Chapter 58 Surgical Decision-Making and Treatment Options for Chiari Malformations in Children
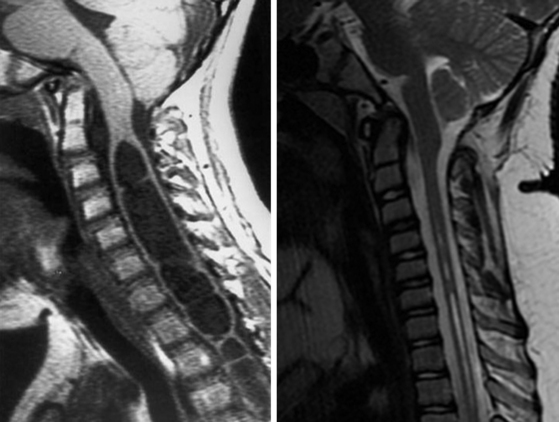
Significant herniation of the cerebellar tonsils through the foramen magnum is termed the Chiari malformation, type I (CMI). Individuals with CMI may present with symptoms, or this entity may be found incidentally. Headache is the most common presenting symptom, but hydrocephalus is found less than 10% of patients. One common presentation is scoliosis, which is found in 10% to 20% of these patients. Syringomyelia, which may or may not be the underlying etiology of the scoliosis, is present in 12% to 85% of patients.1–3 Currently, surgical treatment of the CMI is the only available treatment for symptomatic patients.
This chapter discusses the current literature regarding two aspects of surgical decision-making for children with CMI. The first is the current opinion regarding when surgery is indicated. The second is the decision regarding what operation to perform. In the absence of hydrocephalus, the first-line surgical management for CMI is a posterior fossa decompression (PFD) through a midline suboccipital craniectomy and removal of the posterior arch of the atlas with or without dural opening. If hydrocephalus is present, appropriate cerebrospinal fluid (CSF) diversion should be undertaken prior to any other surgical intervention. This may be accomplished through the insertion of a ventriculoperitoneal shunt or performance of an endoscopic third ventriculostomy.4 These points are widely accepted; however, there is controversy regarding the role of opening the dura as a component of PFD. This chapter discusses the merits and shortcomings of PFD with and without dural opening. When a dural opening is chosen, the surgeon may elect to perform further maneuvers, such as cerebellar tonsillar reduction or resection. Few studies directly compare dural opening with and without additional intradural maneuvers. This chapter therefore does not specifically address decision-making with regard to the completion of further intradural maneuvers after dural opening.
Decision-Making Regarding When to Operate
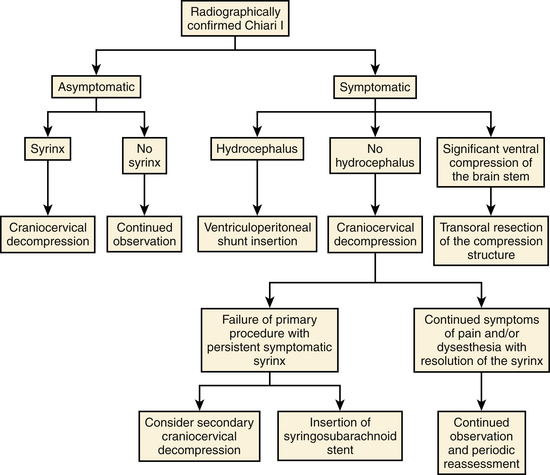
Recent publications regarding operative intervention for CMI demonstrate the varying opinions regarding both when and how to intervene.5–7,9–21 The availability of magnetic resonance imaging (MRI) has resulted in an increased number of asymptomatic patients being diagnosed with CMI.1 In addition to tonsillar herniation, these patients may present with asymptomatic syringomyelia and/or scoliosis. Similarly, patients may present with symptoms referable to any of these three structural lesions. Alden et al. used their experience to design a straightforward algorithm to help guide surgical decision-making.6 Our surgical decision-making tree is shown in Fig. 58-1.
Surgical Intervention for CMI
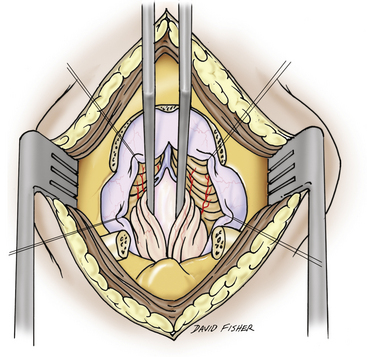
First-line surgical therapy for patients with CMI in the absence of hydrocephalus is PFD via midline suboccipital craniectomy, with appropriate removal of the posterior arch of the atlas with or without dural opening (Figs. 58-2 through 58-4). PFD attempts to reestablish bidirectional CSF flow across the craniocervical junction. This is accomplished through the expansion of the posterior fossa subarachnoid space, thus decompressing the cerebellar tonsils and brain stem. PFD may also eliminate the craniospinal CSF pressure differential that is postulated to contribute to syrinx formation.5 For the remainder of this discussion, the acronym PFD, when used alone, refers to bony suboccipital decompression with dural scoring or splitting but without frank opening of both layers of the dura mater. Most commonly, PFD is undertaken with duraplasty (PFDD) with or without cerebellar tonsil coagulation or resection. Other intradural interventions, such as fourth ventricular stenting and syrinx shunting, have been largely abandoned or are reserved for second- or third-line therapies.6–9
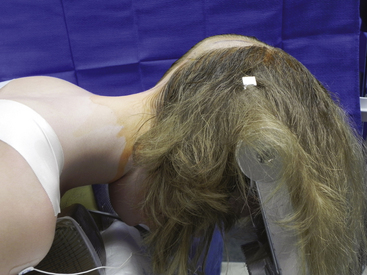
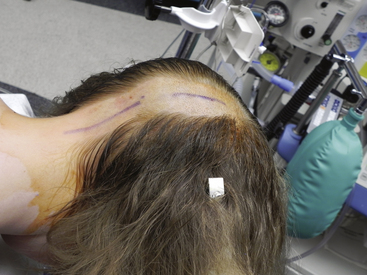
FIGURE 58-3 Skin incisions for PFD. The upper incision is used to harvest a periosteal graft for duraplasty.
Asymptomatic Patients
With regard to asymptomatic patients, survey data collected from the American Association of Neurological Surgeons/Congress of Neurological Surgeons section on pediatric neurosurgery in 19987 demonstrated that 83% of pediatric neurosurgeons would not operate on an asymptomatic child with CMI with or without syringomyelia. However, if the patient subsequently demonstrated asymptomatic syrinx progression, 61% of respondents would intervene. More recent international survey data9 similarly demonstrated that only a small minority (8%) of pediatric neurosurgeons would intervene for a patient with an asymptomatic CMI without syringomyelia. Rates of surgical treatment increased to 28% and 75% when the same patient presented with a thoracic syrinx of 2 and 8 mm in diameter, respectively. Novegno et al.20 conservatively managed 22 asymptomatic or minimally symptomatic children with CMI, 1 of whom had syringomyelia. Over a 5.9-year follow-up period, 17 children (77.3%) remained asymptomatic or had symptom improvement (including the patient with syringomyelia), 2 children (9%) had mild worsening of symptoms, and 3 children (13.6%) required surgical intervention. In our institution, children with asymptomatic CMI without syringomyelia do not undergo operative intervention, while those with a syrinx generally receive treatment. Asymptomatic CMI patients with scoliosis in the absence of syringomyelia are rare and should be evaluated individually.
Symptomatic Patients
There is a consensus that patients with symptomatic CMI merit surgical decompression under most circumstances. The most common presenting symptom of a child with CMI is headache. The subjective and nonspecific nature of this symptom requires neurosurgeons to apply strict criteria for a headache to be attributed to CMI. In most cases, the headache must be posteriorly located, must be of short duration, and should be exacerbated or reproduced with a Valsalva maneuver. Surgical treatment of CMI in children with other headache patterns and no additional symptoms referable to the condition may not achieve satisfactory results. Other common symptoms include neck, shoulder, and back pain; motor and sensory changes in the extremities; and sleep apnea and/or feeding difficulty in younger patients.22
In cases of progressive syringomyelia, Schijman and Steinbok9 reported that 97% of respondents would intervene and that 58% would do so in cases of CMI with progressive scoliosis in the absence of syringomyelia, although such cases are not common. McGirt et al.23 attempted to identify which patients would be most likely to have improvement in symptoms following PFD or PFDD. The authors retrospectively reviewed the clinical response of symptomatic patients who underwent preoperative cine phase-contrast MRI. They concluded that patients with preoperative CSF flow abnormalities, both ventral and dorsal to the caudal brain stem, were less likely to suffer from recurrent symptoms than were patients who lacked such evidence of preoperative CSF flow obstruction.
Decision-Making Regarding Surgical Technique
As previously discussed, once the decision to operate has been made, the surgeon must determine the extent of decompression that will be performed. The goals of surgery in the CMI population include improvement/resolution of symptoms, stabilization/improvement of scoliosis (when present), and radiologic decrease in the extent of syringomyelia (when present). With the postoperative assessment of syringomyelia, there is some debate regarding the extent of diminution that is necessary to demonstrate effective treatment.11,24 This section describes the current evidence regarding PFD with and without dural opening.
Studies Directly Comparing Surgical Techniques
Despite significant literature describing the treatment of CMI, no randomized trials comparing PFD against PFDD have been completed. Among the studies that directly compare the two techniques, only one meta-analysis and two surveys have been completed.7,9,13
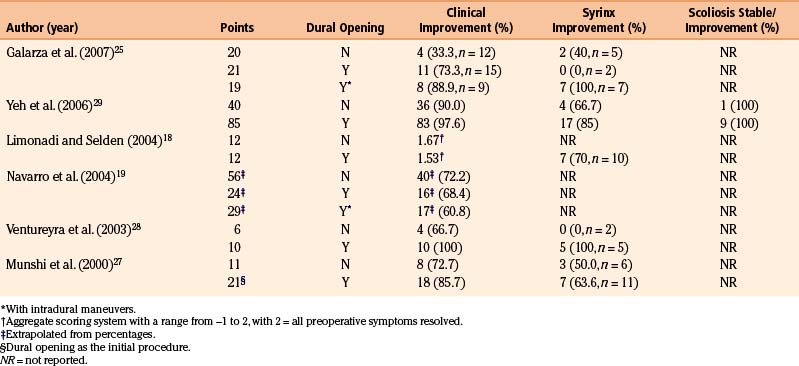
Durham and Fjeld-Olenec13 published a meta-analysis of studies that directly compare cohorts of pediatric patients who underwent PFD with cohorts who were treated with PFDD. A total of seven studies met their inclusion criteria.18,19,25–29 The authors concluded that patients who undergo duraplasty are less likely to require reoperation (2.1% vs. 12.6%) for persistent or recurrent symptoms but are more likely to suffer CSF-related complications (18.5% vs. 1.8%). There was no statistical difference in clinical outcomes between the two groups, specifically with regard to symptom improvement and syringomyelia. In summary, rates of clinical improvement were 65% in the PFD patients and 79% in the PFDD patients. Rates of radiologic syrinx improvement were influenced by small numbers in some studies but were 56% in the PFD patients and 87% in those undergoing PFDD. The authors appropriately acknowledged that their conclusions were limited by the patient selection methods of the studies they examined. Among the seven papers, five studies used intraoperative ultrasound to help determine whether or not to perform a dural opening.18,19,26,28,29 The inherent subjectivity of this technique limits the degree to which the findings of each work may be generalized. Additionally, no study included a randomization or blinding process.
Using expert opinion as an indicator of current practice, Haroun et al.7 reported that 25% of survey respondents would perform PFD for children with symptomatic CMI, 32% recommended PFDD, and 55% recommended further intradural manipulations (some respondents chose more than one intervention). International survey data published by Schijman and Steinbok9 demonstrated that 76% of pediatric neurosurgeons always open the dura mater for treating CMI.
Studies of PFD without Dural Opening
Electrophysiologic evidence suggests that neurologic improvement can be achieved without a dural opening. Groups from Columbia University and Ohio State University found that improved conduction of nerve impulses through the brain stem occurs after bony decompression rather than after dural opening.30–32 As mentioned previously, multiple groups have used intraoperative ultrasound findings to aid their decision-making with regard to dural opening in children with CM-I.10,19,29,33 Yeh et al.29 found that factors that were associated with adequate decompression without duraplasty included age of less than 1 year. Factors that were more likely to be associated with the need for duraplasty included spinal symptoms (motor, sensory, or scoliosis) and a greater magnitude of tonsillar descent.
Clinical Outcome
The majority of studies that report rates of clinical and radiologic improvement following PFD also include patients who were treated with PFDD and have therefore been referenced in the preceding section. Two Italian reports demonstrated excellent results in series of patients who underwent PFD alone.11,34 Genitori et al.34 reported the results of their experience using PFD in 26 patients. Among 16 patients (61.5%) without syringomyelia, 13 (81.3%) had complete symptom resolution and the remaining 3 (18.8%) had partial resolution. Among 10 patients with syringomyelia, symptoms improved or resolved in all cases with the exception of one of three cases of scoliosis (33.3%) and one of five cases of sensory loss (20%). Rates of complete symptom resolution, however, ranged from 25% (sensory loss) to 100% (vertigo). Two patients (7.7%) required reoperation. The authors acknowledged that it is difficult to draw definitive conclusions from their study due to the small numbers in each group.
Caldarelli et al.11 reviewed their experience with PFD in 30 children. After a mean follow-up period of 4.7 years, 28 patients (93.3%) demonstrated a “significant improvement in their clinical condition.”
Syrinx Resolution
In the PFD groups of the studies included in their meta-analysis, Durham and Fjeld-Olenec13 reported an aggregate 56.3% (9 of 16 patients) rate of syrinx reduction, which was not statistically different from the 87.0% (40 of 46 patients) rate calculated for the PFDD group. As previously mentioned, in the series of Genitori et al.,34 10 of 26 patients (38.5%) patients presented with syringomyelia. Following PFD, the syrinx disappeared in 8 (80%). The remaining 2 children (7.7%) had initial clinical improvement but required further surgery, with duraplasty, for persistent syringomyelia. Syringomyelia was present preoperatively in 12 of 30 patients (40%) in Caldarelli et al.’s report.11 Postoperatively, half of these patients (6) had a decrease in the size of the syrinx. Two patients (6.7%) demonstrated recurrent symptoms and postoperative syrinx growth (one de novo and another who had a syrinx preoperatively) and required reoperation. The authors argued that, in the context of clinical improvement, radiologic change (either in syrinx size or in posterior fossa subarachnoid volume) was not mandatory for a successful result. Wetjen et al.24 also argued that the absence of syrinx distention is more important than complete collapse.
Scoliosis Improvement
In two studies that describe outcomes with regard to scoliosis following PFD without duraplasty, Genitori et al.34 reported improvement in 2 of 3 patients and Caldarelli et al.11 reported mild improvement in 2 of 2 patients. In their series of 21 patients with CMI, syringomyelia, and scoliosis, Attenello et al.35 described a single patient who underwent PFD. This patient had progression of scoliosis and underwent PFDD reoperation.
Studies of PFD with Dural Opening
Clinical Outcome
In a series of PFDD in 130 patients, Tubbs et al.3 reported relief of preoperative symptoms in 83% of patients. Headache did not resolve in 12%, and perioperative complications occurred in 2.3%. Smaller series using PFDD (with or without further intradural manipulation) reported clinical improvement rates of 92% to 100% and complication rates of 0% to 16.7%.12,14,15,17,30,31,36–38 In the context of a comparison of duraplasty materials (autograft vs. synthetic allograft), Attenello et al.39 demonstrated mild to moderate symptom recurrence at 16 months follow-up in 14 of 67 patients (20.9%), with 4 of these (6.0%) requiring revision decompression. A pseudomeningocele observed on imaging was identified in 10 patients (17%), but only 1 of these became symptomatic. A total of 5 patients (7%) suffered CSF-related complications: 2 (3%) with CSF leakage and 2 (3%) with aseptic meningitis.
Syrinx Resolution
Reported rates of syrinx improvement in children following PFDD range from 55% to 100%.3,10,14,17,30,31,36–39 The variability in these results may stem partly from the small sample size of some studies and additionally from the absence of a standard definition of “improvement” when considering syrinx size (Table 58-1). As previously mentioned, Durham and Fjeld-Olenec13 found an overall rate of 87% syrinx reduction in the PFDD arms of studies that directly compared PFD with PFDD.
Following initial PFDD, Tubbs et al.3 found that only 8 of 75 syringes (10.7%) did not improve. Of these, 7 syringes (87.5%) improved with repeat PFDD40 (Fig. 58-5). No radiographic parameters predicted failure of syrinx response. As such, we maintain a policy of repeat PFDD in cases of persistent syringomyelia. Attenello et al.10 retrospectively examined the syrinx response rate following hindbrain decompression in 49 consecutive children with CMI, 46 of whom underwent PFDD and 3 of whom underwent PFD alone. They reported a 55% overall syrinx improvement rate at 14 months, with 26 of the 46 (56.5%) in the PFDD group improving. Of 5 patients (10.2%) who underwent repeat decompression, 1 (2.0%) was due to syrinx expansion at 5 months after PFDD. Of the 49 patients, 39 (79.6%) had preoperative symptoms attributable to syringomyelia. Of these, 21 patients (54%) experienced symptom resolution. Complications included aseptic meningitis and wound breakdown in 2 patients (4%) and pseudomeningocele in 1 (2%). None of these complications required reoperation. In the PFD group, 2 of the 3 did not have syrinx improvement. One required reoperation with PFDD, and another underwent spinal fusion for scoliosis.
The expected time to and magnitude of syrinx improvement following PFDD remain incompletely understood. Although a subjectively significant decrease in the syrinx size on the first postoperative imaging examination is reassuring to the surgeon, complete resolution of the fluid collection is likely unnecessary11,24 and syringes may dissipate after a prolonged interval.41
Scoliosis Progression
In the vast majority of cases, scoliosis in the context of CMI is associated with syringomyelia.42 Although the exact mechanism through which the syrinx produces scoliosis remains unknown, it is believed that lower motor neuron injury imbalances innervation to the trunk musculature, predisposing the patient to scoliosis.43–45 Consistent with this theory, multiple authors have reported scoliosis improvement following PFDD in children with CMI. Reported rates of scoliosis improvement are 0% to 73%, and rates of progression are 18% to 72%,3,8,46–53 demonstrating that PFDD may slow or halt scoliosis progression in some cases—although there remains a considerable risk of progression. In some series, younger children (younger than 8-10 years) have been less likely to suffer from scoliosis progression.8,46,47,52,53 Female gender and a smaller presenting Cobb angle have also been associated with improved outcomes; however, Brockmeyer et al.46 found that a decrease in syrinx size did not necessarily correlate with scoliosis improvement. In 20 pediatric patients with CMI-associated scoliosis treated by PFDD, Attenello et al.35 reported that 8 (40%) demonstrated a postoperative improvement of their scoliosis and 9 (45%) progressed. The authors reported that an increased magnitude of the scoliosis curve at presentation was predictive of scoliosis progression, as were thoracolumbar junction scoliosis and a lack of postoperative radiologic syrinx response.
Summary
The Case for PFD
Electrophysiologic data support the assertion that effective PFD occurs with bony decompression and dural scoring, not necessarily requiring dural opening.30–32 Although rates of reoperation for persistent or recurrent symptoms are higher with PFD, the technique is attractive because it minimizes potential surgical complications. Theoretical complications that are avoided with PFD include pseudomeningocele, chemical meningitis, bacterial meningitis, arterial injury, venous sinus bleeding, stroke, and hydrocephalus. Multiple studies have demonstrated that CSF-related complication rates are lower for PFD than for PFDD.18,19,27,29 Additionally, nondural opening PFD is likely to require less operative time.18 The case for PFD is also strengthened because no study directly comparing PFD and PFDD has demonstrated a statistically significant difference in clinical outcomes between the two techniques. Furthermore, while many studies have demonstrated a trend toward a greater pace and magnitude of syrinx collapse following PFDD, the clinical significance of this result has yet to be clearly defined,11,24 making syrinx resolution a questionable measure of efficacy regarding hindbrain decompression for CMI. Lastly, the use of PFD does not preclude patients from undergoing further decompression should this become necessary (Table 58-2).
| PFD | PFDD |
|---|---|
| Lower CSF-related complication rate | Lower reoperation rate |
| Shorter operative time | Better radiological outcomes |
| Optional shorter incision | Inspection possible of arachnoid veils/scarring |
| ± adequate syrinx decompression | ± better clinical outcomes |
The Case for PFDD
Although direct comparisons have not demonstrated a statistically significant difference in clinical outcomes, the large majority of studies reporting the clinical efficacy of PFDD for the most common presentations have demonstrated rates of improvement superior to those of PFD.26–29 Durham and Fjeld-Olenec13 demonstrated a significantly lower rate of reoperation following PFDD versus PFD (2.1% vs. 12.6%). Although their meta-analysis also demonstrated a higher complication rate with PFDD, it must be acknowledged that multiple groups have reported very low complication rates with PFDD.3,15,17 Additionally, at least 12% of patients with CMI and syringomyelia who undergo PFD are likely to require reoperation, because without opening the dura, it is not possible to release arachnoid veils that have been described at the foramen of Magendie in some patients.40 Lastly, in some circumstances, such as rapidly progressive neurologic decline or scoliosis, there is little debate that PFDD is necessary.54 As such, PFDD is the treatment that offers the greatest likelihood of improving the presenting signs and symptoms in any given child.
Conclusions
Selection of the most appropriate surgical technique for CMI is not yet guided by type I or II evidence. Although most authors agree that the combination of hindbrain decompression through suboccipital craniectomy and removal of the posterior arch of the atlas is the best first-line treatment for CMI, the use of duraplasty remains debated. Current data leave the treating surgeon to choose between a procedure that is more likely to require reoperation (PFD) and one that is more likely to result in perioperative CSF-related complications (PFDD). The frequency of these suboptimal outcomes (12.6% and 18.5%, respectively, in Durham and Fjeld-Olenec’s meta-analysis13) does little to clarify which approach is most appropriate for a given patient. Furthermore, wide ranges of clinical efficacy and complications have been reported. Lastly, the spectrum of presentation in children with CMI extends from asymptomatic to potentially life-threatening symptomatology. It is therefore not surprising that no single surgical approach is universally recommended. As such, if a particular neurosurgeon has significant problems with dural opening, then PFD is an attractive option. If, however, PFDD is associated with hospital discharge in fewer than 3 days and very low reoperation rate (<1%), then adding the additional steps of opening the dura, looking for IV ventricular outlet obstruction, and grafting the dura to prevent chemical meningitis has great appeal.
Anderson R.C., Dowling K.C., Feldstein N.A., Emerson R.G. Chiari I malformation: potential role for intraoperative electrophysiologic monitoring. J Clin Neurophysiol. 2000;20:65-72.
Durham S.R., Fjeld-Olenec K. Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari malformation type I in pediatric patients: a meta-analysis. J Neurosurg Pediatr. 2008;2:42-49.
Haroun R.I., Guarnieri M., Meadow J.J. Current opinions for the treatment of syringomyelia and Chiari malformations: survey of the Pediatric Section of the American Association of Neurological Surgeons. Pediatr Neurosurg. 2000;33:311-317.
Hayhurst C., Osman-Farah J., Das K., Mallucci C. Initial management of hydrocephalus associated with Chiari malformation type I–syringomyelia complex via endoscopic third ventriculostomy: an outcome analysis. J Neurosurg. 2008;108:1211-1214.
Oldfield E.H., Muraszko K., Shawker T.H., Patronas N.J. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg. 1994;80:3-15.
Schijman E., Steinbok P. International survey on the management of Chiari I malformation and syringomyelia. Childs Nerv Syst. 2004;20:341-348.
Tubbs R.S., McGirt M.J., Oakes W.J. Surgical experience in 130 pediatric patients with Chiari I malformations. J Neurosurg. 2003;99:291-296.
1. Aitken L.A., Lindan C.E., Sidney S., et al. Chiari type I malformation in a pediatric population. Pediatr Neurol. 2009;40:449-454.
2. Hankinson T.C., Klimo P.Jr., Feldstein N.A. Chiari malformations, syringohydromyelia and scoliosis. Neurosurg Clin N Am. 2007;18:549-568.
3. Tubbs R.S., McGirt M.J., Oakes W.J. Surgical experience in 130 pediatric patients with Chiari I malformations. J Neurosurg. 2003;99:291-296.
4. Hayhurst C., Osman-Farah J., Das K., Mallucci C. Initial management of hydrocephalus associated with Chiari malformation type I–syringomyelia complex via endoscopic third ventriculostomy: an outcome analysis. J Neurosurg. 2008;108:1211-1214.
5. Oldfield E.H., Muraszko K., Shawker T.H., Patronas N.J. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg. 1994;80:3-15.
6. Alden T.D., Ojemann J.G., Park T.S. Surgical treatment of Chiari I malformation: indications and approaches. Neurosurg Focus. 2001;11:E2.
7. Haroun R.I., Guarnieri M., Meadow J.J. Current opinions for the treatment of syringomyelia and Chiari malformations: survey of the Pediatric Section of the American Association of Neurological Surgeons. Pediatr Neurosurg. 1998;33:311-317.
8. Ozerdemoglu R.A., Transfeldt E.E., Denis F. Value of treating primary causes of syrinx in scoliosis associated with syringomyelia. Spine (Phila Pa 1976). 2003;28:806-814.
9. Schijman E., Steinbok P. International survey on the management of Chiari I malformation and syringomyelia. Childs Nerv Syst. 2004;20:341-348.
10. Attenello F.J., McGirt M.J., Gathinji M., et al. Outcome of Chiari-associated syringomyelia after hindbrain decompression in children: analysis of 49 consecutive cases. Neurosurgery. 2008;62:1307-1313. discussion 1313
11. Caldarelli M., Novegno F., Vassimi L., et al. The role of limited posterior fossa craniectomy in the surgical treatment of Chiari malformation type I: experience with a pediatric series. J Neurosurg. 2007;106:187-195.
12. Danish S.F., Samdani A., Hanna A. Experience with acellular human dura and bovine collagen matrix for duraplasty after posterior fossa decompression for Chiari malformations. J Neurosurg. 2006;104:16-20.
13. Durham S.R., Fjeld-Olenec K. Comparison of posterior fossa decompression with and without duraplasty for the surgical treatment of Chiari malformation type I in pediatric patients: a meta-analysis. J Neurosurg Pediatr. 2008;2:42-49.
14. Ellenbogen R.G., Armonda R.A., Shaw D.W., Winn H.R. Toward a rational treatment of Chiari I malformation and syringomyelia. Neurosurg Focus. 2000;8:E6.
15. Feldstein N.A., Choudhri T.F. Management of Chiari I malformations with holocord syringohydromyelia. Pediatr Neurosurg. 1999;31:143-149.
16. Gambardella G., Caruso G., Caffo M., et al. Transverse microincisions of the outer layer of the dura mater combined with foramen magnum decompression as treatment for syringomyelia with Chiari I malformation. Acta Neurochir (Wien). 1998;140:134-139.
17. Hoffman C.E., Souweidane M.M. Cerebrospinal fluid–related complications with autologous duraplasty and arachnoid sparing in type I Chiari malformation. Neurosurgery. 2008;62:156-160. discussion 160-151
18. Limonadi F.M., Selden N.R. Dura-splitting decompression of the craniocervical junction: reduced operative time, hospital stay, and cost with equivalent early outcome. J Neurosurg. 2004;101:184-188.
19. Navarro R., Olavarria G., Seshadri R., et al. Surgical results of posterior fossa decompression for patients with Chiari I malformation. Childs Nerv Syst. 2004;20:349-356.
20. Novegno F., Caldarelli M., Massa A., et al. The natural history of the Chiari Type I anomaly. J Neurosurg Pediatr. 2008;2:179-187.
21. Piatt J.H. Surgical management of the Chiari malformation type I: the way forward. J Neurosurg Pediatr. 2008;2:50-51. discussion 51
22. Tubbs R.S., Lyerly M.J., Loukas M. The pediatric Chiari I malformation: a review. Childs Nerv Syst. 2007;23:1239-1250.
23. McGirt M.J., Atiba A., Attenello F.J., et al. Correlation of hindbrain CSF flow and outcome after surgical decompression for Chiari I malformation. Childs Nerv Syst. 2008;24:833-840.
24. Wetjen N.M., Heiss J.D., Oldfield E.H. Time course of syringomyelia resolution following decompression of Chiari malformation type I. J Neurosurg Pediatr. 2008;1:118-123.
25. Galarza M., Sood S., Ham S. Relevance of surgical strategies for the management of pediatric Chiari type I malformation. Childs Nerv Syst. 2007;23:691-696.
26. McGirt M.J., Attenello F.J., Datoo G., et al. Intraoperative ultrasonography as a guide to patient selection for duraplasty after suboccipital decompression in children with Chiari malformation type I. J Neurosurg Pediatr. 2008;2:52-57.
27. Munshi I., Frim D., Stine-Reyes R., et al. Effects of posterior fossa decompression with and without duraplasty on Chiari malformation–associated hydromyelia. Neurosurgery. 2000;46:1384-1389. discussion 1389-1390
28. Ventureyra E.C., Aziz H.A., Vassilyadi M. The role of cine flow MRI in children with Chiari I malformation. Childs Nerv Syst. 2003;19:109-113.
29. Yeh D.D., Koch B., Crone K.R. Intraoperative ultrasonography used to determine the extent of surgery necessary during posterior fossa decompression in children with Chiari malformation type I. J Neurosurg. 2006;105:26-32.
30. Anderson R.C., Dowling K.C., Feldstein N.A., Emerson R.G. Chiari I malformation: potential role for intraoperative electrophysiologic monitoring. J Clin Neurophysiol. 2003;20:65-72.
31. Anderson R.C., Emerson R.G., Dowling K.C., Feldstein N.A. Improvement in brainstem auditory evoked potentials after suboccipital decompression in patients with Chiari I malformations. J Neurosurg. 2003;98:459-464.
32. Zamel K., Galloway G., Kosnik E.J. Intraoperative neurophysiologic monitoring in 80 patients with Chiari I malformation: role of duraplasty. J Clin Neurophysiol. 2009;26:70-75.
33. McGirt M.J., Attenello F.J., Atiba A., et al. Symptom recurrence after suboccipital decompression for pediatric Chiari I malformation: analysis of 256 consecutive cases. Childs Nerv Syst. 2008;24:1333-1339.
34. Genitori L., Peretta P., Nurisso C. Chiari type I anomalies in children and adolescents: minimally invasive management in a series of 53 cases. Childs Nerv Syst. 2000;16:707-718.
35. Attenello F.J., McGirt M.J., Atiba A., et al. Suboccipital decompression for Chiari malformation–associated scoliosis: risk factors and time course of deformity progression. J Neurosurg Pediatr. 2008;1:456-460.
36. Alzate J.C., Kothbauer K.F., Jallo G.I., Epstein F.J. Treatment of Chiari I malformation in patients with and without syringomyelia: a consecutive series of 66 cases. Neurosurg Focus. 2001;11:E3.
37. Krieger M.D., McComb J.G., Levy M.L. Toward a simpler surgical management of Chiari I malformation in a pediatric population. Pediatr Neurosurg. 1999;30:113-121.
38. Park J.K., Gleason P.L., Madsen J.R. Presentation and management of Chiari I malformation in children. Pediatr Neurosurg. 1997;26:190-196.
39. Attenello F.J., McGirt M.J., Garces-Ambrossi G.L., et al. Suboccipital decompression for Chiari I malformation: outcome comparison of duraplasty with expanded polytetrafluoroethylene dural substitute versus pericranial autograft. Childs Nerv Syst. 2009;25:183-190.
40. Tubbs R.S., Webb D.B., Oakes W.J. Persistent syringomyelia following pediatric Chiari I decompression: radiological and surgical findings. J Neurosurg. 2004;100:460-464.
41. Doughty K.E., Tubbs R.S., Webb D., Oakes W.J. Delayed resolution of Chiari I-associated hydromyelia after posterior fossa decompression: case report and review of the literature. Neurosurgery. 2004;55:711.
42. Tubbs R.S., Doyle S., Conklin M., Oakes W.J. Scoliosis in a child with Chiari I malformation and the absence of syringomyelia: case report and a review of the literature. Childs Nerv Syst. 2006;22:1351-1354.
43. Huebert H.T., MacKinnon W.B. Syringomyelia and scoliosis. J Bone Joint Surg Br. 1969;51:338-343.
44. Isu T., Chono Y., Iwasaki Y., et al. Scoliosis associated with syringomyelia presenting in children. Childs Nerv Syst. 1992;8:97-100.
45. Williams B. Orthopaedic features in the presentation of syringomyelia. J Bone Joint Surg Br. 1979;61-B:314-323.
46. Brockmeyer D., Gollogly S., Smith J.T. Scoliosis associated with Chiari I malformations: the effect of suboccipital decompression on scoliosis curve progression: a preliminary study. Spine (Phila Pa 1976). 2003;28:2505-2509.
47. Eule J.M., Erickson M.A., O’Brien M.F., Handler M. Chiari I malformation associated with syringomyelia and scoliosis: a twenty-year review of surgical and nonsurgical treatment in a pediatric population. Spine (Phila Pa 1976). 2002;27:1451-1455.
48. Farley F.A., Puryear A., Hall J.M., Muraszko K. Curve progression in scoliosis associated with Chiari I malformation following suboccipital decompression. J Spinal Disord Tech. 2002;15:410-414.
49. Flynn J.M., Sodha S., Lou J.E., et al. Predictors of progression of scoliosis after decompression of an Arnold Chiari I malformation. Spine (Phila Pa 1976). 2004;29:286-292.
50. Ghanem I.B., Londono C., Delalande O., Dubousset J.F. Chiari I malformation associated with syringomyelia and scoliosis. Spine (Phila Pa 1976). 1997;22:1313-1317. discussion 1318
51. Hida K., Iwasaki Y., Koyanagi I., Abe H. Pediatric syringomyelia with Chiari malformation: its clinical characteristics and surgical outcomes. Surg Neurol. 1999;51:383-390. discussion 390-381
52. Muhonen M.G., Menezes A.H., Sawin P.D., Weinstein S.L. Scoliosis in pediatric Chiari malformations without myelodysplasia. J Neurosurg. 1992;77:69-77.
53. Sengupta D.K., Dorgan J., Findlay G.F. Can hindbrain decompression for syringomyelia lead to regression of scoliosis? Eur Spine J. 2000;9:198-201.
54. Feldstein NA. Less is more: the argument for decompression without dural opening, in Annual Meeting of the Congress of Neurological Surgeons. New Orleans, LA, 2009.