CHAPTER 53 Surgical approaches to the orbit
Introduction
Historically, orbital surgery has been performed by general surgeons, ophthalmic surgeons and, in particular, neurosurgeons; indeed, Walter Dandy – the highly influential neurosurgeon – suggested that, ‘all orbital surgery should be performed through the transcranial route’ and that ‘such surgery results in less morbidity than the commonly-used orbital approach’1. During the second half of the 20th century there was a shift towards less traumatic techniques for orbitotomy, avoiding craniotomy when a disease was solely intraorbital – this leading to the development of incisions and approaches with much better esthetic results. Clearly, a neurosurgical approach remains imperative where a disease straddles the cranio-orbital junction (such as with lesions passing through the superior orbital fissure or optic canal), and a maxillofacial or head-and-neck surgeon is best experienced to deal with orbital diseases involving the paranasal sinuses or extending widely outside the orbit.
Practical and anatomical considerations
The risk of injury to orbital function varies significantly at different depths within the orbit, there being variations within each zone. The risk of collateral injury is low during surgery in the anterior one-third of the orbit, alongside the globe – the risk probably being greatest in the trochlear area and to the palpebral lobe of the lacrimal gland. The most feared risk whilst operating in the middle-third of the orbit is the (relatively rare) visual loss due to ischemia of the optic nerve or optic nerve head, but the ciliary ganglion is probably the most susceptible structure in this sector – a persistent mydriasis being a not uncommon inconvenience after removal of retrobulbar tumors. Due to crowding of highly important structures in the orbital apex, operating in the posterior third of the orbit carries a high risk of complication – many being serious or permanent2; complete visual loss is quite common with removal of ‘peanut’ tumors wedged in the apex, especially where the tumor lies below the optic nerve, alongside the ophthalmic artery. Complete ophthalmoplegia is common after operating near the superior orbital fissure, this often recovering dramatically at about 3 months after surgery, but sometimes leaving a selective neuropraxia.
Preoperative and postoperative counseling
Apart from difficult access or the encroachment of fat into the operative field, it is intraoperative hemorrhage that creates the greatest difficulty and risk during surgery within the orbital depths; heat generated by the use of bipolar diathermy increases inflammation, the latter probably being contributory to early postoperative visual loss due to vasospasm at the orbital apex2. It is, therefore, very important to take a thorough history for any medications that might affect hemostasis3 – this including over 40 brand-named versions of amino-salicylic acid (ASA) and also many foodstuff and ‘herbal’ remedies. All herbal remedies, garlic, ginger, ginseng and gingko should be avoided completely before surgery. If safe to do so, patients should be encouraged to avoid ASA for at least 3–4 weeks before orbital surgery, non-steroidal anti-inflammatory drugs for at least 2 weeks, and other anti-platelet medications for at least 10–12 days. Special arrangements will be required for patients requiring warfarin anticoagulation for metallic heart valves and for patients with recently implanted arterial stents4. Medical conditions, such as systemic hypertension or diabetes, should be well controlled in all patients listed for routine orbital surgery, and a preoperative assessment by the anesthetist is appropriate where hypotensive general anesthesia will be required for surgery.
The six principal orbitotomies
Anterior orbitotomy through the upper eyelid skin crease
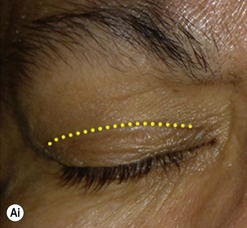
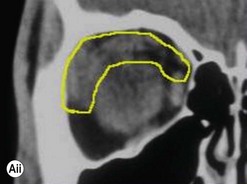
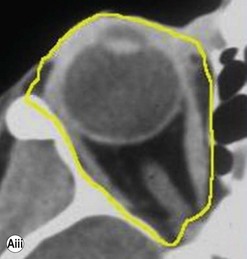
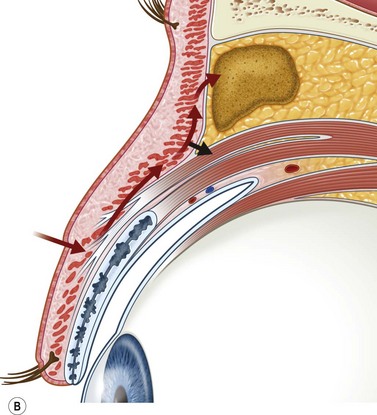
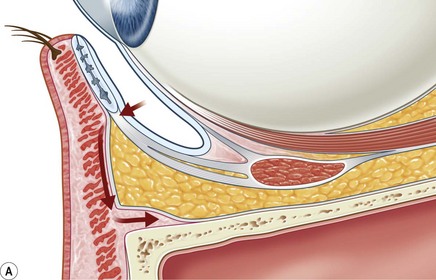
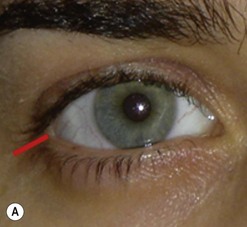
Incisions in the upper eyelid skin crease heal without visible scar and provide access to the upper two-thirds of the orbit, if necessary right back to the superior orbital fissure and orbital apex (Fig. 53.1A). Intraconal lesions lying above or medial to the optic nerve are reached by retracting the superior rectus/levator muscle complex either medially or laterally during the surgery.
The position for the main upper lid crease is determined by ‘nudging’ the eyelid upwards and the skin and orbicularis muscle incised for a suitable length along it, with meticulous hemostasis being maintained at all stages. For non-lacrimal masses, the plane between the orbicularis and septum is dissected upwards for 3–5 mm before opening the septum to reveal orbital fat (Fig. 53.1B); this upward dissection prior to entering the orbit avoids later ptosis due to direct damage to the anterior levator complex. The septum is button-holed centrally and then divided medially and/or laterally, according to the quadrant in which orbital exploration is needed – the quadrant determined by imaging and also by gentle intraorbital palpation during the surgery. A closed pair of blunt-tipped scissors should be directed towards the mass, through the fat, and then opened widely to reveal the orbital depths; before withdrawing the scissors, the plane and depth of exploration are maintained by passing a pair of 12–16 mm retractors alongside the opened scissors. The orbital mass is typically revealed after repeating this maneuver several times, the surgical assistant maintaining the space with suitable, but gentle, retraction. If a well-defined mass, it should be carefully separated from surrounding tissues with gentle diathermy and division of any bridging vascular pedicles. Unless debulking is planned, a large incisional biopsy should be taken from ill-defined masses – this preferably being achieved with a single grip of the tissues specimen so as to reduce crush artifacts. A vacuum drain should be placed where there has been surgery within the deep orbit, as accumulation of extracellular inflammatory fluid at the apex may cause a major rise in tissue pressure – with associated risk of optic nerve ischemia and visual loss; to avoid a visible scar, such drains may conveniently be passed through the brow.
Anterior orbitotomy through the lower lid swinging flaps
The approach to the lower two-thirds of the orbit can be through several routes, of which the direct skin incision in the ‘tear-trough’ is effectively redundant, being sometimes complicated by a prominently visible scar, fistula, or adhesions to the underlying bone. Likewise, the subciliary blepharoplasty incision, whilst useful for removal of excessive lid skin, tends to be unnecessarily vascular, thereby hindering orbital exploration, and scarring5.
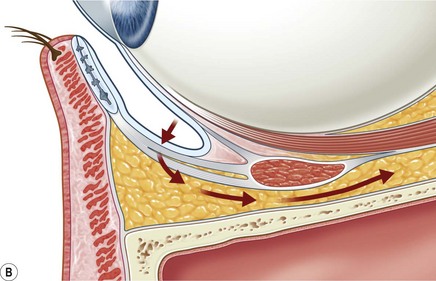
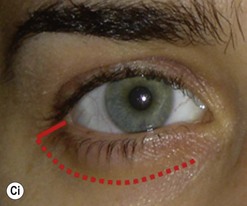
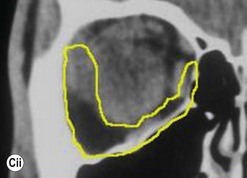
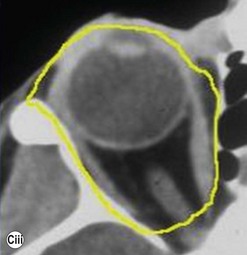
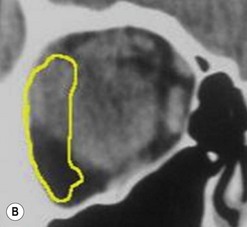
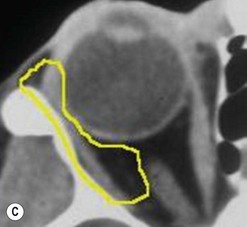
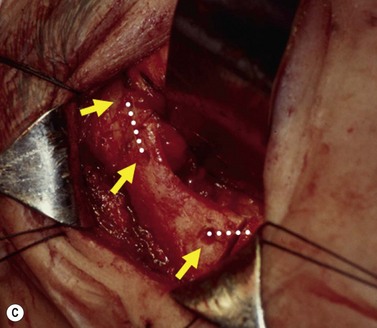
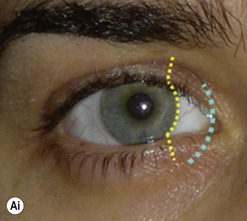
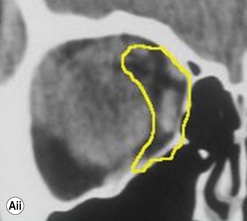
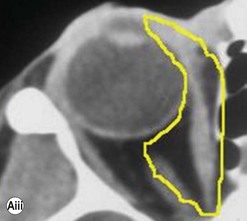
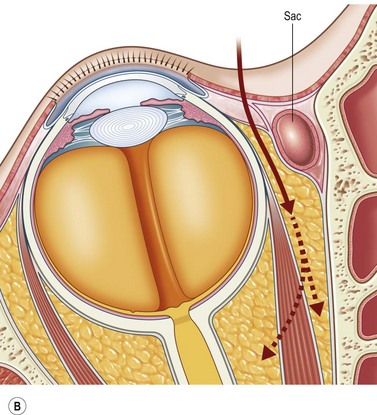
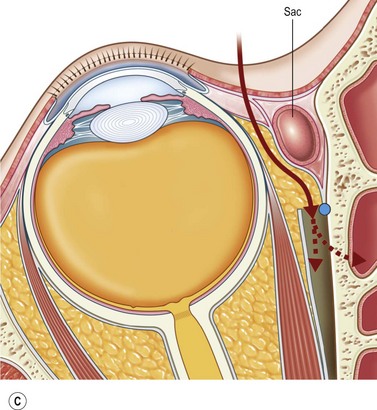
The contemporary workhorse for inferior orbitotomy is the lower lid swinging flap5,6, of which the authors now identify two types: the ‘high’ flap, with the conjunctival incision 1 mm below the inferior border of the lower tarsus (Fig. 53.2A), provides excellent access (via a preseptal plane) to the inferior orbital rim and is suitable for almost all surgery along the orbital floor and medial wall – be it for inferomedial decompression or for repair of fractures. The ‘low’ lower lid swinging flap – whereby the internal incision is based in the depths of the lower fornix (Fig. 53.2B) – enters the postseptal extraconal fat pad and, from here, the surgeon can access all except the superomedial part of both the intraconal and extraconal space (Fig. 53.2C). A fundamental understanding of the two types of lower lid swinging flap, not highlighted in the original description for this approach6, leads to a rational application of these highly valuable techniques.
The ‘low’ lower lid swinging flap
This postseptal route provides excellent access to the lateral, inferior, and – if needed – medial extraconal space and may be used to biopsy or resect large inflammatory masses, tumors, and vascular anomalies in these areas (Fig. 53.3). Masses straddling an expanded inferior orbital fissure may also be debulked right back and into the pterygo-palatine fossa.
Lateral canthotomy approach to anterior orbitotomy
Biopsy of a tissue or tissues lying in the lateral half of the orbit may be achieved through a lateral canthotomy incision, without disconnection of either the upper or lower limbs of the canthal tendon (Fig. 53.4); this has the advantage of a completely hidden scar, no major disturbance of the lateral fixation of either lid, and a very short operative time.
It is also possible to perform a large decompression of the lateral wall through this incision, typically giving a 3–7 mm reduction in proptosis. After undermining the tissues widely, both above and below the canthotomy, the periosteum overlying the lateral orbital rim is exposed and the superficial temporal fascia divided along the posterior edge of the lateral orbital rim. The periosteum is raised forwards over the orbital rim, past the arcus marginalis, and raised widely across the lateral wall of the orbit – dividing the vascular bridges as necessary. The lateral orbital wall is then fenestrated back to the marrow space of the greater wing of the sphenoid, either with preservation of an anterior bar of the lateral rim or – less desirably – by complete removal of the lateral wall. The orbital periosteum is incised widely just behind the arcus marginalis (Fig. 53.5), with three posteriorly directed incisions – one superomedial and one inferolateral to the orbital lobe of the lacrimal gland (thereby allowing the orbital lobe to prolapse back into the upper part of the decompression window) and the third at the lowest part of the fenestration. The fat septa in the inferotemporal quadrant are disrupted widely to encourage a marked prolapse of intraconal and extraconal fat into the area of bone removal, and the periosteum is then closed around the remaining bone rim and to the superficial temporal fascia. A vacuum drain is placed into the intraconal space and subtemporalis space, and the canthotomy is repaired in layers with soluble sutures.
Bone-swinging lateral orbitotomy
Moving the lateral orbital wall outwards during surgery (lateral orbitotomy) allows an increased access to the orbital apex, with a wider and less oblique view of this area, and with a shorter path length. With advent of the ‘low’ lower lid swinging flap, the need for lateral orbitotomy to remove retrobulbar lesions – such as mid-orbital cavernous hemangiomas – has markedly decreased; lateral orbitotomy remains, however, valuable for the intact removal of large lacrimal gland lesions and for removing ‘peanut lesions’ wedged into the orbital apex. Many past techniques were based on an extended brow incision7, but the laterally extended upper lid incision gives excellent access8 through a much shorter incision, with better cosmesis and no thinning of the brow hairs as was frequent after an incision based in the eyebrow. In addition, most contemporary surgeons recognize that it is sufficient to swing the orbital wall out laterally on the temporalis muscle, rather than completely remove the bone during surgery; this former technique has the advantages of retaining a vascular supply to the bone, having a quicker closure, and avoiding any risk of dropping the bone fragment outside the surgical field during surgery.
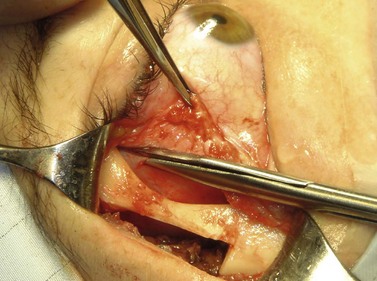
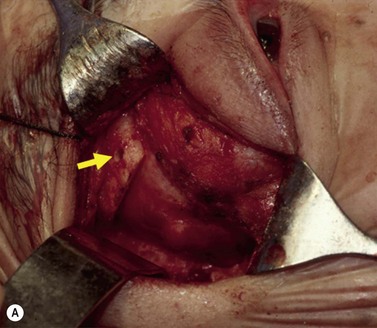
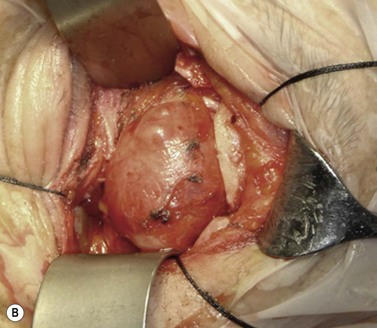
A skin-crease incision in the lateral two-thirds of the upper lid is extended for about 1–1.5 cm into the inferolateral rhytid and the preseptal plane followed out to the orbital rim. The periosteum is incised 6–7 mm outside the rim, from the lateral one-third of the upper rim to the level of the inferior orbital rim, and the periosteal leaf raised anteriorly, over the orbital rim and backwards across the lateral orbital wall (dividing any vascular bridges as necessary). Two parallel antero-posterior saw cuts are made at the upper and lower ends of the lateral osteotomy, drill holes placed either side of the cuts and, using the same drill, the inner aspect of the lateral wall fragment weakened 1 cm behind the rim. With a large retractor in place to protect the orbital contents, the lateral fragment is out-fractured on the temporalis muscle, with relieving incisions being made (superiorly along the superficial temporalis fascia and inferiorly into the muscle) to facilitate this outward swinging of the bone (Fig. 53.6A). The remaining ragged posterior edge is trimmed with bone-nibblers to increase the access posteriorly and to facilitate replacement of the mobilized bone fragment, and the sphenoidal marrow space treated with bone-wax if there is significant bleeding.
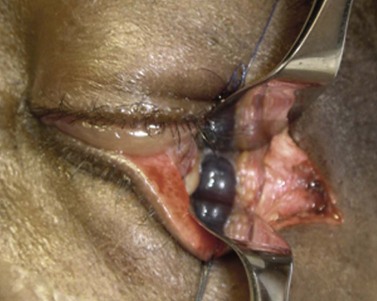
The orbit is opened by incision of the periosteum immediately behind the arcus marginalis and with a posteriorly directed incision just inferior to the lower border of the lacrimal gland. The orbital procedure is performed – typically either intact excision of a large pleomorphic adenoma of the lacrimal gland or dermoid (Fig. 53.6B), or resection of an apical tumor or large intraconal mass – and meticulous hemostasis achieved with bipolar diathermy. The laterally-displaced bone is swung back into place and fixed with a 4-0 absorbable suture passed through the drill holes (Fig. 53.6C), a vacuum drain positioned into the depths of the orbit and the drain taken exteriorly through the lateral brow-line. The periosteum and deep tissues are closed with a 4-0 or 5-0 absorbable suture and the skin incision closed with a running 6-0 nylon suture.
Retrocaruncular medial orbitotomy
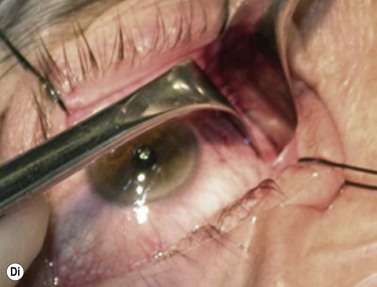
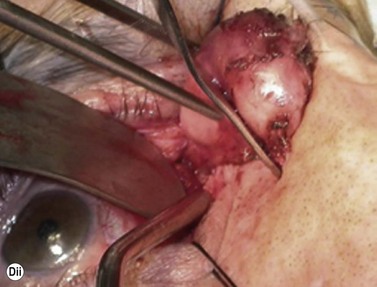
Although frequently described as a transcaruncular approach9,10 to the medial part of the orbit, the retrocaruncular approach provides excellent access to the ethmoid sinuses, the medial half of the orbital floor, and extraconal structures within the medial part of the orbit (Fig. 53.7A); as this approach avoids mobilization of the lacrimal sac, is rapid in access and closure, and has no visible scar, it is vastly superior to the Lynch incision that was commonly used in the past. The retrocaruncular approach is particularly valuable where inferomedial orbital decompression is required for compressive optic neuropathy; indeed, in patients with major systemic medical disease it is even possible to perform this procedure under local anesthesia, without any form of sedation.
Gentle conjunctival diathermy is applied to the back edge of the caruncle and continued for 1 cm into the inferior fornix, caution being taken to avoid damage to the inferior canaliculus. The conjunctiva is opened along the incision, and closed scissors are directed 45° posteromedially, along the under surface of the posterior lacrimal fascia, to reach the posterior lacrimal crest (Fig 53.7B). At this stage the plane of dissection is intraperiosteal (see Fig. 53.7B) and, for ethmoidectomy or repair of fractures, the extraperiosteal plane has to be entered at a point about 5 mm behind the posterior lacrimal crest (Fig. 53.7C); a 12–16 mm malleable retractor, bent to slightly more than a right angle, provides retraction of the orbital fat and periosteum. Bipolar diathermy is used to tease the fat away from the anterior part of the lamina papyracea (and orbital floor if necessary), and then the periosteum is opened about 5 mm behind the posterior lacrimal crest, so that the extraperiosteal space is gained more posteriorly; the upper limit for peeling periosteum from the underlying bone is clearly defined by the anterior and posterior neurovascular bundles, and the lower limit can be taken as far laterally as the infraorbital nerve canal.
The appropriate intraorbital procedure, for example resection of medial orbital dermoid (Fig. 53.7D), repair of fracture, or decompression may be performed, although fat encroachment into the operative field can prove to be very awkward in some patients. The conjunctival incision is readily closed with two 7-0 soluble sutures, one at the upper and one at the lower border of the caruncle.
Transconjunctival orbitotomy
For masses closely applied behind the globe, the intraconal space may be explored through a conjunctival peritomy – this being extended for between 120 and 180° with radial relieving incisions at either end (see Fig. 53.7A). It is possible to biopsy retrobulbar tissues or deliver a large mass through such an incision, although in many cases a more ‘panoramic’ view of the lesion may be available using an upper lid incision or lower lid swinging flap.
Summary
With these six basic approaches, all of which respect the periocular esthetics, it is generally possible to deal with all solely orbital disease; indeed, there appears to be little or no contemporary indication for other approaches – such as a lid-split orbitotomy11 or bicoronal flap12,13 – in such cases. However, where a mixture of orbital and cranial or midfacial disease is present, these patients are best treated by the appropriate surgeons with use of more extensive incisions – such as midfacial degloving, a lateral rhinotomy or a bicoronal flap.
1 Dandy W. Results following the transcranial attack of orbital tumors. Arch Ophthalmol. 1941;25:191-196.
2 Rose GE. The ‘devil’s touch’; visual loss and orbital surgery. A synopsis of the Mustardé Lecture, 2006. Orbit. 2007;26:147-158.
3 Hass AN, Penne RB, Stefanyszyn MA, et al. Incidence of postblepharoplasty orbital haemorrhage and associated visual loss. Ophthal Plast Reconstr Surg. 2004;20:426-432.
4 O’Donnell M, Kearon C. Perioperative management of oral anticoagulation. Cardiol Clin. 2008;26:299-309.
5 De Riu G, Meloni SM, Gobbi R, et al. Subciliary versus swinging eyelid approach to the orbital floor. J Craniomaxillofac Surg. 2008;36:439-442.
6 McCord CDJr, Moses JL. Exposure of the inferior orbit with fornix incision and lateral canthotomy. Ophthalmic Surg. 1979;10:53-63.
7 McNab AA, Wright JE. Lateral orbitotomy – a review. Aust N Z J Ophthalmol. 1990;18:281-286.
8 Harris GJ, Logani SC. Eyelid crease incision for lateral orbitotomy. Ophthal Plast Reconstr Surg. 1999;15:9-16.
9 Garcia GH, Goldberg RA, Shorr N. The transcaruncular approach in repair of orbital fractures: a retrospective study. J Craniomaxillofac Trauma. 1998;4:7-12.
10 Shorr N, Bayliss HI, Goldberg RA, et al. Transcaruncular approach to the medial orbit and orbital apex. Ophthalmol. 2000;107:1459-1463.
11 Kersten RC, Kulwin DR. Vertical lid split orbitotomy revisited. Ophthal Plast Reconstr Surg. 1999;15:425-428.
12 Kalmann R, Mourits MP, van der Pol JP, et al. Coronal approach for rehabilitative orbital decompression in Graves’ ophthalmopathy. Br J Ophthalmol. 1997;81:41-45.
13 Linnet J, Hegedus L, Bierre P. Results of a neurosurgical two-wall orbital decompression in the treatment of severe thyroid associated orbitopathy. Acta Ophthalmol Scand. 2001;79:49-52.