurg. 1999;91(6):1020-1026.
24. Lemole G.M.Jr., Henn J.S., et al. Modifications to the orbitozygomatic approach. Technical note. J Neurosurg. 2003;99(5):924-930.
25. Coscarella E., Vishteh A.G., et al. Subfascial and submuscular methods of temporal muscle dissection and their relationship to the frontal branch of the facial nerve. Technical note. J Neurosurg. 2000;92(5):877-880.
26. Spetzler R.F., Hadley M.N., et al. Aneurysms of the basilar artery treated with circulatory arrest, hypothermia, and barbiturate cerebral protection. J Neurosurg. 1988;68(6):868-879.
27. Lawton M.T., Quinones-Hinojosa A., et al. Combined microsurgical and endovascular management of complex intracranial aneurysms. Neurosurgery. 2003;52(2):263-274. discussion 274–275
28. Allen G.S., Ahn H.S., et al. Cerebral arterial spasm: a controlled trial of nimodipine in patients with subarachnoid hemorrhage. N Engl J Med. 1983;308(11):619-624.
29. Barker F.G.2nd, Ogilvy C.S. Efficacy of prophylactic nimodipine for delayed ischemic deficit after subarachnoid hemorrhage: a metaanalysis. J Neurosurg. 1996;84(3):405-414.
30. Sen J., Belli A., et al. Triple-H therapy in the management of aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2003;2(10):614-621.
31. Kassell N.F., Peerless S.J., et al. Treatment of ischemic deficits from vasospasm with intravascular volume expansion and induced arterial hypertension. Neurosurgery. 1982;11(3):337-343.
32. Bejjani G.K., Bank W.O., et al. The efficacy and safety of angioplasty for cerebral vasospasm after subarachnoid hemorrhage. Neurosurgery. 1998;42(5):979-986. discussion 986–987
33. Weir B., Grace M., et al. Time course of vasospasm in man. J Neurosurg. 1978;48(2):173-178.
34. Klopfenstein J.D., Kim L.J., et al. Comparison of rapid and gradual weaning from external ventricular drainage in patients with aneurysmal subarachnoid hemorrhage: a prospective randomized trial. J Neurosurg. 2004;100(2):225-229.
35. Landolt A.M., Yasargil M.G., et al. Disturbances of the serum electrolytes after surgery of intracranial arterial aneurysms. J Neurosurg. 1972;37(2):210-218.
36. Alexander M.P., Freedman M. Amnesia after anterior communicating artery aneurysm rupture. Neurology. 1984;34(6):752-757.