CHAPTER 365 Surgical Approaches to Intracranial Aneurysms
Surgical Anatomy
Detailed surgical anatomy is beyond the scope of this chapter. The reader is referred to scholarly reviews that describe the relevant vascular and surgical anatomy and that of surrounding osseous and neural structures needed for aneurysm surgery by Rhoton (see Chapter 2), de Oliveira, Yasargil, and colleagues.1–5
Preoperative Considerations
Neurodiagnostic Studies
CT Angiography
CT angiography (CTA) uses a rapid injection of iodinated contrast and image acquisition during the arterial phase. During CTA, the tomographic data is collected in a three-dimensional array and then is reconstructed to provide anatomic information about the aneurysm and adjacent vessels. Reconstruction algorithms include maximum intensity projection (MIP), shaded surface display (SSD), volume rendering (VR), and ray-sum projection (RSP). These reconstructions provide a 3-D appearance to the aneurysm and vascular anatomy. Several clinical series demonstrate that CTA reconstruction can provide useful information about aneurysm morphology that may guide management decisions including when to triage patients to surgical or endovascular procedures.6,7 For example CTA is useful when a patient with a large intracranial hemorrhage (ICH) is evaluated and can eliminate the need for conventional DSA in the rapidly deteriorating patient. The information obtained from CTA also can supplement DSA images and provide useful information about the aneurysm lumen, presence of thrombosis, anatomic detail about the aneurysm neck, vascular relationships and bony landmarks, and in some instances may offer an aneurysm perspective that better approximates the surgical approach and view than conventional DSA.
Angiography
Four-vessel angiography in multiple projections remains the “gold standard” for diagnosis and treatment planning. Five features need to be evaluated: (1) the aneurysm’s vessel of origin; (2) aneurysm size, shape, and relationship to parent and adjacent arteries; (3) the presence and location of vasospasm; (4) adjacent vessel displacement because this suggests mass effect (e.g., ICH or partial aneurysm thrombosis [i.e., the aneurysm dimension is larger than that seen on DSA]); and (5) the presence of other aneurysms or vascular abnormalities. When multiple aneurysms are present after a SAH, several radiological features can be used to decide which lesion ruptured (Table 365-1). Spin, rotational, and three-dimensional DSA with appropriate algorithms can display 3-D data in an orientation similar to intraoperative views.8 This is particularly useful for large and complex aneurysms.
| If the site of rupture cannot be reliably identified, all aneurysms may need to be treated starting with the one most likely to have ruptured. The role of MRI to identify site of rupture remains ill defined. | |
| Exclude extradural aneurysms | |
| CT | Focal clot accumulation |
| Angiographic features | Focal vasospasm Focal mass effect (perianeurysmal hematoma) Aneurysm change on serial angiogram |
| Aneurysm morphology | Active bleeding with contrast extravasation Multilobulated or irregular Larger size Daughter aneurysm (nipple) |
| Focal clinical signs | |
Anesthesia
There is no standard anesthetic regimen for all aneurysm surgery and the technique should be individualized (see Chapter 21). There are several basic principles9,10 and goals (Table 365-2). First, anesthesia should be titratable and short acting to permit a prompt controlled wakeup. Some commonly used agents include infusions of remifentanil, propofol, or inhaled anesthetics such as sevoflurane or desflurane. Second, drugs that reduce cerebral blood flow (CBF) or increase intracranial pressure (ICP) should be avoided. Third, careful blood pressure control is necessary. In particular, large changes in blood pressure that cause an increase in transmural pressure and so increase aneurysm rupture risk need to be avoided during induction. Normotension usually is preferred at surgery; however, mean arterial blood pressure should be increased by 10% to 20% from the baseline if temporary arterial occlusion is applied. Consequently, invasive blood pressure monitoring is necessary in each patient. Fourth, arterial blood gases should be checked during surgery to obtain adequate oxygenation and to maintain PaCO2 levels between 30 and 35 mm Hg. Lower levels of PaCO2 may decrease CBF, particularly in patients with vasospasm. The role of hypothermia and other neuroprotective strategies for aneurysms remains unclear.11–15
TABLE 365-2 Perioperative Anesthetic Goals During Cerebral Aneurysm Surgery
| Prevent aneurysm rupture | Avoid hypertension Avoid rapid ICP decrease with hyperventilation |
| Prevent cerebral ischemia | Maintain adequate cerebral perfusion pressure (70 to 80 mm Hg) Optimize oxygen delivery (Hgb, PaO2) Decrease cerebral metabolic demand (pharmacologic suppression, prevent hyperthermia) |
| Maintain euvolemia | Monitor CVP, urine output, blood loss Normal saline replacement |
| Optimize surgical exposure | CSF drainage Cranial venous drainage Hyperventilation Osmotic diuretics |
| Monitor electrolytes/glucose | Prevent hyperglycemia |
| Cardiac stability | Control blood pressure with short-acting agents EKG monitor—arrhythmias |
Brain Relaxation
The incidence of injury from brain retraction is estimated at 5% in intracranial aneurysm procedures16 and is more likely when the brain is swollen (e.g., poor-grade SAH) or when a large complex aneurysm is treated. Excessive retraction can cause ischemia or contusions. The need for retraction can be reduced through adequate exposure and proper brain relaxation that can be accomplished by several methods.
Positioning
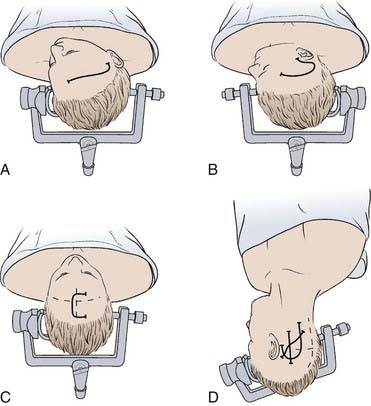
Proper positioning can facilitate aneurysm exposure, reduce the risk of retraction injury, and improve surgeon comfort. Aneurysm morphology and the type of incision and bone flap determine the specific head position; specific positions for each approach are reviewed below. Proper head position should allow the brain to naturally fall away from the surgical trajectory; this enhances exposure and reduces the need for brain retraction (Fig. 365-1). Many aneurysms are approached through a pterional craniotomy or modifications of this. For this craniotomy, patients are in a supine position with slight hip flexion and knees bent. The neck should not be rotated or flexed in such a manner that it obstructs venous return. For most pterional craniotomies, the head is positioned such that the frontal lobe falls away from the floor of the anterior fossa and the temporal lobe falls away from the sylvian fissure (i.e., the malar eminence is highest).
Craniotomy Selection
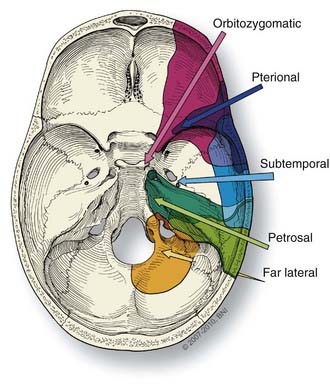
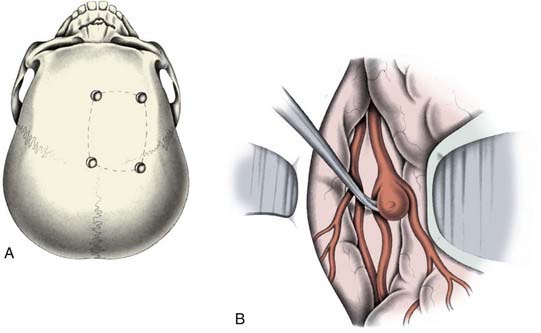
Selection of the correct approach enhances the safety and efficacy of aneurysm occlusion. The type and size of the craniotomy (Fig. 365-2) chosen for a specific aneurysm is influenced by four factors: (1) the aneurysm type (e.g., location and size); (2) aneurysm configuration and anatomy of the associated vessels and surrounding osseous and neural structures; (3) the patient’s clinical status; and (4) surgeon preference. Of these factors, the type of aneurysm and its configuration most influence what craniotomy is used. A three-dimensional understanding of the relevant neuroanatomy is needed to plan the appropriate trajectory to the aneurysm and the possible trajectories of any clip application.18 While unnecessary large craniotomies should be avoided, adequate bone removal is essential to reduce the need for brain retraction or even placement of retractors.
Anterior Circulation
Pterional Craniotomy
Most anterior circulation aneurysms can be approached through a pterional craniotomy, including aneurysms of the ICA, posterior communicating artery (PcomA), anterior communicating artery (AcomA), and middle cerebral artery (MCA). Specific lesions may require minor modifications (e.g., orbitozygomatic approach for some AcomA aneurysms or anterior clinoidectomy for carotid-opthalmic artery aneurysms). For aneurysms that involve the proximal ICA (i.e., paraclinoid region), exposure of the cervical ICA is recommended for proximal control. Because the pterional approach is frequently used in aneurysm surgery it is discussed in detail (Fig. 365-3).
Scalp and Soft Tissue
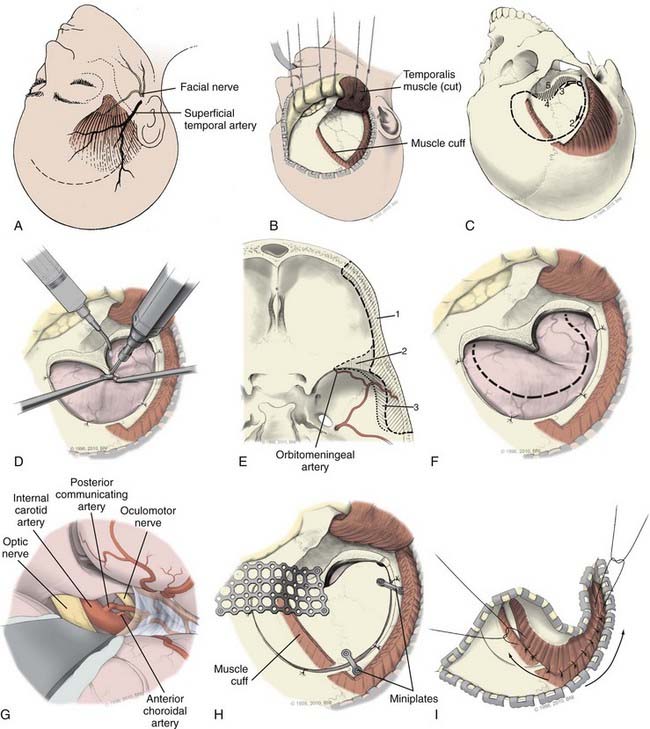
A curvilinear frontal-temporal skin incision is made that starts just anterior to the tragus at the root of the zygoma (Fig. 365-3A). It is carried superiorly to the temporal crest and curved forward to the midline just behind the hairline. While the bone flap does not need to extend to the midline, the skin incision should just cross the midline. This allows the scalp flap to be retracted anterior and inferior to expose fully the frontal zygomatic junction and pterion. The scalp and temporalis muscle can be elevated together and retracted forward using low-profile perforating towel clips or fishhooks that then are secured with rubber bands to bars from the Greenberg system connected to the Mayfield or to the Sugita system when used. A small fascial cuff may be left along the temporalis muscle insertion for later closure (Fig. 365-3B). The pericranium should be separately mobilized if the frontal sinus is likely to be opened during the craniotomy (e.g., with a more medial exposure for some AcomA aneurysms). Extension of the incision inferiorly (below the zygoma root) or subgaleal dissection antero-inferiorly should be avoided, to prevent injury to the facial nerve, which is found about 1.5 cm below the zygomatic arch origin19 and crosses about 2 cm anterior to its origin in an oblique superior-anterior direction. The facial nerve frontal branch that innervates the frontalis muscle courses in the subgaleal fat pad; interfascial dissection of the triangular fat pad or elevation of the temporalis muscle and scalp flap together may help avoid nerve injury. Care should be taken to preserve the superficial temporal artery with at least one of its major branches to supply the temporalis muscle or, in some cases, for a bypass procedure.
Skull
After placement of a bur hole and in the presence of a lumbar drain, careful CSF drainage is initiated. About 20 mL of CSF should be initially drained with subsequently larger (50 to 200 mL is not uncommon) volumes achieved. If extensive sylvian fissure dissection is likely, CSF drainage should be minimized. A single bur hole in the temporal bone at the root of the zygoma is generally all that is required. This single bur hole craniotomy is less likely to be associated with cosmetic defects than multiple bur hole craniotomies. Some surgeons place a bur hole in the pterion. In older patients, the dura is frequently adherent to the skull and frequently torn in the anterior medial frontal region; consequently, multiple bur holes are used in the elderly. The craniotomy (Fig. 365-3C) is C-shaped in an arc “around” the pterion and anterior clinoid process (ACP). The dura is dissected free at the bur hole and then with a high-speed drill, the skull is cut forward in the subtemporal region until the sphenoid wing. Then in a separate cut, the skull is opened in a curvilinear fashion in the frontal-temporal region (i.e., the cut extends superior to the temporal crest and then anterior to the midpupillary region of the supraorbital region (foramen of supraorbital nerve). This cut may extend more medial for an AcomA aneurysm. The opening then proceeds posteriorly to the pterion and sphenoid wing flat along the anterior fossa floor. The supraorbital ridge is preserved for later reconstruction. The drill may not cut all the way through the pterion and in these cases the bone in this region can be thinned and then fractured through the pterion.
Skull Base
Once the bone flap is removed, the dura is dissected free from the anterior fossa skull base. With a high-speed bur, the pterional bone is drilled away to remove the lesser wing of the sphenoid, the ACP base, and flatten the bony prominences of the anterior fossa floor, the inner table of the inferior frontal bone over the supraorbital ridge and the squasmal temporal bone (Fig. 365-3D and E). Orbitomeningeal artery bleeding indicates proximity to the superior orbital fissure (SOF); this artery can be coagulated and divided. For some MCA aneurysms, subtemporal bone may need removal to expose the middle fossa floor. Extensive bone removal creates a flat corridor with ample space under the frontal and temporal lobes. The extradural drilling can be carried down to the ACP, which can be removed before the dural opening to enhance exposure of supraclinoid ICA aneurysms.20 Alternatively the ACP can be removed after the dura is opened (Fig. 365-4). The craniotomy edges then are lined with hemostatic agents and tack-up sutures are placed.
Dural Opening and Brain Preparation
The dura is opened in a C-shaped fashion centered on the pterion and ACP (Fig. 365-3F) and retracted laterally to expose the inferior frontal lobe and anterior sylvian fissure. It is held out of the surgical field with sutures. If there has been adequate bone removal, the optic nerve and ICA at the skull base can now be approached with little brain retraction. First the brain should be protected. This is particularly important after a SAH where its surface may be friable and easily contused. We place large squares of Gelfoam under the dura in the more posterior aspect of the bony opening to prevent direct brain injury and to prevent the run down of blood into the subdural space as brain relaxation and CSF drainage proceeds. The brain surface is covered with moist surgical cottonoids or Telfa strips.
Initial Vascular Dissection
The frontal lobe is mobilized gently close the sylvian fissure. This exposes the olfactory tract and may be done with a No. 5 sucker against a cottonoid, if there is adequate brain relaxation and bone exposure. If the brain remains swollen (e.g., after severe SAH), additional relaxation with further CSF drainage or barbiturates may be needed. When extensive sylvian fissure dissection is planned (e.g., with an MCA aneurysm), we prefer not to drain too much CSF because the fissure is easier to split when it is filled with CSF. Using sharp, careful arachnoid dissection, the fissure may be split from lateral to medial or medial to lateral depending on aneurysm location and morphology and length of the ICA and MCA (M1) segments, but usually on the medial (frontal) side of the sylvian veins. The extent of sylvian fissure dissection also depends on aneurysm location and brain condition. Transsylvian veins should be preserved, in particular those that drain into the sphenoparietal sinus, to limit venous congestion. The goal of initial dissection is to define the proximal ICA and optic nerve and then open arachnoid over these structures (i.e., the anterior and medial portions of the chiasmatic and carotid cisterns) using a sharp arachnoid knife (Fig. 365-3G). This allows further CSF drainage and brain relaxation such that the frontal lobe falls away from the skull base. The surgical approach thereafter depends on the aneurysm to be treated. A detailed description of how aneurysms in different locations should be dissected or occluded is beyond the scope of this chapter. A brief review of select techniques is provided.
Carotid Ophthalmic Artery and Paraclinoid ICA Aneurysms
Aneurysms that involve the ICA as it winds around the ACP (paraclinoid aneurysms) all involve or are near the carotid ring, ACP, optic strut, and optic nerves. Proximal control is important and therefore the ipsilateral neck should be included into the sterile field to provide access to the cervical carotid bifurcation for proximal ICA control, retrograde suction decompression (Fig. 365-5), or a saphenous vein bypass graft when required.21 There should be a low threshold for neck opening throughout the case. We usually obtain cervical ICA exposure in all patients with a clinoidal ICA aneurysm, a complex or giant aneurysm, or aneurysm of the ophthalmic segment.
Extradural ACP Removal
Before the dura is opened, the posterior half of the roof and lateral wall of the orbit and the sphenoid ridge over the SOF are removed to define the orbital portion of the optic nerve. The ACP then is internally cavitated using a small diamond bur, and the remaining thin remnants are removed with small rongeurs and curets. Bleeding is controlled with a combination of bone wax, Surgicel, and Gelfoam.20,22 Slight elevation of the head will frequently result in arrest of venous bleeding.
Intradural ACP Removal
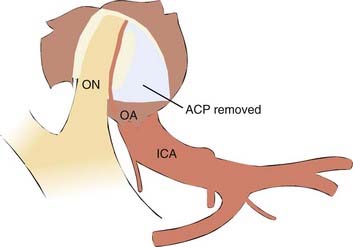
The roof and lateral wall of the orbit and the sphenoid ridge are removed extradurally. Once the dura is opened, the sylvian fissure is split widely from lateral to medial to expose the ICA and the ACP. A longitudinal dural incision then is made from the ACP to the resected edge of the medial sphenoid ridge. The falciform ligament is cut to decompress the optic nerve. The ACP and optic canal roof and lateral wall then are thinned with a small diamond bur, and the remaining bone removed with small rongeurs. Irrigation is necessary to prevent thermal injury and to clear bone dust while drilling. Finally, the optic strut is drilled to expose the anterior border of the ICA clinoidal segment. This exposes the ophthalmic artery (OA) and permits the optic nerve to be mobilized superiorly off the OA/ICA (see Fig. 365-4). Bleeding from the cavernous sinus can be controlled by Surgicel or Gelfoam.
Posterior Communicating Artery
Posterior ICA Wall Aneurysms
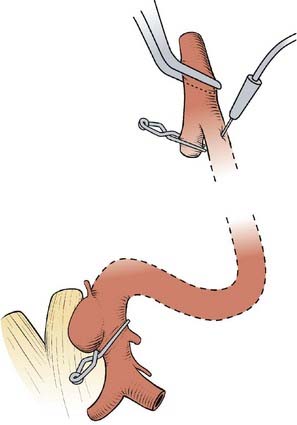
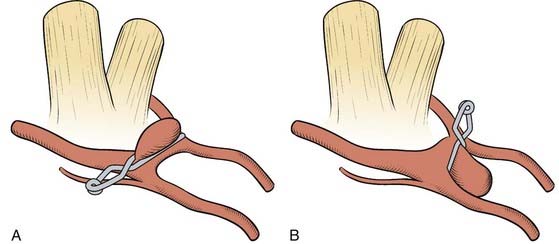
There is usually no identifiable neck in these aneurysms and they may involve ICA branches. In addition, when the surgeon first approaches these aneurysms, it appears that the entire artery is involved, similar to a fusiform aneurysm. Ideally these lesions are occluded with angled fenestrated clips placed over the ICA that is left in the fenestration (i.e., “reconstructing” the ICA lumen). Sometimes multiple clips are necessary (Fig. 365-6). Exposure of the cervical ICA and use of a temporary ICA occlusion in the neck is useful as is intraoperative angiography or fluorescein angiography.
Anterior Cerebral and Anterior Communicating Artery Aneurysms
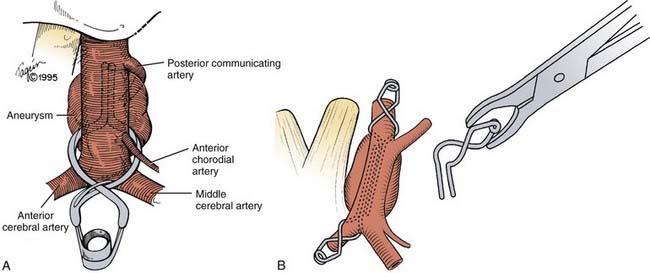
The usual pterional craniotomy is extended more medial and the sphenoid wing and anterior fossa floor are flattened. An orbital zygomatic (OZ) approach may be helpful for superior-oriented AcomA aneurysms. Aneurysm dissection and occlusion occurs under the frontal lobe; wide sylvian opening improves frontal lobe mobility. The initial dissection then occurs on the anterior surface of the A1 segment taking care to avoid the recurrent artery of Heubner. The key to successful AcomA aneurysm occlusion is complete appreciation of the AcomA complex anatomy; aneurysm morphology influences how this may be achieved. The AcomA is formed by the confluence of the two A1s to form the anterior aspect of the Circle of Willis and generally is on the optic chiasm at the lamina terminalis level. The AcomA marks the origin of the A2 segments bilaterally. Small perforating vessels arise from the A1 and A2 segments and the AcomA; these supply the hypothalamus, anterior perforating substance, dorsal optic chiasm, suprachiasmatic area, anterior third ventricle, frontal lobe, and gyrus rectus. The recurrent artery of Heubner arises at, or near, the A1 and A2 junction and has a retrograde course posterior or superior to the ipsilateral A1 and sometimes may be identified before the A1 during microdissection. In addition, frontal-polar branches need to be defined, and ideally both optic nerves seen. A gyrus rectus resection adjacent to the AcomA complex can be helpful, especially with superior-oriented aneurysms, and can help visualize the A2s. Further dissection to define all the vessels then depends on aneurysm orientation.23 AcomA aneurysms can project at any angle in three-dimensional space; however, the projection is best understood in an anterior-posterior or superior-inferior view of perpendicular two-dimensional planes. There is a difference between the perspective in true anatomic space and the pterional surgical exposure; (e.g., an aneurysm that projects anteriorly may appear superiorly directed in the surgical field. Angiographic perspective further differs, and depends on the image projection and obliquity. To understand dissection, AcomA aneurysms may be classified into one of four projections based on their orientation in true anatomic space (Fig. 365-7):
Middle Cerebral Artery Aneurysms
Closure
To assess successful aneurysm clipping (complete aneurysm obliteration and parent vessel patency) may be difficult through direct external inspection alone. In particular this is difficult for paraclinoid and complex aneurysms. Once certain that the aneurysm is occluded, without adjacent vessel compromise or compression of neural structures, the aneurysm can be punctured with a 23- or 25-gauge needle to confirm absent blood flow in the dome. Microvascular Doppler also can be used to confirm aneurysm occlusion and flow in the vessels. Intraoperative angiography (DSA or fluorescein) is invaluable for large or complex aneurysms particularly those of the paraclinoid region. Residual blood clot in the cisterns or in the brain parenchyma is removed when possible using gentle irrigation. Papaverine-soaked Gelfoam can be placed on narrowed exposed vessels. The operative field and brain are carefully inspected under the microscope at different angles to ensure complete hemostasis and the field irrigated. Dissected brain surfaces or an ICH cavity, if present, are covered with Surgicel. When the ACP is removed any communication with sphenoid sinus can be occluded using a small muscle plug, Gelfoam, or wax. Tack-up sutures are placed before dural closure to ensure that the needle does not injure the brain and the dura closed using 4/0 Nurolon suture. Dural defects can be corrected with pericranium or artificial dural substitutes. If the frontal sinus was entered during the craniotomy it should be covered with a vascularized pericranium flap. The bone is replaced using low-profile plates and screws and the pterional region reconstructed with mesh cranioplasty to limit indentation associated with temporalis atrophy (see Fig. 365-3H). The temporalis muscle is reattached with 3-0 or 2-0 Vicryl sutures and then the temporalis fascia is resutured (see Fig. 365-3I). The subgaleal space is again irrigated with saline to clean blood clots, bone, and tissue debris and a subgaleal drain placed. The galea is closed with inverted 2-0 Vicryl sutures and the skin is closed with a running nylon suture or with staples.
Modifications of the Pterional Approach
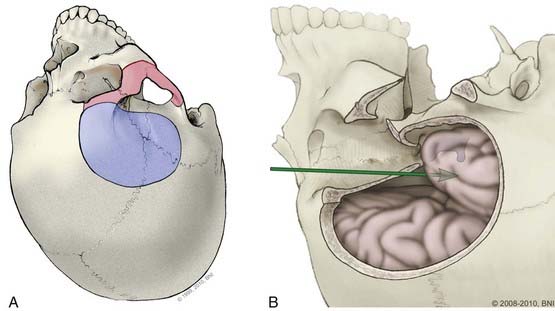
In some cases, the traditional pterional craniotomy can be modified to improve the surgical exposure, limit brain retraction, and enhance access to difficult lesions. An orbital-zygomatic osteotomy, specifically removing the orbital rim and superior and lateral orbital walls, can expand the operative corridor.19,24,25 This modification allows the surgeon to “look-up’ from the skull base and avoid frontal lobe retraction (Fig. 365-8). An extended orbital-zygomatic approach, in which a subtemporal decompression also is performed, can increase the inferior exposure. These approaches are discussed in more detail in the section on Posterior Circulation aneurysms.
Frontal Parasagittal
This approach is used for distal anterior cerebral artery (ACA), specifically pericallosal and callosal marginal, aneurysms,23 which comprise about 2% to 3% of intracranial aneurysms (Fig. 365-9). The patient is placed supine with the head flexed about 20 degrees but not to compromise venous drainage. We check this by placing two fingers between the chin and chest. The head is positioned straight, or slightly angled toward the contralateral side. A bicoronal skin incision behind the hairline or U-shaped frontal incision, with the base of the U just on the contralateral side of the sagittal sinus may be used. A right-sided approach is preferred for the right-handed surgeon. The bone flap is rectangular in shape and should extend past midline; this helps reduce frontal lobe retraction because the falx can be retracted. Frameless stereotaxy may be a useful adjunct to ensure that the bone opening is anterior enough to allow proximal control. The dura is opened so that it remains hinged on the superior sagittal sinus. The approach is interhemispheric; bridging veins therefore need to be preserved. In a patient with multiple aneurysms we avoid simultaneous pterional and parasagittal craniotomies.
Posterior Circulation
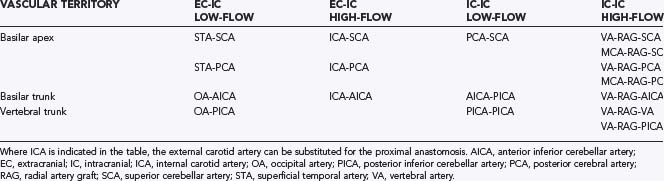
About 10% to 15% of intracranial aneurysms are located in the posterior circulation where they occur most often at the basilar bifurcation, followed by the origins of the superior cerebellar artery (SCA) and posterior inferior cerebellar artery (PICA). Dissections and fusiform aneurysms are more common in the posterior than in the anterior circulation. Many posterior circulation aneurysms are difficult to access because of the deep midline location of the vertebrobasilar system, confinement by the clivus and petrous pyramids, and the close relationship to the cranial nerves. Consequently, as endovascular techniques have advanced, direct surgery on posterior circulation aneurysms now is less frequent. There are several surgical approaches to these lesions defined by the exposed vascular territory (basilar apex, basilar trunk, and vertebral trunk) and surgical trajectory (anterosuperior, lateral, and posteroinferior; Table 365-3). In addition, each approach includes variations in how deep obstacles (e.g., the tentorium or posterior clinoid process) are managed to provide adequate exposure. Surgical morbidity is frequent and often planned (e.g., transcochlear approach), therefore careful consideration should be made by a team of neurovascular and endovascular surgeons about whether and when to treat and how best to occlude a posterior circulation aneurysm before surgery is scheduled. In this section we review the various surgical approaches according to the three main vascular territories.
Basilar Bifurcation (Apex)
Between 5% and 8% of intracranial aneurysms are located at the basilar bifurcation or apex (BB). There are several surgical approaches to these aneurysms (Table 365-4); the extended orbitozygomatic approach provides the greatest exposure and flexibility of trajectories. Careful choice of an approach is critical to surgical success and is, in large part, influenced by aneurysm morphology including: (1) aneurysm site and size, (2) exact origin of the sac, (3) fundus projection and size, (4) clival level of the bifurcation, (5) distance from the sagittal midline, and (6) distance from the clivus. Other factors include: ICA length and direction, presence of other aneurysms, temporal lobe swelling, and intraventricular hemorrhage (IVH). For most BB aneurysms, a right-sided approach is preferable. A left-sided approach is recommended when there is: (1) a left third nerve palsy and right hemiparesis and (2) a coexistent left-sided anterior circulation aneurysm, and both can be repaired through the same craniotomy. A left-sided approach may be optimal with an aneurysm that is oriented to the left.
Subtemporal
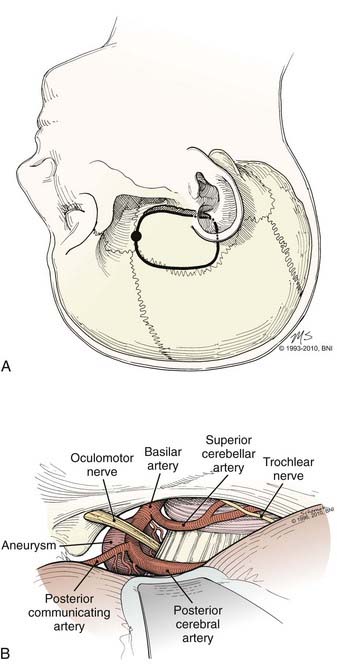
The subtemporal approach proceeds from a lateral trajectory under the temporal lobe and along the middle fossa floor.26 This approach provides direct lateral access along what often is the widest axis of the aneurysm neck and often the optimal direction for clip placement. The area behind the aneurysm, including the perforators, whose preservation is essential, usually is seen best from this approach (Fig. 365-10). The approach also may be suitable for some distal basilar artery (BA), SCA, and posterior cerebral artery (PCA) aneurysms. In general, a right-sided approach is used (but see previous discussion). A relaxed brain achieved through a combination of techniques is crucial when using a subtemporal approach, including direct CSF removal from the opened cisterns, once the temporal lobe is elevated. There are several disadvantages to the subtemporal approach: (1) the operating field is small; (2) excess temporal lobe retraction may be necessary; (3) the ipsilateral P1 lies between the surgeon and the aneurysm, which may limit dissection or clip application; (4) the aneurysm, particularly when large, needs to be retracted to see the opposite P1; and (5) a high-lying bifurcation may be difficult to approach.
The patient is placed in the lateral decubitus or in the supine position with a shoulder roll. The head is rotated until the midline plane (superior sagittal sinus) is parallel to the floor, and the vertex is angled 15 to 20 degrees downward to achieve a line of sight parallel to the floor of the middle fossa. One of two incisions may be used: a 7- to 10-cm linear incision that extends up from a point 1 cm anterior to the tragus at the zygomatic arch or a question mark that starts just anterior to the tragus and curves above the ear to the superior temporal line (Fig. 365-10). A 4- × 4-cm craniotomy is made and temporal squamosal bone removed inferiorly with a bur until flush with the middle fossa floor. Under the operating microscope, a self-retaining retractor is positioned to elevate the temporal lobe and expose the tentorial incisura. Dissection then is directed between the medial temporal lobe and the tentorial edge. The arachnoid is opened to enter the interpeduncular and ambient cisterns, and cranial nerve (CN) III is identified. The edge of the tentorium can be pulled laterally with a tacking suture between CNs III and IV to enlarge the opening into the interpeduncular cistern. The tentorium also may be divided behind the entry of CN IV for additional inferior exposure. The SCA and PCA then are followed medially to the basilar apex with minimal third nerve manipulation.
Pterional-Transsylvian Approach
The pterional approach is more anterolateral than a subtemporal trajectory and provides a better overall anatomic view including better visualization of the opposite P1. However, the posterior perforating arteries are difficult to see and the ICA is in the center of the field and it and the PcomA can be an obstacle to the basilar apex. Posterior-oriented and low-lying BB aneurysms also are difficult to see. The basic approach is similar to a pterional craniotomy for anterior circulation aneurysms. A frontotemporal craniotomy is made that extends medially to the supraorbital nerve foramen and inferiorly to the floor of the anterior cranial fossa. The craniotomy is centered on the pterion that then is drilled (see Fig. 365-3) to remove the lesser wing of the sphenoid, the ACP base, flatten the orbital roof and inner table of the inferior frontal bone over the superior orbital rim, and remove squamosal temporal bone inferiorly to the middle fossa floor. This wide bone removal helps provide a flat anterior fossa and anterior middle fossa trajectory.
Orbitozygomatic-Pterional Approach
The orbitozygomatic approach adds two steps to the pterional approach: (1) soft tissue dissection to expose the orbitozygomatic unit (the orbital rim, orbital roof, lateral orbital wall, and zygomatic arch), and (2) osteotomies to free it, (i.e., the superior and lateral portions of the orbit [Figs. 365-8 and 365-11] are removed). This approach is useful for a high bifurcation and provides a more anterior trajectory, a higher view above the posterior clinoid process, and greater space in the operative corridor than a standard pterional craniotomy.
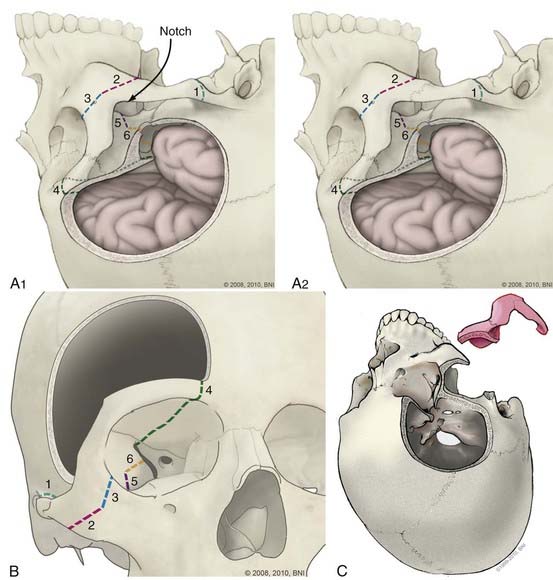
The orbitozygomatic unit is released by six osteotomies (Fig. 365-11) made with a reciprocating saw. First the zygomatic root is cut. The temporomandibular joint is avoided. A fixation plate is secured to the zygoma and registered to improve repair cosmesis. The second and third cuts are across the zygomatic bone; from the inferolateral margin halfway across to the lateral orbital rim, and then from the inferior orbital fissure to the same end point. The resulting V in the zygomatic bone allows the fragment to be secured into position when replaced. Fourth, the medial orbital roof, just lateral to the supraorbital notch is cut. The fifth cut crosses the posterior orbital roof, approximately 2.5 cm posterior to the frontal bone inner table (to preserve the orbital roof), and finishes laterally in the sphenoid ridge and pterion. The final cut crosses the lateral orbital wall to connect the previous cut with the inferior orbital fissure. The orbitozygomatic unit then is removed as a single piece, and additional bone is removed over the orbital apex around the superior orbital fissure. A dural flap based over the orbit is tented forward with tacking sutures to depress the globe gently. The intradural part of this approach is similar to a pterional approach. To widen the view, the choroidal fissure is opened and the temporal pole mobilized laterally.
Surgical Approaches to the Basilar Trunk
The basilar trunk may best be approached through a lateral trajectory. The primary obstacle is the petrous bone. Lateral approaches to the basilar trunk require a presigmoid corridor made by drilling through the temporal bone. The extent of bone removal varies from a retrolabyrinthine resection (petrous-sparing) to a radical transpetrous approach (transcochlear approach) and a combined approach. This decision depends in part on the patient’s condition and whether hearing sacrifice is warranted. Other approaches to the midbasilar artery (extended middle fossa approach, retrolabyrinthine-transsigmoid approach, and transoral approach) are less frequently used. Although the transoral approach27 provides access to the midbasilar artery, this anterior approach generally has been replaced by more lateral approaches because it is associated with high morbidity (meningitis).
Transpetrosal Approaches
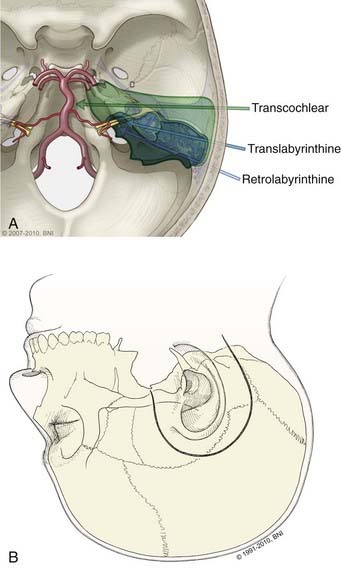
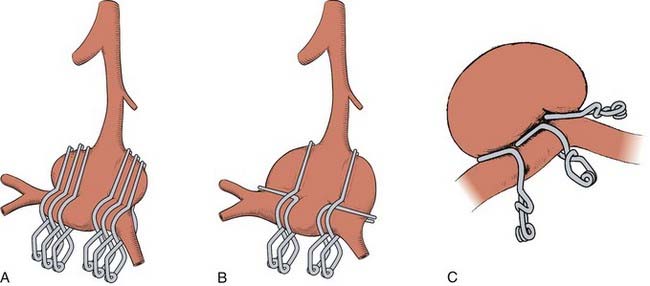
These lateral approaches provide proximal and distal control of the basilar artery but are best suited for small aneurysms because the aneurysm dome often is between the surgeon and the aneurysm neck, or cranial nerves limit instrument maneuverability. Transpetrosal approaches expose the basilar trunk from a lateral trajectory through presigmoid corridors in the petrous bone categorized into three variations based on an increasing extent of resected bone: retrolabyrinthine, translabyrinthine, and transcochlear (Fig. 365-12). The retrolabyrinthine approach removes temporal bone between the semicircular canals anteriorly and the posterior fossa dura on the posterior aspect of the temporal bone.28 The semicircular canals are skeletonized, but not violated. In the translabyrinthine approach, exposure is increased further anteriorly to the internal auditory canal (IAC) and the semicircular canals are removed (i.e., hearing is sacrificed).29 The seventh nerve is left in its bony sheath to protect it. The petrous bone is almost completely removed in the transcochlear approach. The facial nerve canal is opened, the nerve transposed posteriorly to access the cochlea that then is removed.30
Retrolabyrinthine Approach
This approach may be used for smaller basilar trunk aneurysms. The patient is positioned supine with a shoulder roll to reduce neck rotation, and the head is positioned in a Mayfield head holder with the midline parallel to the floor and inclined slightly downward. The head is flexed slightly and the shoulder taped caudally to improve access. The mastoid bone becomes the highest point in the surgical field. The skin is incised 1 cm anterior to the tragus and 2 cm above the zygoma, curving gently around the ear to the mastoid tip (see Fig. 116-8B), and the scalp flap is retracted anteriorly.
Combined Supratentorial and Infratentorial Approach
The various transpetrosal approaches sometimes are inadequate for large or giant basilar trunk aneurysms because the surgical corridor is confined by the residual petrous bone anteriorly, the tentorium superiorly, and the sigmoid sinus posteriorly. The exposure then can be enhanced through division of the tentorium, and posterior mobilization of the sigmoid sinus29,30 that is designed to compensate for less presigmoid exposure. The petrosectomy then becomes the cornerstone of a combination approach that relaxes the superior and posterior barriers. The two critical additions are: (1) a supratentorial and infratentorial craniotomy that crosses the transverse sinus, and (2) division of the tentorium that provides communication between the supratentorial and infratentorial compartments. Very little brain retraction is needed then to expose the medial petrous and clival regions and associated neurovascular structures. The combined approach works with any of the petrosectomy variations but typically is used with a retrolabyrinthine approach instead of a transcochlear approach when it is desired to preserve seventh and eighth nerve function.
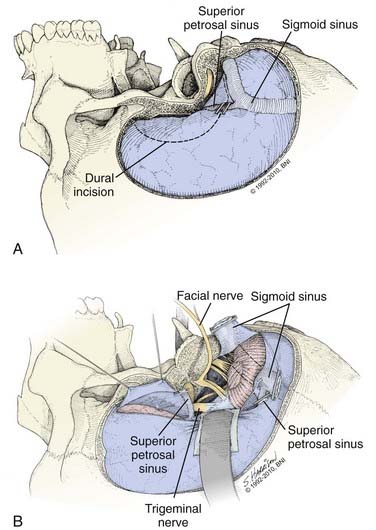
When the temporal bone drilling is complete, an edge of middle fossa dura above the petrosectomy defect and an edge of posterior fossa dura behind the sigmoid sinus are exposed. These serve as bur holes for a subtemporal-suboccipital craniotomy that crosses the transverse sinus. Once the bone flap is removed, a large dural surface is exposed, and the transverse, sigmoid, and superior petrosal sinuses are visible (Fig. 365-13). The brain is relaxed and the dura is incised anteriorly over the temporal lobe and curves posterior and inferior to the superior petrosal sinus below, where it enters the sigmoid sinus. A second dural incision is made inferiorly anterior to the sigmoid sinus, curving up to the superior petrosal sinus. The superior petrosal sinus is divided; the vein of Labbé is preserved when the dura is opened. Although rarely needed, the sigmoid sinus can be sacrificed when the contralateral transverse and sigmoid sinuses are patent and communicate with the ipsilateral sinuses. A test occlusion can be made to measure sigmoid sinus pressure after the superior petrosal sinus is divided; pressure should not increase by more than 10 mm Hg. If these angiographic and hemodynamic criteria are met, the sigmoid sinus can be divided below its confluence with the superior petrosal sinus. The vein of Labbé consistently enters the transverse sinus above this junction and so will drain contralaterally. Next, the tentorium is incised medially to the tentorial hiatus and posterior to the fourth nerve to connect the supratentorial and infratentorial compartments. The posterior temporal lobe is elevated without traction on the vein of Labbé, which is tethered to the transverse sinus. This provides wide exposure along the skull base from the foramen magnum to the dorsum sellae, with little need for brain retraction; the petrous region, clivus, brainstem, cranial nerves, and posterior circulation vessels now are seen easily.
Extended Middle Fossa Approach
The middle fossa approach was developed to remove small, intracanalicular acoustic neuromas and preserve the hearing apparatus.31 The approach was adapted to basilar aneurysms by skeletonizing the IAC and removal of the medial petrous apex. It is suitable for select small basilar aneurysms because the bony corridor created is narrow and the fifth, seventh, and eighth nerves can limit the view of the vertebrobasilar junction and reduce working space. This approach has several names, including the extended middle fossa approach, Kawase’s approach, and rhomboid approach.32,33 The extended middle fossa approach has two differences from other lateral transpetrosal approaches: (1) it reaches the basilar artery from a more anterolateral and superior trajectory in front of the otic capsule and (2) the posterolateral temporal bone is intact.
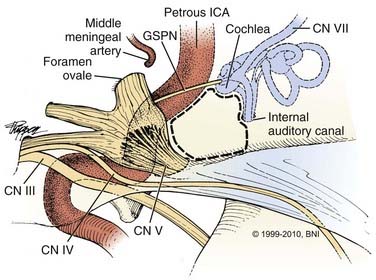
The key to this approach to the basilar artery is removal of the medial petrous apex or Kawase’s triangle (Fig. 365-14). This is really a quadrangle of bone formed by the IAC posteriorly, the GSPN laterally, the lateral margin of the trigeminal nerve and ganglion anteriorly, and the medial edge of the petrous bone medially.32 The inferior petrosal sinus marks the inferior boundary. For the procedure the patient is positioned supine, with the head positioned as for a subtemporal approach. A question mark or horseshoe-shaped incision is used, and the skin and temporalis muscle flaps are reflected anteriorly. A 5- × 5-cm craniotomy is made in the squamosal portion of the temporal bone, two-thirds anterior and one-third posterior to the external auditory canal. The middle fossa floor is exposed, the dura elevated medially to the petrous ridge, where a self-retaining retractor is placed with its tip over the lip of the ridge. The middle meningeal artery is followed along the dura to the foramen spinosum, where it is coagulated and divided. The GSPN is identified about 1 cm medially; it originates from the seventh nerve geniculate ganglion and runs superficially along the middle fossa floor in an anteromedial direction. Lateral to the GSPN is Glasscock’s triangle, through which the petrous ICA can be exposed as it runs horizontally toward the cavernous sinus.
Surgical Approaches to the Vertebral Trunk
Midline Suboccipital Approach
This approach is suitable for aneurysms of the distal PICA after it courses around the anterior and lateral medulla, proximal vertebral lesions, or for PICA bypass procedures.34 The midline approach does not provide good exposure of aneurysms located distally on the vertebral artery near the typical PICA origin.
Far Lateral Approach
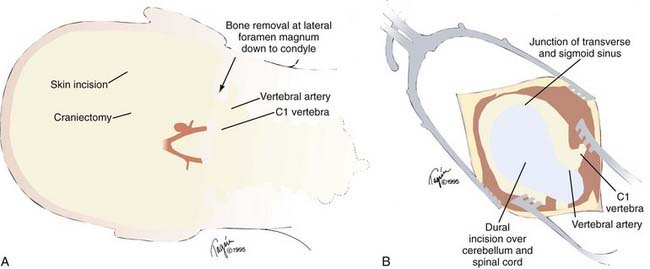
The far lateral approach provides wide exposure of the vertebral trunk and anterolateral brainstem and is the most common approach to vertebral trunk aneurysms35–37 because most are unilateral and extend beyond the region that can be accessed through a midline exposure. Anterior inferior cerebellar artery (AICA), PICA, and some proximal basilar lesions also can be treated with this approach or modifications of it.38 Vertebral artery exposure is improved by shifting the exposure corridor laterally by bone resection in the angle between the lower medulla and cerebellum. This creates a surgical corridor along the vertebral artery axis that requires minimal cerebellar retraction. The outer cranial exposure is enhanced by resection of the C1 posterior arch and the posteroinferior skull base (including the posterolateral foramen magnum, posterior half of the occipital condyle, and jugular tubercle). Synonyms for the far lateral approach include the lateral suboccipital approach, extreme lateral approach, and extreme lateral inferior transcondylar exposure (ELITE). The approach requires operating around and through the lower cranial nerves.
A hockey-stick incision is made that begins in the cervical midline over the C5 spinous process, extends cephalad to the inion, courses laterally along the superior nuchal line to the mastoid bone, and finishes inferiorly at the mastoid tip (Fig. 365-15). The midline nuchal ligament is identified to split the paraspinous musculature in this avascular plane. A cut just below the superior nuchal line detaches the paraspinous muscles, which are mobilized inferolaterally to expose the occipital bone and foramen magnum. This also creates a cuff for muscle reattachment during closure and mobilization rather than muscle transection and reduces postoperative pain. Exposure is continued to the C2 spinous process. The vertebral artery as it courses from the transverse foramen of the C1 lateral mass, through the sulcus arteriosus of the C1 vertebral arch, to its dural entry point is identified and protected. The lateral epidural venous plexus often causes bleeding; it is best preserved by blunt dissection.
The dural incision (Fig. 365-15B) curves from the cervical midline, across the circular sinus, and laterally to the craniotomy edge. An inferior cut laterally under C1 mobilizes the flap farther against the bone-opening margin. The cisterna magna is opened under the microscope, and the arachnoid layers are reflected. The proximal vertebral artery is prepared for proximal control just as it penetrates the dura to keep temporary clips, if used, out of the surgical corridor. To do this the dentate ligament is divided. The tonsillomedullary fissure is dissected and the ipsilateral cerebellar tonsil moved away from the medulla to expose the trajectory along the vertebral artery that is dissected from proximal to distal. The PICA also is dissected distal to proximal. These converging lines lead to the PICA origin. The vertebral artery can be followed distally to the vertebrobasilar junction if needed.
Extended Far Lateral Approach
The far lateral exposure can be extended superiorly by occipital bone removal to the junction of the transverse and sigmoid sinuses.39 This retrosigmoid addition enables the CPA to be entered, and large vertebral artery aneurysms and their efferent arteries can be accessed. The trajectories from the far lateral and retrosigmoid approaches are almost perpendicular (i.e., the retrosigmoid extension does not improve exposure but rather provides an additional vantage of anatomy to help clarify the anatomy and operative strategy). The approach is identical to the far lateral technique, but the superolateral edge of the craniotomy is defined by drilling through the mastoid bone to define the transverse-sigmoid junction. The sigmoid sinus is skeletonized to the jugular bulb. The subsequent far lateral bone opening then connects to this exposed dura, and the craniotomy flap is enlarged. The dura is opened with a flap based on the sigmoid sinus. When tacked anteriorly, the flap pulls the sinus forward to open the route into the CPA. The far lateral dural flap then connects with this flap.
Far Lateral Combined Supratentorial and Infratentorial (Combined-Combined) Approach
Some giant aneurysms that involve the vertebro-basilar junction or lower basilar trunk require an exposure that spans the entire length of the posterior fossa from the petrous apex to the foramen magnum. The far lateral approach, when used with the combined supratentorial and infratentorial approach, exposes the entire petroclival region.40,41 This joins the transpetrosal, subtemporal, and far lateral approaches, and overcomes the limitations of a transpetrosal approach or an extended far lateral approach alone.
Aneurysm Occlusion
Aneurysm Exposure
There are several basic principles to be understood during aneurysm exposure: (1) aneurysms usually arise at the branch site on the parent artery; (2) aneurysms arise at turns or curves in an artery; (3) aneurysms point in the direction that blood would flow if the curve at the aneurysm site was not present (i.e., in the direction of maximal hemodynamic thrust); and (4) there often are perforating arteries near most aneurysms that need to be preserved.42 Exposure is best performed under the operating microscope using sharp microdissection. The afferent and efferent vessels need to be exposed to such an extent that in the event of an intraoperative rupture a temporary clip can be placed across them. In some instances, such as a proximal ICA aneurysm, this means that the ICA in the neck must be exposed to provide proximal control. The cervical ICA is exposed well above the bifurcation to reduce the risk of disturbing any cervical atherosclerotic disease.
Temporary Artery Occlusion
Temporary occlusion should be kept to a minimum but appropriate use of temporary artery occlusion is an important adjunct during aneurysm surgery. Temporary occlusion of the proximal arteries during surgery will, in most instances, reduce aneurysm fundus pressure. This may improve the safety of aneurysm neck dissection and reduce the risk of intraoperative rupture or help reduce the increased morbidity and mortality that may be associated with intraoperative aneurysm rupture.43,44 Temporary occlusion also allows thrombectomy, endaneurysmectomy, and aneurysmorrhaphy to treat giant and complex aneurysms.45
There are several basic tenets when temporary occlusion is used: (1) temporary vessel occlusion should be used selectively and the risk of rupture versus ischemia be balanced; (2) perforating vessels’ patency must be maintained; (3) hypotension during temporary occlusion should to be avoided;47 instead mild hypertension helps collateral flow; (4) safe occlusion time varies with aneurysm location, patient age, and clinical condition (Table 365-5); (5) intermittent reperfusion may increase tolerable occlusion time; and (6) neuroprotection is recommended. When temporary occlusion is planned or thought likely, intraoperative electrophysiological monitors are necessary. The role of cardiac standstill and global circulatory arrest techniques are beyond the scope of this chapter.
| TOTAL DURATION OF TEMPORARY CLIPPING | NUMBER OF PATIENTS | RADIOGRAPHIC EVIDENCE OF CEREBRAL INFARCTION |
|---|---|---|
| <14 minutes | 49 | 0 |
| 14-21 minutes | 27 | 19% |
| 22-30 minutes | 15 | 33% |
| >30 minutes | 9 | 100% |
Modified from Samson D, Batjer HH, Bowman G, et al. A clinical study of the parameters and effects of temporary arterial occlusion in the management of intracranial aneurysms. Neurosurgery. 1994;34(1):22-28.
“Neuroprotection” or “cerebral protection” is the use of pharmacologic agents or the manipulation of physiologic parameters to increase resistance to potential damage from temporary focal ischemia. This is best done before occlusion. The most common strategy is to decrease the metabolic demand typically through pharmacologically induced EEG burst suppression. Mild hypertension, to increase CBF and promote collateral circulation, or hypothermia frequently is induced during temporary occlusion. The role of hypothermia during aneurysm surgery, however, is unclear.11,12 A wide variety of pharmacologic agents that inhibit specific pathways in the ischemic cascade can reduce infarct volume after focal ischemia in animal models. Barbiturates are the most commonly used agent in aneurysm surgery. Etomidate, propofol, isoflurane, mannitol, or the “Sendai cocktail” (mannitol, phenytoin, and dexamethasone) among others also are used.13–15 The strongest predictor of cerebral infarction during temporary occlusion is occlusion time: the risk of infarction increases with the duration of vascular occlusion (see Table 365-5).47,48 The electrical silence that precedes irreversible neuronal damage is the basis for intraoperative EEG and SSEP monitoring (i.e., it provides information about “safe time limits” of temporary artery occlusion during aneurysm surgery).47
Clip Placement
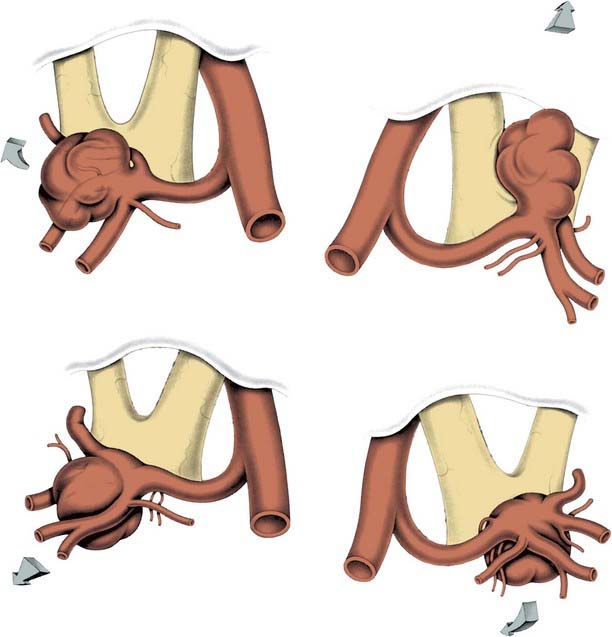
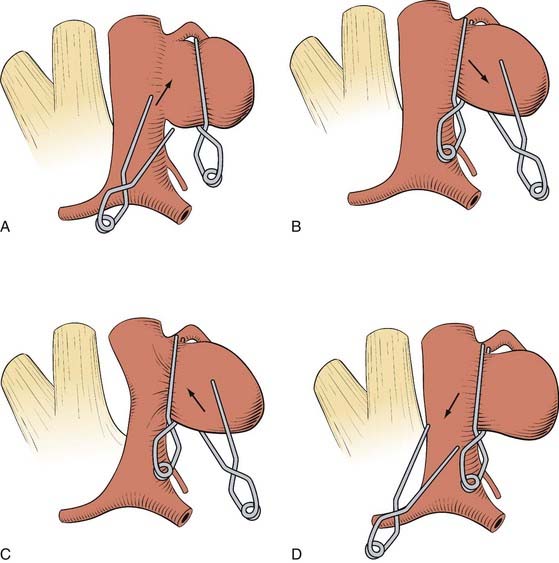
How a clip is applied or what clip is used to occlude the aneurysm is dictated by the particular anatomy of the aneurysm. By studying the preoperative imaging, the surgeon should have a reasonable idea what to expect when the aneurysm is exposed and what clip type and position they would expect to use. Once the afferent and efferent vessels and aneurysm neck have been exposed, a trial pass can be made with a blunt microdissector on each side of the neck in the proposed path of the clip blades to ensure a clip can be placed safely and to free any arachnoid. Irrigation can help prevent the instrument sticking to the vessels. An appropriate permanent clip then is selected and slowly applied while the assistant keeps the aneurysm moist. The goal of clip placement is not to close the aneurysm per se; rather the goal is to reconstruct the vessel wall (Fig. 365-16). For example, if the aneurysm neck is large compared with the overall aneurysm size, the clip may need to be placed parallel to the parent vessel to prevent vessel compromise (Fig. 365-17). The clip blades should be just long enough to close the aneurysm neck; too long a clip may cause inadvertent injury to surrounding neurovascular structures (e.g., the third nerve during PcomA aneurysm occlusion). Most aneurysms can be occluded with a single clip, but for larger aneurysms, a series of clips may need to be stacked, placed in series, or in opposition to reconstruct the vessel (Fig. 365-18). A fenestrated clip can be used to maintain efferent, afferent, or overlying vessel patency if the vessel is in the way of clipping the aneurysm neck.49 Many large aneurysms may have a significant amount of atherosclerosis at their base and make it difficult to place a clip. In this circumstance, a second “booster” clip placed on the first clip may help to increase the closing force. Alternatively, the aneurysm may be opened under temporary occlusion and much like a carotid endarterectomy, the atherosclerosis removed, enabling the clip blades to close. Retrograde suction decompression techniques or direct aneurysm puncture and aspiration may also help aneurysm collapse.21
Intraoperative Rupture
Intraoperative aneurysm rupture can complicate between 5% and 20% of procedures, depending on how it is classified. It is associated with increased morbidity and mortality particularly when there is associated hypotension or induced hypotension to control bleeding.46 Most intraoperative ruptures occur during dissection or clip application but a small number may occur during induction or initial exposure.43 Factors associated with an increased risk of rupture include SAH, lower initial Hunt and Hess grade,44,50 and perhaps blunt dissection or attempted aneurysm occlusion before the aneurysm neck is well defined. Often the consequences of rupture (i.e., size of the hole in the aneurysm) associated with blunt dissection may be worse than sharp dissection.
Giant Aneurysms
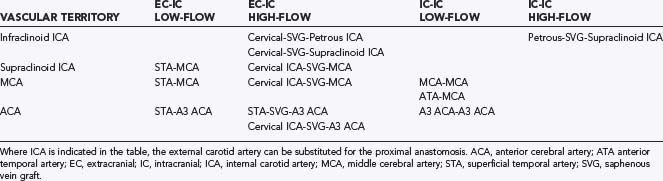
Giant saccular aneurysms and complex fusiform or dolichoectatic aneurysms that lack a clippable neck require alternative techniques that can alter the selection of a surgical approach. These alternative techniques include proximal occlusion of the parent artery (hunterian ligation), aneurysmal trapping, revascularization procedures, aneurysmal excision with reanastomosis, use of hypothermic circulatory arrest, endovascular techniques, or no treatment. Proximal artery occlusion can be considered when blood flow in the collateral arteries is adequate. If the aneurysm is occluded by trapping or excision, a bypass graft may be needed to replace flow. Revascularization procedures are designed to reconstitute blood flow in a major artery before or immediately after surgical occlusion to reduce the risk of ischemic damage. Tables 365-6 and 365-7 summarize revascularization options. The surgical procedures to then “occlude” the aneurysm often are no different from what is required for direct aneurysm occlusion.
Ideal clip application often is hindered when there is atherosclerosis within the aneurysm wall or neck because the atherosclerotic plaque may lead to downward clip migration with resultant partial vessel lumen occlusion or may prevent complete aneurysm neck occlusion. Sequential application and readjustment of aneurysm clips may correct this. If the vessel lumen is compromised, an additional clip can be placed distal to the original clip, which subsequently is removed to restore parent artery patency. This often requires thrombectomy and aneurysmorrhaphy. If complete aneurysm neck obliteration is prevented, a second clip can be applied distal and in parallel to the first, to promote neck occlusion by additional closing pressure. A fenestrated clip also can allow aneurysm neck collapse by encircling the atheroma. These complex aneurysms frequently call for “reconstruction” with multiple clips or tandem clipping in parallel or at right angles (see Figs. 365-16 through 365-18).
Agid R, Lee SK, Willinsky RA, et al. Acute subarachnoid hemorrhage: using 64-slice multidetector CT angiography to “triage” patients’ treatment. Neuroradiology. 2006;48:787-794. Epub 9-29-2006
Baldwin HZ, Spetzler RF, Wascher TM, et al. The far-lateral combined supra- and infratentorial approach: clinical experience. Acta Neurochir (Wien). 1995;134:155-158.
Batjer H, Samson D. Intraoperative aneurysmal rupture: incidence, outcome, and suggestions for surgical management. Neurosurgery. 1986;18:701-707.
Batjer HH, Samson DS. Retrograde suction decompression of giant paraclinoidal aneurysms. Technical note. J Neurosurg. 1990;73:305-306.
Chalif DJ. Surgical treatment of anterior cerebral artery aneurysms. In: Le Roux PD, Winn HR, Newell DW, editors. Management of Cerebral Aneurysms. Philadelphia: Saunders; 2004:763-794.
Day JD, Fukushima T, Giannotta SL. Cranial base approaches to posterior circulation aneurysms. J Neurosurg. 1997;87:544-554.
Dolenc VV. A combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg. 1985;62:667-672.
Elijovich L, Higashida RT, Lawton MT, et al. Predictors and outcomes of intraprocedural rupture in patients treated for ruptured intracranial aneurysms: the CARAT study. Stroke. 2008;39:1501-1506.
Frietsch T, Kirsch JR. Strategies of neuroprotection for intracranial aneurysms. Best Pract Res Clin Anaesthesiol. 2004;18:595-630.
Heros RC. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg. 1986;64:559-562.
Hudgins RJ, Day AL, Quisling RG, et al. Aneurysms of the posterior inferior cerebellar artery. A clinical and anatomical analysis. J Neurosurg. 1983;58:381-387.
Jones TH, Morawetz RB, Crowell RM, et al. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981;54:773-782.
Kawase T, Toya S, Shiobara R, et al. Transpetrosal approach for aneurysms of the lower basilar artery. J Neurosurg. 1985;63:857-861.
Lawton MT, Daspit CP, Spetzler RF. Transpetrosal and combination approaches to skull base lesions. Clin Neurosurg. 1996;43:91-112.
Lee LA, Lam AM. Anesthesia for patients with intracranial aneurysms. In: LeRoux PD, Winn HR, Newell DW, editors. Management of Cerebral Aneurysms. Philadelphia: Saunders; 2004:531-546.
Rhoton ALJr. The three neurovascular complexes in the posterior fossa and vascular compression syndromes (honored guest lecture). Clin Neurosurg. 1994;41:112-149.
Rhoton ALJr. Aneurysms. Neurosurgery. 2002;51(4 suppl):S121-S158.
Samson D, Batjer HH, Bowman G, et al. A clinical study of the parameters and effects of temporary arterial occlusion in the management of intracranial aneurysms. Neurosurgery. 1994;34:22-28.
Sekhar LN, Bucur SD. Cranial base approaches to large and giant aneurysms. Tech Neurosurg. 1998;4:133-152.
Spetzler RF, Daspit CP, Pappas CTE. The combined supra- and infratentorial approach for lesions of the petrous and clival regions: experience with 46 cases. J Neurosurg. 1992;76:588-599.
Spetzler RF. Subtemporal transtentorial approach. J Neurosurg. 2006;104:855-856.
Tedeschi H, Rhoton ALJr. Lateral approaches to the petroclival region. Surg Neurol. 1994;41:180-216.
Thines L, Taschner C, Lejeune JP, et al. Surgical views from three-dimensional digital subtraction angiography for the planning of aneurysm surgery. J Neuroradiol. 2007;34:205-211.
Todd MM, Hindman BJ, Clarke WR, et al. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med. 2005;352:135-145.
Wada K, Arimoto H, Ohkawa H, et al. Usefulness of preoperative three-dimensional computed tomographic angiography with two-dimensional computed tomographic imaging for rupture point detection of middle cerebral artery aneurysms. Neurosurgery. 2008;62:126-132.
Yang I, Lawton MT. Clipping of complex aneurysms with fenestration tubes: application and assessment of three types of clip techniques. Neurosurgery. 2008;62:378-379.
Zabramski JZ, Kiris T, Sankhla S, et al. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998;89:336.
1 Yasargil MG. Microneurosurgery, Vol I. Stuttgart, Germany: Georg Thieme Verlag. 1984.
2 Ferreira MAT, de Oliveira E, Tedeschi H, et al. Microsurgical anatomy of the anterior cerebral circulation. In: Le Roux P, Newell DW, Winn HR, editors. Management of Cerebral Aneurysms. Philadelphia: Elsevier Science; 2004:27-50.
3 Rhoton AL, Mussi ACM, Wen HT. Microsurgical anatomy of the posterior circulation. In: Le Roux P, Newell DW, Winn HR, editors. Management of Cerebral Aneurysms. Philadelphia: Saunders; 2004:51-68.
4 Gibo H, Lenkey C, Rhoton ALJ. Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg. 1981;55:560-574.
5 Rhoton ALJr. The three neurovascular complexes in the posterior fossa and vascular compression syndromes (honored guest lecture). Clin Neurosurg. 1994;41:112-149.
6 Agid R, Lee SK, Willinsky RA, et al. Acute subarachnoid hemorrhage: using 64-slice multidetector CT angiography to “triage” patients’ treatment. Neuroradiology. 2006;48:787-794. Epub 9-29-06
7 Velthuis BK, van Leeuwen MS, Witkamp TD, et al. Computerized tomography angiography in patients with subarachnoid hemorrhage: from aneurysm detection to treatment without conventional angiography. J Neurosurg. 1999;91:761.
8 Thines L, Taschner C, Lejeune JP, et al. Surgical views from three-dimensional digital subtraction angiography for the planning of aneurysm surgery. J Neuroradiol. 2007;34(3):205-211.
9 Lee LA, Lam AM. Anesthesia for patients with intracranial aneurysms. In: LeRoux PD, Winn HR, Newell DW, editors. Management of Cerebral Aneurysms. Philadelphia: Saunders; 2004:531-546.
10 Pasternak JJ, Lanier WL. Neuroanesthesiology update. J Neurosurg Anesthesiol. 2009;21:73-97. (review)
11 Todd MM, Hindman BJ, Clarke WR, et al. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med. 2005;352:135-145.
12 Hindman BJ, Bayman EO, Pfisterer WK, et al. The intraoperative hypothermia for aneurysm surgery trial: no association between intraoperative hypothermia or supplemental protective drug and neurologic outcomes in patients undergoing temporary clipping during cerebral aneurysm surgery: findings from the intraoperative hypothermia for aneurysm surgery trial. Anesthesiology. 2010;112:86-101.
13 Drummond JC. Brain protection during anesthesia: a reader’s guide. Anesthesiology. 1993;79:877-880.
14 Mantz J, Degos V, Laigle C. Recent advances in pharmacologic neuroprotection. Eur J Anaesthesiol. 2010;27:6-10. (review)
15 Frietsch T, Kirsch JR. Strategies of neuroprotection for intracranial aneurysms. Best Pract Res Clin Anaesthesiol. 2004;18:595-630.
16 Andrews RJ, Bringas JR. A review of brain retraction and recommendations for minimizing intraoperative brain injury. Neurosurgery. 1993;33:1052-1063.
17 Samadani U, Huang JH, Baranov D, et al. Intracranial hypotension after intraoperative lumbar cerebrospinal fluid drainage. Neurosurgery. 2003;52:148-151.
18 Wada K, Arimoto H, Ohkawa H, et al. Usefulness of preoperative three-dimensional computed tomographic angiography with two-dimensional computed tomographic imaging for rupture point detection of middle cerebral artery aneurysms. Neurosurgery. 2008;62:126-132.
19 Zabramski JZ, Kiris T, Sankhla S, et al. Orbitozygomatic craniotomy. Technical note. J Neurosurg. 1998;89:336.
20 Dolenc VV. A combined epi- and subdural direct approach to carotid-ophthalmic artery aneurysms. J Neurosurg. 1985;62:667-672.
21 Batjer HH, Samson DS. Retrograde suction decompression of giant paraclinoidal aneurysms. Technical note. J Neurosurg. 1990;73:305-306.
22 Dolenc VV. A combined transorbital-transclinoid and transsylvian approach to carotid-ophthalmic aneurysms without retraction of the brain. Acta Neurochir Suppl. 1999;72:89-97.
23 Chalif DJ. Surgical treatment of anterior cerebral artery aneurysms. In: Le Roux PD, Winn HR, Newell DW, editors. Management of Cerebral Aneurysms. Philadelphia: Saunders; 2004:763-794.
24 Lemole GMJr, Henn JS, Zabramski JM, et al. Modifications to the orbitozygomatic approach. Technical note. J Neurosurg. 2003;99:924-930. (review)
25 Smith RR, Al-Mefty O, Middleton TH. An orbitocranial approach to complex aneurysms of the anterior circulation. Neurosurgery. 1989;24:385-391.
26 Spetzler RF. Subtemporal transtentorial approach. J Neurosurg. 2006;104:855-856.
27 Uttley D, Moore A. Skull base approaches for aneurysm occlusion. In: Le Roux PD, Winn HR, Newell DW, editors. Management of Cerebral Aneurysms. Philadelphia: Saunders; 2004:643-657.
28 Spetzler RF, Hamilton MG, Daspit CP. Petroclival lesions. Clin Neurosurg. 1991;41:62-82.
29 Sekhar LN, Bucur SD. Cranial base approaches to large and giant aneurysms. Tech Neurosurg. 1998;4:133-152.
30 Lawton MT, Daspit CP, Spetzler RF. Transpetrosal and combination approaches to skull base lesions. Clin Neurosurg. 1996;43:91-112.
31 House WF, Shelton C. Middle fossa approach for acoustic tumor removal. Otolaryngol Clin North Am. 1992;25:347-359.
32 Kawase T, Toya S, Shiobara R, et al. Transpetrosal approach for aneurysms of the lower basilar artery. J Neurosurg. 1985;63:857-861.
33 Day JD, Fukushima T, Giannotta SL. Microanatomical study of the extradural middle fossa approach to the petroclival and posterior cavernous sinus region: description of the rhomboid construct. Neurosurgery. 1994;34:1009-1016.
34 Hudgins RJ, Day AL, Quisling RG, et al. Aneurysms of the posterior inferior cerebellar artery. A clinical and anatomical analysis. J Neurosurg. 1983;58:381-387.
35 Heros RC. Lateral suboccipital approach for vertebral and vertebrobasilar artery lesions. J Neurosurg. 1986;64:559-562.
36 Spetzler RF, Grahm TW. The far-lateral approach to the inferior clivus and the upper cervical region: technical note. BNI Q. 1990;6:35-38.
37 Sen CN, Sekhar LN. An extreme lateral approach to the intradural lesions of the cervical spine and foramen magnum. Neurosurgery. 1990;27:197-204.
38 Day JD, Fukushima T, Giannotta SL. Cranial base approaches to posterior circulation aneurysms. J Neurosurg. 1997;87:544-554.
39 Tedeschi H, Rhoton ALJr. Lateral approaches to the petroclival region. Surg Neurol. 1994;41:180-216.
40 Baldwin HZ, Spetzler RF, Wascher TM, et al. The far-lateral combined supra- and infratentorial approach: clinical experience. Acta Neurochir (Wien). 1995;134:155-158.
41 Spetzler RF, Daspit CP, Pappas CTE. The combined supra- and infratentorial approach for lesions of the petrous and clival regions: experience with 46 cases. J Neurosurg. 1992;76:588-599.
42 Rhoton ALJr. Aneurysms. Neurosurgery. 2002;51(4 suppl):S121-S158.
43 Batjer H, Samson D. Intraoperative aneurysmal rupture: incidence, outcome, and suggestions for surgical management. Neurosurgery. 1986;18:701-707.
44 Lawton MT, Du R. Effect of the neurosurgeon’s surgical experience on outcomes from intraoperative aneurysmal rupture. Neurosurgery. 2005;57:9-15.
45 McDermott MW, Durity FA, Borozny M, et al. Temporary vessel occlusion and barbiturate protection in cerebral aneurysm surgery. Neurosurgery. 1989;25:54-61.
46 Giannotta SL, Oppenheimer JH, Levy ML, et al. Management of intraoperative rupture of aneurysm without hypotension. Neurosurgery. 1991;28:531-536.
47 Jones TH, Morawetz RB, Crowell RM, et al. Thresholds of focal cerebral ischemia in awake monkeys. J Neurosurg. 1981;54:773-782.
48 Samson D, Batjer HH, Bowman G, et al. A clinical study of the parameters and effects of temporary arterial occlusion in the management of intracranial aneurysms. Neurosurgery. 1994;34:22-28.
49 Yang I, Lawton MT. Clipping of complex aneurysms with fenestration tubes: application and assessment of three types of clip techniques. Neurosurgery. 2008;62:378-379.
50 Elijovich L, Higashida RT, Lawton MT, et al. Predictors and outcomes of intraprocedural rupture in patients treated for ruptured intracranial aneurysms: the CARAT study. Stroke. 2008;39:1501-1506.