Chapter 132 Surgery of the Sympathetic Nervous System
Sympathectomy procedures involve interrupting thoracic or lumbar sympathetic pathways to provide relief from autonomically mediated syndromes. Currently the most common disorder treated is essential hyperhidrosis,1,2 but sympathectomies have been used extensively in the past to treat various pain syndromes, including complex regional pain syndrome (CRPS; also known as reflex sympathetic dystrophy), causalgia, vascular insufficiency pain syndromes, and Raynaud phenomenon.3–6 More recently, pain syndromes have been treated less frequently with ablative sympathectomy procedures because of the limited success with sympathectomy and potential improvement in outcomes with neurostimulation techniques.7,8 This chapter reviews the history, relevant anatomy and physiology, surgical indications, and techniques of the open and closed sympathectomy procedures as well as the more recent endoscopic techniques, with particular regard to perioperative management, complications, and outcomes of the different sympathectomy procedures.
Clinical Syndromes
Neuropathic and Ischemic Pain
Chronic pain syndromes9,10 such as causalgia and reflex sympathetic dystrophy (now referred to as CRPS) are thought to arise from peripheral nerve trauma that is usually ill defined. They also include several other related syndromes (e.g., phantom pain, shoulder-hand syndrome, posttraumatic neuralgia). Characteristic symptoms are burning pain, edema, and trophic skin changes in the extremity. Temperature changes are frequently noted in the affected extremity. Ischemic pain syndromes including Raynaud phenomenon and other vasculitic disorders typically have episodes of severe, painful skin blanching, primarily of the hands and fingertips.11 Cold temperature or emotional response may exacerbate these episodes, and extreme cases may result in ischemia and gangrenous ulceration of the digits. The initial treatment is avoidance of cold and administration of α-adrenergic blocking agents, which are useful for less severe cases. Sympathectomy procedures have been used extensively in the past and can provide significant initial relief from severe pain and digital ulcers. However, the long-term outcomes of sympathectomy for relieving the episodic vasospasms associated with chronic pain syndromes and Raynaud phenomenon are less optimal.
Anatomy and Physiology
The autonomic nervous system includes both the sympathetic and the parasympathetic nervous systems. The sympathetic system mediates the “fight-or-flight” responses such as pupillary dilation, tachycardia, bronchial dilation, increased muscle blood flow, and the release of adrenergic agents from the adrenal glands. It is a two-neuron disynaptic system in which responses are mediated through autonomic ganglia, with the ultimate regulation occurring in the hypothalamus. Anatomically, the sympathetic nervous system has outflow in the thoracic and upper lumbar regions of the spinal cord. Preganglionic fibers from the intermediolateral cell column exit the spinal cord through the ventral nerve roots into spinal nerves and enter the paravertebral chain ganglia, coursing through the myelinated white rami communicantes.3,4 Once in the ganglia, the presynaptic neuron can (1) synapse with a postganglionic neuron and exit as a gray ramus to the viscera, (2) synapse with a postganglionic neuron and exit as a gray ramus in a segmental nerve, (3) travel up or down the sympathetic chain, (4) stimulate the adrenal gland, or (5) exit the sympathetic chain in the splanchnic nerves and enter peripherally located ganglia such as the mesenteric ganglia. Postganglionic fibers travel in peripheral nerves or along arteries to reach their target organs. Afferent autonomic fibers travel from receptors though the dorsal spinal roots to enter the spinal cord, where they can trigger reflexes through spinal cord interneurons and efferent autonomic fibers.
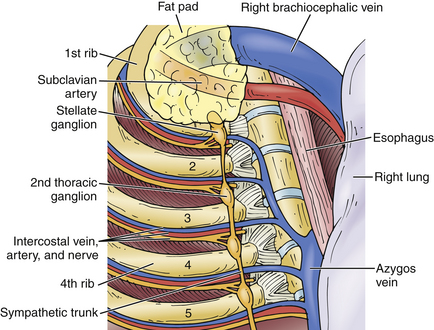
The autonomic ganglia are variable in size, number, and location. There are generally three cervical ganglia (superior, middle, and inferior). The lowest cervical ganglia can fuse with the highest thoracic ganglia to form the stellate or cervicothoracic ganglion.3,4 Pupillary dilation occurs as a result of sympathetic output from the spinal cord ciliospinal center of Budge. The preganglionic fibers exit the spinal cord at the T1 and T2 levels and travel through the thoracic, stellate, and middle cervical ganglia to synapse in the superior cervical ganglia; the postganglionic fibers then enter the sympathetic plexus surrounding the carotid artery and travel along the third, fifth, and sixth cranial nerves to enter the orbit and pass through the ciliary ganglion to the pupillary dilators via the long anterior ciliary nerves. A lesion anywhere along this course is manifested by pupillary miosis, anhidrosis (loss of sympathetic innervation to the sweat glands of the face), ptosis (loss of innervation of the superior tarsal musculature), and, occasionally, enophthalmos. The thoracic ganglia correlate with the corresponding thoracic level, as do the upper lumbar ganglia.
Sexual and urinary function are also influenced by the autonomic nervous system.12 Sympathetic efferent innervation to the bladder arises from the lower thoracic and upper lumbar levels. The efferent nerves travel through a series of ganglia in the sacral region, and the postganglionic fibers travel to the vesicular plexus via the hypogastric nerves. There is also sympathetic stimulation involved in both erection and ejaculation in male patients.
Preoperative Evaluation
Medical treatment of autonomically mediated syndromes is theoretically useful and may have potential in limited cases. Medications that produce systemic sympathetic blockade include phenoxybenzamine, which blocks the α-adrenergic receptors.13,14 Alpha blockade is associated with frequent complications, including hypotension, miosis, and loss of ejaculatory function, but it is an effective test treatment for causalgia-type symptoms.
Neuromodulation
Spinal cord stimulation is increasingly being used to treat CRPS. The exact mechanisms by which it works require further study.15 Broad reviews have found it to be both effective and cost effective.16
Open Sympathectomy Techniques
Open sympathectomy techniques have been used to treat hyperhidrosis effectively, but have less efficacy for treating pain syndromes, particularly given the need for highly invasive procedures. These approaches do not require specialized endoscopic equipment and techniques. The open techniques are generally known to most practicing physicians.
Cervical and Cervicothoracic Approaches
Operative Techniques
Cervical or cervicothoracic sympathectomy procedures can be performed using several techniques. These include ventral supraclavicular or transaxillary approaches or dorsal costotransversectomy approaches.17,18 Ventral approaches provide good exposure of the sympathetic chain but carry risks of injury to the brachial plexus and pleural cavity.
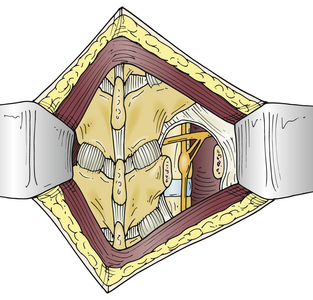
The most common open approach for upper thoracic and stellate ganglionectomy is the dorsal approach, where a T2 ganglionectomy has been performed for hyperhidrosis and CRPS. The patient is placed in the prone position on the operating table after general anesthesia. A 4- to 5-cm midline incision over T2 and T3 and the paraspinal muscles is retracted to expose and remove the T2 transverse process and proximal rib of T3 (Fig. 132-1), with preservation of the intercostal nerves. The lateral surface of the vertebral body is then exposed by elevating the parietal pleura carefully to expose the sympathetic chain. The ganglia are usually clearly visible and easily dissected from the chain, which is then cauterized with bipolar electrocautery above and below the ganglia, and the ganglion is resected (Fig. 132-2). The ganglion can be sent to the pathology unit for histologic confirmation. A similar procedure is performed on the opposite side for patients with bilateral palmar hyperhidrosis.
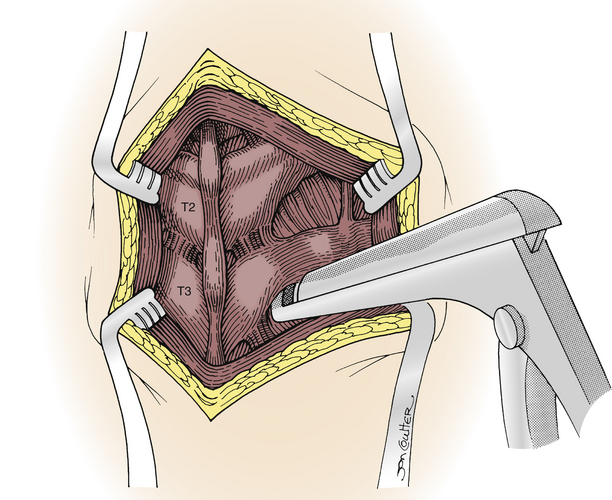
FIGURE 132-1 Dorsal T2 sympathectomy approach with removal of T2 transverse process and proximal T3 rib.
In the transaxillary ventral approach described by Atkins,17 the patient is in a lateral position with the arm extended forward. An incision is made in the second intercostal space from the latissimus dorsi to the pectoralis musculature. The pleural cavity is entered and the ribs slowly retracted. The lung is partially deflated, the sympathetic chain is located, and the appropriate ganglion is identified and removed. The transthoracic endoscopic approach developed in recent years has become the most frequently used method today.
Complications
The most common complications after ventral (or dorsal) cervical and thoracic sympathectomy include Horner syndrome, pneumothorax, pneumonia, wound infection and dehiscence, failure of the procedure adequately to relieve the preoperative symptoms, regrowth of the sympathetic chain, cerebrospinal fluid leak, and spinal cord injury. Horner syndrome is avoided by limiting the resection to the second thoracic ganglion and avoiding injury to the first thoracic and stellate ganglia.19 Horner syndrome may be only a temporary effect caused by stretching of the chain. Pneumothorax is rare and requires placement of a small-tube thoracostomy. Hardy and Bay3 recommend placement of a 12-Fr red rubber catheter in the pleural cavity if a pleural laceration is noted intraoperatively; negative suction is applied to the catheter with positive-pressure ventilation, and the catheter is then quickly removed and the wound closed. Postoperative chest radiographs should be obtained for all patients to ensure that pneumothorax has not occurred.
Lumbar Approaches
Operative Technique
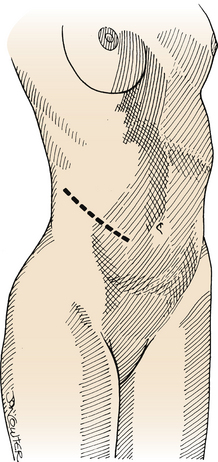
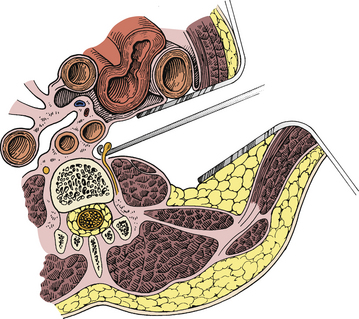
The lumbar sympathectomy procedure2,20 is performed with the patient in the supine position. A 10- to 12-cm incision is made in the flank from the tip of the 11th rib toward the anterior superior iliac spine (Fig. 132-3). The abdominal muscles are split longitudinally with the fibers into the retroperitoneal space and the fat is then bluntly divided with digital dissection to the ventrolateral aspect of the spine, where the lumbar sympathetic chain is located in the gutter between the psoas muscle and the spinal column (Fig. 132-4). The chain can be tethered by lumbar arterial branches on the left and lumbar veins on the right that must be mobilized and often divided to allow access to the lumbar sympathetic chain. The L2-4 ganglia can then be resected. Occasionally the vena cava must be partially mobilized to gain access to the sympathetic chain on the right side. Closure is obtained by reapproximating the fascia with absorbable suture and the subcutaneous layer and skin with absorbable sutures or staples.
Complications
Complications of open ventral lumbar sympathectomy include ileus, infection, injury to intraperitoneal contents, wound complications, impotence, ureteral injury, and vascular injury. Impotence can occur even with unilateral disruption or injury to the sympathetic chain and is difficult to predict or prevent; all male patients should be warned of this potential adverse outcome. The ureter is at risk during all retroperitoneal exposures; however, injury can be avoided by visualizing the ureter, which is usually on the psoas muscle lateral to the sympathetic chain. Injury to the ureter can be difficult to detect and may result in flank pain and tenderness. Some patients may experience thigh pain for several weeks after a lumbar sympathectomy, the etiology of which is unclear but may be due to muscle retraction.
Endoscopic Sympathectomy Techniques
Thoracoscopic Approach for Cervical and Thoracic Sympathectomy
Endoscopic thoracic sympathectomy for the upper extremity was originally reported in 1951 by Kux,21 who described treatment of hyperhidrosis with excellent results. There has been significant recent interest in these minimally invasive procedures.22 The attractiveness of minimally invasive treatment of autonomically mediated syndromes affecting the upper extremities is due to the development of endoscopic techniques. The most frequent indication for thoracic sympathectomy is hyperhidrosis.23–27 Less frequently, CRPS, causalgia, Raynaud phenomenon, postamputation syndrome (phantom pain), and refractory cardiac tachyarrhythmias28–30 are also treated with sympathectomy. Endoscopic techniques provide both a panoramic and magnified view for precise identification of the sympathetic chain and adjacent structures, allowing definition of the anatomy for resection of the sympathetic chain. This procedure has become the preferred method of thoracic sympathectomy, and extensive clinical experience has resulted in improvements in patient satisfaction and reduced hospital stays and costs. Because thoracoscopic procedures have a distinct learning curve, it is necessary to understand intrathoracic anatomy and gain initial endoscopic experience with a thoracoscopic surgeon.
Palmar hyperhidrosis is the main indication for thoracic sympathectomy with minimally invasive techniques. Severe axillary sweating (with or without palmar hyperhidrosis) has also been treated with good results. Some patients with facial hyperhidrosis have also been treated successfully with a sympathectomy procedure. The precise sympathectomy procedure for each of these clinical syndromes with regard to which ganglionic levels are to be resected remains somewhat speculative. Most authors agree that only a T2 and T3 sympathectomy will effectively treat palmar hyperhidrosis. However, some authors recommend either a T2 or T3 sympathectomy and cite a lowered incidence of compensatory sweating, but this has not been clearly substantiated. Axillary sweating is thought to be best treated with sympathectomy at T3, T4, and T5 for good results. Facial sweating is treated with a T2 sympathectomy just below the stellate ganglion.31
Patients with pain syndromes and vasculitis or Raynaud phenomenon may also respond to an endoscopic T2, T3, and/or T4 sympathectomy, but results are less optimal.32 Spinal stimulation appears to have largely replaced sympathectomy for these indications, for several reasons8: (1) pain symptoms have a high incidence of recurrence after sympathectomy, (2) stimulation is a nonablative procedure, (3) stimulation is usually a technically less demanding procedure than a sympathectomy, (4) stimulation is a reversible procedure, and (5) stimulation can be modulated after the procedure is completed.
Recent reports also describe the utility and selective efficacy of pulsed radiofrequency for more selective ablation of the lumbar sympathetic chain.33 However, the most recent (2010) Cochrane review found only one high-quality study directly comparing chemical with radiofrequency lumbar sympathectomy for CRPS, a study by Manjunath et al. in 2008 that demonstrated no difference in outcomes based on the technique of neural ablation.34,35
Patients with cardiac tachyarrhythmias have also been treated with sympathectomy procedures. Studies have demonstrated effectiveness in treating stress-related malignant tachyarrhythmias that may be related to disproportionate left and right sympathetic innervation. Patients treated inadequately with medical therapy can be considered for a left thoracoscopic sympathectomy; however, improved medical therapy has nearly eliminated the use of sympathectomy for this indication.3–6
Operative Technique
Anesthesia and Positioning
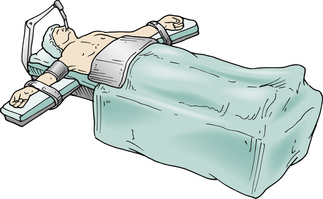
The patient requires general anesthesia and placement of a double-lumen endotracheal tube to allow collapse of the lung on the operated side. The supine position (Fig. 132-5) is used, and the head of the table can be elevated and rotated.
Ports and Port Placement
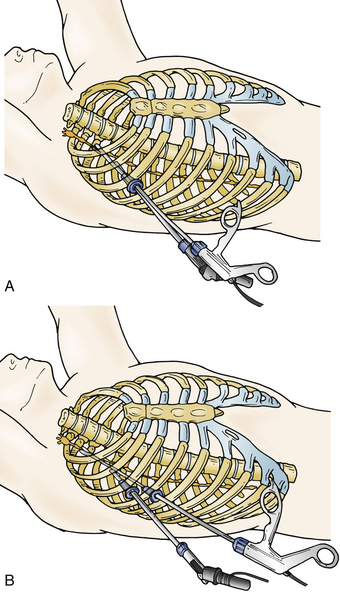
A soft, flexible thoracic endoscopic port is inserted through a 2-cm chest wall incision, similar to the placement of a chest tube. A single port (Fig. 132-6A), or occasionally two ports (Fig. 132-6B), can be used, and both the endoscope and other working instruments can be placed in the port.36–39 The port is placed in the third or fourth intercostal space in the midaxillary line while the anesthesiologist deflates the ipsilateral lung.
Operative Procedure
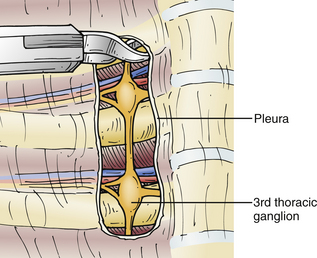
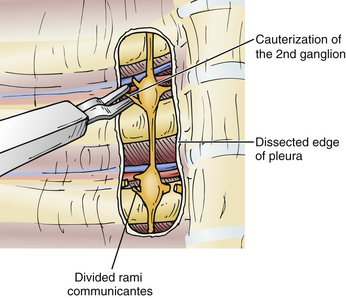
The sympathectomy procedure begins with an incision in the pleura overlying the sympathetic chain at the T3 level, using the curved scissors (Fig. 132-7). The pleural incision is extended in a rostral direction over the sympathetic chain to T2 (Fig. 132-8), and the sympathetic ganglia and chain at T2 and T3 are then cauterized (Fig. 132-9). The nerve of Kuntz can often be identified as a large branch arising from the T2 ganglion and coursing laterally to the brachial plexus, likely providing much of the sympathetic innervation to the upper extremity. Thus, denervation of the T2 ganglion is important.40 Horner syndrome is best prevented by avoiding injury or traction to the stellate ganglion, which can occur during the dissection and denervation of the T2 ganglion. Once the sympathectomy has been completed, a portion of the sympathetic ganglion can be sent for pathologic evaluation; the surgical site is then irrigated and hemostasis confirmed. A red rubber catheter is inserted through the port and aspirated while the lung is reinflated by the anesthesiologist; the port is then removed and the incision closed with absorbable sutures and Steri-Strips.
Complications
Surgical complications41–43 from thoracoscopic sympathectomy are few, and most do not require intervention. They include (1) pneumothorax, (2) Horner syndrome, (3) subcutaneous emphysema, (4) pleural effusions, (5) segmental atelectasis, and (6) intercostal neuralgia. Postoperative pneumothorax usually results from inadequate reinflation of the collapsed lung; however, a small apical pneumothorax can be observed and will spontaneously resolve. Horner syndrome results from injury to the stellate ganglion and is infrequent and usually transient. Injury to the intercostal nerves during port placement or pressure applied during the procedure may result in intercostal neuralgia. The incidence is minimized by using soft, flexible thoracic endoscopic ports rather than the rigid laparoscopic ports.
Compensatory hyperhidrosis is increased sweating, usually in the truncal area, that occurs after a sympathectomy procedure.44 Facial anhidrosis can also occur.45 The incidence is probably higher than previously recognized and has been reported to range from 10% to 90%. The severity is highly variable, ranging from mild to severe, and cannot be determined before surgery. Some authors suggest that a more limited sympathectomy of T2 alone may reduce the incidence of compensatory sweating, whereas others claim that only sympathectomy of T3 reduces the incidence.46 Increasing data are emerging to indicate that more limited (i.e., T2) sympathectomy may have a lower incidence of compensatory hyperhidrosis.47 Resympathectomy is also an option for both primary failure and recurrent hyperhidrosis, with success rates varying from 96% for unilateral primary failures to 50% for bilateral primary failure. Licht et al.’s study of 669 redo endoscopic sympathectomies found the reoperation to be 75% effective in recurrent palmar hyperhidrosis.48 However, none of these theories and observations have been clearly substantiated. More recently, clipping of the sympathetic chain has been performed in hopes of creating a reversible lesion.49,50 Reconstruction of the sympathetic chain has been reported, but no long-term data are available.44
Lumbar Endoscopic Sympathectomy
Endoscopic techniques have been used for sympathectomy in the lumbar region and are described by Onimus et al.51 and others52,53; they are similar to an open lumbar sympathectomy except for being much less invasive (see Figs. 132-3 and 132-4). A 1- to 2-cm incision is made in the flank below the tip of the 11th rib, and the retroperitoneum is dissected by finger. Similar to the open procedure, a tissue expander or optical trocar can be used to dissect and expose the psoas and the lumbar sympathetic chain. An endoscopic portal is inserted, and CO2 insufflation maintains the space for the procedure. A second portal is placed for instrumentation. The sympathectomy then proceeds as in the open procedure.
Radiofrequency and Chemical Destruction of the Sympathetic Chain
Percutaneous placement of a needle into or near a sympathetic ganglion allows destruction of the ganglion by injection of a neurolytic agent (i.e., alcohol, phenol), or a radiofrequency lesion can be created to achieve sympathectomy. Fluoroscopic or CT guidance can be used for localization.54 These techniques can be performed without general anesthesia, with local anesthetics and mild sedation in older patients, and allow immediate evaluation of the results. They can often be performed on an outpatient basis and obviate the need for expensive and complicated endoscopic technology or large surgical incisions with open procedures. Although some authors report excellent results with these techniques, not all surgeons can achieve these outcomes, and in general the short- and long-term results have been less successful than with open or endoscopic procedures, presumably because of inadequate denervation.
The percutaneous radiofrequency thoracic sympathectomy technique has been well described and documented by Wilkinson.55,56 The patient is positioned prone on a fluoroscopic table and a radiofrequency lesioning needle is placed in a paramedian location and directed between the ribs, with the needle tip at the rib head and the sympathetic ganglion at the T2-3 level. Stimulation is used to confirm the final needle position, and a radiofrequency pulse is generated to create a lesion. A success rate of more than 90% was noted at 3-year follow-up. Complications, including pneumothorax, occur at a low rate.
Chemical sympathectomy with phenol (6% to 10%) is described by Gybels and Sweet.57 It has been used with fluoroscopic guidance primarily in the lumbar region to treat vaso-occlusive disease. Most patients obtain fair early relief of pain, but results deteriorate over time. Complications are infrequent and include groin pain and ureteral injuries.
Bandyk D.F., Johnson B.L., Kirkpatrick A.F., et al. Surgical sympathectomy for reflex sympathetic dystrophy syndromes. J Vasc Surg. 2002;35:269-277.
Barolat G., Schwartzman R., Woo R. Epidural spinal cord stimulation in the management of reflex sympathetic dystrophy. Stereotact Funct Neurosurg. 1989;53:29-39.
Johnson J.P., Patel N.P. Uniportal and biportal endoscopic thoracic sympathectomy. Neurosurgery. 2002;51(Suppl 5):79-83.
Singh B., Moodley J., Ramdial P.K., et al. Pitfalls in thoracoscopic sympathectomy: mechanisms for failure. Surg Laparosc Endosc Percutan Tech. 2001;11:364-367.
Wang Y.C., Sun M.H., Lin C.W., et al. Anatomical location of T2-3 sympathetic trunk and Kuntz nerve determined by transthoracic endoscopy. J Neurosurg. 2002;96(Suppl 1):68-72.
1. Adar R., Kurchin A., Zweig A., et al. Palmar hyperhidrosis and its surgical treatment. Ann Surg. 1977;186:34-41.
2. Munro P.A.G. Sympathectomy: an anatomical and physiological study with clinical applications. London: Oxford University Press; 1959.
3. Hardy R.W., Bay J.W. Surgery of the sympathetic nervous system. In: Schmidek H.H., Sweet W.H., editors. Operative neurosurgical techniques. ed 2. Orlando, FL: Grune and Stratton; 1988:1271.
4. Kleinert H.E., Norberg H., McDonough J.J. Surgical sympathectomy: upper and lower extremity. In: Omer G.E.Jr., Spinner M., editors. Management of peripheral nerve problems. Philadelphia: WB Saunders; 1980:284.
5. Malone P.S., Cameron A.E.P., Rennie J.A. The surgical treatment of upper limb hyperhidrosis. Br J Dermatol. 1986;115:81-84.
6. Rutherford R.B. Role of sympathectomy in the management of vascular disease. In: Moore W.S., editor. Vascular surgery: a comprehensive review. Philadelphia: WB Saunders; 1993:300.
7. Barolat G., Schwartzman R., Woo R. Epidural spinal cord stimulation in the management of reflex sympathetic dystrophy. Stereotact Funct Neurosurg. 1989;53:29-39.
8. Oakley J.C., Prager J.P. Spinal cord stimulation: mechanisms of action. Spine (Phila Pa 1976). 2002;27:2574-2578.
9. Bandyk D.F., Johnson B.L., Kirkpatrick A.F., et al. Surgical sympathectomy for reflex sympathetic dystrophy syndromes. J Vasc Surg. 2002;35:269-277.
10. Bergan J.J., Conn J.Jr. Sympathectomy for pain relief. Med Clin North Am. 1968;52:147-159.
11. Matsumoto Y., Ueyama T., Endo M., et al. Endoscopic thoracic sympathectomy for Raynaud’s phenomenon. J Vasc Surg. 2002;36:57-61.
12. Burchiel K.J., Burns A.S. Summary statement: pain, spasticity, and bladder and sexual function after spinal cord injury [comment]. Spine (Phila Pa 1976). 2001;26(Suppl 24):S161.
13. Ghostine S.Y., Comair Y.G., Turner D.M., et al. Phenoxybenzamine in the treatment of causalgia: report of 40 cases. J Neurosurg. 1984;60:1263-1268.
14. Weiner N. Drugs that inhibit adrenergic nerves and block adrenergic receptors. In: Gilman A.G., Goodman L.S., Gilman A., editors. Goodman and Gilman’s pharmacological basis of therapeutics. ed 6. New York: MacMillan; 1980:176.
15. Prager J.P. What does the mechanism of spinal cord stimulation tell us about complex regional pain syndrome? Pain Med. 2010;11:1278-1283.
16. Bala M.M., Riemsma R.P., Nixon J., et al. Systematic review of the (cost)-effectiveness of spinal cord stimulation for people with failed back surgery syndrome. Clin J Pain. 2008;24:741-756.
17. Atkins H.J.B. Sympathectomy by the axillary approach. Lancet. 1954;1:538-539.
18. Kurchin A., Zweig A., Adar R., et al. Upper dorsal sympathectomy for palmar primary hyperhidrosis by the supraclavicular approach. World J Surg. 1977;1:667-674.
19. Kisch B. Horner’s syndrome, an American discovery. Bull Hist Med. 1951;25:284-288.
20. Baker D.M., Lamerton A.F. Operative lumbar sympathectomy for severe lower limb ischaemia: still a valuable treatment option. Ann R Coll Surg Engl. 1994;76:50-53.
21. Kux E. The endoscopic approach to the vegetative nervous system and its therapeutic possibilities. Dis Chest. 1951;20:139-147.
22. Drott C., Göthberg G., Claes G. Endoscopic procedures of the upper thoracic sympathetic chain: a review. Arch Surg. 1993;128:237-241.
23. Adams D.C.R., Wood S.J., Tulloh B.R., et al. Endoscopic transthoracic sympathectomy: experience in the south west end of England. Eur J Vasc Surg. 1992;6:558-562.
24. Banerjee A.K., Edmondson R., Rennie J.A. Endoscopic transthoracic electrocautery of the sympathetic chain for palmar and axillary hyperhidrosis. Br J Surg. 1990;77:1435.
25. Byrne J., Walsh T.N., Hederman W.P. Endoscopic transthoracic electrocautery of the sympathetic chain for palmar and axillary hyperhidrosis. Br J Surg. 1990;77:1046-1049.
26. Chen H.J., Liang C.L., Lu K. Associated change in plantar temperature and sweating after transthoracic endoscopic T2-3 sympathectomy for palmar hyperhidrosis. J Neurosurg. 2001;95(Suppl 1):58-63.
27. Claes G., Göthberg G., Drott C. Endoscopic transthoracic electrocautery of the sympathetic chain for palmar and axillary hyperhidrosis [comment]. Br J Surg. 1991;78:760.
28. Crampton R. Preeminence of the left stellate ganglion in the long QT syndrome. Circulation. 1979;59:769-778.
29. Ouriel K., Moss A.J. Long QT syndrome: an indication for cervicothoracic sympathectomy. Cardiovasc Surg. 1995;3:475-478.
30. Schwartz P.J., Periti M., Malliani A. The long QT syndrome. Am Heart J. 1975;89:378-390.
31. Lin T.S., Fang H.Y. Transthoracic endoscopic sympathectomy for craniofacial hyperhidrosis: analysis of 46 cases. J Laparoendosc Adv Surg Tech A. 2000;10:243-247.
32. Ahn S.S., Machleder H.I., Concepcion B., et al. Thoracoscopic cervicodorsal sympathectomy: preliminary results. J Vasc Surg. 1994;20:511-517. discussion 517–519
33. Akkoc Y., Uyar M., Oncu J., et al. Complex regional pain syndrome in a patient with spinal cord injury: management with pulsed radiofrequency lumbar sympatholysis. Spinal Cord. 2008;46:82-84.
34. Straube S., Derry S., Moore R.A., et al. Cervico-thoracic or lumbar sympathectomy for neuropathic pain and complex regional pain syndrome. Cochrane Database Syst Rev. 2010;7:CD002918.
35. Manjunath P.S., Jayalakshmi T.S., Dureja G.P., et al. Management of lower limb complex regional pain syndrome type 1: an evaluation of percutaneous radiofrequency thermal lumbar sympathectomy versus phenol lumbar sympathetic neurolysis—a pilot study. Anesth Analg. 2008;106:647-649.
36. Johnson J.P., Patel N.P. Uniportal and biportal endoscopic thoracic sympathectomy. Neurosurgery. 2002;51(Suppl 5):79-83.
37. Johnson J.P., Obasi C., Hahn M.S., et al. Endoscopic thoracic sympathectomy. J Neurosurg. 1999;91(Suppl 1):90-97.
38. Lin T.S., Kuo S.J., Chou M.C. Uniportal endoscopic thoracic sympathectomy for treatment of palmar and axillary hyperhidrosis: analysis of 2000 cases. Neurosurgery. 2002;51(Suppl 5):84-87.
39. Vanaclocha V., Saiz-Sapena N., Panta F. Uniportal endoscopic superior thoracic sympathectomy. Neurosurgery. 2000;46:924-928.
40. Wang Y.C., Sun M.H., Lin C.W., et al. Anatomical location of T2-3 sympathetic trunk and Kuntz nerve determined by transthoracic endoscopy. J Neurosurg. 2002;96(Suppl 1):68-72.
41. Lin T.S., Wang N.P., Huang L.C. Pitfalls and complication avoidance associated with transthoracic endoscopic sympathectomy for primary hyperhidrosis (analysis of 2200 cases). Int J Surg Invest. 2001;2:377-385.
42. Plas E.G., Fugger R., Herbst F., et al. Complications of endoscopic thoracic sympathectomy. Surgery. 1995;118:493-495.
43. Singh B., Moodley J., Ramdial P.K., et al. Pitfalls in thoracoscopic sympathectomy: mechanisms for failure. Surg Laparosc Endosc Percutan Tech. 2001;11:364-367.
44. Telaranta T. Secondary sympathetic chain reconstruction after endoscopic thoracic sympathicotomy. Eur J Surg Suppl. 1998;580:17-18.
45. Kao M.C., Chen Y.L., Lee Y.S., et al. Craniofacial hyperhidrosis treated with video endoscopic sympathectomy. J Clin Laser Med Surg. 1994;12:93-95.
46. Leseche G., Castier Y., Thabut G., et al. Endoscopic transthoracic sympathectomy for upper limb hyperhidrosis: limited sympathectomy does not reduce postoperative compensatory sweating. J Vasc Surg. 2003;37:124-128.
47. Miller D.L., Bryant A.S., Force S.D., et al. Effect of sympathectomy level on the incidence of compensatory hyperhidrosis after sympathectomy for palmar hyperhidrosis. J Thorac Cardiovasc Surg. 2009;138:581-585.
48. Licht P.B., Clausen A., Ladegaard L. Resympathicotomy. Ann Thorac Surg. 2010;89:1087-1090.
49. Beatty R.A. The use of clip-suture in thoracic sympathectomy: technical note. J Neurosurg. 1994;81:482.
50. Lin T.S., Huang L.C., Wang N.P., et al. Video-assisted thoracoscopic T2 sympathetic block by clipping for palmar hyperhidrosis: analysis of 52 cases. J Laparoendosc Adv Surg Tech A. 2001;11:59-62.
51. Onimus M., Papin P., Gangloff S. Extraperitoneal approach to the lumbar spine with video assistance. Spine (Phila Pa 1976). 1996;21:2491-2494.
52. Kathouda N., Wattanasirichaigoon S., Tang E., et al. Laparoscopic lumbar sympathectomy. Surg Endosc. 1997;11:257-260.
53. Watarida S., Shiraishi S., Fujimura M., et al. Laparoscopic lumbar sympathectomy for lower-limb disease. Surg Endosc. 2002;16:500-503.
54. Chuang K.S., Liou N.H., Liu J.C. New stereotactic technique for percutaneous thermocoagulation of upper thoracic ganglionectomy in cases of palmar hyperhidrosis. Neurosurgery. 1988;22:600-604.
55. Wilkinson H.A. Percutaneous radiofrequency upper thoracic sympathectomy: a new technique. Neurosurgery. 1984;15:811-814.
56. Wilkinson H.A. Percutaneous radiofrequency upper thoracic sympathectomy. Neurosurgery. 1996;38:715-725.
57. Gybels J.M., Sweet W.H. Neurosurgical treatment of persistent pain: physiological and pathological mechanisms of human pain. Pain Headache. 1989;11:1-402.