CHAPTER 362 Surgical Decision Making for the Treatment of Intracranial Aneurysms
Overview: The Status of the Patient and the Natural History of the Aneurysm
The status of the patient and the natural history of the aneurysm must be considered together. Many patient-related factors will determine outcome irrespective of the technical treatment of the aneurysm. Choosing the treatment modality that is safest and most efficient for each individual patient is an important therapeutic decision. In addition, a variety of factors such as whether the aneurysm is intact or ruptured, aneurysm size and location, the patient’s age and medical condition, and associated factors such as intracerebral hemorrhage (ICH), intraventricular hemorrhage (IVH), and clinical grade after aneurysm rupture can all influence the outcome of attempted aneurysm occlusion. It is important therefore to consider these various factors before treating each patient. Taken together, the natural history of the patient and the natural history of the particular aneurysm must be considered in evaluating the overall approach to treatment. For example, the treatment of a small basilar tip aneurysm in a 40-year-old and in an 80-year-old will differ despite the similarity in the lesion. Thus exclusive focus on either the patient or the aneurysm is not an optimal management strategy. Patient considerations and the natural history of cerebral aneurysms are comprehensively reviewed in Chapter 354 and Chapter 360.
Neuroradiological Evaluation
These studies include computed tomography (CT), a variety of magnetic resonance imaging (MRI) sequences, CT angiography (CTA), MR angiography (MRA), and cerebral angiography. Digital subtraction angiography (DSA) is the current “gold standard” to evaluate aneurysms. Noninvasive techniques are evolving rapidly and CTA in particular frequently can provide a useful complement to DSA. A combination of imaging techniques often is necessary, particularly for more complex aneurysms; this permits accurate preoperative planning and a full understanding of the relevant anatomy and these studies will often be critical in determining timing (e.g., presence of intracerebral clot in a poor grade patient), selection of treatment options (e.g., endovascular versus open surgery) and surgical approaches (e.g., low-versus high-lying basilar tip aneurysm; atretic A-1 in an anterior communicating aneurysm). For surgical planning, CTA with three-dimensional reconstructions and spin, rotational, and three-dimensional DSA (3D-DSA) data in an orientation that reproduces the neurosurgeon’s intraoperative view is a valuable adjunct.1 This is particularly useful for large and complex aneurysms. The following features need to be evaluated: (1) the aneurysm’s parent vessel; (2) aneurysm size, shape, and relationship to parent and adjacent arteries; (3) the presence and location of vasospasm; (4) adjacent vessel displacement consistent with mass effect (e.g., partial aneurysm thrombosis [i.e., the aneurysm is larger than that seen on DSA]); and (5) the presence of other aneurysms or vascular abnormalities. The reader should consult Chapter 359 and Chapter 360 for more detailed information.
Unruptured and Ruptured Aneurysms: Impact on Decision Making
Unruptured Aneurysm
Efficacy of Treatment
There are few studies that evaluate the success rate of surgery in achieving complete occlusion of unruptured aneurysms and the long-term subsequent angiographic reoccurrence or SAH. Tsutsumi and coworkers2 followed 115 patients for almost 9 years after their unruptured aneurysm had been clipped and found only 1 patient (0.8% over 8.8 years or <0.01% per year) suffered a SAH from regrowth of the original aneurysm. Most studies are focused on patients initially having a SAH or a mixed population of both intact and ruptured aneurysm patients. Extrapolation from surgical series after a SAH suggests that the complete occlusion rate is high and that such occlusion is not perfect, but protective (see discussion later).
Surgical Risk
Surgical outcome after repair of unruptured aneurysms has been described in several case series dealing with unruptured aneurysms. Most case series3–9 demonstrate that the morbidity and mortality associated with surgical treatment of unruptured aneurysms is very low; about 5% of patients die or are disabled. By contrast, findings from the International Study of Unruptured Intracranial Aneurysms (ISUIA), a prospective study including 1172 patients, 85% of whom underwent surgery, suggested that overall surgical risk was much higher; the combined morbidity and mortality approached 15% at 1 year.10 In particular, surgical risk was greater than the risk of hemorrhage among patients with an incidental aneurysm less than 10 mm in diameter. In ISUIA II in-hospital mortality was 1.8% and adverse outcomes were observed in 8.9% of survivors.11 Administrative discharge data from New York and California suggest mortality is between 2.5% and 3% and adverse events occur in 21.3% to 22.4% of patients.12,13
Several authors have performed meta-analyses of surgical series to clarify surgical risk14; the majority suggest that the combined morbidity and mortality is between 3% and 7%. For example, King and associates14 performed a meta-analysis of 28 clinical series containing 733 patients; overall, 4.1% of patients were disabled and 1% died. Similarly, Raaymakers and coworkers15 performed a meta-analysis from a Medline search between 1966 and 1996 and identified 61 studies involving 2460 patients. Surgical morbidity was 10.9% and mortality 2.6%; the risk of a poor outcome decreased in more recent years. It is difficult, however, to derive meaningful conclusions by combining the various surgical series for several reasons: (1) most are reported by surgeons with an inherent bias; (2) unruptured aneurysms of various sizes and location are included in each series; (3) the technique of aneurysm occlusion, such as wrapping or clipping, is not always described; (4) the effectiveness of surgery is rarely described because postoperative angiography is rarely performed; (5) description of outcome measures is lacking; and (6) neuropsychological and quality of life assessment is lacking. It must be recognized that much of the data on results of aneurysm surgery also come from high volume centers and single-center studies, and publication bias in particular must be considered when applying the reported risks of aneurysm surgery to a broader population.16 In addition administrative data often describe a discharge condition rather than a 3- or 6-month outcome.
Factors That Are Associated with Surgical Outcome
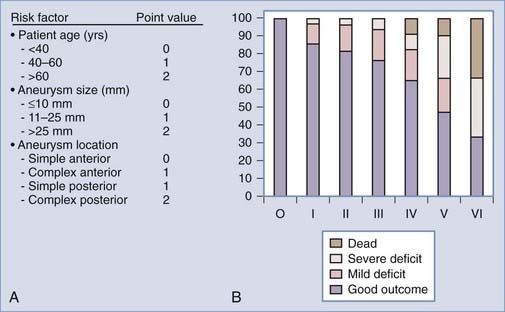
Increased aneurysm size is the most important factor associated with surgical complications and poor outcome (Fig. 362-1).7,17,18 Overall, aneurysm size greater than 25 mm has a fourfold increased risk compared with a 5-mm aneurysm.19 For example, Wirth and associates8 demonstrated that significant operative morbidity complicated surgery in 2% of unruptured aneurysms less than 5 mm, 7% of aneurysms between 6 and 15 mm, and 14% of aneurysms greater than 16 mm in diameter. Similarly, Solomon and coworkers7 observed a favorable outcome in 83% of patients who underwent surgical repair of giant unruptured aneurysms. By contrast, 99% of patients who had aneurysms less than 10 mm in diameter experienced good outcomes. The association between increased aneurysm size and poor outcome may be explained, in part, by the aneurysm’s intimate association with small perforators, the broad aneurysm neck, intraluminal thrombosis, or atherosclerosis in the aneurysm neck or dome.
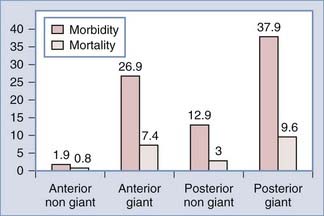
FIGURE 362-1 Surgical risk for unruptured intracranial aneurysms.
(The data are from 61 studies including 2460 patients treated between 1966 and 1996 and reviewed in Raaymakers TW, Rinkel G, Limburg M, and coworkers. Mortality and morbidity of surgery for unruptured intracranial aneurysms. A meta-analysis. Stroke. 1998;29:1531-1538.)
Repair of unruptured posterior circulation aneurysms, particularly giant basilar bifurcation aneurysms, is associated with increased surgical risk (Fig. 362-1).15,19 For example, Solomon and associates7 observed that giant unruptured basilar aneurysms were associated with 50% surgical morbidity and mortality. By contrast, the morbidity and mortality for giant anterior circulation unruptured aneurysms was 13%. Reasonable surgical results can be expected with nongiant unruptured posterior circulation aneurysms.6 However, when compared with similar size lesions in the anterior circulation, there is a nearly tenfold increase in risk.19,20 In the anterior circulation, anterior communicating artery and ICA bifurcation lesions appear to have the greatest risk.8
Several other aneurysm features identified on angiogram such as aneurysm orientation, wide aneurysm neck, atherosclerosis, and calcification in the aneurysm neck are associated with increased risk. For example, we have observed a significant increase in surgical complications among posterior oriented basilar bifurcation aneurysms.21 Similarly, there is an increased risk among posterior superior oriented anterior communicating artery aneurysms. Calcification in the aneurysm neck often is associated with poor outcome, in part, because many of these lesions are large or giant aneurysms. In addition, multiple clips frequently are necessary to occlude the aneurysm, leading to an increased incidence of cerebral embolism. Calcification may be removed by endarterectomy; despite this, surgical results remain poor because the remaining wall for clip placement is often very friable and thin. Preoperative CT scanning can help identify neck calcification. Hypothermic circulatory arrest should be considered in the reconstruction of some heavily calcified aneurysms.
There are several patient-related factors including advanced age, ischemic cerebrovascular disease, and medical conditions such as diabetes mellitus that increase the risk of unruptured aneurysm surgery.7,8,17,19 A recent retrospective, single-center, multivariate analysis by Ogilvy and coworkers demonstrated that after aneurysm size and location, patient age is the next most important risk factor of poor outcome.18 In addition, in some patients such as those with severe cardiac or pulmonary disease, the anesthetic risk of surgery may carry a great immediate risk whereas in others, such as patients with advanced malignancy, any potential benefit is negated by a reduced life expectancy.
Which Patient with an Unruptured Aneurysm Should Be Treated?
In 1994 and 200922,23 the American Heart Association published guidelines for the treatment of unruptured intracranial aneurysms. Although there are no randomized trials, the consensus reports recommended occlusion of unruptured aneurysms greater than 5 to 7 mm among patients with acceptable surgical risk.
Since then several studies have improved our understanding of the natural history and surgical risk and alternate treatments for unruptured aneurysms have evolved. Management should be tailored to the patient’s risk and the physician’s own experience. Microsurgical, endovascular, and alternative strategies such as vessel occlusion with or without bypass or no treatment other than observation and clinical follow-up should be considered, specific to the patient and the aneurysm. Khanna and associates19 have attempted to do this by creating a grading scale for unruptured aneurysms (Fig. 362-2). This scale was derived from a retrospective analysis of 172 patients who underwent treatment of unruptured aneurysms and then validated prospectively in 50 patients. The incidence of poor outcome progressively increased from 0% in grade 0 to 66% in grade IV patients. Garrett and associates24 recently performed a comprehensive literature review (10,545 patients) and using mathematical models created an online open access program to quantify the benefit of surgery on unruptured aneurysms according to age, size, and location. Small basilar and supraclinoid aneurysms (5 mm) only had an operative benefit in young patients (40 years). By contrast, larger aneurysms (10 to 15 mm) had an operative benefit for a wider age range. There was no age and size combination that provided an operative benefit for cavernous aneurysms.
The case fatality rate for aneurysmal SAH remains high,25 and it is recognized that the main determinant of outcome is the severity of the initial bleed. It is therefore postulated that if SAH could be prevented before aneurysm rupture, poor outcomes theoretically may be avoided. However, because few asymptomatic aneurysms actually rupture and because all aneurysm treatment carries some risk, the management of patients with an unruptured aneurysm remains controversial.22 Based on the available literature, we recommend the following patients with unruptured aneurysms should be treated: (1) SAH from another aneurysm, (2) symptomatic aneurysm, (3) aneurysms more than 7 to 10 mm in nearly all patients with a life expectancy of 12 or more years, and (4) aneurysms more than 5 mm if the patient is young or middle-aged. These recommendations depend on collaboration between a highly experienced cerebrovascular team of microneurosurgeons and endovascular neurosurgeons at a tertiary medical center with a high case volume. Small, incidental aneurysms less than 5 mm in diameter should be managed conservatively in most cases; the exception is when there is a positive family history. In addition patients who smoke and are hypertensive may be considered for treatment when the aneurysm is small. In select patients when both treatment and natural history carry very high risks (e.g., those with giant aneurysms), nonoperative management also may be elected. When aneurysm occlusion is not recommended, patients should be counseled about risk factors and serial imaging (CTA or MRA) used to follow the aneurysm because an increase in size or a morphology change appears to represent an indication for treatment. For a small aneurysm (e.g., 4 mm) a 1-mm change in diameter represents a doubling of the aneurysm volume. This size change, however, may be difficult to detect and it is not precisely defined whether growth always proceeds rupture. In addition, how frequently imaging is required is not clear. Consequently recommendation of serial imaging is somewhat controversial (we obtain serial noninvasive imaging every 6 months).
The Ruptured Aneurysm
Aneurysm Rebleeding
Rebleeding is a major cause of poor outcome after SAH and is currently the most treatable cause of poor outcomes and will be a determinate factor in the timing of treatment (see later discussion). Approximately 70% to 90% of patients who rebleed die. Untreated, between 20% and 30% of aneurysms rerupture within the first 30 days and then at a rate of approximately 3% to 5% per year.26 The risk of rebleeding is greatest on day 1 (4%)—it may also complicate about 10% of patients in the ambulance or at the referring hospital before admission to the treating hospital.27 Recent evidence suggests that the risk of “ultra early rebleeding” (within 24 hours of SAH) may be as high as 15%.27,28 Rebleeding then occurs at a constant rate of between 1% and 2% per day during the subsequent 4 weeks.29,30 Poor clinical grade, abnormal hemostatic parameters, higher rates of intracerebral hematoma or intraventricular hematoma and posterior circulation aneurysms appear to be associated with a greater risk of rebleeding.27,31,32 Some studies suggest that ventricular drainage may be associated with rebleeding; however, when preoperative ventriculostomy is followed by early treatment of the ruptured aneurysm, the rebleeding risk is not increased by the ventriculostomy.33 Blood pressure must be monitored and controlled; however, the relationship of elevated blood pressure to rebleeding is not well elucidated, and control of hypertension must be balanced against the risk of cerebral infarction.22,27,32,34 The major goal in SAH treatment is aneurysm obliteration to prevent rebleeding. Aneurysm rebleeding may be reduced by antifibrinolytic administration, but can only be prevented by direct obliteration using surgical or endovascular techniques. No well-controlled studies exist that answer whether blood pressure control in acute SAH influences rebleeding. There are two important questions: when should surgery be performed and which patients should undergo surgical or endovascular aneurysm occlusion?
Timing of Aneurysm Obliteration
Early surgery eradicates the risk of rebleeding and appears to be associated with improved outcome. For example, among the 722 patients treated at 27 North American centers in the International Cooperative Study on the Timing of Aneurysm Surgery, a prospective epidemiologic, but nonrandomized study found early surgery significantly improved outcome.35 Adjusted overall outcome demonstrated that 70.9% of patients undergoing surgery between 0 and 3 days after aneurysm rupture experienced a good recovery, whereas 62.9% of patients enjoyed a similar outcome if surgery was performed after 14 days. Brilstra and associates36 examined how effective aneurysm clipping was at reducing poor outcome associated with rebleeding: a risk reduction of 19% was observed in patients who had surgery rather than conservative management.
For each patient with a ruptured aneurysm, there are several factors, including aneurysm-related, hemorrhage-related, and patient-related factors, that influence whether to operate acutely or in a delayed fashion. Among patient-related factors, neurological grade and age are the most important. Poor grade patients are at greater risk for rebleeding and vasospasm.37,38 Early aneurysm occlusion should therefore be achieved. Although cerebral swelling is more frequent, early surgery in poor grade patients is not associated with a greater risk of surgical complications than in good grade patients.39 Alternatively, those patients who demonstrate significant swelling on admission CT scan may be excellent candidates for endovascular occlusion. Advanced age is associated with poor outcome; however, this should not exclude the patient from early surgery, in part because elderly patients are more likely to have intracerebral hematomas and a decreased cerebrovascular reserve that increases their risk for delayed ischemia.40 Similarly these patients may be excellent candidates for endovascular aneurysm occlusion. Early surgery may be useful in those patients at high risk for vasospasm, such as those with thick SAH on CT, in part because the role of hypervolemic therapy and angioplasty may be best when the aneurysm is secured. Delayed surgery may be preferable for complex lesions such as giant aneurysms or those where prolonged periods of temporary occlusion are expected to achieve aneurysm occlusion.
Several lines of evidence demonstrate that surgery within 3 days of SAH is associated with improved outcome among patients with ruptured anterior circulation aneurysms. For example, a single randomized study has addressed surgical timing in good clinical grade (Hunt and Hess I-III) patients following anterior circulation aneurysm rupture.41 At 3 months, independent outcomes were observed in 91.5% of patients undergoing surgery within 3 days, 78.6% between 4 and 7 days, and 80% undergoing surgery greater than 8 days after a SAH. Nonrandomized studies that describe concurrent cohorts or historical controls and large clinical series35,42–48 have observed a tendency for patients undergoing early surgery to experience better outcomes.
The timing of aneurysm obliteration for posterior circulation aneurysms is less well defined. In addition, because many of these patients now undergo endovascular procedures, the question may be less relevant. Most information that favored delayed surgery came from specialized referral centers. This may bias results because epidemiologic studies demonstrate that patients with posterior circulation aneurysms are 3 times more likely to die before reaching the hospital or within the first 48 hours of a SAH than patients with anterior circulation lesions.49–51 Several recent studies suggest that early surgery may reduce morbidity and mortality.52–54 For example, Hillman and associates53 prospectively reviewed 59 cases of ruptured posterior fossa aneurysms treated during 1 year. Fifty percent of patients scheduled for late surgery (n = 26) made a good recovery, whereas 72% scheduled for early surgery (n = 23) made a good recovery. Intraoperative complications were equally frequent in early or delayed surgery. When surgery is indicated rather than endovascular occlusion, we recommend early surgery for ruptured posterior circulation aneurysms except for those lesions that may present technical difficulty, such as large posterior-oriented basilar bifurcation aneurysms.
Earlier aneurysm occlusion using surgical or endovascular techniques has reduced the impact of rebleeding on outcome.32 In addition, early aneurysm occlusion permits more aggressive and early management of cerebral vasospasm with hemodynamic therapy and interventional management. Increased time to treatment, however, remains associated with increased rates of preoperative rebleeding. There is evidence that time from SAH to treatment is shorter in patients who have their aneurysm occluded with endovascular techniques. For example, in the International Subarachnoid Aneurysm Trial (ISAT), the mean time to treatment was 1.1 days for endovascular occlusion and 1.8 days for surgery. Consequently there were fewer preoperative rebleeds in the endovascular group.55,56 Some investigators28,57 advocate short-term antifibrinolytic therapy (e.g., ξ-aminocaproic acid [EACA]) started at diagnosis and administered for a maximum of 72 hours to reduce rebleeding while waiting for treatment.
Efficacy of Aneurysm Surgery
Aneurysm surgery is effective; overall more than 90% of aneurysms undergoing surgical clip occlusion are completely obliterated at surgery.39,58–62 For example, Feurerberg and associates60 retrospectively examined 715 patients operated on between 1970 and 1980. Twenty-seven patients (3.8%) had incomplete obliteration on follow-up angiography; only 1 patient rebled during 266 person-years of follow-up. David and associates59 observed that the rate of complete occlusion on postoperative angiography was 91.8%. The rate of aneurysm recurrence was 0.5% and there were no hemorrhages among completely occluded aneurysms. The annual hemorrhage rate was 1.9% among incompletely occluded aneurysms. However, there are very few studies that report the routine use of postoperative angiography, suggesting that many patients may not undergo postoperative evaluation. This may be important because several clinical series suggest that even when a surgeon believes the operative result is satisfactory, vessel occlusion or aneurysm remnants may be found on 5% of postoperative angiograms.61,63–65
What factors are associated with failure to occlude the aneurysm? Aneurysmal remnants and vessel occlusion are more likely when a large aneurysm or cerebrovascular atherosclerosis is identified or multiple attempts to place aneurysm clips are made.61 For example, Solomon and associates,7 describing the surgical treatment of 202 unruptured aneurysms, observed that increased aneurysm size is associated with poor technical results. Among giant aneurysms only 60% underwent clip occlusion at surgery. By contrast, clip occlusion was achieved in 85% of lesions greater than 10 mm and 93% of lesions less than 10 mm. Postoperative angiographs were obtained in two thirds of the patients; complete aneurysm occlusion was observed in greater than 90% of the small aneurysms but only 54% of the giant aneurysms. During surgery, inadequate brain relaxation is associated with poor technical results. For example, Giannotta and Litofsky66 retrospectively reviewed 524 patients, including 20 who required reoperation for inadequately treated aneurysms. Fourteen of the reoperations were attributed to failure to obtain a slack brain or inadequate bone exposure at the initial surgery.
The Poor Grade Patient
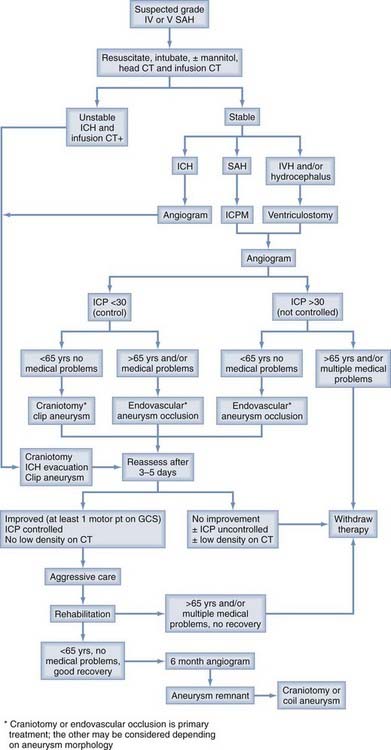
Between 20% and 40% of patients admitted to a hospital after aneurysm rupture are in poor clinical condition (Hunt and Hess grades IV and V). The association between poor outcome and poor clinical grade following a SAH is well described. However, the published data suggest that an aggressive policy may provide these patients their best chance of recovery (Fig. 362-3). This treatment includes rapid resuscitation and transport to a hospital, intracranial pressure (ICP) control, early aneurysm occlusion, prophylaxis against delayed cerebral ischemia, and care in a dedicated neurointensive care unit. In addition the use of noninvasive physiologic assessment such as transcranial Doppler, single photon emission computed tomography (SPECT), and invasive monitors for ICP and cardiac hemodynamics are necessary to guide therapy. Control of ICP is important25 and some poor grade patients may benefit from a decompressive craniectomy.67,68 Other monitors, such as microdialysis or brain oxygen offer promise but are still relatively clinically unproved.69 We routinely monitor brain oxygen tension (PbtO2) to help prevent brain hypoxia because this is associated with worse outcome.70 Epidemiologic studies demonstrate that up to 15% of the patients die before reaching the hospital and 30% die within the first 48 hours of aneurysm rupture.49,50,71,72 To be successful, therefore, an aggressive approach requires an organized multidisciplinary, proactive critical care approach that starts with in-the-field resuscitation and immediate ICP control. Alternate, but less successful, strategies include treatment of select patients, or delayed treatment when clinical improvement is observed.37,73,74
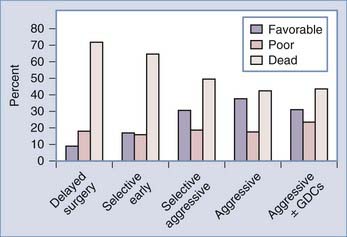
FIGURE 362-3 Overall management outcome for Hunt and Hess grade IV or V patients.
(Modified from Table 21-3 in Le Roux P, Winn HR. Management of the ruptured aneurysm. In: Le Roux P, Newell D, Winn HR, eds. Management of Cerebral Aneurysms. Philadelphia: Elsevier; 2004:303-333.)
Aggressive management requires a large commitment of resources; should all poor grade patients be treated? In the individual poor grade patient, admission clinical and radiographic findings are often insufficient to accurately predict outcome.37,73–77 For example, predicting outcome based only on admission findings may result in withholding treatment from 30% of the grade IV and V patients who subsequently experience favorable outcomes.74 Consequently we believe that management should be initiated in most patients. Continued observation, including an ICP monitor and follow-up CT scan, helps define outcome. Most poor grade patients who do well are able to follow commands within 5 days of aneurysm rupture whereas those who die may do so within the same time frame.73,74,77 In addition, patients who do poorly frequently develop intractable intracranial hypertension and progressive low density changes on follow-up CT scan.37,74 A suggested management algorithm for poor grade patients is illustrated in Fig. 362-4.
Endovascular occlusion of the acutely ruptured aneurysm is an attractive alternative to surgery for poor grade patients. However, the role of endovascular therapy in these patients has only been described in limited clinical series and very few poor grade patients were included in the ISAT trial.55,56,78–82 The few case series published suggest that endovascular therapy is a reasonable option in some poor grade patients, and that combined endovascular and open surgical therapies may be beneficial, particularly in the presence of ICH. For example, Suzuki and associates83 described 80 Hunt and Hess grade IV and 31 grade V patients, including 7 whose aneurysm was coiled and then had a craniotomy for hematoma evacuation. Overall mortality was 32.4% and a favorable outcome (mRS 1-2) was observed in 43.8% of grade IV and 12.9% of grade V patients.
A potential advantage of endovascular therapy for poor grade patients is that it may be physiologically less stressful because brain retraction and vessel dissection is not required. Consequently, endovascular therapy may be the preferable treatment for poor grade patients when extensive cerebral swelling is seen on CT scan or ICP is not controlled. In addition, endovascular therapy may be useful in elderly patients where long-term coil stability may be less relevant (Fig. 362-5). However, in the elderly, outcome may be less than expected after GDC treatment, in large part because there is 3 times greater risk of thrombo-embolic complications from atherosclerosis.84 Surgery, however, should be the primary treatment in young patients, when the ruptured aneurysm is associated with an ICH, the aneurysm is associated with mass effect, or the aneurysm, such as a wide-necked lesion, is not suitable for coil occlusion. There has been limited comparison of the use of endovascular techniques for poor grade patients to other management strategies. Inamasu and associates,82 using historical controls, observed no benefit to using endovascular techniques over a conservative policy; what determined outcome was not how the aneurysm was occluded, but rather in what condition the patient was and how the consequences of the SAH were managed. Groden and associates85 compared outcome in patients treated using surgical (n = 20), endovascular (n = 20), or by both modalities (n = 1); there was no difference in outcome.
Intracerebral Hemorrhage
ICH increases mortality after SAH.86–88 A single randomized study89 and several clinical series demonstrate a tendency for improved patient outcome when emergency ICH evacuation is performed, particularly when simultaneous aneurysm obliteration is achieved.75,90,91 Factors such as young age, better clinical grade, and small ICH volume (<25 mL) are associated with better outcome. However many comatose patients, including those with brainstem compression and large ICH can experience a favorable outcome if rapidly resuscitated and operated on within a few hours of aneurysm rupture.75 We obtain angiography on patients with suspected aneurysmal ICH provided they are neurologically stable. In the unstable patient, however, even single-vessel angiography may cause a life-threatening delay. Infusion CT scan, or CTA92–95 obtained immediately after the head CT scan, are useful in these patients; both techniques are rapid and can detect greater than 90% of aneurysms greater than 3 to 5 mm in diameter. In the neurologically unstable patient we then proceed to craniotomy, ICH evacuation, and aneurysm obliteration based on the CT angiogram alone (see Fig. 362-4). With this strategy we have found that 30% of moribund patients with clinical and CT evidence of brainstem compression after aneurysmal ICH are independent and living at home with only mild disability 6 months later.75 The role of endovascular aneurysm occlusion and then ICH evacuation83,96 and the role of decompressive craniectomy are attractive management strategies but their use is not yet well defined in SAH.
Acute Intraventricular Hemorrhage and Hydrocephalus
Acute hydrocephalus (ventricular enlargement within 72 hours) and IVH often are observed after aneurysm rupture, particularly in poor grade patients and those with thick subarachnoid blood on CT,97–100 although increased ICP also is seen in as many as 50% of good grade patients.25 External ventricular drainage (EVD) is recommended, particularly when the patient has depressed consciousness: 40% to 80% have some degree of improvement after the procedure.97,100 Several clinical series describe good results using EVD for hydrocephalus or IVH following aneurysm rupture provided early aneurysm occlusion is achieved.98,101 Attempts to remove casted intraventricular blood through craniotomy or the infusion of urokinase, however, do not appear to improve outcome.102,103 “Routine” EVD use in grade IV and V patients is advocated; the role in grade III patients is less certain.104 One recent study found a significant reduction in poor outcomes and clinical vasospasm using a lumbar drain placed intraoperatively and kept for 24 or more hours; however, this finding has not been replicated and the study has several methodological limitations.105 Clinical improvement within 24 hours of starting EVD suggests a favorable outcome; however, clinical improvement after ventricular drainage is not always associated with a favorable outcome.76,77,99,100 In addition, many patients who do not improve can undergo surgery with satisfactory results. Several authors have observed that EVD increases the risk of aneurysm rebleeding97,101,106,107 or impairs natural mechanisms that arrest aneurysm rupture.108 Ventricular drainage should therefore be performed carefully to prevent altering aneurysm transmural pressure that may precipitate rebleeding. Acute hydrocephalus is often associated with vasospasm, as well as intracranial hypertension and reduced cerebral blood flow (CBF);25,109–111 ventricular drainage should therefore be accompanied by early aneurysm occlusion to allow effective use of hyperdynamic therapy and angioplasty.
Chronic hydrocephalus is observed in about 25% who survive aneurysm rupture and its neurological and/or medical sequela.110 Half the patients with acute hydrocephalus eventually require a ventriculoperitoneal shunt. Factors associated with hydrocephalus include older age, increased ventricular size, and IVH at admission; poor clinical grade; preexisting hypertension; alcoholism; female sex; increased aneurysm size; pneumonia; and meningitis.110 The rate of shunt dependency is associated with a worse clinical grade, a higher Fisher computed tomographic grade, IVH, repeat SAH, and aneurysms arising at the anterior communicating artery. The need for a permanent shunt can be reduced by EVD, including long tunneled catheters, serial lumbar punctures, and perhaps by lamina terminalis fenestration at craniotomy.97,104,112 Though several series suggest that whether an aneurysm is occluded using surgery or endovascular techniques does not influence the subsequent risk for hydrocephalus,113–115116 a recent meta-analysis by de Oliveira and associates113 found significantly greater risk for shunt dependency after coiling than after clipping. Though coiling may not cause shunt-dependent hydrocephalus, it appears clipping may offer a reduced risk, possibly through clot evacuation. The implications of these studies are limited, however, because clot evacuation was not quantified in any of the studies and, at least in the study by de Oliveira and associates, IVH was significantly more common in the coiled group, which may represent a significant confounder.113
Ruptured Aneurysms and Early Vasospasm
Vasospasm is associated with poor outcome; between 10% and 15% of patients will have angiographic evidence of vasospasm within 48 hours of aneurysm rupture.117 What management is appropriate for these patients? Recent experimental and clinical observations suggest that surgical manipulation of blood vessels may not exacerbate the arterial narrowing seen after SAH.118–121 For example, in patients undergoing preoperative and postoperative angiography, surgical timing does not alter the incidence of angiographic vasospasm,119 or correlate with infarction, provided prophylactic hypervolemia is instituted early.121 Qureshi and associates117 in an analysis of 296 patients in the placebo arm of a multicenter trial found that early vasospasm is associated with subsequent poor outcome and an increased risk of symptomatic vasospasm. However, early surgery reduced the risk of poor outcome in these patients. We have found that prompt aneurysm obliteration, followed by immediate angioplasty for patients having symptomatic vasospasm and an unsecured aneurysm is feasible and offers a reasonable chance of neurologic recovery to these patients who might otherwise progress to cerebral infarction.122 The success of angioplasty is less dependent on angiographic success but rather on early intervention.123 In poor grade patients who cannot be reliably evaluated clinically, prophylactic angioplasty may be feasible in select patients with severe vasospasm.124 These patients may be selected for treatment based on cerebral blood flow studies or microdialysate analysis of extracellular metabolites for delayed ischemia.125
Surgical Complications After SAH
Surgical complications, such as intraoperative aneurysm rupture, major vessel occlusions, cerebral contusion, or ICH are associated with about 10% of the morbidity and mortality after SAH.39,45,65,126–132 Factors such as inexperience or poor surgical technique may play a role.127,131 However, several series suggest that surgical complications after SAH are primarily associated with aneurysm location, size, and morphology.61,64,128–130,132,133 In particular, large or giant aneurysms, aneurysms with atherosclerotic necks, or aneurysms located at the basilar bifurcation or anterior communicating artery are more frequently associated with surgical complications. The Cooperative Study and several studies comparing patient cohorts or historical controls suggest that although brain swelling is more common during early surgery, surgical timing is not associated with surgical complications.42,43,46,129 Similarly we found that the incidence of surgical complications is not influenced by clinical grade.39 However, it is not clear whether the impact of a surgical complication in a poor grade patient has greater adverse impact than the same complication in a good grade patient. Similarly, whether retraction of the swollen brain results in neuropsychological or cognitive deficits that may not occur in delayed surgery when the brain is less swollen, is not defined. However, recent data using neuropsychologic testing demonstrate no association between timing of surgery and neuropsychological outcomes.52,134–141
Where Should Patients with Intracranial Aneurysms Be Managed?
The association between surgical volume and outcome is well described for a variety of surgical procedures. In particular, policies that discourage coronary artery bypass surgery at low-volume hospitals are credited with a substantial decline in mortality associated with this procedure in New York and Canada.142 Similarly, the development of level I trauma centers is associated with better patient outcome and a reduction in preventable mortality.143–146 The management of aneurysmal SAH has several parallels with trauma and there is a growing body of evidence that patients with cerebral aneurysms should be managed in specialized centers by experienced practitioners.12,13,141,147–150 Several years ago, Solomon and associates150 and Taylor and associates,151 who incorporated all hospitals in a geographic region, observed that patient mortality, both for ruptured and unruptured aneurysms, was significantly lower in institutions that frequently performed aneurysm surgery. Both authors observed that teaching hospitals fared better than nonteaching hospitals. More recent studies based on administrative data have observed a significant difference in outcomes between high- and low-volume centers.12,13,149 For example, Berman and associates13 examined data from 1995 to 2000 at hospitals across New York and found among 5963 patients with an intracranial aneurysm who were treated with surgery or endovascular therapy that hospitals that performed more than 35 annual aneurysm procedures had lower death rates than low-volume hospitals. This effect was modest for ruptured aneurysms and greatest among unruptured aneurysms: for each additional 10 cases performed annually, the adverse outcome odds ratio (OR) was 0.85 for ruptured aneurysms and 0.89 for unruptured aneurysms. However, few patients (<5%) are transferred to high-volume centers.12 A cost benefit analysis performed on the same California dataset suggested that transfers to high-volume centers would result in significant improvements in quality-adjusted life years at an acceptable economic cost.148 In addition, the availability of both endovascular and surgical means of aneurysm occlusion within an institution appears to improve overall institutional outcome, though in at least one large study this effect was smaller than that of procedural volume.13,152
There are limitations to these various studies which are largely from secondary sources or administrative data. Nevertheless, the findings are compelling and, particularly in light of the findings from the International Subarachnoid Aneurysm Trial,55,56 suggest that all SAH patients should be treated in centers with a particular interest in cerebrovascular disorders and neurointensive care and where both endovascular and neurosurgical techniques are available. In addition there should be a dedicated neurointensive care unit where patients are cared for in a team-based comanagement approach.153 However, because there are significant risks of transferring critically ill patients between centers, the true improvement in outcome is difficult to estimate and further study is required.154 There will still be patients whose only chance of survival will be immediate attention at local facilities (e.g., a patient with a large temporal lobe clot after an SAH from a ruptured aneurysm that evolves into a herniation syndrome). The role of tranexamic acid, a short-acting antifibrinolytic agent, to reduce the incidence of early rebleeding during transfer requires further study.28
Special Circumstances
Age
Many studies suggest advanced age is associated with poor outcome after SAH. However, other studies demonstrate that young and old people in the same clinical condition experience similar outcomes.52,135–141 There are several important caveats when examining the association between age and outcome: (1) older patients are frequently excluded from active treatment; this influences outcome; (2) older patients are more often in poor clinical grade, which has a greater impact on outcome than age;139,155,156 and (3) other variables such as hypertension or atherosclerosis are common in elderly patients; these factors may have an independent adverse effect on outcome or in multivariate analysis replace the effect of age.45,74,138,157 Although no randomized trial has specifically addressed the role of active treatment in elderly patients, several lines of evidence suggest that surgically treated patients do better than conservatively treated patients after aneurysm rupture.140,158 In ISAT, subgroup analysis of 278 good grade SAH patients with small anterior circulation aneurysms found that between 42% and 86% of patients had a 1-year favorable outcome that depended in part on aneurysm location and treatment type. The data also suggests that endovascular techniques should probably be the favored treatment for ruptured internal carotid and posterior communicating artery aneurysms, whereas elderly patients with ruptured middle cerebral artery aneurysms benefit from surgery.159 Consequently, we believe that withholding treatment on the grounds of advanced age may not always be justified. Instead, the decision to treat an elderly patient after a SAH should be considered in light of the natural history of the disease and the patient’s overall physiologic condition and associated risk factors. Atherosclerosis in the aneurysm and associated vessels frequently increases the technical risk of aneurysm occlusion in the elderly. Consequently these patients may be best treated at specialized centers.160
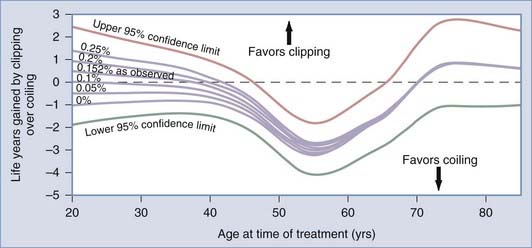
Although good results among patients older than 60 years old undergoing surgical repair of unruptured aneurysms are reported, advanced age is a risk factor.7,10,19,160 This increased risk is due in part to more medical complications among older patients. In addition, older patients are more likely to have atherosclerotic or calcified aneurysms or adjacent vessels both of which increase surgical risk.39 Very old patients may not benefit from unruptured aneurysm treatment. Decision analysis studies suggest that surgical treatment of unruptured aneurysms is beneficial provided that the patient’s life expectancy is greater than 13 years.161,162 Quality of life studies suggest that the quality of life is lengthened by 4 years in 40-year-old patients, 2.4 years in 50-year-old patients, 1.3 years in 60-year-old patients, and by 0.6 years in 70-year-old patients.163 These various studies make certain assumptions about surgical risk and risk of aneurysm rupture but suggest overall that surgery may be appropriate in a 64-year-old man or a 68-year-old woman.161 Patients older than 70 years old, particularly those with small asymptomatic aneurysms or giant posterior circulation aneurysms associated with few symptoms, should probably be followed with serial MRI, MRA, and CT angiography. In these patients, treatment probably offers little over the natural history of these lesions and should only be considered if symptoms progress or aneurysm growth is documented.
Pregnancy
The management of intracranial aneurysms during pregnancy is complicated by the physiologic changes that accompany pregnancy and by potential risks to the fetus when investigating and treating the mother. Intracranial aneurysms are not commonly discovered during pregnancy, in part because the risk of hemorrhage is greatest during the late third trimester and delivery.164 Investigation of the pregnant woman with an aneurysm is similar to that for a nonpregnant female. However, the abdomen should be shielded with lead to reduce the risk of ionizing radiation to the fetus. In general, pregnant patients with ruptured aneurysms are treated the same as nonpregnant women with certain caveats. For example, during surgery temporary clips rather than hypotension are preferred to reduce the risk of fetal hypoperfusion. Similarly care should be taken when mannitol is administered because its use can lead to maternal hypoperfusion and subsequent uterine hypoperfusion, or fetal hyperosmolality. Hyperventilation can cause acid-base shifts and decreased oxygen delivery to the fetus. Once the aneurysm is occluded, pregnancy is allowed to proceed to term. If the patient is in good neurological condition, she should be able to deliver vaginally. When labor begins or is expected to begin immediately after aneurysm occlusion, then cesarean section is preferred. If aneurysm rupture occurs during labor, simultaneous craniotomy and cesarean section may be necessary; the cesarean section should be performed first so that the fetus is not exposed to prolonged anesthesia.165 Surgery should be deferred until the second trimester when an unruptured aneurysm is discovered during the first trimester, in part, to reduce potential drug teratogenic effects during treatment. Other medications such as anticonvulsants or calcium channel blockers should be avoided or used cautiously. A C-section may be preferable when an unruptured aneurysm is discovered late in pregnancy, particularly if the aneurysm becomes symptomatic or has enlarged. A vaginal delivery, using epidural anesthesia to shorten the second stage of labor, is acceptable in asymptomatic patients with an unruptured aneurysm. When needed and when appropriately performed, surgery for both the mother and fetus appears to be safe.166
Pediatric Aneurysms
Aneurysms in children are rare. In contrast to adults, posterior circulation, dissecting, giant, infectious, or traumatic aneurysms are more frequent.167–169 In small children, the operating room should be warmed and care taken to prevent intraoperative rupture because the total blood volume is relatively small.
Mycotic Aneurysms
Mycotic or infective aneurysms comprise between 2% and 6% of intracranial aneurysms in adults. These lesions often are associated with infective endocarditis or immunologic compromise.170 Fungal infections, particularly aspergillosis and candida, may be associated with proximal aneurysms. Aneurysms associated with bacterial infections, most commonly streptococci and Staphylococcus aureus, usually are located on distal middle cerebral artery (MCA) branches. All infective aneurysms require 4 to 6 weeks of culture-directed antimicrobial therapy whether the aneurysm is occluded or not. Antimicrobial therapy alone may be used to treat some infective aneurysms; between 30% and 50% resolve or decrease in size. The lesions require close follow-up with serial angiography and blood cultures. When aneurysm surgery is required, any hemodynamically significant cardiac lesion should be corrected before aneurysm obliteration. However, when the aneurysm is associated with an abscess or a hematoma, aneurysm surgery may be necessary first. During surgery the surgeon must be prepared for aneurysm excision and parent vessel anastomosis, bypass, or a ligation procedure. A short course of preoperative antibiotics may make the aneurysm and parent vessels less friable. The role of endovascular techniques to occlude infective aneurysms is not defined, in part because of concerns about placing a foreign body in an infected friable sac.
Traumatic Aneurysms
Less than 1% of all intracranial aneurysms are traumatic. Traumatic aneurysms usually are associated with penetrating head injury or contiguous skull fracture and are most frequent in the MCA region after low-velocity shrapnel injuries or stab wounds. However, basal skull fractures may be associated with basal traumatic aneurysms.171,172 The majority of traumatic aneurysms occur within 2 to 3 weeks of the original injury and are false aneurysms.170 A definable neck is rare and the lesions are prone to intraoperative rupture. Consequently the surgeon should be prepared to trap or excise the lesion. Encircling clips may also be useful. The surgical approach therefore must provide proximal and distal control and access to the superficial temporal artery or saphenous vein for a bypass if required. Endovascular techniques are feasible in some traumatic aneurysms173 and can effectively temporize a patient while the acute sequelae of the head injury resolve.174
Aneurysms and Arteriovenous Malformations
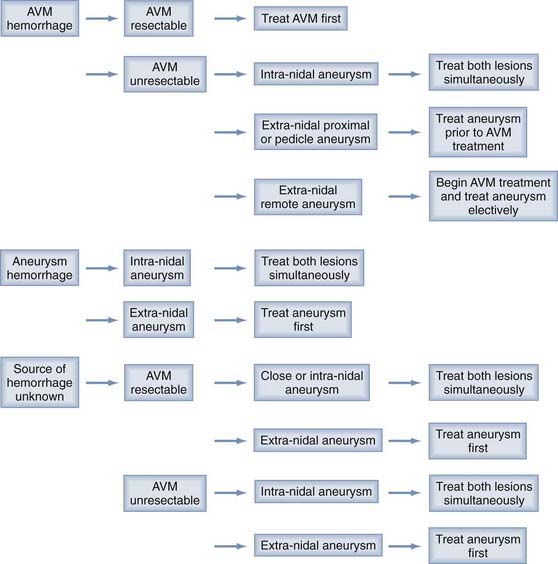
Between 3% and 14% of aneurysms and arteriovenous malformations (AVMs) are associated with an aneurysm.175 In some instances the aneurysms may arise because of hemodynamic stress. There are several different types of aneurysms associated with AVMs: (1) type I dysplastic or remote aneurysms are located at some distance from the AVM and appear structurally unrelated to major inflow vessels; (2) type II proximal aneurysms arise from the circle of Willis or proximal portion of a major feeding vessel; (3) type III pedicular aneurysms are located on the middle portion of a major feeding pedicle; whereas (4) type IV intranidal lesions are found within the AVM. The natural history of these various aneurysms may be different and so require different treatment strategies.176 A management protocol for AVMs and associated aneurysms occurring with hemorrhage is illustrated in Figure 362-5. In general when the lesions appear in the absence of hemorrhage, treat the aneurysm first because of the higher morbidity associated with aneurysm rupture. A multimodality approach including microsurgery, endovascular techniques, and Gamma Knife sterotactic radiosurgery (GK SRS) is preferred.
Coexistent Carotid Artery Disease
Smoking and hypertension are important risk factors for atherosclerotic carotid artery disease and also for intracranial aneurysms. Héman and associates in a review of more than 4000 patients found that between 3% and 4% of patients had both internal carotid artery (ICA) stenosis greater than 50% and an aneurysm; the prevalence of aneurysms greater than 5 mm was 0.9%.177 Most authors recommend treatment of the symptomatic lesion first when a patient has both carotid artery disease and an intracranial aneurysm.160 When both lesions are asymptomatic, treatment should be directed at the lesion with a worse natural history.178 An association between a carotid endarterectomy and an increased risk of aneurysm rupture has not been clearly demonstrated.
Fusiform Aneurysms
Fusiform intracranial aneurysms occur most frequently at the skull base, particularly in the vertebrobasilar system. These aneurysms are characterized by circumferential dilation, elongation, and tortuosity of cerebral arteries and are associated with atherosclerosis and dolichoectasia. The vessel dilation can cause turbulence, damage to branching vessels, and thrombus formation. The majority of patients with fusiform aneurysms have ischemic symptoms. In these patients, provided there is no SAH, antiplatelet therapy or anticoagulation may improve outcome. Some fusiform aneurysms, however, require surgery; this can usually be accomplished by circumferential wrapping techniques.179,180 Aneurysm wrapping should be followed by anticoagulation. Fusiform aneurysms of the vertebral artery may be best treated with endovascular Hunterian ligation under full heparinization or by surgical Hunterian ligation when precise ligation is necessary to prevent inclusion of vital perforators. Endovascular techniques using stents also show promise for fusiform aneurysms.181,182
Microaneurysms
Microaneurysms are less than 3 mm in diameter. They are often broad based relative to their height and so do not lend themselves to clips or GDC coils. Unruptured microaneurysms, particularly if asymptomatic, require close follow-up. Treatment of ruptured microaneurysms is uncertain. Short-term angiographic follow-up suggests that bipolar electrocoagulation and reinforcement with muslin gauze may be a reasonable treatment.179,180,183
Giant Aneurysms
Giant aneurysms represent approximately 4% of intracranial aneurysms. Untreated the 2-year mortality of giant aneurysms is between 60% and 100%. This poor prognosis warrants aggressive management, preferably in tertiary referral centers. There are two treatment goals: prevent aneurysm rupture and relief of mass effect. Direct clipping often is the preferred treatment method; however, surgical results by direct clipping for giant aneurysms are worse than for small aneurysms even when using sophisticated techniques such as bypass or cardiac standstill.45,184–188 Overall, surgery on giant aneurysms is associated with a procedure-related mortality of between 5% and 15%, whereas 70% to 86% of patients experience a favorable outcome.184–186,189–191 The best surgical option for many giant aneurysms, particularly serpentine lesions or those with wide necks, may be aneurysm trapping, aneurysmorrhaphy, or parent vessel occlusion and bypass if needed.186,190 Ischemic consequences of parent artery occlusion may be predicted by a temporary balloon test occlusion.192 However, ischemic sequelae may still occur in those who tolerated test occlusion, even if an extracranial-intracranial arterial bypass is performed.193 In some instances mass effect can be alleviated by parent vessel occlusion. Consequently, surgery should be delayed after aneurysm rupture to evaluate the cerebral circulation and hemodynamic reserve, and allow disturbed autoregulation to recover.190
There are three important technical considerations during surgery for giant aneurysms: (1) wide exposure, (2) proximal and distal control, and (3) clip reconstruction. Wide exposure can be obtained using skull base techniques. For giant anterior circulation aneurysms, an orbitozygomatic approach may be preferable to the pterional approach. The orbitozygomatic approach can be useful for lesions such as proximal giant ICA (ophthalmic, superior hypophyseal, paraclinoid) aneurysms that may require anterior clinoidectomy, cavernous lesions, or when an upward viewing angle is required for lesions such as giant anterior communicating or ICA bifurcation aneurysms that tend to project upward. Deep bypass procedures also are facilitated by the orbitozygomatic approach. For posterior circulation aneurysms, exposures such as the orbitozygomatic, various transpetrosal approaches (retrolabyrinthine, presigmoid, translabyrinthine, or transcochlear), the far lateral or extreme lateral approach or a combination of approaches depending on aneurysm location may be needed.185 Dividing the basilar artery into fifths helps determine which approach is suitable. An orbitozygomatic approach is reasonable for lesions involving the upper two fifths of the basilar artery. For lesions involving the middle fifth, transpetrosal approaches are preferable, whereas far—or extreme—lateral approaches are suitable for aneurysms of the lower two fifths of the basilar artery and the intradural vertebral artery. When the lesion straddles these zones, a combination of approaches is recommended.
Proximal and distal vascular control is important during repair of giant aneurysms; it permits reduction of aneurysm size and so improves visualization of the surrounding anatomy. Similarly an aneurysm with intraluminal thrombosis can be opened and the clot removed. Cerebral protection, such as barbiturates, is necessary. Skull base techniques facilitate vascular control. For some proximal ICA giant aneurysms, exposure of the cervical or petrous carotid artery may be helpful, whereas endovascular techniques or hypothermic circulatory arrest may be useful for posterior circulation giant aneurysms. Surgical aneurysm clipping requires a favorable neck; many giant aneurysms have wide necks, whereas fusiform aneurysms have no intervening neck.194 Therefore clip reconstruction to recreate the normal anatomy rather than clip occlusion often is required to occlude giant aneurysms. Single clips may be inadequate; instead, several shorter clips or fenestrated clips placed serially along the aneurysm neck may be a better choice.
The Residual Aneurysm
What should be done with a residual aneurysm found on postoperative angiogram? Whereas some residual aneurysms may thrombose, even small residual aneurysms may regrow and bleed. Overall the incidence of rebleeding from residual ruptured aneurysms is estimated to be less than 0.5% per year59,60,64,195,196 and the majority of hemorrhages after treatment are reported in patients who on postprocedure angiography have incompletely occluded aneurysms. However, aneurysms with a broad-based residua frequently enlarge and appear to have a nearly fourfold greater risk of subsequent hemorrhage.59 These lesions should therefore be repaired when identified on postoperative angiograms. Small residual dog ears may be observed; however, careful long-term follow-up is important. For example, Giannotta and Litofsky197 reported an average interval of 10.5 years between the original surgery and subsequent rebleeding among inadequately treated ruptured aneurysms. How small residual unruptured aneurysms should be managed is less certain. The lesions may be followed using angiography or spiral CT angiography; aneurysm obliteration is necessary when aneurysm growth is observed.198 Risk factors for aneurysm growth or rupture, such as hypertension or cigarette smoking, should be treated.199
Endovascular Aneurysm Occlusion
The International Subarachnoid Aneurysm Trial (ISAT), which randomized patients to clipping or coiling and demonstrated a morbidity and mortality benefit at 1 year with coiling over surgical clipping in patients for whom either clipping or coiling were deemed appropriate, combined with improved technology and a growing body of experience has resulted in significant increases in the rates of GDC coil use at many institutions.56,200–202 The limitations and applicability of this trial will be discussed; however, it is clear that the appropriate selection of surgical or endovascular aneurysm occlusion requires a collaborative approach between neurosurgeons, endovascular surgeons, and interventional radiologists and an understanding of the anatomic, physical, and physiologic characteristics of both techniques. The selection of one treatment over the other requires a careful consideration of both patient- and aneurysm-specific factors. Anecdotal reports suggest that the collaboration between neurosurgeons and endovascular surgeons (or interventional neuroradiologists) will increase the number of patients who can safely be treated either de novo or after one technique fails.201,203–205 In this section we briefly review factors that influence selection of endovascular techniques for aneurysm occlusion so that patients may be appropriately selected for endovascular or surgical techniques.
Safety and Results of Endovascular Aneurysm Occlusion
Several clinical series have established that endovascular aneurysm occlusion using GDC coils (i.e., endosaccular occlusion) is feasible and safe).206–212 In expert hands complete aneurysm occlusion or greater than 90% occlusion is observed in approximately 50% to 90% of aneurysms, respectively, using endovascular techniques.11,203,207,212–214 In contrast, greater than 90% of aneurysms are completely occluded using surgical techniques.59,61,62 Technical complications, including aneurysm perforation, distal embolization, parent vessel occlusion, or coil migration may be observed in between 9% and 30% of patients. Overall permanent complications of embolization occur in 4% to 7% of patients.* In a meta-analysis of 1256 patients, Brilstra and associates213 described the procedural risk of coil embolization: aneurysmal perforation occurred in 2.4% and ischemic complications in 8.5%. These complications were associated with permanent deficits in 3.7%. In the International Study of Unruptured Intracranial Aneurysms,11 procedural (30-day) mortality was 2.0% and disability was 7.4% after aneurysm coiling.
Long-term Stability and the Implications of Residual Aneurysms
Incomplete aneurysm occlusion after endosaccular coiling is frequent, and recurrent filling is seen in between 15% and 50% of aneurysms on angiograms obtained 6 months to several years after treatment.80,206,213,219–226 For example, Ferns and associates in a review of 46 studies that included 8161 coiled aneurysms found 20.8% of aneurysms reopened and 10.3% were retreated.227 This recurrence may be seen in both partially and completely occluded aneurysms after coil embolization.80,207,226,228 In a large North American series, Murayama and associates followed 818 patients with 916 coiled aneurysms over 11 years.223 They excluded data from the first 5 years (considering this the learning curve). Among the 665 aneurysms in 558 patients treated during the last 6 years small aneurysms (4 to 10 mm diameter) with small necks (<4 mm), had a 1.1% recurrence; in small aneurysms with wide necks (>4 mm in diameter), recurrence occurred in 7.5% of completely occluded aneurysms and 29.4% of incompletely coiled aneurysms. In large aneurysms (11 to 25 mm in diameter), recurrence was 30% in completely occluded aneurysms and 44% of incompletely coiled aneurysms. Sixty percent of incompletely occluded giant aneurysms (>25 mm in diameter) and 42% of completely occluded giant anerysms recurred.
Rates of retreatment with repeat GDC insertion or surgery reported for patients who have undergone coiling and long-term follow-up in large single-center series and multicenters range from 5% to 20%, and appear to be consistently higher than those treated with surgery, which may increase potential risk to the patient.80,229–233 In ISAT, 17.4% of patients receiving primary endovascular coiling required retreatment, compared with 3.8% of the 1012 patients who had their aneurysms surgically clipped.232 Failure to achieve complete occlusion and recurrence rates seem to be closely related to aneurysm morphology, in particular large aneurysms (>10 mm) and posterior circulation aneurysms seem to recur more frequently because of coil compaction.227 In addition, patients, particularly females, who continue to smoke have a greater risk of aneurysm recurrence after coil treatment.234 Endovascular retreatment (and its potential risks), however, may not always negate the early benefit of endovascular techniques.229
The need for complete angiographic occlusion is debated. However, the SAH rate after coil embolization of ruptured aneurysms is described in several case series and is reported to be between 1% and 3% per year.* In ISAT, a large randomized trial to compare surgery and endovascular therapy, the reported 1-year rehemorrhage rate was 2.9% in aneurysms treated with endovascular therapy and 0.9% with surgical clipping.55,56 The risk of rerupture may be greater in posterior circulation aneurysms: 1.4% annual rerupture rate208,238 and higher in giant aneurysms. For example, Lempert and associates observed that 33% of giant aneurysms, 4% of large aneurysms, and no small aneurysms had new hemorrhage during an average of 3.5 years of follow-up.238 The rebleed rate also is associated with how successful occlusion was; in the Cerebral Aneurysm Rerupture After Treatment (CARAT) study the risk of rebleed was 0.6 rehemorrhages per 100 person-years for completely occluded aneurysms and 15 rehemorrhages per 100 person-years for partially occluded lesions.233 Similarly, Byrne and associates206 treated 317 patients within 30 days of a SAH; the median follow-up was 22.3 months. Thirty-eight aneurysm recurrences were observed; 3 (8%) rebled. By contrast, 1 (0.4%) of 221 aneurysms that appeared angiographically stable after at least 11 months rebled. Rerupture appears to be more common in the first year56,206,233,235,239 and mortality is frequent with rerupture. For example, Sluzewski and van Rooij observed among 431 patients who had a ruptured aneurysm coiled, that all patients who rebled (1.4%) died.240 These shortcomings of coil embolization (endosaccular aneurysm occlusion) have led to questions about the long-term durability and, for the more complex and larger aneurysms, the long-term efficacy of the technique. Consequently, parent vessel reconstruction is preferred when appropriate or surgery is advocated in some young patients. Overall, the published data suggest that complete endovascular occlusion is protective and that regrowth and rebleeding are frequent if not inevitable consequences of incomplete aneurysm occlusion. Few data are available to define the appropriate timing of follow-up imaging. However, if only a 6-month postprocedure angiogram is performed, approximately half the aneurysm recurrences will be missed (i.e., regular follow-up angiography until at least 3 years after coil treatment is advisable).224,226
Aneurysm occlusion using endosaccular techniques or surgery is different; this difference may have significant implications for residual aneurysms. In the clipped aneurysm residual, the walls are closely apposed and the remaining aneurysm is completely excluded from the circulation. By contrast, using endosaccular techniques the coils keep the remnant’s walls apart. In addition, although experimental models of coiled aneurysms demonstrate that the aneurysm neck becomes entirely occluded by organized thrombus and that the free luminal surface is covered by endothelium,210,241 endothelialization is not observed in coiled aneurysms obtained at autopsy or surgery.228,242 These various factors mean that any intra-aneurysmal thrombus or coil is exposed to circulating blood, which may allow compaction of the coils or flow around the coil’s periphery into the aneurysm sac. Devices such as Pipeline (i.e., parent vessel reconstruction) appear to address these “biologic” shortcomings of endosaccular coils.
Patients with incompletely occluded coiled aneurysms consequently require repeat angiography, coil embolization, or surgery. Are these safe or cost-effective? Angiography is relatively safe in patients harboring cerebral aneurysms; less than 1% suffer a stroke.61 In addition, angiograms performed for the surveillance of coiled intracranial aneurysms are reported to have a very low complication rate (0.43%).243 However, in the presence of atherosclerotic disease, the stroke risk is increased fourfold. Contrast enhanced MRA or CT angiography may prove useful to follow aneurysm remnants.244,245 There is limited but growing experience with surgical treatment of coiled aneurysms. Small clinical series suggest that a coiled aneurysm is not a simple surgical lesion.66,228,246 Aneurysm obliteration may be easily obtained if the aneurysm is partially packed with coils but difficult with more complete packing, in part because there is insufficient space between the coils and the parent vessel for clip placement.203,246 Other factors associated with technical difficulty include the relationship between coil width and compaction height (C/H <2.5, or a wedge angle <90 degrees).247 Procedural mortality and permanent morbidity is 9%. A bypass strategy for unclippable coiled aneurysms therefore may be preferable to thrombectomy, coil extraction, and clip reconstruction in some patients.
Which Aneurysm Can Be Successfully Occluded Using Endosaccular Techniques?
Coil embolization is the most frequent endovascular technique. In general, when using endosaccular coil embolization, morphologic results are good in small aneurysms with small necks and those at a right angle to blood flow (Table 362-1, Fig. 362-6). Coil procedures appear less effective in large- or wide-necked aneurysms,* particularly when considered relative to the size of the aneurysm.251,252 Neck diameters of less than 5 mm and a ratio of neck diameter to the largest aneurysm dimension of less than 0.5 are associated with better outcomes (i.e., less complications and greater likelihood of complete occlusion by coil embolization). For example, Murayama and associates reported results for 916 aneurysms in 818 patients treated over 11 years and found in small aneurysms (4 to 10 mm) with small necks (<4 mm) complete occlusion could be achieved in 75.4%, compared with only 41.2% of small aneurysms with wide necks, 40.4% of large aneurysms (11 to 25 mm), and 26% of giant aneurysms (>25 mm).223 Overall successful occlusion rate was 55%. Greater coil packing at the initial procedure may improve long-term aneurysm obliteration of large aneurysms but at the potential risk of increased complications at the time of procedure, particularly vessel occlusion or thromboembolism. Stent-assisted coil embolization is a feasible method for the endovascular treatment of wide-necked intracranial aneurysms. In patients with acutely ruptured wide-necked intracranial aneurysms, technical success is reported to be 72% with this technique.253 Wide aneurysm necks generally play a less significant role in surgery because the parent vessel can be reconstructed using fenestrated and angled clips. Aneurysm location also may influence the success of the procedure. For example, superior hypophyseal aneurysms are more amenable to coils than carotid-opthalmic aneurysms because the catheter tends to follow the curve of the siphon into the aneurysm because of its location within the concavity of the carotid artery.254 Cavernous internal carotid artery aneurysms are relatively easily treated with endovascular rather than surgical strategies255 and endovascular therapy is preferred now for most posterior circulation aneurysms.256 However, microsurgery still remains a preferable primary therapy for some aneurysms located on the superior cerebellar artery, P1 posterior cerebral artery, distal anteroinferior cerebellar artery, and posteroinferior cerebellar artery aneurysms.257 Because of their morphology, MCA artery aneurysms may be difficult to treat with coil embolization258,259 and so open microsurgery often is preferred. Very small aneurysms (<2 to 3 mm) also may be technically difficult to treat using GDC coils;211 however, comparative studies have not evaluated the impact of size on outcome.
TABLE 362-1 Factors That May Limit Successful Endosaccular Aneurysm Occlusion Using GDC Coils
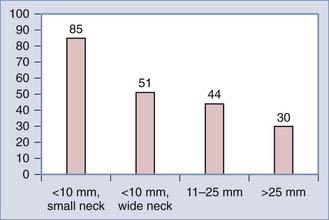
FIGURE 362-6 Complete occlusion of incidental unruptured aneurysms using GDC coils (n = 120).
(Modified from Murayama Y, Vinuela F, Duckwiler G, et al. Embolization of incidental cerebral aneurysms by using the Guglielmi detachable coil system. J Neurosurg. 1999;90:207-214.)
There are several additional considerations when endovascular techniques are used. First, GDC coils do not appear to be suitable for many MCA aneurysms.258,260 Second, endovascular techniques cannot be used exclusively in the setting of ICH that requires treatment, though some authors have reported success with a combined approach.83 Third, patients who undergo endovascular procedures often require anticoagulation or antiplatelet agents.212 The impact of these medications in aneurysm patients has not been studied in detail; however, the use of long-term anticoagulation doubles the risk of a poor outcome after aneurysm rupture. For example, Rinkel and associates261 evaluated 141 patients after a SAH. Among 15 taking anticoagulants, 14 died or were dependent at 3-month follow-up, whereas 62 of the 126 patients not taking anticoagulants suffered a similar fate. It is uncertain, however, whether these findings apply to short-term anticoagulation or to antiplatelet agent use. Clinical series suggest that anticoagulation (e.g., use of the glycoprotein IIb-IIIa inhibitor Abciximab combined with heparin, acetylsalicylic acid, and clopidogrel) is a safe rescue therapy in thromboembolic events that complicate aneurysm coil embolization.262 Antiplatelet agents, unlike ischemic stroke, do not appear to provide any additional benefit to patients after SAH.263
Many of these limitations may eventually be overcome by technological advances. The likelihood of aneurysm recurrence after endovascular treatment may be further reduced with biologically active coils; however, the results thus far have been disappointing.264–268 Newer techniques such as balloon remodeling appear to improve the results of endovascular occlusion of wide-necked aneurysms; initial case series suggest that greater than 80% of wide-necked aneurysms can be occluded using this technique.269–271 The development of neck-bridge devices, 3-D coils,272 embolic liquid polymers,273,274 stents,181,182,275–282 or use of balloon-assisted coiling techniques may solve some of the problems associated with treating large aneurysms.283 Ethylene vinyl alcohol is an embolic liquid material that forms a polymer cast on contact with aqueous solutions, and has thus far been used primarily in patients who have failed coiling or clipping and has shown initially high rates of occlusion and low recanalization rates.273,274 Stenting technology appears promising to both expand the scope of aneurysms amenable to endovascular therapies and possibly limit the use of coils (and complications thereof), yet it carries with it its own risks, such as in-stent stenosis and increased risk of intraprocedural hemorrhage, and may require postprocedural antiplatelet therapy, which would limit its utility in treating patients with ruptured aneurysms.181,182,275–280 Both stenting and embolic liquid polymers have been used primarily in unruptured aneurysms and still require further study.
Endovascular Techniques After SAH
Observational studies suggest that outcome after occlusion of ruptured aneurysms using GDC coils is similar to conventional microsurgery in the short term and can prevent early rebleeding.* In particular, these various studies suggest that endovascular techniques provide protection against rebleeding in the first few months when rebleeding is most frequent.206,212,284 For example, Vinuela and associates212 described short-term follow-up after coil occlusion of 401 ruptured aneurysms. Half the aneurysms were incompletely occluded; overall, 4.5% rebled within 6 months. Whereas these results are worse than surgery, they may represent an improvement on the natural history because 75% of the aneurysms were treated within 7 days of SAH. Angiographic data demonstrate that overall 50% of ruptured aneurysms can be completely occluded using endovascular techniques.213,221 Incomplete treatment is frequent, and recurrent filling is seen in between 15% and 50% of aneurysms on angiograms obtained 6 months to several years after treatment.80,206,213,219–221,223–225 This recurrence may be seen in both partially and completely occluded aneurysms after coil embolization.80,207,226,228 Rates of retreatment with GDC or surgery reported for patients who have undergone coiling and long-term follow-up in large single-center series and multicenters range from 5% to 20%, and appear to be consistently higher than those treated with surgery, which may increase the risk to the patient.80,229–233
Contemporary single-center comparisons suggest patients who undergo coil occlusion have worse admission grades. Overall there is a greater risk of incomplete aneurysm occlusion (21.7% versus 7.6%) and vasospasm in the endovascular group (66% versus 52%). Overall 3-month outcomes, however, are similar.285 Multicenter studies (i.e., ISAT, a randomized clinical trial) demonstrate a moderate outcome benefit at 1 year but not at 5 years for patients who undergo coiling rather than clip procedures.286 These results apply best to patients in good clinical grade with small anterior circulation aneurysms.
Endovascular occlusion of the acutely ruptured aneurysm is an attractive alternative to surgery for poor grade patients. However, the role of endovascular therapy in these patients has only been described in limited clinical series and very few poor grade patients were included in the ISAT trial.55 Malisch and associates80 treated nine poor grade patients using coils; all nine patients died or experienced a poor outcome. By contrast, Kinugasa and associates78 used cellulose acetate polymer and cisternal tPA in 12 grade III-V patients. Eight patients experienced a favorable outcome; however, 7 partially thrombosed aneurysms required subsequent surgery. Kremer and associates79 described 40 poor grade patients whose aneurysms were occluded using GDC coils; 5 of these patients were referred greater than 23 days after aneurysm rupture. Sixteen (40%) patients experienced a good outcome; there was tendency for better outcome among posterior circulation aneurysms. During the same time, 23 patients who underwent surgery for aneurysmal ICH were treated but not included in the analysis. Van Loon and associates81 treated 11 consecutive patients in World Federation of Neurosurgical Societies (WFNS) grade V using early endovascular treatment and aggressive vasospasm and ICP management. Two patients died from increased intracranial pressure and 2 had early rebleeding after the coiling and required further treatment. Overall, 4 patients had good outcomes at 12-month follow-up. Recently, Suzuki and associates83 observed a favorable outcome (mRS 1-2) in 43.8% of grade IV and 12.9% of grade V patients treated with endovascular techniques (35.1% of grades IV and V combined), with a 32.4% overall mortality.
What Is the Role of Endovascular Techniques for Unruptured Aneurysms?
The decision to use surgical or endovascular occlusion should be based on aneurysm morphology, patient age, and medical condition. Because complete aneurysm occlusion is achieved in only half the aneurysms and the risk of recanalization with present interventional techniques,11,212 surgical occlusion rather than endosaccular occlusion is preferred provided the patient is in reasonable medical condition and particularly if young. While uncertainties remain about the role of endovascular techniques,287 newer flow diverting devices, stents, and later generation coils are likely to increase the number of unruptured aneurysms that can be repaired using endovascular techniques. Clipping and coiling for unruptured aneurysms have not been compared in any randomized trials although a number of studies are underway to evaluate the results of coiling in this population, including the TEAM and ATENA trials.288,289 Pierot and associates267 for the AETNA investigators recently described 649 patients with 1100 aneurysms; 739 were treated. Aneurysms were treated in most cases (98.4%) with coils alone (54.5%), balloon remodeling (37.3%), or stents (7.8%). Endovascular treatment failed in 4.3% of aneurysms.267 This and other clinical case series suggest that unruptured aneurysms can be occluded using endovascular techniques with low mortality (about 1%) and morbidity (3% to 5%). The most frequent complications are thromboembolic events (10%) that may cause ischemic symptoms.290,291 Treatment-related aneurysm perforation complicates about 2.5% of patients; 60% of these patients die. In addition, not all aneurysms can be occluded and over time there is a decrease of the occlusion rate in all sizes but most pronounced in giant aneurysms; on follow-up angiogram less than 70% of aneurysms remain completely occluded.291 Consequently the risk of rupture for giant aneurysms is as high as expected in observational studies. In some institutions unruptured intracranial aneurysms are considered for coil embolization first if accessibility, neck width, and fundus-to-neck ratio are appropriate. If not, then the aneurysms are treated surgically. When this strategy was used, Aghakhani and associates observed (in 176 patients with 238 aneurysms) that the morbidity rate was 0.56% for aneurysms less than 10 mm in patients younger than 65 years old.292 In a prospective study of 101 patients where coiling was used as first-line therapy and surgical clipping only when coiling was inappropriate, Raftopoulos and associates observed improved outcomes in the surgical clipping group.293 This may only apply to MCA aneurysms.
Endovascular Techniques for Giant Aneurysms
Giant aneurysms are a formidable challenge no matter what technique is selected and so are best approached in a collaborative multidisciplinary fashion. In some centers endovascular therapy is a first choice237 and a variety of techniques are available (Table 362-2). The various clinical series that describe endosaccular treatment of giant aneurysms suggest that most lesions require multiple procedures and that the immediate neurological results are similar to surgery.212,214,215,294 For example, Gruber and associates294 treated 31 giant or large aneurysms in 30 patients; the procedure mortality and morbidity was 6.7% and 13.3%, respectively. Recently Jahromi and associates described the long-term clinical and radiologic outcome of 39 consecutive giant aneurysms, including 10 ruptured aneurysms treated with endovascular repair in 38 patients at two tertiary referral centers.295 At angiographic follow-up, a mean of 21.5 months, 36% of aneurysms were completely occluded and 64% were greater than 95% occluded. The parent vessel was preserved in three quarters of the treated aneurysms and stents were used in 25 lesions. On average, 1.9 ± 1.1 sessions were required to treat each aneurysm and 20% of treatment sessions resulted in permanent morbidity. At long-term follow-up (mean 24.8 months), 29% of patients had died and 26% had worsened neurological function from baseline.
TABLE 362-2 Endovascular Strategies to Occlude Giant Aneurysms
There are several potential disadvantages using endosaccular occlusion techniques for giant aneurysms. First, morphologic results are poor because very few giant aneurysms can be completely occluded,80,215,250,294 in large part because many have wide necks. Second, symptoms from the aneurysm are improved in only half the patients and in some mass effect can be aggravated.66,80,214,294 Third, greater than half the giant aneurysms occluded using endosaccular coil techniques recur during follow-up and require recoiling.80,294 Finally, rebleeding complicates a third of giant aneurysms that have been coiled.80 Alternatively, endovascular techniques may be used for parent vessel occlusion with or without bypass. In the future, intravascular stents may improve endovascular treatment of giant aneurysms. Stents may uncouple the aneurysm from the circulation, leading to thrombosis, or may aid in packing coils in wide-necked aneurysms by providing a scaffold to prevent coil herniation.296 Though further developments in stent technology may improve outcomes in these patients, the literature is still largely limited to case reports and small series.181,182,275–282 These observations suggest that surgery, including direct aneurysm occlusion or bypass, may be the first line of treatment for giant aneurysms for the moment. However as more experience with Pipeline and other stents is obtained, endovascular strategies will increasingly be used.
Collaboration and Comparison
In the past, endovascular techniques were used to occlude aneurysms because of “anticipated surgical difficulty,” “medical contraindications,” or “failed surgery.” Today, however, endovascular techniques, in particular GDC coils and stents, should be considered a complement to surgical techniques and the primary treatment for some aneurysms and patients. The appropriate selection of surgical or endovascular aneurysm occlusion requires a collaborative approach between neurosurgeons and endovascular surgeons or interventional radiologists and an understanding of the anatomic, physical, and physiologic characteristics of both techniques. The selection of one treatment over the other requires a careful consideration of both patient- and aneurysm-specific factors. Several reports suggest that the collaboration between neurosurgeons and interventional radiologists will increase the number of patients who can safely be treated either de novo or after one technique fails203–205,297,298 and it is clear that an “exclusive” surgical or endovascular approach is not feasible.260 This collaborative effort is important particularly with more complex aneurysms such as posterior circulation and giant ruptured aneurysms.299 In addition, administrative data suggest that when both techniques are available, overall institutional outcome may be improved.152 Others, however, in a small retrospective analysis using historical controls have not observed this benefit.300 Finally, some aneurysms, because of their fusiform or complex wide-necked structure, giant size, or involvement with critical perforating or branch vessels, are not amenable to direct surgical clipping or endovascular coil treatment. In some of these patients, a combined neurosurgical-neuroendovascular approach is likely to increase the number of good outcomes.301
There are two randomized clinical trials (RCT) that compare endovascular and surgical techniques after a SAH. The first study randomly assigned patients with ruptured aneurysms to surgical (n = 57) or endovascular (n = 52) treatment.236 Significantly better primary angiographic results were achieved after surgery in patients with anterior cerebral artery aneurysms and after endovascular treatment in those with posterior circulation aneurysms. Clinical and neuropsychological outcome at 12 months after treatment were similar. Outcome was predicted by similar variables such as symptomatic vasospasm, poorer Hunt and Hess grade, need for permanent shunt, and larger size of the aneurysm. The conclusions from this study, however, are limited by the small sample size. The second RCT, the International Subarachnoid Aneurysm Trial (ISAT), was a multicenter trial based primarily in the United Kingdom and Europe; 9559 patients were assessed and 2143 randomly assigned to neurosurgical clip ligation or GDC coil treatment.55,56 There are many limitations to this study and the results can only be applied to about 20% of patients after SAH. Nevertheless, this study demonstrated a modest outcome benefit at 1 year to GDC occlusion of select aneurysms when both surgical or endovascular techniques are suitable with an absolute risk reduction of 6.9% in favor of endovascular techniques. Longer follow-up suggests that this benefit, however, may be lost particularly in patients younger than 40 years old (Fig. 362-7) because the incidence of rebleeding was greater among aneurysms treated using GDC coils.239 Among 2004 ISAT patients followed for a mean period of 9 years, the proportion of patients with a MRS score of 2 or less (i.e., those who are independent) conditional on survival at 5 years was 626 of 755 (83%) in the endovascular group and 584 of 713 (82%) in the neurosurgical group.286 Mortality was greater in the surgical group but when mortality and severe morbidity were combined there was equivalence in outcome.
Raja and associates recently performed a systematic review of two prospective randomized trials, 23 prospective observational studies, 20 retrospective observational studies, and 2 studies that used both prospective and retrospective data to compare endovascular and microsurgical therapy.302 In 18 studies, outcome was equivalent in the coiled and clipped patients; 18 studies favored endovascular techniques; 10 studies favored microsurgery, and 1 study did not reach a conclusion. Taken together, these various data described in the preceding sections emphasize three important points. First, all patients who have suffered a SAH should be treated in institutions where both endovascular and neurosurgical techniques are available. Second, neurovascular surgeons and interventionists must cooperate to determine which procedure, or combination of procedures, is the most likely to succeed for each patient. Aneurysm morphology appears to be closely associated with the success and risk of endovascular and surgical techniques. Therefore, careful collaborative study of imaging studies to determine aneurysm size and configuration, presence of intra-aneurysmal thrombosis, the relationship between the aneurysm and surrounding structures, aneurysm wall thickness and neck morphology are essential.303 Finally, the benefit associated with either GDC coils or surgery is likely to be modest and so studies addressing the use of these techniques will require a large population to demonstrate any treatment effect. An absolute risk reduction of 8% to 10% in an RCT often is considered the minimally important difference (MID) (i.e., the smallest beneficial effect that would be considered clinically meaningful and that would lead physicians to recommend a new therapy to their patients). In ISAT, the absolute risk reduction at 1 year was 6.9%.
Bardach NS, Zhao NS, Gress DR, et al. Association between subarachnoid hemorrhage outcomes and number of cases treated at California hospitals. Stroke. 2002;33:1851-1856.
Bardach NS, Olson SJ, Elkins JS, et al. Regionalization of treatment for subarachnoid hemorrhage: a cost-utility analysis. Circulation. 2004;109:2207-2212.
Batjer H, Samson D. Intraoperative aneurysmal rupture: incidence, outcome, and suggestions for surgical management. Neurosurgery. 1986;18:701-707.
Bederson JB, Connolly ESJr, Batjer HH, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994-1025•.
Bederson JB, Awad IA, Weibers DO, et al. Recommendations for the management of patients with unruptured intracranial aneurysms: a statement for healthcare professionals from the Stroke Council of the American Heart Association. Stroke. 2000;31:2742-2750.
Broderick JP, Brown RDJr, Sauerbeck L, et al. Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke. 2009;40:1952-1957.
Broderick JP, Brott TG, Duldner JE, et al. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342-1347.
Ingall T, Asplund K, Mahonen M, et al. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke. 2000;31:1054-1061.
International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725-1733.
Johnston SC. Effect of endovascular services and hospital volume on cerebral aneurysm treatment outcomes. Stroke. 2000;31:111-117.
Johnston SC, Dowd CF, Higashida RT, et al. Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms: the cerebral aneurysm rerupture after treatment (CARAT) study. Stroke. 2008;39:120-125.
Kassell NF, Torner JC, Haley ECJr, et al. The international cooperative study on the timing of aneurysm surgery: part 1: overall management results. J Neurosurg. 1990;73:18-36.
Kowalski RG, Claasen J, Kreiter RT, et al. Initial misdiagnosis and outcome after subarachnoid hemorrhage. JAMA. 2004;291:866-869.
Le Roux P, Elliott JP, Newell DW, et al. Predicting outcome in poor grade subarachnoid hemorrhage: a retrospective review of 159 aggressively managed patients. J Neurosurg. 1996;85:39-49.
Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009;64:632-642.
Mitchell P, Kerr R, Mendelow AD, et al. Could late rebleeding overturn the superiority of cranial aneurysm coil embolization over clip ligation seen in the international subarachnoid aneurysm trial? J Neurosurg. 2008;108:437-442.
Molyneux A, Kerr R, Stratton I, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
Molyneux AJ, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809-817.
Molyneux AJ, Kerr RS, Birks J, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the international subarachnoid aneurysm trial (ISAT): long-term follow-up. Lancet Neurol. 2009;8:427-433.
Pierot L, Spelle L, Vitry F. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke. 2008;39:2497-2504.
Raja PV, Huang J, Germanwala AV, et al. Microsurgical clipping and endovascular coiling of intracranial aneurysms: a critical review of the literature. Neurosurgery. 2008;62:1187-1202.
Ringer AJ, Lanzino G, Veznedaroglu E, et al. Does angiographic surveillance pose a risk in the management of coiled intracranial aneurysms? A multicenter study of 2243 patients. Neurosurgery. 2008;63:845-849.
van Doormaal TP, Van der Zwan A, Verweij BH, et al. Treatment of giant and large internal carotid artery aneurysms with a high-flow replacement bypass using the excimer laser-assisted nonocclusive anastomosis technique. Neurosurgery. 2008;62(6 Suppl 3):1411-1418.
Waldron JS, Halbach VV, Lawton MT. Microsurgical management of incompletely coiled and recurrent aneurysms: trends, techniques, and observations on coil extrusion. Neurosurgery. 2009;64(5 Suppl 2):301-315.
White PM, Raymond J. Endovascular coiling of cerebral aneurysms using “bioactive” or coated-coil technologies: a systematic review of the literature. AJNR Am J Neuroradiol. 2009;30:219-226.
1 Thines L, Taschner C, Lejeune JP, et al. Surgical views from three-dimensional digital subtraction angiography for the planning of aneurysm surgery. J Neuroradiol. 2007;34:205-211.
2 Tsutsumi K, Ueki K, Morita A, et al. Risk of aneurysm recurrence in patients with clipped cerebral aneurysms: results of long-term follow-up angiography. Stroke. 2001;32:1191-1194.
3 Deruty R, Gelissou-Guyotat I, Mottolese C, et al. Surgical management of unruptured intracranial aneurysms. Personal experience with 37 cases and discussion of the indications. Acta Neurohir (Wien). 1992;119:35-41.
4 Eskesen V, Rosenørn J, Schmidt K, et al. Clinical features and outcome in 48 patients with unruptured intracranial saccular aneurysms: a prospective consecutive study. Br J Neurosurg. 1987;1:47-52.
5 Heiskanen O. Risks of surgery for unruptured intracranial aneurysms. J Neurosurg. 1986;65:451-453.
6 Rice BJ, Peerless SJ, Drake CG. Surgical treatment of unruptured aneurysms of the posterior circulation. J Neurosurg. 1990;73:165-173.
7 Solomon RA, Fink ME, Pile-Spellman J. Surgical management of unruptured intracranial aneurysms. J Neurosurg. 1994;80:440-446.
8 Wirth FP, Laws ERJr, Piepgras D, et al. Surgical treatment of intracranial aneurysms. Neurosurgery. 1983;12:507-511.
9 Moroi J, Hadeishi H, Suzuki A, et al. Morbidity and mortality from surgical treatment of unruptured cerebral aneurysms at Research Institute for Brain and Blood Vessels-Akita. Neurosurgery. 2005;56:224-231.
10 International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. N Engl J Med. 1998;339:1725-1733.
11 Wiebers DO, Whisnant JP, Huston JIII, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110.
12 Bardach NS, Zhao S, Gress DR, et al. Association between subarachnoid hemorrhage outcomes and number of cases treated at California hospitals. Stroke. 2002;33:1851-1856.
13 Berman MF, Solomon RA, Mayer SA, et al. Impact of hospital-related factors on outcome after treatment of cerebral aneurysms. Stroke. 2003;34:2200-2207.
14 King JT, Berlin JA, Flamm ES. Morbidity and mortality from elective surgery for asymptomatic, unruptured, intracranial aneurysms: a meta-analysis. J Neurosurg. 1994;81:837-842.
15 Raaymakers TW, Rinkel G, Limburg M, et al. Mortality and morbidity of surgery for unruptured intracranial aneurysms. A meta-analysis. Stroke. 1998;29:1531-1538.
16 Yoshimoto Y. Publication bias in neurosurgery: lessons from series of unruptured aneurysms. Acta Neurochir (Wien). 2003;145:45-48.
17 Connolly ES, Solomon RA. Management of symptomatic and asymptomatic unruptured aneurysms. Neurosurg Clin N Am. 1998;9:509-524.
18 Ogilvy CS, Carter BS. Stratification of outcome for surgically treated unruptured intracranial aneurysms. Neurosurgery. 2003;52:82-87.
19 Khanna RK, Malik GM, Qureshi N. Predicting outcome following surgical treatment of unruptured intracranial aneurysms: a proposed grading system. J Neurosurg. 1996;84:49-54.
20 Drake CG. Management of cerebral aneurysm. Stroke. 1981;12:273-283.
21 Le Roux P, Sethi R, Grant G, et al. Factors associated with surgical complications for basilar bifurcation aneurysms: an analysis of 101 patients. J Neurosurg. 1998;88:391A.
22 Bederson JB, Connolly ES, Batjer H, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994-1025.
23 Mayberg MR, Batjer HH, Dacey R, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. Stroke. 1994;25:2315-2338.
24 Garrett MC, Komotar RJ, Starke RM, et al. Theoretical model for quantifying the operative benefit of unruptured cerebral aneurysms by location, age and size. Future Neurol. 2010;5:27-35.
25 Heuer GG, Smith MJ, Elliott JP, et al. Relationship between intracranial pressure and other clinical variables in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;101:408-416.
26 Winn HR, Richardson AE, Jane JA. The long-term prognosis in untreated cerebral aneurysms. I. The incidence of late hemorrhage in cerebral aneurysm: a 10-year evaluation of 364 patients. Ann Neurol. 1977;1:358-370.
27 Ohkuma H, Tsurutani H, Suzuki S. Incidence and significance of early aneurysmal rebleeding before neurosurgical or neurological management. Stroke. 2001;32:1176-1180.
28 Hillman J, Fridriksson S, Nilsson O, et al. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. J Neurosurg. 2002;97:771-778.
29 Kassell NF, Torner JC. Aneurysmal rebleeding: a preliminary report from the cooperative aneurysm study. Neurosurgery. 1983;13:479-481.
30 Rosenorn J, Eskesen V, Schmidt K, et al. The risk of rebleeding from ruptured intracranial aneurysms. J Neurosurg. 1987;67:329-332.
31 Fuji Y, Takeuchi S, Sasaki O, et al. Ultra-early rebleeding in spontaneous subarachnoid hemorrhage. J Neurosurg. 1996;84:35-42.
32 Naidech AM, Janjua N, Kreiter KT, et al. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Arch Neurol. 2005;62:410-416.
33 McIver JI, Friedman JA, Wijdicks EF, et al. Preoperative ventriculostomy and rebleeding after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002;97:1042-1044.
34 Rose JC, Mayer SA. Optimizing blood pressure in neurological emergencies. Neurocrit Care. 2004;1:289-299.
35 Haley ECJr, Kassell NF, Torner JC. The international cooperative study on the timing of aneurysm surgery: the North American experience. Stroke. 1992;23:205-214.
36 Brilstra EH, Algra A, Rinkel GJ, et al. Effectiveness of neurosurgical clip application in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002;97:1036-1041.
37 Le Roux P, Winn HR. The poor grade aneurysm patient. Acta Neurochir Suppl. 1999;72:7-26.
38 Richardson AE, Jane JA, Yashon D. Prognostic factors in the untreated course of posterior communicating aneurysms. Arch Neurol. 1966;14:172-176.
39 Le Roux P, Elliott JP, Newell DW, et al. The incidence of surgical complications is similar in good and poor grade patients undergoing repair of ruptured anterior circulation aneurysms: a retrospective review of 355 patients. Neurosurgery. 1996;38:887-895.
40 Lanzino G, Kassell N, Germanson T. Age and outcome after aneurysmal subarachnoid hemorrhage: why do elderly patients fare worse? J Neurosurg. 1996;85:410-418.
41 Ohman J, Heiskanen O. Timing of operation for ruptured supratentorial aneurysms: a prospective randomized study. J Neurosurg. 1989;70:55-60.
42 Chyatte D, Forde N, Sundt T. Early versus late intracranial aneurysm surgery in subarachnoid hemorrhage. J Neurosurg. 1988;69:326-331.
43 Disney L, Weir B, Petruk K. Effect on management mortality of a deliberate policy of early operation on supratentorial aneurysms. Neurosurgery. 1987;20:695-701.
44 Hernesniemi J, Vapalahti M, Niskanen M, et al. One year outcome in early aneurysm surgery: a 14 year experience. Acta Neurochir (Wien). 1993;122:1-10.
45 Kassell NF, Torner JC, Haley C, et al. The international cooperative study on the timing of aneurysm surgery: part 1: overall management results. J Neurosurg. 1990;73:18-36.
46 Milhorat TH, Krautheim M. Results of early and delayed operations for ruptured intracranial aneurysms in two series of 100 consecutive patients. Surg Neurol. 1986;26:123-128.
47 Miyaoka M, Sato K, Ishii S. A clinical study of the relationship of timing to outcome of surgery for ruptured cerebral aneurysms. A retrospective analysis of 1622 cases. J Neurosurg. 1993;79:373-378.
48 Rosenorn J, Eskesen V, Schmidt K, et al. Clinical features and outcome in 1076 patients with ruptured intracranial saccular aneurysms: a prospective consecutive study. Br J Neurosurg. 1987;1:33-46.
49 Schievink WI, Wijdick EFM, Piepgras DG, et al. The poor prognosis of ruptured intracranial aneurysms of the posterior circulation. J Neurosurg. 1995;82:791-795.
50 Schievink WI, Wijdicks EFM, Parisi JE, et al. Sudden death from aneurysmal subarachnoid hemorrhage. Neurology. 1995;45:871-874.
51 Laidlaw JD, Siu KH. Ultra-early surgery for aneurysmal subarachnoid hemorrhage: outcomes for a consecutive series of 391 patients not selected by grade or age. J Neurosurg. 2002;97:247-258.
52 Hernesniemi J, Vapalahti M, Niskanen M, et al. Management outcome for vertebrobasilar artery aneurysms by early surgery. Neurosurgery. 1992;31:857-862.
53 Hillman J, Saveland H, Jakobsson KE, et al. Overall management outcome of ruptured posterior fossa aneurysms. J Neurosurg. 1996;85:33-38.
54 Peerless SJ, Hernesniemi JA, Gutman FB, et al. Early surgery for ruptured vertebrobasilar aneurysms. J Neurosurg. 1994;80:643-649.
55 Molyneux A, Kerr R, Stratton I, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002;360:1267-1274.
56 Molyneux AJ, Kerr RS, Yu LM, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366:809-817.
57 Starke RM, Kim GH, Fernandez A, et al. Impact of a protocol for acute antifibrinolytic therapy on aneurysm rebleeding after subarachnoid hemorrhage. Stroke. 2008;39:2617-2621.
58 Acevedo JC, Turjman F, Sindou M. Postoperative angiography in surgery for intracranial aneurysm: propsective study in consecutive series of 267 operated cases. Neurochirurgie. 1997;43:275-284.
59 David CA, Vishteh G, Spetzler RF, et al. Late angiographic follow-up of surgically treated aneurysms. J Neurosurg. 1999;91:396-401.
60 Feuerberg I, Linquist C, Lindqvist M, et al. Natural history of postoperative aneurysmal rests. J Neurosurg. 1987;66:30-34.
61 Le Roux P, Elliott JP, Eskridge JM, et al. Risks and benefits of diagnostic angiography following aneurysm surgery: a retrospective analysis of 597 studies. Neurosurgery. 1998;42:1248-1255.
62 Rauzzino MJ, Quinn CM, Fischer W. Angiography after aneurysm surgery: indications for selective angiography. Surg Neurol. 1998;49:32-41.
63 Drake CG, Allcock JM. Postoperative angiography and the slipped clip. J Neurosurg. 1973;39:683-689.
64 Drake CG, Friedman AH, Peerless SJ. Failed aneurysm surgery: reoperation in 115 cases. J Neurosurg. 1984;61:848-856.
65 MacDonald RL, Wallace C, Kestle JRW. Role of angiography following aneurysm surgery. J Neurosurg. 1993;79:826-832.
66 Litofsky NS, Vinuela F, Giannotta SL. Progressive visual loss after electrothrombosis treatment of a giant intracranial aneurysm: case report. Neurosurgery. 1994;34:548-551.
67 D’Ambrosio AL, Sughrue ME, Yorgason J, et al. Decompressive hemicraniectomy for poor-grade aneurysmal subarachnoid hemorrhage patients: clinical outcome and quality of life assessment. Neurosurgery. 2004;56:12-20.
68 Smith ER, Carter BS, Ogilvy CS. Proposed use of prophylactic decompressive craniectomy in poor-grade aneurysmal subarachnoid hemorrhage patients presenting with associated large Sylvian hematomas. Neurosurgery. 2002;51:117-124.
69 Komotar RJ, Schmidt JM, Starke RM, et al. Resuscitation and critical care of poor-grade subarachnoid hemorrhage. Neurosurgery. 2009;64:397-410.
70 Ramakrishna R, Stiefel M, Udoteuk J, et al. Brain oxygen tension and outcome in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2008;109:1075-1082.
71 Broderick JP, Thoma GB, Dudler JE, et al. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342-1347.
72 Pakarinen S. Incidence, etiology and prognosis of primary subarachnoid hemorrhage. A study based on 589 cases diagnosed in a defined population during a defined period. Acta Neurol Scand. 1967;43(Suppl 29):1-128.
73 Bailes JE, Spetzler RF, Hadley MN, et al. Management morbidity and mortality of poor grade aneurysm patients. J Neurosurgery. 1990;72:559-566.
74 Le Roux P, Elliott JP, Newell DW, et al. Predicting outcome in poor grade subarachnoid hemorrhage: a retrospective review of 159 aggressively managed patients. J Neurosurg. 1996;85:39-49.
75 Le Roux P, Dailey AT, Newell DW, et al. Emergent aneurysm clipping without angiography in the moribund patient with intracerebral hemorrhage: the use of infusion computed tomography scans. Neurosurgery. 1993;33:189-197.
76 Nowak G, Schwachenwald R, Arnold H. Early management in poor grade aneurysm patients. Acta Neurochir (Wien). 1994;126:33-37.
77 Steudel WI, Reif J, Voges M. Modulated surgery in the management of ruptured intracranial aneurysm in poor grade patients. Neurol Res. 1994;16:49-53.
78 Kinugasa K, Kamata I, Hirotsune N, et al. Early treatment of subarachnoid hemorrhage after preventing rerupture of an aneurysm. J Neurosurg. 1995;83:34-41.
79 Kremer C, Groden C, Hansen HC, et al. Outcome after endovascular treatment of Hunt and Hess grade IV or V aneurysms. Comparison of anterior versus posterior circulation. Stroke. 1999;30:2617-2622.
80 Malisch T, Guglielmi G, Vinuela F, et al. Intracranial aneurysms treated with Guglielmi detachable coil: midterm clinical results in a consecutive series of 100 patients. J Neurosurg. 1997;87:176-183.
81 van Loon J, Waerzeggers Y, Wilms G, et al. Early endovascular treatment of ruptured cerebral aneurysms in patients in very poor neurological condition. Neurosurgery. 2002;50:457-465.
82 Inamasu J, Nakamura Y, Saito R, et al. Endovascular treatment for poorest-grade subarachnoid hemorrhage in the acute stage: has the outcome been improved? Neurosurgery. 2002;50:1199-1206.
83 Suzuki S, Jahan R, Duckwiler GR, et al. Contribution of endovascular therapy to the management of poor-grade aneurysmal subarachnoid hemorrhage: clinical and angiographic outcomes. J Neurosurg. 2006;105:664-670.
84 Sedat J, Dib M, Lonjon M, et al. Endovascular treatment of ruptured intracranial aneurysms in patients aged 65 years and older: follow-up of 52 patients after 1 year. Stroke. 2002;33:2620-2625.
85 Groden C, Kremer C, Regelsberger J, et al. Comparison of operative and endovascular treatment of anterior circulation aneurysms in patients in poor grades. Neuroradiology. 2001;43:778-783.
86 Hauerberg J, Eskesen V, Rosenorn J. The prognostic significance of intracerebral hematoma as shown on CT scanning after aneurysmal subarachnoid hemorrhage. Br J Neurosurg. 1994;8:333-339.
87 O’Sullivan MG, Sellar R, Statham PF, et al. Management of poor grade patients after subarachnoid haemorrhage: the importance of neuroradiological findings on clinical outcome. Br J Neurosurgery. 1996;10:445-452.
88 Rosen DS, Macdonald RL, Huo D, et al. Intraventricular hemorrhage from ruptured aneurysm: clinical characteristics, complications, and outcomes in a large, prospective, multicenter study population. J Neurosurg. 2007;107:261-265.
89 Heiskanen O, Poranen A, Kuurne T, et al. Acute surgery for intracerebral hematomas caused by rupture of an intracranial arterial aneurysm. A prospective randomized study. Acta Neurochir. 1988;90:81-83.
90 Pasqualin A, Bazzan A, Cavazzani P, et al. Intracranial hematomas following aneurysmal rupture: experience with 309 cases. Surg Neurol. 1986;25:6-17.
91 Wheelock B, Weir B, Watts R, et al. Timing of surgery for intracerebral hematomas due to aneurysm rupture. J Neurosurg. 1983;58:476-481.
92 Anderson G, Steinke D, Petruk K, et al. Computed tomographic angiography versus digital subtraction angiography for the diagnosis and early treatment of ruptured intracranial aneurysms. Neurosurgery. 1999;45:1315-1322.
93 Hsiang JNK, Liang EY, Lam JMK, et al. The role of computed tomography angiography in the diagnosis of intracranial aneurysms and emergent aneurysm clipping. Neurosurgery. 1996;38:481-487.
94 Murai Y, Takagi R, Ikeda Y, et al. Three-dimensional computerized tomography angiography in patients with hyperacute intracerebral hemorrhage. J Neurosurg. 1999;91:424-431.
95 Newell DW, Le Roux PD, Dacey RG, et al. CT infusion scanning for the detection of cerebral aneurysms. J Neurosurg. 1989;71:175-179.
96 Niemann DB, Wills AD, Maartens NF, et al. Treatment of intracerebral hematomas caused by aneurysm rupture: coil placement followed by clot evacuation. J Neurosurg. 2003;99:843-847.
97 Hasan D, Vermeulen M, Wijdicks EFM, et al. Management problems in acute hydrocephalus after subarachnoid hemorrhage. Stroke. 1989;20:747-753.
98 Milhorat TH. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1987;20:15-20.
99 Mohr G, Ferguson G, Khan M, et al. Intraventricular hemorrhage from ruptured aneurysm: retrospective analysis of 91 cases. J Neurosurg. 1983;58:482-487.
100 Rajshekhar V, Harbaugh RE. Results of routine ventriculostomy with external ventricular drainage for acute hydrocephalus following subarachnoid haemorrhage. Acta Neurochir (Wien). 1992;115:8-14.
101 Raimondi AJ, Torres H. Acute hydrocephalus as a complication of subarachnoid hemorrhage. Surg Neurol. 1973;1:23-26.
102 Shimoda M, Oda S, Shibata M, et al. Results of early surgical evacuation of packed intraventricular hemorrhage from aneurysm rupture in patients with poor-grade subarachnoid hemorrhage. J Neurosurg. 1999;91:408-414.
103 Todo T, Usui M, Takakura K. Treatment of severe intraventricular hemorrhage by intraventricular infusion of urokinase. J Neurosurg. 1991;74:81-86.
104 Komotar RJ, Hahn DK, Kim GH, et al. The impact of microsurgical fenestration of the lamina terminalis on shunt-dependent hydrocephalus and vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2008;62:123-132.
105 Klimo P, Kestle JR, MacDonald JD, et al. Marked reduction of cerebral vasospasm with lumbar drainage of cerebrospinal fluid after subarachnoid hemorrhage. J Neurosurg. 2004;100:215-224.
106 Pare L, Delfino R, Leblanc R. The relationship of ventricular drainage to aneurysmal rebleeding. J Neurosurg. 1992;76:422-427.
107 van Gijn J, Hijdra A, Wijdicks E, et al. Acute hydrocephalus after aneurysmal subarachnoid hemorrhage. J Neurosurg. 1985;63:355-362.
108 Voldby B, Enevoldsen E. Intracranial pressure changes during aneurysm rupture: recurrent hemorrhages. J Neurosurg. 1982;56:784-789.
109 Claassen J, Bernardini GL, Kreiter K, et al. Effect of cisternal and ventricular blood on risk of delayed cerebral ischemia after subarachnoid hemorrhage: the Fisher scale revisited. Stroke. 2001;32:2012-2020.
110 Sheehan JP, Polin RS, Sheehan JM, et al. Factors associated with hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1999;45:1120-1128.
111 Black P. Hydrocephalus and vasospasm after subarachnoid hemorrhage from ruptured intracranial aneurysms. Neurosurgery. 1986;18:12-16.
112 Tomasello F, d’Avella D, de Divitiis O. Does lamina terminalis fenestration reduce the incidence of chronic hydrocephalus after subarachnoid hemorrhage? Neurosurgery. 1999;45:827-832.
113 de Oliveira JG, Beck J, Seifert V, et al. Assessment of flow in perforating arteries during intracranial aneurysm surgery using intraoperative near-infrared indocyanine green videoangiography. Neurosurgery. 2007;61:63-72.
114 Sethi H, Moore A, Dervin J, et al. Hydrocephalus: comparison of clipping and embolization in aneurysm treatment. J Neurosurg. 2000;92:991-994.
115 Varelas P, Helms A, Sinson G, et al. Clipping or coiling of ruptured cerebral aneurysms and shunt-dependent hydrocephalus. Neurocrit Care. 2006;4:223-228.
116 Gruber A, Reinprecht A, Bavinski G, et al. Chronic shunt dependent hydrocephalus after early surgery and early endovascular treatment of ruptured intracranial aneurysms. Neurosurgery. 1999;44:503-512.
117 Qureshi AI, Sung GY, Suri MA, et al. Prognostic value and determinants of ultra-early angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 1999;44:967-974.
118 Findlay JM, MacDonald RL, Weir BKA, et al. Surgical manipulation of primate cerebral arteries in established vasospasm. J Neurosurg. 1991;75:425-532.
119 MacDonald RL, Wallace MC, Coyne TJ. The effect of surgery on the severity of vasospasm. J Neurosurg. 1994;80:433-439.
120 Origitano TC, Wascher TM, Reichman OH, et al. Sustained increased cerebral blood flow with prophylactic hypertensive hypervolemic hemodilution (Triple-H therapy) after subarachnoid hemorrhage. Neurosurgery. 1990;27:729-740.
121 Solomon RA, Onesti ST, Klebanoff L. Relationship between the timing of aneurysm surgery and the development of delayed cerebral ischemia. J Neurosurg. 1991;75:56-61.
122 Le Roux P, Newell DW, Eskridge J, et al. Severe symptomatic vasospasm: the role of immediate postoperative angioplasty. J Neurosurg. 1994;80:224-229.
123 Eskridge J, McAuliffe W, Song JK, et al. Balloon angioplasty for the treatment of vasospasm: results of the first 50 cases. Neurosurgery. 1998;42:510-517.
124 Muizelaar JP, Zwienenberg M, Rudisill N, et al. The prophylactic use of transluminal balloon angioplasty in patients with Fisher grade 3 subarachnoid hemorrhage: a pilot study. J Neurosurg. 1999;91:424-431.
125 Nilsson O, Brandt L, Ungerstedt U, et al. Bedside detection of brain ischemia using intracerebral microdialysis: subarachnoid hemorrhage and delayed ischemic deterioration. Neurosurgery. 1999;45:1176-1185.
126 Allcock JM, Drake CG. Postoperative angiography in cases of ruptured intracranial aneurysm. J Neurosurg. 1963;20:752-759.
127 Batjer H, Samson D. Intraoperative aneurysmal rupture: incidence, outcome, and suggestions for surgical management. Neurosurgery. 1986;18:701-707.
128 Giannotta SL, Oppenheimer JH, Levy ML, et al. Management of intraoperative rupture of aneurysm without hypotension. Neurosurgery. 1991;28:531-536.
129 Kassell NF, Torner JC, Jane J, et al. The international cooperative study on the timing of aneurysm surgery. Part 2. Surgical results. J Neurosurg. 1990;73:37-47.
130 Ljunggren B, Saveland H, Brandt L. Causes of unfavorable outcome after early aneurysm operation. Neurosurgery. 1983;13:629-633.
131 Maurice-Williams R, Kitchen ND. Ruptured intracranial aneurysms—learning from experience. Br J Neurosurg. 1994;8:519-527.
132 Schramm J, Cedzich C. Outcome and management of intraoperative aneurysm rupture. Surg Neurol. 1993;40:26-30.
133 Sundt TM, Whisnant JP. Subarachnoid hemorrhage from intracranial aneurysms. Surgical management and natural history of disease. N Engl J Med. 1978;299:116-122.
134 Mavaddat N, Sahakian BJ, Hutchinson PJ, et al. Cognition following subarachnoid hemorrhage from anterior communicating artery aneurysm: relation to timing of surgery. J Neurosurg. 1999;91:402-407.
135 Brouwers PJAM, Dippel DWJ, Vermeulen M, et al. Amount of blood on computed tomography as an independent predictor after aneurysm rupture. Stroke. 1993;24:809-814.
136 Hugosson R. Intracranial arterial aneurysms: consideration on the upper age limit for surgical treatment. Acta Neurochir (Wien). 1973;28:157-164.
137 Longstreth WTJr, Nelson LM, Koepsell TD, et al. Clinical course of spontaneous subarachnoid hemorrhage: a population-based study in King County, Washington. Neurology. 1993;43:712-718.
138 Niskanen MM, Hernesniemi JA, Vapalahti MP, et al. One-year outcome in early aneurysm surgery: prediction of outcome. Acta Neurochir. 1993;123:25-32.
139 O’Sullivan MG, Dorwartod N, Whittle IR, et al. Management and long-term outcome following subarchnoid haemorrhage and intracranial aneurysm surgery in elderly patients: an audit of 199 consecutive cases. Br J Neurosurg. 1994;8:23-30.
140 Rosenorn J, Eskesen V, Schmidt K. Age as a prognostic factor after intracranial aneurysm rupture. Br J Neurosurg. 1987;1:335-341.
141 Taylor B, Harries P, Bullock R. Factors affecting outcome after surgery for intracranial aneurysm in Glasgow. Br J Neurosurg. 1991;5:591-600.
142 Grumbach K, Anderson GM, Luft HS, et al. Regionalization of cardiac surgery in the United States and Canada: geographic access, choice, and outcomes. JAMA. 1995;274:1282-1288.
143 Mendeloff J, Cayten C. Trauma systems and public policy. Annu Rev Public Health. 1991;12:401-424.
144 Roy P. The value of trauma centers: a methodologic review. Can J Surg. 1987;30:7-22.
145 Shackford S, Mackersie R, Hoyt D, et al. Impact of a trauma system on outcome of severely injured patients. Arch Surg. 1987;122:523-527.
146 Smith JJr, Martin L, Young W, et al. Do trauma centers improve outcome over non-trauma centers: the evaluation of regional trauma care using discharge abstract data and patient management categories. J Trauma. 1990;30:1533-1538.
147 Fridriksson S, Hillman J, Landtblom AM, et al. Education of referring doctors about sudden onset headache in subarachnoid hemorrhage. A prospective study. Acta Neurol Scand. 2001;103:238-242.
148 Bardach NS, Olson SJ, Elkins JS, et al. Regionalization of treatment for subarachnoid hemorrhage: a cost-utility analysis. Circulation. 2004;109:2207-2212.
149 Cross CTIII, Tirschwell DL, Clarke MA, et al. Mortality rates after subarachnoid hemorrhage: variations according to hospital case volume in 18 states. J Neurosurg. 2003;99:810-817.
150 Solomon RA, Mayer SA, Tarmey JJ. Relationship between the volume of craniotomies for cerebral aneurysm performed at New York state hospitals and in-hospital mortality. Stroke. 1996;27:13-17.
151 Taylor CL, Yuan Z, Selman WR, et al. Mortality rates, hospital length of stay, and the cost of treating subarachnoid hemorrhage in older patients: institutional and geographical difference. J Neurosurg. 1997;86:583-588.
152 Johnston SC. Effect of endovascular services and hospital volume on cerebral aneurysm treatment outcomes. Stroke. 2000;31:111-117.
153 Josephson SA, Douglas VC, Lawton MT, et al. Improvement in intensive care unit outcomes in patients with subarachnoid hemorrhage after initiation of neurointensivist co-management. J Neurosurg. 2010;112:626-630.
154 Gordon HS, Rosenthal GE. Impact of interhospital transfers on outcomes in an academic medical center. Implications for profiling hospital quality. Med Care. 1996;34:295-309.
155 Gerber CJ, Lang DA, Neil-Dwyer G, et al. A simple scoring system for accurate prediction of outcome within four days of a subarachnoid hemorrhage. Acta Neurochir (Wien). 1993;122:11-22.
156 Inagawa T. Management outcome in the elderly patient following subarachnoid hemorrhage. J Neurosurg. 1993;78:554-561.
157 Le Roux P, Elliott JP, Downey L, et al. Improved outcome following rupture of anterior circulation aneurysms: a retrospective 10 year review of 224 good grade patients. J Neurosurg. 1995;83:394-402.
158 Fridriksson S, Hillman J, Saveland H, et al. Intracranial aneurysm surgery in the 8th and 9th decades of life: impact on population-based management outcome. Neurosurgery. 1995;37:627-632.
159 Ryttlefors M, Enblad P, Kerr RS, et al. International subarachnoid aneurysm trial of neurosurgical clipping versus endovascular coiling: subgroup analysis of 278 elderly patients. Stroke. 2008;39:2720-2726.
160 Elliott JP, Le Roux PD, Ransom G, et al. Predicting length of hospital stay and cost by aneurysm grade on admission. J Neurosurg. 1996;85:388-391.
161 Leblanc R, Worsley KJ. Surgery for unruptured asymptomatic aneurysms. A decision analysis. Can J Neurol Sci. 1995;22:30-35.
162 King JT, Glick HA, Mason TJ, et al. Elective surgery for asymptomatic, unruptured, intracranial aneurysms: a cost-effctiveness analysis. J Neurosurg. 1995;83:403-412.
163 Chang HS, Kirino T. Quantification of operative benefit for unruptured aneurysms. A thereotical approach. J Neurosurg. 1995;83:413-420.
164 Stoodley M, Macdonald RL, Weir BKA. Pregnancy and intracranial aneurysms. Neurosurg Clin N Am. 1998;9:549-556.
165 Kriplani A, Relan S, Misra NK. Ruptured intracranial aneurysm complicating pregnancy. Int J Gynaecol Obstet. 1995;48:201-206.
166 Cohen-Gadol AA, Friedman JA, Friedman JD, et al. Neurosurgical management of intracranial lesions in the pregnant patient: a 36-year institutional experience and review of the literature. J Neurosurg. 2009;111:1150-1157.
167 Meyer F, Sundt T, Fode N. Cerebral aneurysms in childhood and adolescence. J Neurosurg. 1989;70:420-425.
168 Norris JS, Wallace MC. Pediatric intracranial aneurysms. Neurosurg Clin N Am. 1998;9:557-563.
169 Lasjaunias P, Wuppalapati S, Alvarez H, et al. Intracranial aneurysms in children aged under 15 years: review of 59 consecutive children with 75 aneurysms. Childs Nerv Syst. 2005;21:437-450.
170 Kumar M, Kitchen ND. Infective and traumatic aneurysms. Neurosurg Clin N Am. 1998;9:577-586.
171 Haddad FS, Haddad GF, Taha J. Traumatic intracranial aneurysms caused by missiles. Their presentation and management. Neurosurgery. 1991;28:1-7.
172 Keick CK, de Villiers JC. Vascular lesions due to transcranial stab wounds. Neurosurgery. 1984;60:42.
173 Cohen JE, Gomori JM, Segal R, et al. Results of endovascular treatment of traumatic intracranial aneurysms. Neurosurgery. 2008;63:476-485.
174 Bell RS, Vo AH, Roberts R, et al. Wartime traumatic aneurysms: acute presentation, diagnosis, and multimodal treatment of 64 craniocervical arterial injuries. Neurosurgery. 2010;66:66-79.
175 Cockroft K, Thompson RC, Steinberg GK. Aneurysms and arteriovenous malformations. Neurosurg Clin N Am. 1998;9:565-576.
176 Perata HJ, Tomsick TA, Tew JM. Feeding artery pedicle aneurysms: association with parenchymal hemorrhage and arteriovenous malformations. J Neurosurg. 1994;8:631-634.
177 Heman LM, Jongen LM, van der Worp HB, et al. Incidental intracranial aneurysms in patients with internal carotid artery stenosis: a CT angiography study and a meta-analysis. Stroke. 2009;40:1341-1346.
178 Mackey WC, O’Donnell TF, Callow AD. Carotid endarterectomy in patients with intracranial vascular disease: short-term risk and long-term outcome. J Vasc Surg. 1989;10:432-438.
179 Bederson J, Zambramski J, Spetzler RF. Treatment of fusiform intracranial aneurysms by circumferential wrapping with clip reinforcement. Technical note. J Neurosurg. 1992;77:478-480.
180 Sugita K, Kobayashi S, Inoue T. New angled fenestrated clips for fusiform vertebral artery aneurysms. J Neurosurg. 1981;54:346-350.
181 Fiorella D, Albuquerque FC, McDougall CG. Preliminary experience using the Neuroform stent for the treatment of cerebral aneurysms. Neurosurgery. 2004;54:6-16.
182 Fiorella D, Woo HH, Albuquerque FC, et al. Definitive reconstruction of circumferential, fusiform intracranial aneurysms with the pipeline embolization device. Neurosurgery. 2008;62:1115-1120.
183 Nussbaum ES, Erickson DL. The fate of intracranial microaneurysms treated with bipolar electrocoagulation and parent vessel reinforcement. Neurosurgery. 1999;45:1172-1175.
184 Lawton MT, Daspit CP, Spetzler RF. Technical aspects and recent trends in the management of large and giant midbasilar artery aneurysms. Neurosurgery. 1997;41:513.
185 Lawton MT, Spetzler R. Surgical strategies for giant intracranial aneurysms. Neurosurg Clin N Am. 1998;9:725-742.
186 Solomon RA, Baker CJ. Direct surgical approaches to giant intracranial aneurysms. Neurosurg Q. 1992;2:1-27.
187 Spetzler RF, Hadley MN, Rigamonti D, et al. Aneurysms of the basilar artery treated with circulatory arrest, hypothermia, and barbiturate cerebral protection. J Neurosurg. 1988;68:868-879.
188 Symon L, Vajda J. Surgical experience with giant intracranial aneurysms. J Neurosurg. 1984;61:1009-1028.
189 Drake CG. Giant intracranial aneurysms. Experience with surgical treatment in 174 patients. Clin Neurosurg. 1979;26:12-96.
190 Piepgras DG. Management of incidental intracranial aneurysms. Clin Neurosurg. 1989;35:511-518.
191 Sundt T, Piepgras D, Fode N, et al. Giant intracranial aneurysms. Clin Neurosurg. 1991;37:116-154.
192 Linskey ME, Jungreis CA, Yonas H, et al. Stroke risk after abrupt internal carotid artery sacrifice: accuracy of preoperative assessment with balloon test occlusion and stable xenon-enhanced CT. AJNR Am J Neuroradiol. 1994;15:829-843.
193 Abruzzo T, Joseph GJ, Owens DS, et al. Prevention of complications resulting from endovascular carotid sacrifice: a retrospective assessment. Neurosurgery. 2000;46:910-916.
194 Anson J, Lawton M, Spetzler R. Characteristics and surgical treatment of dolichoectatic and fusiform aneurysms. J Neurosurg. 1996;84:185.
195 Drake CG, Vanderlinden RG. The late consequences of incomplete surgical treatment of cerebral aneurysms. J Neurosurg. 1967;27:226-238.
196 Lin T, Fox AT, Drake CG. Regrowth of aneurysm sacs from residual neck following aneurysm clipping. J Neurosurgery. 1989;70:556-560.
197 Giannotta SL, Litofsky NS. Reoperative management of intracranial aneurysms. J Neurosurg. 1995;83:387-393.
198 Juvela S, Porras M, Heiskanen O. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. J Neurosurg. 1993;79:174-182.
199 Le Roux P, Winn HR. Management of cerebral aneurysms: how can current management be improved? Neurosurg Clin N Am. 1998;9:421-433.
200 Gnanalingham KK, Apostolopoulos V, Barazi S, et al. The impact of the international subarachnoid aneurysm trial (ISAT) on the management of aneurysmal subarachnoid haemorrhage in a neurosurgical unit in the UK. Clin Neurol Neurosurg. 2006;108:117-123.
201 Lanzino G, Fraser K, Kanaan Y, et al. Treatment of ruptured intracranial aneurysms since the international subarachnoid aneurysm trial: practice utilizing clip ligation and coil embolization as individual or complementary therapies. J Neurosurg. 2006;104:344-349.
202 Koebbe CJ, Veznedaroglu E, Jabbour P, et al. Endovascular management of intracranial aneurysms: current experience and future advances. Neurosurgery. 2006;59:S93-S102.
203 Gurian JH, Martin NA, King WA, et al. Neurosurgical management of cerebral aneurysms following unsuccessful or incomplete endovascular embolization. J Neurosurg. 1995;83:843-853.
204 Khayata MH, Spetzler RF, Moov JJA, et al. Combined surgical and endovascular treatment of a giant vertebral artery aneurysm in a child. J Neurosurg. 1994;81:304-307.
205 Thielen KR, Nichols DA, Fulcham JR, et al. Endovascular treatment of cerebral aneurysms following incomplete clipping. J Neurosurg. 1997;87:184-189.
206 Byrne JV, Sohn MJ, Molyneux AJ. Five-year experience in using coil embolization for ruptured intracranial aneurysms: outcomes and incidence of late rebleeding. J Neurosurg. 1999;90:656-663.
207 Casasco AE, Aymard A, Gobin P, et al. Selective endovascular treatment of 71 intracranial aneurysms with platinum coils. J Neurosurg. 1993;79:3-10.
208 Eskridge J, Song J. Endovascular embolization of 150 basilar tip aneurysms with Guglielmi detachabel coils: results of the Food and Drug Administration multicenter clinical trial. J Neurosurg. 1998;89:81-86.
209 Graves VB, Strother CM, Duff TA, et al. Early treatment of ruptured aneurysms with Guglielmi detachable coils: effect on subsequent bleeding. Neurosurgery. 1995;37:640-648.
210 Guglielmi G, Viñuela F, Sepetka I, et al. Electrothrombosis of saccular aneurysms via endovascular approach. Part 1: electrochemical basis, technique, and experimental results. J Neurosurg. 1991;75:1-7.
211 Raymond J, Roy D, Boianowski M, et al. Endovascular treatment of acutely ruptured and unruptured aneurysms of the basilar bifurcation. J Neurosurg. 1997;86:211-219.
212 Vinuela F, Duckwiler G, Mawad M. Guglielmi detachable coil embolization of acute intracranial aneurysm: perioperative anatomical and clinical outcome in 403 patients. J Neurosurg. 1997;86:475-482.
213 Brilstra E, Rinkel G, van der Graaf Y, et al. Treatment of intracranial aneurysms by embolization with coils: a systematic review. Stroke. 1999;30:470-476.
214 Halbach VV, Higashida RT, Down CF, et al. The efficacy of endosaccular aneurysm occlusion in alleviating neurological deficits produced by mass effect. J Neurosurg. 1994;80:659-666.
215 Byrne JV, Adams CBT, Kerr R, et al. Endosaccular treatment of inoperable intracranial aneurysms with platinum coils. Br J Neurosurg. 1995;9:585-592.
216 Nelson PK. Neurointerventional management of intracranial aneurysms. Neurosurg Clin N Am. 1998;9:879-885.
217 Pierot L, Boulin A, Castaings L, et al. Selective occlusion of basilar artery aneurysms using controlled detachable coils: report of 35 cases. Neurosurgery. 1996;38:948-954.
218 Vanninen R, Kovisto T, Saari T, et al. Ruptured intracranial aneurysms: acute endovascualr treatment with electrolytically detachable coils—a prospective randomized study. Radiology. 1999;211:325-336.
219 Tateshima S, Murayama Y, Gobin YP, et al. Endovascular treatment of basilar tip aneurysms using Guglielmi detachable coils: anatomic and clinical outcomes in 73 patients from a single institution. Neurosurgery. 2000;47:1332-1342.
220 Cognard C, Weill A, Spelle L, et al. Long-term angiographic follow-up of 169 intracranial berry aneurysms occluded with detachable coils. Radiology. 1999;212:348-356.
221 Hayakawa M, Murayama Y, Duckwiler GR, et al. Natural history of the neck remnant of a cerebral aneurysm treated with the Guglielmi detachable coil system. J Neurosurg. 2000;93:561-568.
222 Murayama Y, Malisch T, Guglielmi G, et al. Incidence of cerebral vasospasm after endovascular treatment of acutely ruptured aneurysms: report on 69 cases. J Neurosurg. 1997;87:830-835.
223 Murayama Y, Song JK, Uda K, et al. Combined endovascular treatment for both intracranial aneurysm and symptomatic vasospasm. AJNR Am J Neuroradiol. 2003;24:133-139.
224 Ng P, Khangure MS, Phatouros CC, et al. Endovascular treatment of intracranial aneurysms with Guglielmi detachable coils: analysis of midterm angiographic and clinical outcomes. Stroke. 2002;33:210-217.
225 Thornton J, Debrun GM, Aletich VA, et al. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery. 2002;50:239-249.
226 Raymond J, Guilbert F, Weill A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003;34:1398-1403.
227 Ferns SP, Sprengers ME, van Rooij WJ, et al. Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke. 2009;40:e523-e529.
228 Mizoi K, Yoshimoto T, Takahashi A, et al. A pitfall in the surgery of a recurrent aneurysm after coil embolization and its histological observation: technical case report. Neurosurgery. 1996;39:165-169.
229 Renowden SA, Benes V, Bradley M, et al. Detachable coil embolisation of ruptured intracranial aneurysms: a single center study, a decade experience. Clin Neurol Neurosurg. 2009;111:179-188.
230 Tait MJ, Critchley GR, Norris JS. How much can be concluded from the international subarachnoid aneurysm trial (ISAT)? Br J Neurosurg. 2007;21:3-6.
231 Pandey AS, Koebbe CJ, Rosenwasser R, et al. Endovascular coile embolization of ruptured and unruptured posterior circulation aneurysms: review of a 10-year experience. Neurosurgery. 2007;60:626-637.
232 Campi A, Ramzi N, Molyneux AJ, et al. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trail (ISAT). Stroke. 2007;38:1538-1544.
233 Johnston SC, Dowd CF, Higashida RT, et al. Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms: the cerebral aneurysm rerupture after treatment (CARAT) study. Stroke. 2008;39:120-125.
234 Ortiz R, Stefanski M, Rosenwasser R, et al. Cigarette smoking as a risk factor for recurrence of aneurysms treated by endosaccular occlusion. J Neurosurg. 2008;108:672-675.
235 Sluzewski M, Bosch JA, van Rooij WJ, et al. Rupture of intracranial aneurysms during treatment with Guglielmi detachable coils: incidence, outcome, and risk factors. J Neurosurg. 2001;94:238-240.
236 Koivisto T, Vanninen R, Hurskainen H, et al. Outcomes of early endovascular versus surgical treatment of ruptured cerebral aneurysms: a prospective randomized study. Stroke. 2000;31:2369-2377.
237 van Rooij WJ, Sluzewski M. Endovascular treatment of large and giant aneurysms. AJNR Am J Neuroradiol. 2009;30:12-18.
238 Lempert TE, Malek AM, Halbach VV, et al. Endovascular treatment of ruptured posterior circulation cerebral aneurysms. Clinical and angiographic outcomes. Stroke. 2000;31:100-110.
239 Mitchell P, Kerr R, Mendelow AD, et al. Could late rebleeding overturn the superiority of cranial aneurysm coil embolization over clip ligation seen in the international subarachnoid aneurysm trial? J Neurosurg. 2008;108:437-442.
240 Sluzewski M, van Rooij WJ. Early rebleeding after coiling of ruptured cerebral aneurysms: incidence, morbidity, and risk factors. AJNR Am J Neuroradiol. 2005;26:1739-1743.
241 Dawson RC, Krisht AF, Barrow DL, et al. Treatment of experimental aneurysms using collagen-coated microcoils. Neurosurgery. 1995;36:133-140.
242 Kwan ESK, Heilman CB, Shucart WA. Enlargement of basilar artery aneurysms following balloon occlusion: “Water-hammer effect”—report of two cases. J Neurosurg. 1991;75:963-968.
243 Ringer AJ, Lanzino G, Veznedaroglu E, et al. Does angiographic surveillance pose a risk in the management of coiled intracranial aneurysms? A multicenter study of 2243 patients. Neurosurgery. 2008;63:845-849.
244 Dorsch NW, Young N, Kingston RJ, et al. Early experience with spiral CT in the diagnosis of intracranial aneurysms. Neurosurgery. 1995;36:230-236.
245 Farb RI, Nag S, Scott JN, et al. Surveillance of intracranial aneurysms treated with detachable coils: a comparison of MRA techniques. Neuroradiology. 2005;47:507-515.
246 Civit T, Auque J, Marchal JC, et al. Aneurysm clipping after endovascular treatment with coils: a report of eight patients. Neurosurgery. 1996;38:955-961.
247 Waldron JS, Halbach VV, Lawton MT. Microsurgical management of incompletely coiled and recurrent aneurysms: trends, techniques, and observations on coil extrusion. Neurosurgery. 2009;64:301-315.
248 Murayama Y, Vinuela F, Duckwiler G, et al. Embolization of incidental cerebral aneurysms by usingthe Guglielmi detachable coil system. J Neurosurg. 1999;90:207-214.
249 Rufenacht D, Mandai S, Levrier O. Endovascular treatment of intracranial aneurysms. AJNR Am J Neuroradiol. 1996;17:1658-1660.
250 Zubillaga AF, Guglielmi G, Vinuela F, et al. Endovascular occlusion of intracranial aneurysms with electrically detachable coils: correlation of aneurysm neck ziae and treatment results. AJNR Am J Neuroradiol. 1994;15:815-820.
251 Turjman F, Massoud TF, Sayre J, et al. Predictors of aneurysmal occlusion in the period immediately after endovascular treatment with detachable coils: a multivariate analysis. AJNR Am J Neuroradiol. 1998;19:1645-1651.
252 Hope JK, Byrne JV, Molyneux AJ. Factors influencing successful angiographic occlusion of aneurysms treated by coil embolization. AJNR Am J Neuroradiol. 1999;20:391-399.
253 Tahtinen OI, Vanninen RL, Manninen HI, et al. Wide-necked intracranial aneurysms: treatment with stent-assisted coil embolization during acute (<72 hours) subarachnoid hemorrhage—experience in 61 consecutive patients. Radiology. 2009;253:199-208.
254 Gurian JH, Vinuela F, Guglielmi G, et al. Endovascular embolization of superior hypophyseal artery aneurysms. Neurosurgery. 1996;39:1150-1156.
255 Halbach VV, Higashida RT, Dowd CF, et al. Cavernous internal carotid artery aneurysms treated with electrolytically detachable coils. J Neuroophthalmol. 1997;17:231-239.
256 Gruber DP, Zimmerman G, Tomsick TA, et al. A comparison between endovascular and surgical management of basilar apex aneurysms. J Neurosurg. 1999;90:868-874.
257 Sanai N, Tarapore P, Lee AC, et al. The current role of microsurgery for posterior circulation aneurysms: a selective approach in the endovascular era. Neurosurgery. 2008;62:1236-1249.
258 Regli L, Uske A, de Tribolet N. Endovascular coil placement compared with surgical clipping for the treatment of unruptured middle cerebral artery aneurysms: a consecutive series. J Neurosurg. 1999;90:1025-1030.
259 Rinne J, Hernesniemi J, Niskanen M, et al. Analysis of 561 patients with 690 middle cerebral artery aneurysms: anatomic and clinical features as correlated to management outcome. Neurosurgery. 1996;38:2-11.
260 Raftopoulos C, Mathurin P, Boscherini D, et al. Prospective analysis of aneurysm treatment in a series of 103 consecutive patients when endovascular embolization is considered the first option. J Neurosurg. 2000;93:175-182.
261 Rinkel GJE, Prins NEM, Algra A. Outcome of aneurysmal subarachnoid hemorrhage in patients on anticoagulant treatment. Stroke. 1997;28:6-9.
262 Ries T, Siemonsen S, Grzyska U, et al. Abciximab is a safe rescue therapy in thromboembolic events complicating cerebral aneurysm coil embolization: single center experience in 42 cases and review of the literature. Stroke. 2009;40:1750-1757.
263 van den Bergh WM, MASH Study Group, Algra A, Dorhout Mees SM, et al. Randomized controlled trial of acetylsalicylic acid in aneurysmal subarachnoid hemorrhage: the MASH Study. Stroke. 2006;37:2326-2330.
264 Abrahams JM, Forman MS, Grady MS, et al. Delivery of human vascular endothelial growth factor with platinum coils enhances wall thickening and coil impregnation in a rat aneurysm model. AJNR Am J Neuroradiol. 2001;22:1410-1417.
265 Abrahams JM, Song C, DeFelice S, et al. Endovascular microcoil gene delivery using immobilized anti-adenovirus antibody for vector tethering. Stroke. 2002;33:1376-1382.
266 Hong L, Miyamoto S, Yamada K, et al. Enhanced formation of fibrosis in a rabbit aneurysm by gelatin hydrogel incorporating basic fibroblast growth factor. Neurosurgery. 2001;49:954-961.
267 Pierot L, Leclerc X, Bonafe A, et al. Endovascular treatment of intracranial aneurysms with matrix detachable coils: midterm anatomic follow-up from a prospective multicenter registry. AJNR Am J Neuroradiol. 2008;29:57-61.
268 Fiorella D, Albuquerque FC, McDougall CG. Durability of aneurysm embolization with matrix detachable coils. Neurosurgery. 2006;58:51-59.
269 Lefkowitz M, Gobin P, Akiba Y, et al. Balloon assited Guglielmi detachable coilng of wide-necked aneurysms: part II: clinical results. Neurosurgery. 1999;45:531-538.
270 Moret J, Cognard C, Weill A. La technique de reconstruction dans la traitment des anevrismes intracranies a collet large. Resultats angiographiques et cliniques a long terms. A propose de 56 cas. J Neuroradiol. 1997;24:30-44.
271 Shapiro B, Babb J, Becske T, et al. Safety and efficacy of adjunctive balloon remodeling during endovascular treatment of intracranial aneurysms: a literature review. AJNR Am J Neuroradiol. 2008;29:1777-1781.
272 Cloft HJ, Joseph GJ, Tong FC, et al. Use of three-dimensional Guglielmi detachable coils in the treatment of wide-necked cerebral aneurysms. AJNR Am J Neuroradiol. 2000;21:1312-1324.
273 Molyneux AJ, Cekirge S, Saatci I, et al. Cerebral aneurysm multicenter European onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. AJNR Am J Neuroradiol. 2004;25:39-51.
274 Cekirge HS, Saatci I, Ozturk MH, et al. Late angiographic and clinical follow-up results of 100 consecutive aneurysms treated with Onyx reconstruction: largest single-center experience. Neuroradiology. 2006;48:113-126.
275 Fiorella D, Albuquerque FC, Woo H, et al. Neuroform in-stent stenosis: incidence, natural history, and treatment strategies. Neurosurgery. 2006;59:34-42.
276 Fiorella D, Kelly ME, Albuquerque FC, et al. Curative reconstruction of a giant midbasilar trunk aneurysm with the pipeline embolization device. Neurosurgery. 2009;64:212-217.
277 Mocco J, Snyder KV, Albuquerque FC, et al. Treatment of intracranial aneurysms with the Enterprise stent: a multicenter registry. J Neurosurg. 2008;110:35-39.
278 Kallmes DF, Ding YH, Dai D, et al. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007;38:2346-2352.
279 Fiorella D, Albuquerque FC, Deshmukh VR, et al. Usefulness of the Neuroform stent for the treatment of cerebral aneurysms: results at initial (3-6-mo) follow-up. Neurosurgery. 2005;56:1191-1201.
280 Benitez RP, Silva MT, Klem J, et al. Endovascular occlusion of wide-necked aneurysms with a new intracranial microstent (Neuroform) and detachable coils. Neurosurgery. 2004;54:1359-1367.
281 Mawad ME, Cekirge S, Ciceri E, et al. Endovascular treatment of giant and large intracranial aneurysms by using a combination of stent placement and liquid polymer injection. J Neurosurg. 2002;96:474-482.
282 Phatouros CC, Sasaki TY, Higashida RT, et al. Stent-supported coil embolization: the treatment of fusiform and wide-neck aneurysms and pseudoaneurysms. Neurosurgery. 2000;47:107-115.
283 Raymond J, Guilbert F, Roy D. Neck-bridge device for endovascular treatment of wide-neck bifurcation aneurysms: initial experience. Radiology. 2001;221:318-326.
284 Byrne JV, Molyneaux AJ, Brennan RP, et al. Embolization of recently ruptured intracranial aneurysms. J Neurol Neurosurg Psychiatry. 1995;59:616-620.
285 Natarajan SK, Sekhar LN, Ghodke B, et al. Outcomes of ruptured intracranial aneurysms treated by microsurgical clipping and endovascular coiling in a high-volume center. AJNR Am J Neuroradiol. 2008;29:753-759.
286 Molyneux AJ, Kerr RS, Birks J, et al. Risk of recurrent subarachnoid haemorrhage, death, or dependence and standardised mortality ratios after clipping or coiling of an intracranial aneurysm in the international subarachnoid aneurysm trial (ISAT): long-term follow-up. Lancet Neurol. 2009;8:427-433.
287 Piepgras DG, Brown RDJr. Management of unruptured intracranial aneurysms: perspectives on endosaccular coiling and persistent uncertainties. Stroke. 2008;39:743-744.
288 Raymond J, Molyneux AJ, Fox AJ, et al. The TEAM trial: safety and efficacy of endovascular treatment of unruptured intracranial aneurysms in the prevention of aneurysmal hemorrhages: a randomized comparison with indefinite deferral of treatment in 2002 patients followed for 10 years. Trials. 2008;9:43.
289 Pierot L, Spelle L, Vitry F. Immediate clinical outcome of patients harboring unruptured intracranial aneurysms treated by endovascular approach: results of the ATENA study. Stroke. 2008;39:2497-2504.
290 Standhardt H, Boecher-Schwarz H, Gruber A, et al. Endovascular treatment of unruptured intracranial aneurysms with Guglielmi detachable coils: short- and long-term results of a single-centre series. Stroke. 2008;39:899-904.
291 Gallas S, Drouineau J, Gabrillargues J, et al. Feasibility, procedural morbidity and mortality, and long-term follow-up of endovascular treatment of 321 unruptured aneurysms. AJNR Am J Neuroradiol. 2008;29:63-68.
292 Aghakhani N, Vaz G, David P, et al. Surgical management of unruptured intracranial aneurysms that are inappropriate for endovascular treatment: experience based on two academic centers. Neurosurgery. 2008;62:1227-1234.
293 Raftopoulos C, Goffette P, Vaz G, et al. Surgical clipping may lead to better results than coil embolization: results from a series of 101 consecutive unruptured intracranial aneurysms. Neurosurgery. 2003;52:1280-1287.
294 Gruber A, Killer M, Bavinski G, et al. Clinical and angiographic results of endosaccular coiling treatment of giant and very large intracranial aneurysms: a 7 year single center experience. Neurosurgery. 1999;45:793-804.
295 Jahromi BS, Mocco J, Bang JA, et al. Clinical and angiographic outcome after endovascular management of giant intracranial aneurysms. Neurosurgery. 2008;63:662-674.
296 Lanzino G, Wakhloo A, Fessler R, et al. Efficacy and current limitations of intravascular stents for intracranial internal carotid, vertebral and basilar artery aneurysms. Neurosurgery. 1999;91:538-546.
297 Asgari S, Doerfler A, Wanke I, et al. Complementary management of partially occluded aneurysms by using surgical or endovascular therapy. J Neurosurg. 2002;97:843-850.
298 Rabinstein AA, Nichols DA. Endovascular coil embolization of cerebral aneurysm remnants after incomplete surgical obliteration. Stroke. 2002;33:1809-1815.
299 Ogilvy CS, Hoh BL, Singer RJ, et al. Clinical and radiographic outcome in the management of posterior circulation aneurysms by use of direct surgical or endovascular techniques. Neurosurgery. 2002;51:14-22.
300 Sturaitis MK, Rinne J, Chaloupka JC, et al. Impact of Guglielmi detachable coils on outcomes of patients with intracranial aneurysms treated by a multidisciplinary team at a single institution. J Neurosurg. 2000;93:569-580.
301 Hoh BL, Putman CM, Budzik RF, et al. Combined surgical and endovascular techniques of flow alteration to treat fusiform and complex wide-necked intracranial aneurysms that are unsuitable for clipping or coil embolization. J Neurosurg. 2001;95:24-35.
302 Raja PV, Huang J, Germanwala AV, et al. Microsurgical clipping and endovascular coiling of intracranial aneurysms: a critical review of the literature. Neurosurgery. 2008;62:1187-1202.
303 Villablanca JP, Martin N, Jahan R, et al. Volume-rendered helical computerized tomography angiography in the detection and characterization of intracranial aneurysms. J Neurosurg. 2000;93:254-264.