CHAPTER 389 Surgical and Radiosurgical Management of Giant Arteriovenous Malformations
Successful treatment of arteriovenous malformations (AVMs) remains one of the more challenging problems faced by neurosurgeons. Giant AVMs represent a rare, but excessively difficult group of AVMs that are often associated with higher treatment morbidity and mortality than their smaller counterparts. The standard definition of a giant AVM is a high-flow, angiographically visible vascular malformation that is greater than 6 cm in maximum diameter. The size alone results in a Spetzler-Martin grade of at least III, and most of these giant AVMs are classified as Spetzler-Martin grade IV or V.1 The size of these giant AVMs almost invariably results in at least a portion of the malformations being located within or immediately adjacent to eloquent regions of the brain, and these lesions often have both deep and superficial venous drainage. Giant AVMs frequently have an arterial supply from multiple vascular distributions, from the anterior as well as the posterior circulation, and in many cases the arterial supply is bilateral.
Clinical Findings/Preoperative Evaluation
Symptoms
The size of giant AVMs and the large amount of arteriovenous shunting within these lesions often result in symptoms unique from those with smaller AVMs. Small vascular malformations are typically manifested as headaches,2,3 seizures,4–6 or hemorrhage.5–9 Giant AVMs can have any of these symptoms but can also cause transient and progressive neurological dysfunction through cerebrovascular “steal.” The large blood volume shunting through these malformations can result in relative hypoperfusion in the surrounding neurological tissue with subsequent ischemia.10–13 This is due to lower blood pressure within the arterial feeders of the AVM than in the surrounding brain, which results in preferential shunting of blood to the AVM away from normal brain tissue.
AVMs have initial hemorrhage rates of 2% to 4% and annual rebleeding rates of approximately 4% to 18%.7,14–17 Mortality from each hemorrhage approximates 10% to 15%,5,8 and up to 50% of patients suffer some form of neurological deficit as a result of bleeding from an AVM.9 Some authors believe that larger AVMs have bleeding rates slightly lower than their smaller counterparts.7,18,19 Other studies have shown no consistent relationship between size and risk for hemorrhage,14,20 and feeding artery pressure (believed to be a risk factor for AVM hemorrhage) did not correlate with AVM size.21 However, giant AVMs with components in the basal ganglia, thalamus, and pineal region may be associated with not only a higher hemorrhage rate22 but also increased morbidity if they bleed, with more than 60% of these patients suffering significant morbidity or death from hemorrhage.23 Angiographic factors that have been shown to correlate with risk for AVM hemorrhage are central venous drainage, periventricular location, and intranidal aneurysms,24,25 as well as a single draining vein25 and stenotic venous drainage.26 By their size alone, giant AVMs are more likely to have a component of central venous drainage and a portion of the AVM adjacent to or within the ventricle.
Indications for and Contraindications to Surgery
Indications for resection of giant AVMs must consider both the natural history of the AVM and the combined risks associated with multimodality treatment for a particular patient.27,28 Most angiographically documented giant AVMs are candidates for treatment, particularly if they have hemorrhaged or are causing significant progressive neurological deficits, disabling headaches, or medically intractable seizures. The risk with surgical resection is generally related to AVM size and location and the complexity of arterial feeders and venous drainage,1 factors that make these giant AVMs a higher risk than smaller AVMs to treat with surgery alone. Larger lesions may require staged surgical resection or multimodality therapy combining microsurgery, embolization, and stereotactic radiosurgery.27–30 Other factors affecting treatment risk include the patient’s clinical condition and age and surgeon experience.
The location of the giant AVM under consideration significantly affects the risk associated with surgical resection.27–32 Because of their size, these larger AVMs often involve eloquent cortex, which significantly increases the risk of resection. Resection of giant cortical AVMs may cause motor or sensory deficits (frontal/parietal) or visual field defects (occipital), memory deficits, and changes in cognition and personality (cingulate gyrus and corpus callosum). Treatment of deep portions of giant AVMs within the basal ganglia and thalamus can result in hemiplegia, sensory deficits, dysphasia, and cognitive and memory deficits.23,33,34 The clinical condition of the patient also has a bearing on surgical resection, with poor-grade patients usually resulting in a poor outcome.29 Younger patients have a better benefit-to-risk ratio for the treatment of giant AVMs for two reasons. First, the brain of a young patient can better tolerate resection of the vascular malformation and has improved recovery after surgery.35 Second, a younger patient translates into longer AVM-free survival on completion of resection.35 Likewise, patients with a significant mass effect from a hemorrhagic clot resulting from AVM rupture should undergo emergency evacuation of the hemorrhage, with resection of the AVM being reserved for a later date. The timing of resection of giant AVMs is similar to that for other AVMs in that resection should be planned under nonemergency conditions to minimize patient morbidity. Resection of the AVM 4 to 6 weeks after hemorrhage, when the blood is liquefied, may improve the ease of resection.8,23,30,36 In many cases, the hematoma cavity has performed a portion of the dissection around the nidus of the AVM. Furthermore, this short interval allows the patient to achieve stabilization or improvement of any neurological deficits.
Failure of stereotactic radiosurgery and embolization is another indication for surgical resection,27,30,32 although in the case of giant AVMs, these treatment modalities are generally used as adjuncts in reducing AVM size before planned microsurgical resection. Most studies indicate that partial obliteration after radiosurgery does not confer protection from AVM rehemorrhage,23,27,29,33,37,38 and hence an alternative therapy should be sought for residual AVMs seen more than 3 years after radiosurgical treatment. Embolization is a useful treatment of giant cortical AVMs,39 but it is less useful in the treatment of deeper arterial feeders (thalamoperforating, choroidal, and lenticulostriate arteries) within the basal ganglia and thalamus. These vessels primarily arise from the parent vessels at right angles, have small lumens, and are thus difficult to canulate.23,33,34 Nonetheless, in selected cases, embolization has been extremely helpful in partially obliterating even basal ganglia and thalamic arterial feeders.29,30,40
Hemodynamics Of Giant Arteriovenous Malformations
All AVMs serve as a shunt between the arterial and venous systems. Pressure within the arterial feeders of AVMs has been shown to be lower than the pressure in normal brain arteries, whereas pressure within the venous channels draining the AVM has been shown to be higher than normal venous pressure. Furthermore, as a result of this low pressure on the arterial side, blood that would normally supply the cortex adjacent to the AVM may preferentially flow to the AVM. This cerebrovascular steal, which may be more pronounced with giant AVMs, is hypothesized to cause ischemic symptoms within the cortex adjacent to the AVM. The arteries within the adjacent cortex respond to this steal phenomenon by dilating their lumen to maintain flow through autoregulation. The extended interval in which these cortical arteries remain dilated results in the loss of their rapid ability to constrict should the blood pressure within these cortical arteries change. When these vessels are subjected to a higher pressure head, as when the low-resistance high-flow giant AVM is removed, they may not have adequate autoregulatory compensation, and edema and hemorrhage in the surrounding brain parenchyma can result. Angiographic characteristics found to be correlated with cerebrovascular steal are angiomatous change, AVM size, and pattern of peripheral venous drainage.41
The process of impaired autoregulation and hemorrhage from cortical vessels adjacent to an AVM after AVM resection is called normal perfusion pressure breakthrough.42 Although this phenomenon is rare, it is believed to occur more frequently in giant AVMs because these malformations shunt a higher volume of blood and thus create more vascular steal from the surrounding cortex.27 AVM features associated with an increased likelihood of normal perfusion pressure breakthrough (other than AVM size) include dilated feeding arteries, poor filling of the surrounding cerebral vasculature on angiograms, clinical symptoms of ischemia from cerebral vascular steal, and physiologic evidence of impaired autoregulation in the brain.31,43 Normal perfusion pressure breakthrough–associated bleeding can be limited by careful control of blood pressure in the immediate postoperative period, staging of resection of the giant AVM, and the judicious use of presurgical embolization or radiosurgery, or both, in an attempt to reduce the size of the arteriovenous shunt.
Evaluation
Angiography
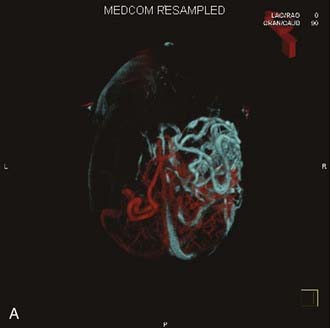
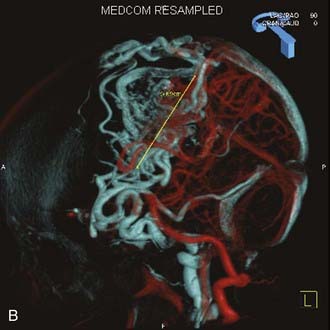
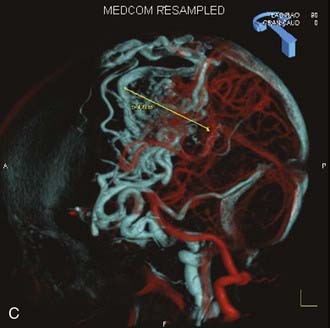
Angiography remains the “gold standard” with respect to evaluation of AVMs. Because of the multiplicity of arterial feeders to these giant AVMs, it is important that a four-vessel angiogram be performed to document all of the arterial supply. Giant AVMs may have a portion of their blood supply from extracranial sources, so both external carotid arteries should be evaluated.44 As with smaller AVMs, multiple views should be obtained to ensure that the neurosurgeon has a complete understanding of the origin of all the arterial feeders, the size and configuration of the AVM, and the number and direction of veins draining the AVM. A complete initial angiogram also allows better comparison with subsequent angiograms obtained during and after treatment. Angiography can also potentially document patients with a substantial amount of cerebrovascular steal as a result of the giant AVM. In these cases, normal cortical vessels may show more limited flow than other vessels at a distance from the AVM. In addition to standard two-dimensional angiography, the more recent development of three-dimensional angiography has improved the ability to identify the true size of the AVM (Fig. 389-1).45
Post-treatment angiograms should be performed to ensure complete obliteration of the AVM, either soon after microsurgical resection of the final portion of the giant AVM or within several years after stereotactic radiosurgery if this modality is used as the final treatment within a staged approach to a giant AVM. It is critical to confirm complete treatment of an AVM, particularly giant AVMs, because leaving a residual AVM component is more problematic and reports suggest that partial treatment does not protect against future hemorrhage.37,38,46–49
Angiography also plays an important role in the treatment of AVMs with stereotactic radiosurgery. Current radiosurgical systems rely on a fusion of angiography and magnetic resonance imaging (MRI) or computed tomography (CT) as a basis for planning treatment. In older radiosurgical systems, angiography is performed with a stereotactic base ring (or bite block) attached to the patient’s head and a fiducial cage mounted to the base ring. The fusion of angiography and MRI/CT allows improved targeting of the nidus while avoiding extraneous radiation doses to the draining veins or the surrounding cortical tissue. Newer image-guided radiosurgery is performed without a stereotactic ring, and in these cases, three-dimensional angiographic images can be directly fused to stereotactic CT and MRI during the treatment-planning process.45
Magnetic Resonance Imaging
Although angiography remain the primary modality for evaluation of giant AVMs, pretreatment MRI provides additional information. MRI shows the relationship of the giant AVM to other intracranial structures, thereby affording the neurosurgeon a better understanding of the anatomy surrounding the AVM. The size and direction of large draining veins can often be noted, as can evidence of previous hemorrhage. MRI is also critical during the planning of stereotactic radiosurgery in conjunction with cerebral angiography. In select cases of giant AVMs with irregular shapes or deep components, intraoperative navigation with MRI-based guidance can be extremely useful. More recent developments in MRI technology have allowed the incorporation of three-dimensional imaging to assess both the AVM nidus itself50–53 and white matter tracts adjacent to the AVM.54–56 Functional MRI has also been used to elucidate the relationship between an AVM and eloquent areas of the brain.57
Xenon Computed Tomography
Xenon CT is rarely used in the evaluation of smaller AVMs, but for larger AVMs with significant surrounding cortical ischemia as a result of steal, this modality may be useful in determining the degree and distribution of hypoperfusion.58 Impaired augmentation indicates failure of autoregulation and may identify patients at high risk for normal perfusion pressure breakthrough bleeding.
Treatment
Because of the relative infrequency of giant AVMs, which are thought to account for 10% of all AVMs,14 and the complexity of arterial feeders and venous drainage, treatment is individualized for each patient. It is rare for these giant AVMs to be treated successfully at a single sitting with one modality, and the best patient outcomes often involve staged treatment with two or more of the treatment modalities available.
Embolization
Because giant AVMs are difficult to treat with microsurgery alone and are too large to be optimal targets for stereotactic radiosurgery, embolization is generally the first course of treatment. Historically, this process consists of using either small polyvinyl alcohol particles31 or cyanoacrylate glue59–62 in an attempt to occlude the feeding arteries. Over the past decade, n-butyl-2-cyanoacrylate (NBCA) has emerged as the main embolic agent of choice because its soft nature after occluding portions of the nidus does not interfere with later surgical resection of the AVM.61 Recently, ethylene vinyl alcohol copolymer (Onyx, Micro Therapnetics, Irvine, CA) has been studied as an embolic agent, with several clinical series documenting the efficacy of this agent.63–67 The primary goal of embolization is to reduce volume and flow in the AVM to facilitate safer and more effective subsequent treatment with microsurgery or radiosurgery (or both). Embolization of deep feeders to the AVM or deep AVM components may help in later surgical resection. Occasionally, embolization alone is used for palliation of symptoms from giant AVMs with no intent of obliterating the entire lesion. Embolization is usually performed in several stages, often three or more, for giant AVMs.44 Staged embolization allows the AVMs to adjust to changes in flow hemodynamics after each embolic treatment; attempts to embolize large portions of a giant AVM in a single stage are frequently associated with increased risk for hemorrhage. Giant AVMs can have a significant external carotid component, and embolization of these vessels often makes the craniotomy opening easier for the surgeon, as well as reduces blood supply to the AVM. Many giant AVMs have arterial supplies from both the anterior and posterior circulation and frequently from both hemispheres, and therefore multiple vessels need to be cannulated in an attempt to achieve optimal embolization. Care must be taken to avoid passing embolic glue or particles into normal cortical vessels to avoid the development of infarcts.
During embolization, patients with giant AVMs are monitored with electrophysiologic potentials, which provides several benefits.39,40 First, such monitoring is particularly useful in identifying when normal cortical vessels are inadvertently occluded. Second, electrophysiologic monitoring during embolization can also provide some insight to the neurosurgeon regarding whether the patient’s brain can tolerate periods of relative hypotension (which may be used later during surgery to reduce bleeding). This is particularly relevant when treating an AVM with “steal” or ischemic symptoms. Furthermore, when a particular vessel is in question, amobarbital sodium (Amytal) can be injected into the vessel before embolization to determine whether the vessel is supplying an area of eloquent cortex and hence would cause a neurological deficit if occluded.68
Some institutions use intraoperative embolization extensively in treating giant AVMs.31 The advantages of this technique are that the risk of reflux into normal vessels is decreased and the surgeon has the opportunity to inject numerous distal vessels inaccessible to the neurointerventionalist in the angiography suite. Intraoperative embolization proceeds once the surgeon has performed the craniotomy and exposed the AVM. Injection of the embolic agent should be performed as close to the AVM nidus as possible to minimize reflux into normal vessels. After temporary occlusion of the proximal portion of the vessel, 27-gauge needles can be introduced and several cubic centimeters of NBCA injected. This process can be repeated for various feeding arteries until flow to the AVM is significantly reduced. After embolization, each feeding artery can be sacrificed with either a small clip or bipolar coagulation.
Stereotactic Radiosurgery
Stereotactic radiosurgery has established itself as successful treatment of certain intracranial AVMs, particularly small or moderate-sized AVMs located in critical brain regions. A number of clinical series using either heavy charged particles (protons and helium ions) or photons (Gamma Knife or linear accelerator) have demonstrated that AVMs less than 3 cm in diameter treated with 20 to 25 Gy equivalents (GyE) have a 3-year obliteration rate of 80% to 95% with a low complication rate (2.5% to 4.5% permanent neurological deficits, 2.5% to 4.5% transient deficits).29,37,38,69–77 However, various limitations of stereotactic focused radiosurgery have become apparent after analyzing the results in treating AVMs larger than 3 cm. These AVMs have a much lower obliteration rate, even after 3 years (33% to 58%), and a higher complication rate (20% to 30%) with treatment doses of 15 to 20 Gy.29,38,70,73,75–79
The primary mechanism of AVM obliteration after radiosurgery involves hyperplasia of the vascular intima80 with progressive narrowing and ultimately occlusion. Such obliteration typically takes place over a 2- to 3-year period. There are three considerations when weighing surgery versus radiosurgery for the treatment of AVMs. First, AVMs larger than 4 cm in diameter have only a 33% to 50% rate of obliteration at 3 years after radiosurgery but a 20% to 30% complication rate with treatment doses of 15 to 20 Gy.38,77 The rate of obliteration is increased for larger AVMs when higher doses are used (25 to 45 Gy); however, the risk for radiation-induced complications increases significantly. Second, the risk for intracranial hemorrhage persists during the interval between treatment and complete obliteration.38,75,77 Third, serial radiographic studies, including cerebral angiography, are necessary to confirm complete obliteration.
Despite these limitations, radiosurgery may be associated with less morbidity than surgical resection in patients with portions of giant AVMs in eloquent brain.28,30 Radiosurgery can be used as a preoperative adjunct to obliterate portions of giant AVMs and thus decrease the size of the remaining nidus for later surgical resection.29 Some authors have recommended staged stereotactic radiosurgery when treating giant AVMs.81 In these cases, multiple radiosurgical treatments are delivered to different portions of the AVM at various intervals to avoid delivering treatment to a single large target. This theoretically reduces the risk for radiation necrosis.
Some patients with giant AVMs require more than one course of stereotactic radiosurgery, and this option has been used in selected patients.29,82 The disadvantages of such an approach are the second latency period of 1 to 3 years before obliteration occurs, the possibility that a second radiosurgical treatment may still not obliterate the AVM, and the risk for radiation-induced injury, which may be higher with a second radiosurgical treatment.
Microsurgery
Another indication for surgical treatment of giant AVMs would involve resection of radiation necrosis. Radiation injury after stereotactic radiosurgery may result in significant mass effect and edema and require prolonged treatment with high doses of corticosteroids. We have observed several patients with steroid dependence as a result of radiation injury who were successfully tapered off corticosteroids after resecting areas of necrosis, as well as some patients who have shown substantial neurological improvement.83
Multimodality Treatment
The complexity of giant AVMs often requires combinations of embolization, stereotactic radiosurgery, and microsurgery to achieve a complete cure. Our experience with large and complex AVMs has shown that such multimodality therapy can reduce patient morbidity and mortality.27,29,30,84 Embolization obviously reduces the volume of nidus requiring resection, but stereotactic radiosurgery delivered several years before surgical resection also produces a benefit. In some patients, partial AVM thrombosis significantly reduced the volume of residual AVM that required surgical resection. More importantly, at surgery the irradiated (but patent) AVMs were found to be much less vascular, even in nonembolized areas, than AVMs not previously irradiated. Radiosurgically treated AVM vessels were easier to occlude with bipolar coagulation, thus facilitating quicker and safer resection with less blood loss.29 Although preoperative angiograms often demonstrate significant residual AVM after radiosurgery, observations at surgery suggest that the prior radiosurgery has obliterated the small-vessel component of the AVM not visible on the angiogram.80 Our success in completely obliterating several previously irradiated AVMs with embolization alone also suggests that prior radiosurgery may thrombose a small-vessel component and leave larger arteriovenous fistulous portions of the AVM.85,86
Special Perioperative Equipment/Techniques
Intraoperative Monitoring
Electrophysiologic monitoring is an extremely valuable aid during procedures for midline or deep AVM resection and improves clinical results.32,87 Monitoring routinely consists of bilateral somatosensory evoked potentials and motor evoked potentials. Continuous monitoring of these sensory and motor pathways has allowed early detection of excessive retraction, manipulation of critical structures, excessive hypotension, or sacrifice of critical nonfeeding arteries, all of which are more likely to occur during resection of giant AVMs than during resection of smaller malformations. In addition, we have found brainstem auditory evoked potentials useful during the resection of posterior fossa AVMs.87 Electrophysiologic monitoring has also proved useful in assisting in determination of the extent of AVM resection when staged procedures are planned. Frequently, changes in electrophysiologic monitoring result in cessation of further AVM resection. When these changes are detected early and surgery halted, potentials usually return to baseline and clinical worsening does not generally occur. Because giant AVMs are often located adjacent to motor and sensory cortex, intraoperative mapping has also proved useful in assessing the resectability of these lesions. Burchiel and colleagues used corticography and stimulation mapping to evaluate motor, sensory, and language cortex, with excellent results.88
Mild Hypothermia
Mild brain hypothermia can be used for cerebral protection. Core body and brain temperature is decreased to 33°C by applying a cooling blanket or using an intravascular catheter cooling system (Innercool Therapies, San Diego, CA).89 This degree of mild brain hypothermia has been shown to provide excellent protection against experimental ischemic and traumatic cerebral injury,90 and we have used it at Stanford for more than 2600 intracranial procedures with good results. Under the operative conditions of AVM resection, this hypothermic technique is safe, feasible, and economical with good clinical outcomes overall.
Intraoperative Angiography
Intraoperative angiograms have been possible with the advent of high-resolution portable angiography and allow the surgeon to determine the completeness of AVM resection.91 If residual AVM is present, the surgeon has an opportunity to complete the resection before closure of the wound. At least two studies have shown that patients are not protected with partial resection of AVMs,4,92 so complete resection remains the goal for all AVMs. In two other studies, 10% to 18% of surgical procedures for resection of AVMs were altered by the results of intraoperative angiography.93,94 We routinely use intraoperative angiography for resection of most giant AVMs to document the completeness of resection and sometimes to assess the extent of residual AVM for deliberate staged surgery.
Intraoperative Doppler Blood Flow Measurements and Ultrasound
Intraoperative ultrasound for measuring blood flow can have several uses. First, ultrasound can determine whether flow through veins is arterialized. Such knowledge allows the surgeon to establish whether the nidus still has a significant arterial source and thus whether the vein in question can safely be transected. Second, directional and quantitative ultrasound can help distinguish feeding arteries from arterialized draining veins, as well as establish flow rates through individual feeding arteries. Nornes and Grip were able to estimate total AVM flow in 9 of 16 patients (range, 150 to >900 mL/min; average, 490 mL/min).12 They were also able to make pressure recordings from feeding arteries at their entrance to the AVM and showed that this pressure was well below systemic arterial blood pressure in all cases (ranging from 40 to 77 mm Hg; average, 56 mm Hg). For deep AVMs, ultrasound can be used to locate the malformation, which can facilitate the planning of cortical incisions, identification of the vessels involved, and determination of the surgical approach.95
Surgical Outcome
There are few published series exclusively consisting of giant AVMs because most authors have included these vascular malformations in larger series of general vascular malformations. Anson and Spetzler reported a series of 32 giant AVMs (all were Spetzler-Martin grade V lesions).31 After combined therapy, 15 patients were clinically improved, 7 were unchanged, and 10 had worsening deficits, although 8 of these 10 deficits were either transient or mild.31 Seven patients required two surgical stages and 4 patients required three surgical stages to complete the AVM resection. Three of the 4 patients undergoing three operations also required presurgical embolization (three courses each) to reduce AVM size.
Heros and coauthors reported 21 patients with giant AVMs in a series of 153 AVM patients.96 Early good or excellent results were achieved in 61% of grade IV patients and 29% of grade V patients. Patients improved with longer follow-up; a 12% morbidity and mortality rate for grade IV giant AVMs and a 38% rate for grade V malformations were demonstrated.
Fifteen patients with AVMs larger than 5 cm were reported in a series of 90 AVM patients by Hernesniemi and Keranen.97 Of these patients, 5 had excellent outcomes, 6 had moderate disabilities, 1 had a severe disability, 1 died, and 2 were lost to follow-up. These authors recommended staged resection and several days of induced systemic hypotension to prevent breakthrough bleeding.
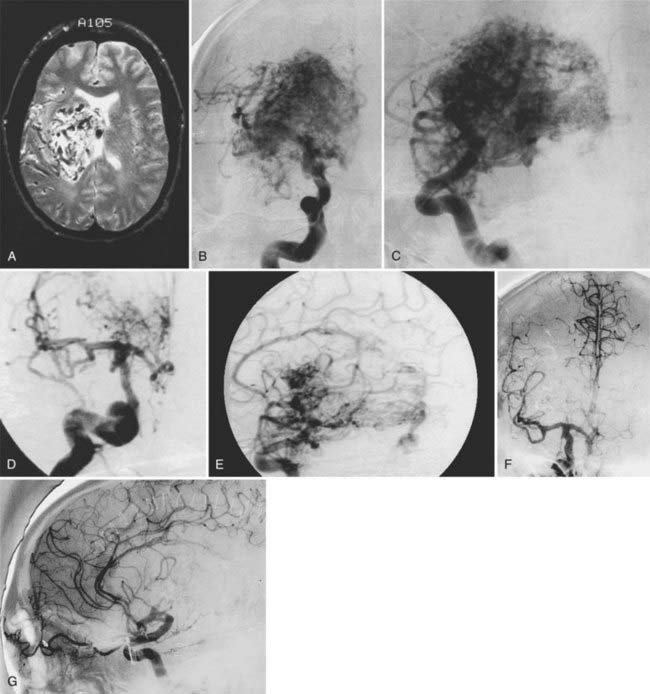
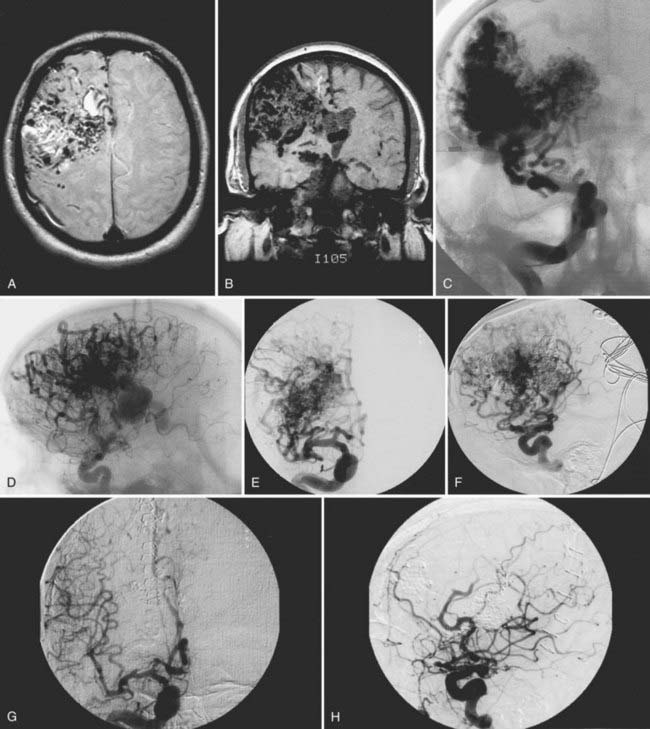
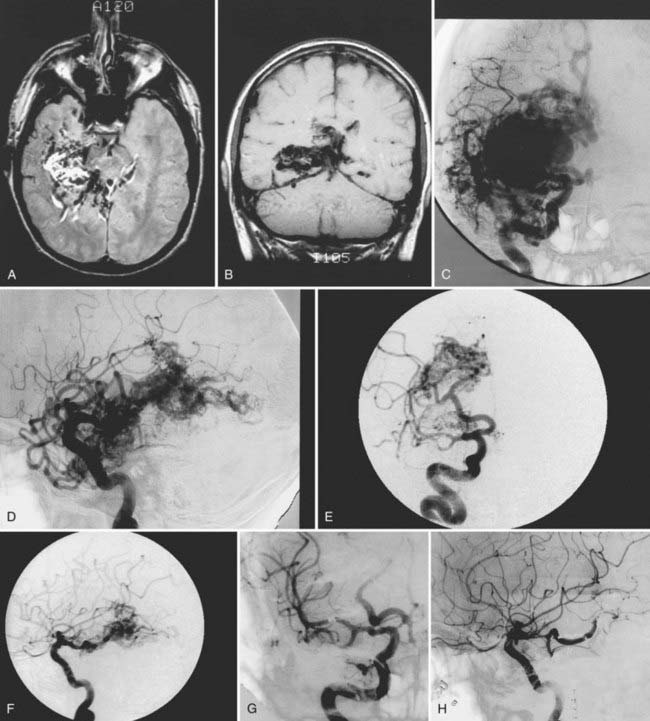
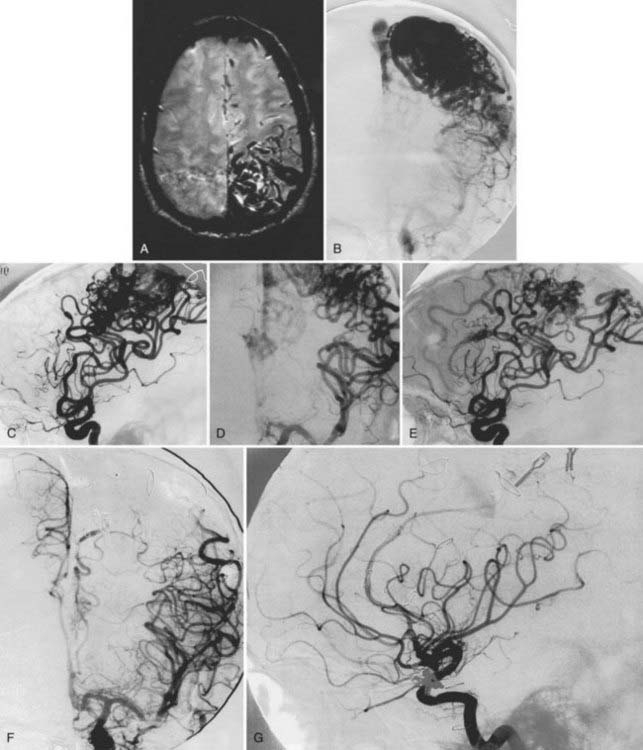
We have treated 53 patients (20 males and 33 females) at Stanford with giant AVMs larger than 6 cm.27 Twenty of these patients (38%) were seen initially with hemorrhage, 8 (15%) with headache, 18 (34%) with seizures, and 7 (13%) with progressive neurological deficits. One patient had a Spetzler-Martin grade III AVM, 9 had Spetzler-Martin grade IV AVMs, and 43 had Spetzler-Martin grade V AVMs. The mean AVM size was 6.8 cm (range, 6 to 15 cm). AVM venous drainage was superficial (n = 7), deep (n = 20), or both (n = 26). At initial evaluation, 31 patients (58%) were graded as being in excellent neurological condition, 17 were graded good (32%), and 5 were graded poor (9%). Treatment included surgery (n = 27; 51%), embolization (n = 52; 98%), or radiosurgery alone or combined with surgery or embolization (n = 47; 89%). Most patients received multimodality treatment consisting of embolization followed by surgery (n = 5), embolization followed by radiosurgery (n = 23), or embolization, radiosurgery, and surgery (n = 23) (Figs. 389-2 to 389-5). Nineteen patients (36%) were completely cured of their giant AVMs, 90% obliteration was achieved in 4 patients (8%), less than 90% obliteration was achieved in 29 patients (55%) who had residual AVMs even after multimodality therapy, and 1 patient was lost to follow-up. Of the 33 patients who either completed treatment or were alive more than 3 years after undergoing their most recent radiosurgery, 19 (58%) were cured of their AVMs. The long-term treatment-related morbidity rate was 15%. The clinical results after a mean follow-up of 37 months were 27 excellent (51%), 15 good (28%), 3 poor (6%), and 8 dead (15%).
Complications
Hemorrhage
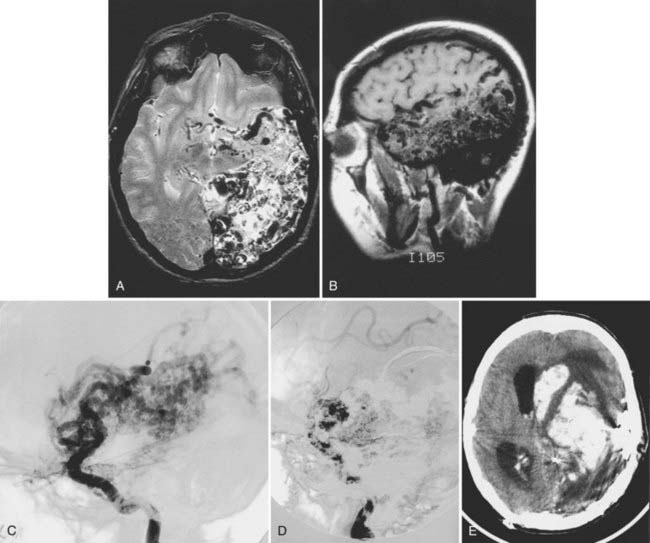
The most devastating complication is postoperative hemorrhage (Fig. 389-6), which fortunately is extremely rare. It can be due to either inadequate hemostasis, failure to completely resect the AVM, or normal perfusion pressure breakthrough. Postoperative hemorrhage can be minimized by careful inspection and hemostasis of the resection bed after removal of the AVM and by a short period of induced hypertension after resection but before closure to check for breakthrough bleeding. Intraoperative angiography and image-guided navigational systems can also aid in confirming complete AVM resection. After surgery, hypotension is maintained in the intensive care unit for 1 to 2 days to permit adjustment of the cerebral vasculature to the new hemodynamics. Normal perfusion pressure breakthrough bleeding can be minimized by staging surgical procedures to allow a gradual change in cortical vasculature flow dynamics after each procedure, followed by several days of deliberate hypotension after final resection of the AVM. Hemorrhage can also occur after embolization of AVMs and occasionally result in the need for emergency craniotomy to remove blood clots.98 This risk is reduced by staging embolization procedures and maintaining a 12-hour period of relative hypotension after the procedure.
Radiosurgical Complications
Adverse sequelae from radiation are broadly classified according to their time of appearance with respect to treatment.99,100 Complications from radiosurgery can be similarly categorized. Acute reactions are defined as occurring during, immediately after, or within the first several days of therapy and primarily consist of nausea and emesis. These symptoms occur in 10% to 16% of patients within 6 hours of treatment.101 Preventive measures include pretreatment with antiemetics and steroids.101 Late reactions occur more than 3 months after therapy and are usually associated with some degree of permanent neurological injury. Because of the large tissue volume of giant AVMs treated by stereotactic radiosurgery, the potential for adverse sequelae is greater than for smaller targets.
Although seizures can occur after stereotactic radiosurgery, they are also associated with the underlying pathology in many patients. Consequently, it is not always clear whether a seizure is directly related to treatment. Kjellberg and coworkers reported a small increase in acute seizure risk in AVM patients treated with proton radiosurgery.47 Similarly, it has been our experience at Stanford that there is a slightly increased risk for seizures within the first 48 hours after radiosurgery, especially when treating large AVMs in eloquent cortex. Post-treatment seizures often correlate with subtherapeutic levels of anticonvulsants in patients who are receiving these agents. We regularly augment standard anticonvulsant doses on the day of radiosurgery to achieve a “high therapeutic” level in patients at risk for post-treatment seizures and have found that this practice minimizes these adverse events during the immediate post-treatment period.
Symptomatic radiation necrosis and edema remain the most debilitating of the late complications caused by stereotactic radiosurgery. Histologic changes in the affected brain consist of neuronal death, gliosis, and both endothelial proliferation and hyalinization.80,102 The reported incidence of radiation necrosis varies between 2.3% and 20%,38,103–105 and it generally occurs 6 to 9 months after treatment, with larger AVM volumes likely to result in increased risk for injury. Current treatment of the edema that accompanies radiation necrosis is corticosteroids. The time course of symptoms and the need for steroids are typically quite protracted, with up to 1 year or longer needed before resolution. In severe cases of necrosis, surgical resection can ameliorate the mass effect and significantly improve the clinical status of some patients.
Ischemia
Embolization and microsurgical resection of AVMs can result in the inadvertent sacrifice of arteries that supply normal cortex. If these arteries are small, it is possible that no obvious neurological deficit will occur. However, when larger vessels are obliterated, either transient or permanent neurological deficits may develop as a result of ischemia. Recent series of patients undergoing embolization have shown an approximately 10% incidence of transient neurological events39 and up to a 6% incidence of permanent neurological disorders.40 In some instances, retrograde thrombosis of feeding arteries may be the cause of the neurological deficits, with one report indicating that neurological deficits occurred in three of five patients in whom retrograde thrombosis was noted on angiography.106
Chang SD, Marcellus ML, Marks MP, et al. Multimodality treatment of giant intracranial arteriovenous malformations. Neurosurgery. 2003;53:1-11.
Fleetwood IG, Marcellus ML, Levy RP, et al. Deep arteriovenous malformations of the basal ganglia and thalamus: natural history. J Neurosurg. 2003;98:747-750.
Spetzler RF, Martin NA, Carter LP, et al. Surgical management of large AVMs by staged embolization and operative excision. J Neurosurg. 1987;67:17-28.
Steinberg GK, Chang SD, Levy RP, et al. Surgical resection of large incompletely treated intracranial arteriovenous malformations following stereotactic radiosurgery. J Neurosurg. 1996;84:920-928.
Steinberg GK, Levy RP, Marks MP, et al. Vascular malformations: Charged-particle radiosurgery. In: Alexander E, Loeffler JS, Lunsford L, editors. Stereotactic Radiosurgery. New York: McGraw-Hill; 1993:122-135.
1 Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986;65:476-483.
2 Martin NA, Vinters HV. Arteriovenous malformations. In: Carter LP, Spetzler RF, Hamilton MG, editors. Neurovascular Surgery. New York: McGraw-Hill; 1995:875-903.
3 Troost BT, Newton TH. Occipital lobe arteriovenous malformations. Clinical and radiologic features in 26 cases with comment on differentiation and migraine. Arch Ophthalmol. 1956;93:250-254.
4 Drake CG. Cerebral arteriovenous malformations: considerations for and experience with surgical treatment in 166 cases. Clin Neurosurg. 1979;26:145-208.
5 Luessenhop AJ. Natural history of cerebral arteriovenous malformations. In: Wilson CB, Stein BM, editors. Intracranial Arteriovenous Malformations. Baltimore: Williams & Wilkins; 1984:12-23.
6 Mohr J. Neurological manifestations and factors related to therapeutic decisions. In: Wilson CB, Stein BM, editors. Intracranial Arteriovenous Malformations. Baltimore: Williams & Wilkins, 1984.
7 Graf CJ, Perret GE, Torner JC. Bleeding from cerebral arteriovenous malformations as part of their natural history. J Neurosurg. 1983;58:331-337.
8 Perrit G, Nishioka H. Report on the Cooperative Study of Intracranial Aneurysms and Subarachnoid Hemorrhage. Section VI. Arteriovenous malformations. An analysis of 545 cases of cranio-cerebral arteriovenous malformations and fistulae reported to the cooperative study. J Neurosurg. 1966;25:467-490.
9 Wilkins RH. Natural history of intracranial vascular malformations: a review. Neurosurgery. 1985;16:421-430.
10 Hachinski V, Norris JW, Cooper PW, et al. Symptomatic intracranial steal. Arch Neurol. 1977;34:149-153.
11 Kusske JA, Kelly WA. Embolization and reduction of the “steal” syndrome in cerebral arteriovenous malformations. J Neurosurg. 1974;40:313-321.
12 Nornes H, Grip A. Hemodynamic aspects of cerebral arteriovenous malformations. J Neurosurg. 1980;53:456-464.
13 Spetzler RF, Selman WR. Pathophysiology of cerebral ischemia accompanying arteriovenous malformations. In: Wilson CB, Stein BM, editors. Intracranial Arteriovenous Malformations. Baltimore: Williams & Wilkins; 1984:24-31.
14 Brown RDJr, Wiebers DO, Forbes G, et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1988;68:352-357.
15 Group AMS. Arteriovenous malformations of the brain in adults. N Engl J Med. 1999;340:1812-1818.
16 Hamilton MG, Spetzler RF. The prospective application of a grading system for arteriovenous malformations. Neurosurgery. 1994;34:2-6.
17 Ondra SL, Troupp H, George ED, et al. The natural history of symptomatic arteriovenous malformations of the brain: a 24-year follow-up assessment. J Neurosurg. 1990;73:387-391.
18 Fults D, Kelly DLJr. Natural history of arteriovenous malformations of the brain: a clinical study. Neurosurgery. 1984;15:658-662.
19 Spetzler RF, Hargraves RW, McCormick PW, et al. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76:918-923.
20 Crawford PM, West CR, Chadwick DW, et al. Arteriovenous malformations of the brain: natural history in unoperated patients. J Neurol Neurosurg Psychiatry. 1986;49:1-10.
21 Hassler W, Steinmetz H. Cerebral hemodynamics in AVM patients: an intraoperative study. J Neurosurg. 1987;67:822-831.
22 Fleetwood IG, Marcellus ML, Levy RP, et al. Deep arteriovenous malformations of the basal ganglia and thalamus: natural history. J Neurosurg. 2003;98:747-750.
23 Lewis AI, Tew JMJr. Management of thalamic–basal ganglia and brain-stem vascular malformations. Clin Neurosurg. 1994;41:83-111.
24 Marks MP, Lane B, Steinberg GK, et al. Hemorrhage in intracerebral arteriovenous malformations: angiographic determinants. Radiology. 1990;176:807-813.
25 Miyasaka Y, Yada K, Ohwada T, et al. An analysis of the venous drainage system as a factor of hemorrhage from arteriovenous malformations. J Neurosurg. 1992;76:239-243.
26 Miyasaka Y, Kurata A, Tokiwa K, et al. Draining vein pressure increases and hemorrhage in patients with arteriovenous malformation. Stroke. 1994;25:504-507.
27 Chang SD, Marcellus ML, Marks MP, et al. Multimodality treatment of giant intracranial arteriovenous malformations. Neurosurgery. 2003;53:1-11.
28 Steinberg GK, Marks MP, Levy RP, et al. Multimodality treatment of vascular malformations in functional brain areas using stereotactic radiosurgery, embolization and microsurgery. In: Yamada S, editor. Arteriovenous Malformations in Functional Brain Areas. Mt. Kisco, NY: Futura; 1999:181-196.
29 Steinberg GK, Chang SD, Levy RP, et al. Surgical resection of large incompletely treated intracranial arteriovenous malformations following stereotactic radiosurgery. J Neurosurg. 1996;84:920-928.
30 Steinberg GK, Marks MP. Intracranial arteriovenous malformations: Therapeutic options. In: Batjer HH, editor. Cerebrovascular Disease. New York: Lippincott-Raven; 1997:727-742.
31 Anson JA, Spetzler RF. Giant arteriovenous malformations. In: Carter LP, Spetzler RF, Hamilton MG, editors. Neurovascular Surgery. New York: McGraw-Hill; 1995:1017-1028.
32 Chang SD, Steinberg GK. Surgical approaches: Interhemispheric. In: Awad IA, Jafar JJ, Rosenwasser RH, editors. Vascular Malformations of the Central Nervous System. New York: Lippincott-Raven; 1999:297-308.
33 Lee JP. Surgical treatment of thalamic arteriovenous malformations. Neurosurgery. 1993;32:498-503.
34 Solomon RA, Stein BM. Interhemispheric approach for the surgical removal of thalamocaudate arteriovenous malformations. J Neurosurg. 1987;66:345-351.
35 Heros RC, Tu Y-K. Unruptured arteriovenous malformations: a dilemma in surgical decision making. Clin Neurosurg. 1986;33:187-212.
36 Gewirtz RJ, Steinberg GK. AVMs of the posterior fossa: evaluation and management. Contemp Neurosurg. 1996;18:1-6.
37 Betti OO, Munari C, Rosler R. Stereotactic radiosurgery with the linear accelerator: treatment of arteriovenous malformations. Neurosurgery. 1989;24:311-321.
38 Steinberg GK, Fabrikant JI, Marks MP, et al. Stereotactic heavy-charged-particle Bragg-peak radiation for intracranial arteriovenous malformations. N Engl J Med. 1990;323:96-101.
39 Paulson RD, Steinberg GK, Norbash AM, et al. Embolization of rolandic cortex arteriovenous malformations. Neurosurgery. 1999;44:479-484.
40 Paulson RD, Steinberg GK, Norbash AM, et al. Embolization of basal ganglia and thalamic arteriovenous malformations. Neurosurgery. 1999;44:996-997.
41 Marks MP, Lane B, Steinberg GK, et al. Vascular characteristics of intracerebral arteriovenous malformations in patients with clinical steal. AJNR Am J Neuroradiol. 1991;12:489-496.
42 Spetzler RF, Wilson CB, Weinstein P, et al. Normal perfusion pressure breakthrough theory. Clin Neurosurg. 1978;25:651-672.
43 Morcos JJ, Heros RC. Supratentorial arteriovenous malformations. In: Carter LP, Spetzler RF, Hamilton MG, editors. Neurovascular Surgery. New York: McGraw-Hill, 1995.
44 Spetzler RF, Martin NA, Carter LP, et al. Surgical management of large AVMs by staged embolization and operative excision. J Neurosurg. 1987;67:17-28.
45 Colombo F, Cavedon C, Francescon P, et al. Three-dimensional angiography for radiosurgical treatment planning for arteriovenous malformations. J Neurosurg. 2003;98:536-543.
46 Kjellberg RN. Proton beam therapy for arteriovenous malformations of the brain, 2nd ed. Schmidek HH, Sweet WH, editors. Operative Neurosurgical Techniques: Indications, Methods, Results, Vol 2. Philadelphia: Saunders. 1988:911-915.
47 Kjellberg RN, Davis KR, Lyons S, et al. Bragg peak proton beam therapy for arteriovenous malformation of the brain. Clin Neurosurg. 1983;31:248-290.
48 Kjellberg RN, Hanamura T, Davis KR, et al. Bragg-peak proton-beam therapy for arteriovenous malformations of the brain. N Engl J Med. 1983;309:269-274.
49 Steiner L. Radiosurgery in cerebral arteriovenous malformations. In: Fein JM, Flamm ES, editors. Cerebrovascular Surgery, Vol 4. New York: Springer-Verlag; 1985:1161-1215.
50 Gauvrit JY, Oppenheim C, Nataf F, et al. Three-dimensional dynamic magnetic resonance angiography for the evaluation of radiosurgically treated cerebral arteriovenous malformations. Eur Radiol. 2006;16:583-591.
51 Heidenreich JO, Schilling AM, Unterharnscheidt F, et al. Assessment of 3D-TOF-MRA at 3.0 tesla in the characterization of the angioarchitecture of cerebral arteriovenous malformations: a preliminary study. Acta Radiol. 2007;48:678-686.
52 Reinacher P, Reinges MH, Simon VA, et al. Dynamic 3-D contrast-enhanced angiography of cerebral tumours and vascular malformations. Eur Radiol. 2007;17(suppl 6):F52-F62.
53 Unlu E, Temizoz O, Albayram S, et al. Contrast-enhanced MR 3D angiography in the assessment of brain AVMs. Eur J Radiol. 2006;60:367-378.
54 Berube J, McLaughlin N, Bourgouin P, et al. Diffusion tensor imaging analysis of long association bundles in the presence of an arteriovenous malformation. J Neurosurg. 2007;107:509-514.
55 Kikuta K, Takagi Y, Nozaki K, et al. Early experience with 3-T magnetic resonance tractography in the surgery of cerebral arteriovenous malformations in and around the visual pathway. Neurosurgery. 2006;58:331-337.
56 Kikuta K, Takagi Y, Nozaki K, et al. Introduction to tractography-guided navigation: using 3-tesla magnetic resonance tractography in surgery for cerebral arteriovenous malformations. Acta Neurochir Suppl. 2008;103:11-14.
57 Stancanello J, Cavedon C, Francescon P, et al. BOLD fMRI integration into radiosurgery treatment planning of cerebral vascular malformations. Med Phys. 2007;34:1176-1184.
58 Marks MP, O’Donahue J, Fabrikant JI, et al. Cerebral blood flow evaluation of arteriovenous malformations with stable xenon CT. AJNR Am J Neuroradiol. 1988;9:1169-1175.
59 Cromwell LD, Harris AB. Treatment of cerebral arteriovenous malformations. A combined neurosurgical and neuroradiological approach. J Neurosurg. 1980;52:705-708.
60 Debrun G, Vinuela F, Fox A, et al. Embolization of cerebral arteriovenous malformations with bucrylate. Experience in 46 cases. J Neurosurg. 1982;56:615-627.
61 Jafar JJ, Davis AJ, Berenstein A, et al. The effect of embolization with N-butyl cyanoacrylate prior to surgical resection of cerebral arteriovenous malformations. J Neurosurg. 1993;78:60-69.
62 Samson D, Ditmore QM, Beyer CW. Intravascular use of isobutyl 2-cyanoacrylate. Part I. Treatment of intracranial vascular malformations. Neurosurgery. 1981;8:43-51.
63 Mounayer C, Hammami N, Piotin M, et al. Nidal embolization of brain arteriovenous malformations using Onyx in 94 patients. AJNR Am J Neuroradiol. 2007;28:518-523.
64 Panagiotopoulos V, Gizewski E, Asgari S, et al. Embolization of intracranial arteriovenous malformations with ethylene-vinyl alcohol copolymer (Onyx). AJNR Am J Neuroradiol. 2009;30:99-106.
65 van Rooij WJ, Sluzewski M, Beute GN. Brain AVM embolization with Onyx. AJNR Am J Neuroradiol. 2007;28:172-177.
66 Velat GJ, Reavey-Cantwell JF, Sistrom C, et al. Comparison of N-butyl cyanoacrylate and onyx for the embolization of intracranial arteriovenous malformations: analysis of fluoroscopy and procedure times. Neurosurgery. 2008;63:ONS73-78.
67 Weber W, Kis B, Siekmann R, et al. Preoperative embolization of intracranial arteriovenous malformations with Onyx. Neurosurgery. 2007;61:244-252.
68 Rauch RA, Vinuela F, Dion J, et al. Preembolization functional evaluation in brain arteriovenous malformations: the ability of superselective Amytal test to predict neurologic dysfunction before embolization. AJNR Am J Neuroradiol. 1992;13:309-314.
69 Alexander E 3rd, Loeffler JS. Radiosurgery using a modified linear accelerator. Neurosurg Clin N Am. 1992;3:167-190.
70 Colombo F, Benedetti A, Pozza F, et al. Linear accelerator radiosurgery of cerebral arteriovenous malformations. Neurosurgery. 1989;24:833-840.
71 Colombo F, Pozza F, Chierego G, et al. Linear accelerator radiosurgery of cerebral arteriovenous malformations: an update. Neurosurgery. 1994;34:14-20.
72 Fabrikant JI, Levy RP, Steinberg GK, et al. Charged-particle radiosurgery for intracranial vascular malformations. Neurosurg Clin N Am. 1992;3:99-139.
73 Friedman WA, Bova FJ. Linear accelerator radiosurgery for arteriovenous malformations. J Neurosurg. 1992;77:832-841.
74 Leksell L. Stereotactic radiosurgery. J Neurol Neurosurg Psychiatry. 1983;46:797-803.
75 Lunsford LD, Kondziolka D, Bissonette DJ, et al. Stereotactic radiosurgery of brain vascular malformations. Neurosurg Clin N Am. 1992;3:79-98.
76 Lunsford LD, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg. 1991;75:512-524.
77 Steiner L, Lindquist C, Adler JR, et al. Clinical outcome of radiosurgery for cerebral arteriovenous malformations. J Neurosurg. 1992;77:1-8.
78 Levy RP, Fabrikant JI, Frankel KA, et al. Stereotactic heavy-charged-particle Bragg peak radiosurgery for the treatment of intracranial arteriovenous malformations in childhood and adolescence. Neurosurgery. 1989;24:841-852.
79 Levy RP, Fabrikant JI, Frankel KA, et al. Charged-particle radiosurgery of the brain. Neurosurg Clin N Am. 1990;1:955-990.
80 Chang SD, Shuster DL, Steinberg GK, et al. Stereotactic radiosurgery of arteriovenous malformations: pathologic changes in resected tissue. Clin Neuropathol. 1997;16:111-116.
81 Firlik AD, Levy EI, Kondziolka D, et al. Staged volume radiosurgery followed by microsurgical resection: a novel treatment for giant cerebral arteriovenous malformations: technical case report. Neurosurgery. 1998;43:1223-1228.
82 Pollock BE, Kondziolka D, Lunsford LD, et al. Repeat stereotactic radiosurgery of arteriovenous malformations: Factors associated with incomplete obliteration. Neurosurgery. 1996;38:318-324.
83 Massengale JL, Levy RP, Marcellus M, et al. Outcomes of surgery for resection of regions of symptomatic radiation injury after stereotactic radiosurgery for arteriovenous malformations. Neurosurgery. 2006;59:553-560.
84 Kelly ME, Guzman R, Sinclair J, et al. Multimodality treatment of posterior fossa arteriovenous malformations. J Neurosurg. 2008;108:1152-1161.
85 Marks MP, Lane B, Steinberg GK, et al. Endovascular treatment of cerebral arteriovenous malformations following radiosurgery. AJNR Am J Neuroradiol. 1993;14:297-303.
86 Steinberg GK, Levy RP, Marks MP, et al. Vascular malformations: Charged-particle radiosurgery. In: Alexander E, Loeffler JS, Lunsford L, editors. Stereotactic Radiosurgery. New York: McGraw-Hill; 1993:122-135.
87 Chang SD, Lopez JR, Steinberg GK. The usefulness of electrophysiologic monitoring during resection of central nervous system vascular malformations. J Stroke Cerebrovasc Dis. 1999;8:412-422.
88 Burchiel KJ, Clarke H, Ojemann GA, et al. Use of stimulation mapping and corticography in the excision of arteriovenous malformations in sensorimotor and language related neocortex. Neurosurgery. 1989;24:322-327.
89 Steinberg GK, Ogilvy CS, Shuer LM, et al. Comparison of endovascular and surface cooling during unruptured cerebral aneurysm repair. Neurosurgery. 2004;55:307-314.
90 Steinberg GK, Grant G, Yoon EJ. Deliberate hypothermia. In: Andrews RJ, editor. Intraoperative Neuroprotection. Baltimore: William & Wilkins; 1996:65-84.
91 Anegawa S, Hayashi T, Torigoe R, et al. Intraoperative angiography in the resection of arteriovenous malformations. J Neurosurg. 1994;80:73-78.
92 Forster DMC, Steiner L, Hakanson S. Arteriovenous malformations of the brain. A long term clinical study. J Neurosurg. 1972;37:562-570.
93 Barrow DL, Boyer KL, Joseph GJ. Intraoperative angiography in the management of neurovascular disorders. Neurosurgery. 1992;30:153-159.
94 Martin N, Doberstein C, Benston J. Intraoperative angiography in cerebrovascular surgery. Clin Neurosurg. 1991;37:312-331.
95 Nornes H, Grip A, Wikeby P. Intraoperative evaluation of cerebral hemodynamics using directional Doppler techniques. Part I: Arteriovenous malformations. J Neurosurg. 1979;50:145-151.
96 Heros RC, Korosue K, Diebold PM. Surgical excision of cerebral arteriovenous malformations: late results. Neurosurgery. 1990;26:570-577.
97 Hernesniemi J, Keranen T. Microsurgical treatment of arteriovenous malformations of the brain in a defined population. Surg Neurol. 1990;33:384-390.
98 Purdy PD, Batjer HH, Samson D. Management of hemorrhage complications from preoperative embolization of arteriovenous malformations. J Neurosurg. 1991;74:205-211.
99 Chang SD, Adler JR. Management of the radiosurgery patient: Causes and treatment of adverse sequalae. In: Meyer J, editor. Radiation Injury: Advancements in Management and Prevention. New York: Karger Medical; 1999:155-165.
100 Sheline GE, Wara WM, Smith V. Therapeutic irradiation and brain injury. Int J Radiat Oncol Biol Phys. 1980;6:1215-1228.
101 Loeffler JS, Siddon RL, Wen PY, et al. Stereotactic radiosurgery of the brain using a standard linear accelerator: a study of early and late effects. Radiother Oncol. 1990;17:311-321.
102 Adams JH, Graham DI. An Introduction to Neuropathology. London: Churchill Livingstone; 1988.
103 McKenzie MR, Souhami L, Caron JL, et al. Early and late complications following dynamic stereotactic radiosurgery and fractionated stereotactic radiotherapy. Can J Neurol Sci. 1993;20:279-285.
104 Steiner L. Stereotactic radiosurgery with the cobalt-60 gamma unit in the surgical treatment of intracranial tumors and arteriovenous malformations. In: Schmidek HH, Sweet . Operative Neurosurgical Techniques: Indications, Methods, Results. Philadelphia: Saunders; 1988:515-529.
105 Sutcliffe JC, Forster DM, Walton L, et al. Untoward clinical effects after stereotactic radiosurgery for intracranial arteriovenous malformations. Br J Neurosurg. 1992;6:177-185.
106 Miyasaka Y, Yada K, Ohwada T, et al. Retrograde thrombosis of feeding arteries after removal of arteriovenous malformations. J Neurosurg. 1990;72:540-545.