Chapter 97 Surgery for Ventricular Arrhythmias
Surgical Methods for Controlling Ventricular Tachyarrhythmias
Indirect Methods
The first reported surgical cure of VT resulted from excision of a left ventricular aneurysm. When aneurysmectomy was used more widely as a treatment for VT, however, it became apparent that this procedure had low efficacy and high mortality rates (often because of intractable VT). CABG was also used in an attempt to control VT, with similarly poor results. These methods generally failed because although aneurysms are frequently present in patients with VT, the excised portion of the aneurysm does not contain critical portions of VT circuits, and VT episodes usually do not result from acute ischemia (which could be corrected by coronary bypass). CABG has definite application in relatively rare cases of polymorphic VT or VF that clearly occurs in the setting of acute ischemia. CABG and aneurysmectomy are both still performed in some cases of VT but only as adjunctive methods along with one of the more direct techniques discussed below. Neural modification, typically with left or bilateral stellate ganglionectomy, has benefit in selected patients with VTAs: patients with long QT syndrome (LQTS) who have recurrent syncope or cardiac arrest despite β-blocker and ICD therapies; patients with recurrent episodes of VF in the absence of SHD (idiopathic VF); and patients with catecholaminergic polymorphic VT. A variety of approaches have been used for the destruction or removal of the left or bilateral stellate ganglia (cervical, thoracotomy incisions). Thoracoscopic techniques are being more widely used for approaching the stellate ganglion, rather than open surgical exposure.1
Direct Methods
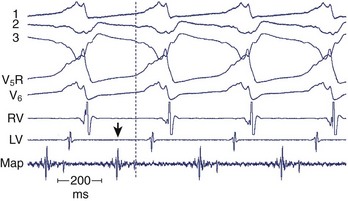
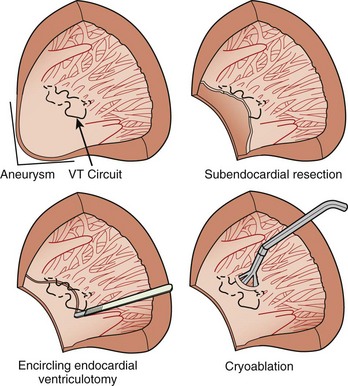
After the failure of CABG and aneurysmectomy to reliably affect VT recurrence, several investigators began using intraoperative electrophysiological mapping studies to understand more about where VT was originating. The rationale was that a site in the ventricle from which VT arose, or exited from a circuit, should precede the onset of each surface QRS complex by greater than 40 ms, or even occur in mid-diastole. Epicardial mapping during VT usually did not reveal sites of activation that met this criterion; endocardial mapping, after ventriculotomy, routinely showed sites activated prior to the QRS during VT (Figure 97-1). These areas were typically near the border between the aneurysm or the dense infarct and more normal muscle. Several means of therapy targeting these regions were devised in the late 1970s, including excision (endocardial resection) an isolating incision (encircling ventriculotomy), and cryoablation. Additional modalities have been used, including intraoperative laser photoablation. Because these hearts have already suffered extensive damage from the infarction that caused the arrhythmia, a cardinal principle of surgical therapy is to remove or destroy only as much tissue as is necessary to eliminate the VT while damaging as little remaining normal myocardium as possible. Mapping tools and techniques have aided in the discrimination between normally and abnormally functioning tissue.
Ventricular Tachycardia
Preoperative Studies
Because most patients undergoing surgery for VT have prior infarction from coronary atherosclerosis, cardiac catheterization with coronary arteriography is an important part of the preoperative evaluation. Coronary stenoses that should undergo bypass grafting can thus be identified and any possible valvular lesions identified. Ventriculography (assessing overall left ventricular function) can be helpful in estimating operative risk. In the presence of coronary stenoses of uncertain flow-limiting significance, stress testing with some form of perfusion imaging can help determine whether bypass grafting of particular arteries would be beneficial. Preoperative electrophysiological study (EPS) is almost always indicated (except in cases of severe left main stenosis or three-vessel disease, in which the rapid heart rates encountered during induced VT could possibly be detrimental). This study can provide information as to how readily inducible VT is as a guide to how vigorous intraoperative stimulation will need to be. Endocardial catheter mapping can also be performed at this time to help focus attention on certain areas that need to be ablated during surgery. However, if mapping is performed in this setting, it is almost always accompanied by ablation attempts; if the ablation successfully eliminates inducibility of one or more morphologies of VT, they no longer need surgical attention. If ablation is not successful, the validity of the mapping information may be called into question. Thus preoperative mapping may have a more limited role than was once thought. Percutaneous access to the pericardial space has been used for epicardial mapping and ablation; this is more frequently necessary in cardiomyopathic cases than in post-MI VT. Recently, a hybrid procedure has been developed in which epicardial catheter mapping has been facilitated by surgical exposure of the pericardial space using a small subxiphoid incision. This is particularly helpful when prior cardiac surgery has resulted in extensive pericardial adhesions that would significantly restrict catheter mobility and access to all areas of the epicardial surface.2
Principles of Cardiac Mapping
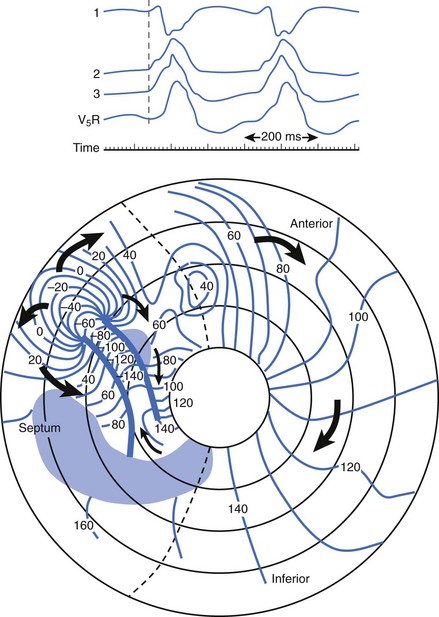
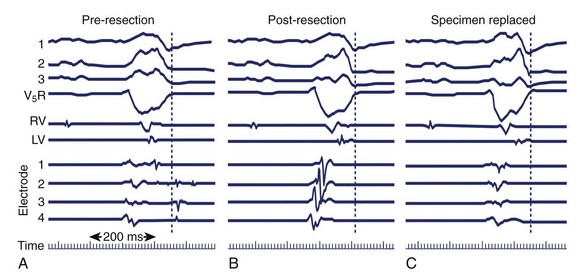
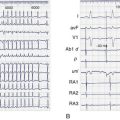
If mapping is to be performed, several pieces of equipment are necessary beyond the usual requirements for open-heart surgery. These include electrodes, a mapping system (consisting of amplifiers and a recording and analysis system), and a stimulator with which to initiate VT. Several commercial mapping systems are available, capable of recording from 64 to 256 electrodes simultaneously, online analysis of activation times, and generation of isochronal maps. Most record onto magnetic or optical media for data archiving. At the time of surgery, the electrocardiogram (ECG) leads from the patient are connected to the mapping system. The heart is exposed through a median sternotomy, and a pericardial cradle is formed. Cannulae are placed for cardiopulmonary bypass, and reference electrodes are inserted and tested. The heart is inspected for regions of infarct or aneurysm, through which the ventricle may be entered without damaging the viable myocardium; sometimes, this area is only evident after the patient is on cardiopulmonary bypass and the left ventricle (LV) has been vented. Cardiopulmonary bypass is then established while maintaining a perfusate temperature of 37° C to 38° C. This is necessary because cardiac electrical activity deteriorates at cooler temperatures and arrhythmias may not be inducible or mappable. Radiant heat loss of the heart surface during mapping may necessitate even slightly higher perfusate temperatures. Epicardial mapping during sinus rhythm or VT can be performed with the heart closed, but this is usually omitted in the interests of time unless specific circumstances suggest its usefulness. In most cases, once the heart-lung machine is running well, the LV is then opened through the previously identified infarct or aneurysm. The endocardial surface is inspected for the presence of any adherent thrombus, which is then removed. An electrode or multipolar electrode array is placed on the endocardial surface in an area of obvious scar tissue. At this time, sinus rhythm mapping can be performed to designate areas with abnormal electrograms indicating damaged myocardium; this step usually adds little and is omitted except in special situations. In most cases, VT is then initiated using previously placed electrodes, and endocardial mapping is performed during VT. Some tissue is activated at every instant during a re-entrant arrhythmia, even during the diastolic interval between discrete QRS complexes; sites from which diastolic activation is recorded during VT are of particular interest, since these regions are often in “protected” corridors that are critical for continued re-entry. Computerized mapping systems are able to quickly display these areas of diastolic activation (Figure 97-2). Because most patients with post-infarct VT have more than one ECG morphology of VT that may arise from the same or different regions, attempts are made to initiate and map other morphologies of VT. Because of time limitations, rigorous entrainment studies (overdrive pacing during VT) to validate the activation mapping data are often not performed. Once all inducible morphologies of VT have been mapped, the electrode array is removed and the ablation portion of the procedure begins. Several different procedures have been used for this purpose (Figure 97-3), including:
After the ablation portion, attempts are usually made to re-initiate VT with the same type of stimulation used during mapping. If VT can still be initiated, mapping is repeated; this usually reveals sites just outside the area ablated or deep to it (intramural). Ablation is performed again (often cryoablation of deeper sites), and cycles of stimulation, mapping and ablation are repeated until VT can no longer be induced. This technique results in postoperative noninducibility of VT approaching 90%. In inferior infarction, cryoablation of the isthmus of the ventricular myocardium between the ventriculotomy and the mitral annulus has increased surgical success rates.8 The ventriculotomy is closed and other procedures performed, such as CABG or valve surgery. If no other procedures are needed, the patient is weaned from cardiopulmonary bypass. Recent innovations in how the ventriculotomy is closed (aneurysmorrhaphy) have led to improvements in postoperative left ventricular function. Patients typically undergo EPS 5 to 7 days after surgery to assess its antiarrhythmic efficacy.
In some cases, no obvious site for making a ventriculotomy to enter the ventricle (no infarct or aneurysm) exists. This is especially true in inferior infarctions and cases in which early reperfusion of the infarct (pharmacologic or mechanical) yielded only a partial success. In these cases, an electrode-studded inflatable latex balloon can be inserted through the mitral orifice after a purse-string left atriotomy. Once the balloon is in the ventricular cavity, it is inflated such that its electrodes contact the endocardial surface. Mapping can then be carried out as indicated above but without ventriculotomy, and ablation can be performed either with electrical energy (DC shock or radiofrequency energy) delivered through appropriately located electrodes, or using a cryoprobe advanced through the mitral orifice as the array had been.9
Efficacy
The success rates for the various surgical ablation procedures vary according to the procedure as well as the definition of “success.” Most data indicate that elimination of all inducible VT at postoperative EPS is the most reliable endpoint of success and that simply eliminating the inducibility of certain VT morphologies (but not all VTs) will not prevent subsequent recurrences of other VTs. Measured against the more stringent standard of eliminating all inducible VTs, most of the procedures discussed above yield success rates from 70% to 90%.10
Operative Risk
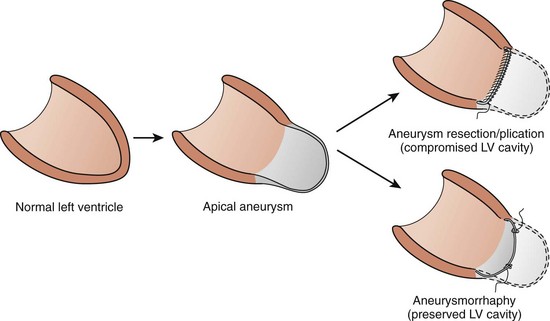
Because of pre-existing extensive myocardial damage, the operative mortality rates of VT surgery are higher than in other forms of elective cardiac surgery. Mortality rates range from 3% to 20% in different patient subsets. Because of this, several investigators have evaluated the risk factors for VT surgery and have identified left ventricular wall motion score, age greater than 65 years, and emergency surgery as factors that increase risk of operative mortality. A major cause of perioperative mortality is heart failure; in the early surgical experience, the aneurysm was removed (or plicated) and its cut edges sutured together. This could result in an inordinately small left ventricular cavity and compromise cardiac output. In the 1990s, a more physiological closure method, introduced by Dor and colleagues, more closely approximated the normal ventricular shape.11 Lower operative mortality rates and better functional capacity have been reported with this method (Figure 97-5).
Current Status
Ventricular Fibrillation
Patients in the first group, with very severe coronary stenoses, normal left ventricular function and wall motion, and no clinical, electrocardiographic or angiographic evidence of prior damage, appear to respond well to revascularization alone. Although a theoretical risk of recurrent VF is always present if graft occlusion or re-stenosis occurs, this has not been a reported clinical problem (with limited numbers of cases). Patients in the second group (with significant coronary stenoses, reduced left ventricular function and prior scar), for whom revascularization is planned, should undergo EPS prior to and following revascularization to determine whether ventricular arrhythmias are inducible with programmed stimulation. If VT or VF can be initiated prior to surgical revascularization but not following the procedure, the chance of arrhythmia recurrence is significantly lower than if the VT or VF can still be initiated postoperatively.12 Although data are limited, it appears that patients in this latter group (persistent arrhythmia inducibility following revascularization) should undergo ICD therapy as well since they have evidence for the ongoing presence of substrate for these arrhythmias. Some patients in this group with myocardial scar and VF have frequent recurrences of arrhythmia in the absence of significant ischemia; some of the patients have responded well to endocardial resection or cryoablation guided by mapping of fractionated electrograms during sinus rhythm. Patients in the third group (cardiomyopathy) cannot be expected to have antiarrhythmic benefit from revascularization and should undergo ICD therapy, as should patients in the fourth group (no evidence of structural disease), who have no other reliable means of preventing arrhythmia recurrences.
key References
Atallah J, Fynn-Thompson F, Cecchin F, et al. Video-assisted thoracoscopic cardiac denervation: A potential novel therapeutic option for children with intractable ventricular arrhythmias. Ann Thorac Surg. 2008;86(5):1620-1625.
Dor V, Sabatier M, Montiglio F, et al. Results of nonguided subtotal endocardiectomy associated with left ventricular reconstruction in patients with ischemic ventricular arrhythmias. J Thorac Cardiovasc Surg. 1994;107(5):1301-1307.
Guiraudon G, Fontaine G, Frank R, et al. Encircling endocardial ventriculotomy: A new surgical treatment for life-threatening ventricular tachycardias resistant to medical treatment following myocardial infarction. Ann Thor Surg. 1978;26:438-444.
Guiraudon GM, Thakur RK, Klein GJ, et al. Encircling endocardial cryoablation for ventricular tachycardia after myocardial infarction: Experience with 33 patients. Am Heart J. 1994;128(5):982-989.
Hargrove WC, Miller JM. Surgery for ischemic ventricular tachycardia—operative techniques and long-term results. Semin Thorac Cardiovasc Surg. 1989;1(1):83-87.
Hargrove WC, Miller JM, Vassallo JA, Josephson ME. Improved results in the operative management of ventricular tachycardia related to inferior wall infarction: Importance of the annular isthmus. J Thorac Cardiovasc Surg. 1986;92(4):726-732.
Josephson ME, Harken AH, Horowitz LN. Endocardial excision: A new surgical technique for the treatment of recurrent ventricular tachycardia. Circulation. 1979;60(7):1430-1439.
Kelly P, Ruskin JN, Vlahakes GJ, et al. Surgical coronary revascularization in survivors of prehospital cardiac arrest: Its effect on inducible ventricular arrhythmias and long-term survival [see comments. J Am Coll Cardiol. 1990;15(2):267-273.
Littmann L, Svenson RH, Chuang CH, et al. Neodymium:YAG contact laser photocoagulation of the in vivo canine epicardium: Dosimetry, effects of various lasing modes, and histology. Lasers Surg Med. 1993;13(2):158-167.
Mickleborough LL, Wilson GJ, Usui A, et al. Surgical treatment of ventricular tachycardia by balloon electric shock ablation: Potential effects on the mitral valve apparatus. J Thorac Cardiovasc Surg. 1992;103(4):629-637.
Page PL, Cardinal R, Shenasa M, et al. Surgical treatment of ventricular tachycardia: Regional cryoablation guided by computerized epicardial and endocardial mapping. Circulation. 1989;80(3):I124-I134.
Soejima K, Couper G, Cooper JM, et al. Subxiphoid surgical approach for epicardial catheter-based mapping and ablation in patients with prior cardiac surgery or difficult pericardial access. Circulation. 2004;110(10):1197-1201.
1 Atallah J, Fynn-Thompson F, Cecchin F, et al. Video-assisted thoracoscopic cardiac denervation: A potential novel therapeutic option for children with intractable ventricular arrhythmias. Ann Thorac Surg. 2008;86(5):1620-1625.
2 Soejima K, Couper G, Cooper JM, et al. Subxiphoid surgical approach for epicardial catheter-based mapping and ablation in patients with prior cardiac surgery or difficult pericardial access. Circulation. 2004;110(10):1197-1201.
3 Josephson ME, Harken AH, Horowitz LN. Endocardial excision: A new surgical technique for the treatment of recurrent ventricular tachycardia. Circulation. 1979;60(7):1430-1439.
4 Guiraudon G, Fontaine G, Frank R, et al. Encircling endocardial ventriculotomy: A new surgical treatment for life-threatening ventricular tachycardias resistant to medical treatment following myocardial infarction. Ann Thor Surg. 1978;26:438-444.
5 Page PL, Cardinal R, Shenasa M, et al. Surgical treatment of ventricular tachycardia: Regional cryoablation guided by computerized epicardial and endocardial mapping. Circulation. 1989;80(3):I124-I134.
6 Guiraudon GM, Thakur RK, Klein GJ, et al. Encircling endocardial cryoablation for ventricular tachycardia after myocardial infarction: Experience with 33 patients. Am Heart J. 1994;128(5):982-989.
7 Littmann L, Svenson RH, Chuang CH, et al. Neodymium:YAG contact laser photocoagulation of the in vivo canine epicardium: Dosimetry, effects of various lasing modes, and histology. Lasers Surg Med. 1993;13(2):158-167.
8 Hargrove WC, Miller JM, Vassallo JA, Josephson ME. Improved results in the operative management of ventricular tachycardia related to inferior wall infarction: Importance of the annular isthmus. J Thorac Cardiovasc Surg. 1986;92(4):726-732.
9 Mickleborough LL, Wilson GJ, Usui A, et al. Surgical treatment of ventricular tachycardia by balloon electric shock ablation: Potential effects on the mitral valve apparatus. J Thorac Cardiovasc Surg. 1992;103(4):629-637.
10 Hargrove WC, Miller JM. Surgery for ischemic ventricular tachycardia—operative techniques and long-term results. Semin Thorac Cardiovasc Surg. 1989;1(1):83-87.
11 Dor V, Sabatier M, Montiglio F, et al. Results of nonguided subtotal endocardiectomy associated with left ventricular reconstruction in patients with ischemic ventricular arrhythmias. J Thorac Cardiovasc Surg. 1994;107(5):1301-1307.
12 Kelly P, Ruskin JN, Vlahakes GJ, et al. Surgical coronary revascularization in survivors of prehospital cardiac arrest: Its effect on inducible ventricular arrhythmias and long-term survival [see comments. J Am Coll Cardiol. 1990;15(2):267-273.