Surgery for Ventricular Arrhythmias
Historical Perspective
In the past, open-heart surgery was not uncommonly used to treat refractory ventricular arrhythmias.1 The predominant population subjected to this management strategy was comprised of patients with healed myocardial infarcts who experienced sustained ventricular tachycardia (VT). The original technique used resection of the dyskinetic/akinetic scarred myocardial tissue (aneurysmectomy) with only modest success (~40%) for long-term arrhythmia control.2 Subsequent mapping studies revealed that critical components of the VT reentrant circuit used the border zone between the dense scar and healthy myocardium. This resulted in the development of subendocardial resection (removing tissue up to a depth of 2-4 mm in the area surrounding the dense aneurysm).3 By using this approach with or without aneurysmectomy, the overall success rate for long-term arrhythmia control improved to 90%.4 Subsequent innovations in mapping tools (multielectrode epicardial shock and endocardial basket) allowed operators to better characterize VT circuits for more effective surgical ablation.5 Nevertheless, a major limitation of the technique remained its highly invasive nature (median sternotomy with cardiopulmonary bypass and ventriculotomy).6 Furthermore, because it was typically performed on sick patients with impaired cardiac function (who experienced recurrent ventricular arrhythmias refractory to multiple antiarrhythmic drugs), there was high periprocedure mortality rate.1,4,5,7 For these reasons, the technique was never widely adopted and it remained confined to a few centers/operators specializing in VT surgery. With the invention of implantable cardioverter defibrillators (ICDs), there was a shift away from surgical VT ablation. Furthermore, because ICDs could be implanted outside the operating room (OR), this technique was widely adopted. At the same time that ICD platforms were becoming smaller and more sophisticated, there were major advances in percutaneous catheter mapping and ablation techniques. The latter allowed electrophysiologists to replicate the surgical VT experience by using a significantly less invasive approach.8 These developments have resulted in the decline of surgical VT ablation, which nowadays is used only rarely. Nevertheless, in a select group of patients, surgical ablation is still an important option for treating refractory ventricular arrhythmias.
Indications: Current and Emerging
Currently, surgical VT ablation is generally the treatment of last resort reserved primarily for patients who have failed a combination of antiarrhythmic drugs and percutaneous catheter ablation attempts.8,9 The experience at the Hospital of The University of Pennsylvania is consistent with this. Over a 3-year period (2007 to 2009), 527 patients underwent 644 VT ablation procedures; structural heart disease was present in 295 (56%). Of these 295, 144 patients (49%) had heart disease categorized as nonischemic based on the lack of a prior infarct and absence of coronary disease. In 8 of these patients (1.5%; 7 men), all with a nonischemic substrate (median left ventricular ejection fraction of 35%), the arrhythmia remained refractory to medications and endocardial/epicardial percutaneous radiofrequency (RF) ablation attempts. These patients eventually underwent surgical VT ablation for arrhythmia control. Six had dilated cardiomyopathy and 2 had longstanding hypertrophic cardiomyopathy.10 In the latter group, arrhythmia was found to originate from the midmyocardium (identified by magnetic resonance imaging [MRI]) of the basal left ventricle (LV), which was markedly thickened (>20 mm). This was impossible to target effectively by percutaneous catheter ablation from the endocardium or epicardium. Consistent with those observations, case reports from other investigators also suggest that ventricular arrhythmias arising from the basal interventricular septum (IVS) are challenging to ablate percutaneously.11 Thus, in patients with VT features manifesting on an electrocardiogram (ECG) that suggest origin from the IVS region, attempts should be made a priori to identify septal scar using MRI, positron emission tomography scan, and transthoracic and/or intracardiac echocardiography, among others (Figure 129-1). Typical ECG features suggesting VT origin from the IVS region include (1) left bundle branch block morphology with superior or inferior axis and early precordial transition (before lead V3) or (2) right bundle branch block morphology with inferior or superior axis and an unusual precordial transition pattern with lead V2 manifesting predominantly negative complexes (rS or QS) compared with leads V1 and V2 (Figure 129-2).12,13 In these cases, especially if a scar is identified in the IVS, it is prudent to make the patient aware of the possibility that percutaneous catheter ablation may not be successful. Similarly, in patients with hypertrophied LV who have ventricular arrhythmias in the setting of midmyocardial scar, consideration should be given to a surgical option if an attempt at percutaneous catheter ablation fails.10 In addition to this, surgical ablation of ventricular arrhythmias should be considered in patients who are undergoing open-heart surgery for other cardiac conditions, such as valve surgery or coronary artery bypass grafting, and have manifested recurrent VT despite antiarrhythmic drug (AAD) therapy and/or percutaneous catheter ablation attempts.
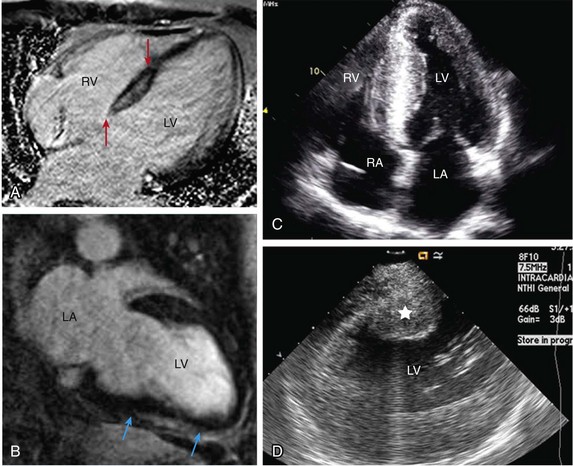
Figure 129-1 Septal scar identified by MRI and echocardiographic imaging . A, Proximal interventricular scar (red arrows). B, Scar in the distal interventricular distribution extending to the apex (blue arrows) identified by MRI. C, Apical 4-chamber transthoracic echocardiographic view showing thickened septum with marked echogenicity representing infiltrative disease process. D, Thickened proximal interventricular septum (star) identified using intracardiac echocardiography in a patient with hypertrophic cardiomyopathy. In all these patients, refractory ventricular arrhythmias originated from the septal substrate, which was targeted during surgical ablation. LA, left atrium; RA, right atrium; RV, right ventricle; LV, left ventricle.
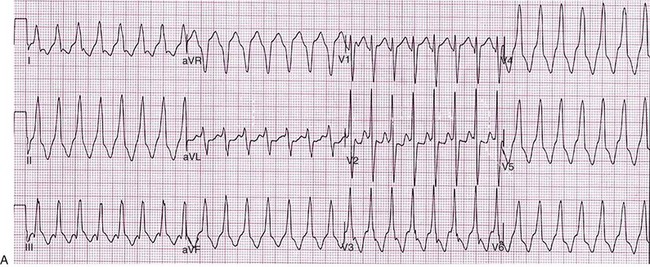
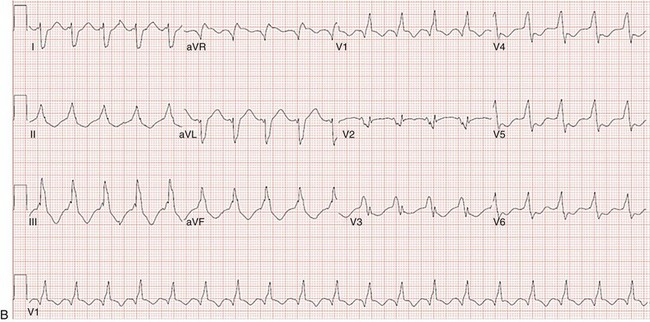
Figure 129-2 A, 12-lead ECG of VT manifesting a left bundle branch block morphology and inferior axis with early transition (≤V3). B, 12-lead ECG of VT manifesting a right bundle branch block morphology, inferior axis, and unusual transition pattern (predominantly positive forces in leads V1, V3-V6 but negative forces in lead V2). These features are characteristic of VT originating from the interventricular septal region.
Accessing the Ventricle: Approaches
Adequate access to all cardiac surfaces is critical to the success of surgical VT ablation. Median sternotomy typically provides the best visualization of the entire heart.1,4,10 The epicardial aspect of the right ventricle (RV), superior IVS, and anterior LV wall are well-visualized through median sternotomy without additional cardiac manipulation (Figure 129-3). However, to adequately inspect the posterolateral LV wall, the heart has to be physically lifted. Although this can cause a drop in cardiac output and blood pressure, proper pericardial and cardiac manipulation as conducted during off-pump coronary artery bypass grafting can facilitate good exposure of the posterolateral LV for inspection and mapping if necessary.14 Actual ablation, particularly cryoablation, is typically performed with cardiopulmonary bypass on an arrested heart to avoid the energy sink of warm blood within the heart. To access the LV endocardium, there are several options. The transaortic approach offers the best visualization of the basal LV, including the IVS region as well as the anterior and lateral LV walls.14,15 To achieve this, a standard aortotomy for aortic valve replacement is performed above the sinotubular junction.10 The aortic valve leaflets are carefully retracted against the sinus of Valsalva and the LV outflow tract is exposed (see Figure 129-3). The IVS is seen immediately under the right coronary aortic valve (AV) cusp. The basal anterior and lateral LV endocardium is visualized under the junction of right-left commissures and the left coronary cusp, respectively (see Figure 129-3). A transatrial, trans–mitral valve (MV) approach offers the best visualization of both papillary muscles, the posterior LV endocardium, and the LV apex.14,16,17 These structures are frequently involved in VT circuits in patients with healed inferior infarcts and can also be the source of VT in patients with nonischemic cardiomyopathy. In patients with mechanical AV and MV, apical ventriculotomy has been used to access the LV endocardium.18 In patients undergoing surgical ventricular aneurysm repair, the site of aneurysmectomy can also be used to access the LV endocardium before aneurysm resection and closure. The RV too can be accessed either via a transatrial, trans-tricuspid valve (TV) approach or directly through the RV free wall.14,18 An important difference between epicardial alone versus additional endocardial cardiac access pertains to cardiopulmonary bypass use.19 When the arrhythmia originates from the RV free wall or the inferior IVS region, access to these locations can be obtained by partial sternotomy, sparing the upper half of the sternum (see Figure 129-3).20 The purported advantages of this approach are shorter postprocedure recovery and more stable sternal healing. The authors have used this approach successfully in a patient undergoing ablation for refractory VT originating from the inferior IVS region and the anterior epicardial LV surface. There are also case series describing access to the epicardium using a surgical window via an epigastric incision after removal of the xyphoid process, or a left anterior thoracotomy using a limited left anterior incision extending from the third to fifth intercostal spaces. Both these techniques have been successfully accomplished in the cardiac electrophysiology (EP) laboratory. However, using this approach in the EP laboratory, only the epicardial aspect of the inferior, anterior, and parts of the lateral cardiac surfaces were accessed.21
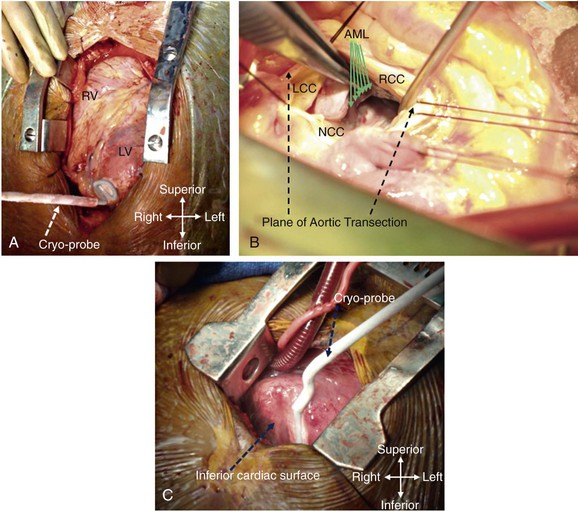
Figure 129-3 Examples of different techniques to expose various cardiac surfaces during surgical VT ablation. A, Cardiac exposure via median sternotomy. B, Transaortic access to LV endocardium. C, Cardiac exposure via partial (inferior) sternotomy. In (A) and (C), the cryoprobe can be seen deployed at the LV apex and the inferior interventricular septal region, respectively. RV, right ventricle; LV, left ventricle; LCC, left coronary cusp; RCC, right coronary cusp; NCC, noncoronary cusp; AML, anterior mitral leaflet.
Mapping: Tools and Techniques
Early attempts at mapping ventricular arrhythmias in the OR used finger-mounted bipolar electrodes that were manipulated to various locations in the chamber of interest. This method was adequate for identifying critical components of the reentrant circuit in patients with healed infarcts.3,4 Subsequently, multipolar meshes and basket catheters were developed, which had the ability to record electrical activity at a higher resolution than the finger-mounted bipolar electrode(s) and thus could provide more comprehensive understanding of the activation sequence during ventricular arrhythmias.22,23 However, interpretation of data required special signal processing, which made these tools cumbersome to use in the OR. With the development of electroanatomic mapping (EAM) techniques, electrophysiologists are now able to create a three dimensional shell of the chamber of interest during ongoing arrhythmia.24 This tool provides more intuitive visualization of the tachycardia circuit vis-à-vis the underlying anatomy and substrate. EAM requires a low-intensity magnetic field around the patient, which requires a special setup. In hybrid ORs that are specifically designed for both percutaneous catheter and open-heart surgical interventions, use of advanced cardiac EP mapping tools including EAM is possible.25 There are case reports describing the use of EAM in hybrid ORs during surgical VT ablation.11,18 To accomplish this, the magnet is set up under the operating table, reference electrodes are sutured to the epicardial surface of either ventricle (exposed via sternotomy), and the mapping catheter is manipulated on or within the chamber of interest. However, to sustain and map ventricular arrhythmias in the OR, the heart usually has to be actively “beating.” In some cases, ventricular arrhythmias can be induced and sustained with the heart connected to cardiopulmonary bypass, but without aortic cross-clamping. However, in these instances, the cardiac temperatures on bypass need to be maintained in the normothermic range. Furthermore, if cryothermy is used for ablation, then the most effective lesions are created on a cooled, cardioplegia-arrested heart. However, it is impossible to sustain ventricular arrhythmias during cold cardioplegia.10,11,22 Even without using cold cardioplegia, reproducible induction and maintenance of the clinical arrhythmia(s) during open heart surgery (in patients under deep anesthesia) can be challenging. The loss of autonomic tone as well as the effects of various anesthetic agents coupled with under-filled hearts can make this hard. An alternative to mapping ventricular arrhythmias in the OR is to perform detailed mapping of these a priori in the cardiac EP laboratory. This is the strategy used for all patients who have undergone surgical VT ablation recently at the authors’ institution.10 In each case, detailed endocardial and, when indicated, epicardial mapping of the arrhythmia was performed in the EP laboratory. Using activation, entrainment, and EAM, the critical components of the reentrant circuit and/or the site of origin of VT (vis-à-vis the underlying substrate) were characterized. These locations were then targeted by conventional RF energy. Although percutaneous RF ablation in these cases was not effective in suppressing the arrhythmias, in the OR the RF lesions were identifiable and served as targets for cryothermy application (Figure 129-4). This precluded the need for additional arrhythmia induction or mapping during open-heart surgical ablation. In the OR (under cold cardioplegia), the cryoprobe was positioned over the RF lesion site and full duration (3 minutes) cryothermy application was made. This procedure typically resulted in a large lesion the induration from which could be palpated on the opposing surface (see Figure 129-4). Thus, cryolesions delivered on the endocardial surface could be palpated from the epicardial aspect and vice versa. As a result, it is possible to make an additional cryothermy application on the opposing cardiac surface to ensure a transmural lesion that would extend through the entire thickness of the targeted LV myocardium.
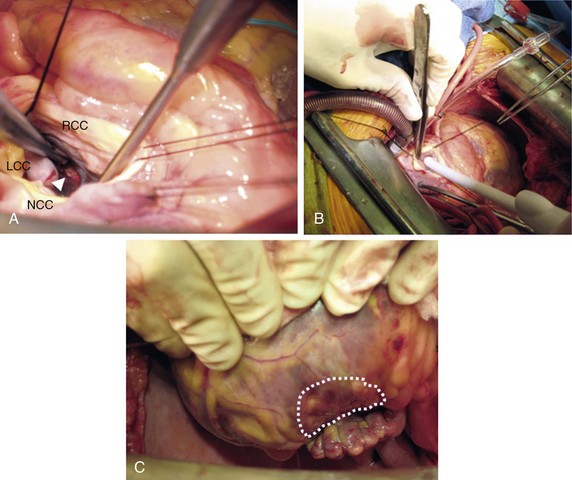
Figure 129-4 The authors’ approach to surgical VT ablation by cryothermy. A, Example in which RF lesions delivered during percutaneous mapping and ablation in the cardiac electrophysiology laboratory on the posterolateral LV endocardial surface were identifiable at the time of surgical ablation (7 days later; white arrowhead). B, This location being targeted by cryothermy (transaortic deployment of cryoprobe tip). The induration created by cryothermal application (3-minute lesion) at this endocardial location was easily palpable on the corresponding epicardial surface (C, dotted enclosed area).
Lesion Creation: Energy Sources
Several energy sources have been used to create effective lesions during surgical ablation of ventricular arrhythmias. These include RF energy, cryothermy, microwave, laser energy, etc. Among these, the largest experience has been with cryothermy. The cryoablation technology used earlier during surgical VT ablation used a stiff handheld probe that was able to achieve tissue temperatures of −60° C.22 Although this was found to be adequate for targeting VT circuits around the aneurysm of healed infarcts, it likely created lesions no larger than 5 mm. Thus, for deeper intramural VT circuits/sources, this may not be an effective energy source.11 In comparison, the newer-generation cryoablation platform can cool to much lower temperatures (−150° C) and is capable of creating larger and deeper lesions (up to 60 mm). In our recent surgical VT ablation experience, the Surgifrost Surgical Cryoablation System (Medtronic CryoCath LP, Quebec, Canada) was used.10 This system uses a flexible metal probe with an adjustable insulation sheath and can be molded to conform to cardiac contours. The system uses Argon gas to achieve rapid cooling to a temperature of −150° C. The standard duration of cryoapplication with this unit is for a maximum of 3 minutes and comprises both the cooling and thawing phases. The best results are achieved under cold cardioplegia, which allows the probe to achieve the lowest tissue temperatures. The malleable probe can be configured into various shapes, which is helpful for ablating at different cardiac locations and surfaces. The entire length of the probe is positioned to create linear lesions when targeting large epicardial scars in the IVS region (Figure 129-5). In instances when the epicardial source of VT is more focal, the probe can be coiled to create large circular lesions (see Figure 129-5). For targeting VT circuits and sources endocardially, just the tip of the probe (without any modifications) can be deployed at the ablation site to create smaller, more precise lesions (see Figure 129-4). Because the entire length of the probe cools during energy application, care has to be taken to avoid making contact of the proximal end of probe with critical cardiac structures (e.g., valve leaflets, conduction system), which can sometimes be frozen inadvertently. This is particularly important when the probe is deployed across the aortic or mitral valves (see Figure 129-4).
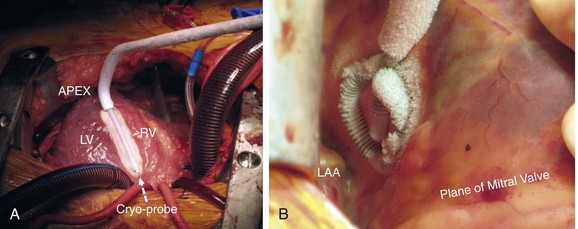
Figure 129-5 Two examples of cryothermy application using the Surgifrost Surgical Cryoablation System (Medtronic CryoCath LP, Quebec, Canada). A, The entire length of the probe (white interrupted arrow) is positioned over the anterior interventricular septum to create a linear lesion. In (B), the probe has been coiled to create a circular lesion over the posterolateral LV surface. During cryothermy application, the entire length of the probe, including the insulation sheath, becomes excessively cold and care has to be taken to avoid contact of the latter with critical cardiac structures. LV, left ventricle; RV, right ventricle; LAA, left atrial appendage.
Conventional RF energy has been infrequently used to create lesions during surgical VT ablation. Typically, RF ablation requires a generator, delivery platform, and impedance pads. These may be cumbersome to deploy in the OR setting. Furthermore, creating lesions using resistive heating may not be as effective in cooled hearts. For these reasons, RF energy is nowadays the preferred modality for surgical ablation of ventricular arrhythmias. In the recent literature, there is only one case series describing successful targeting of VT originating from the posteromedial papillary muscle in two patients with healed infarcts using monopolar RF energy delivered through a cool-tip ablation catheter.17 Laser energy (Nd-YAG, pulsed Argon) has been used for surgical VT ablation in patients with healed infarcts.26,27 In a single-center experience that used pulsed Argon laser energy source, this technique was found to be helpful in targeting VT originating from all LV locations, with excellent long-term arrhythmia-free survival (90% freedom from VT or death at 1 year).27 However, it remains unclear why this technology is no longer used for surgical VT ablation. Microwave has also been used to create cardiac lesions.28 This technology has been used for isolating pulmonary veins and creating linear lesions in patients undergoing surgical AF ablation. A recent case report has also described the efficacy of this technology during surgical VT ablation.29
Although cryothermy seems to be the most popular energy source in patients undergoing surgical ablation of ventricular arrhythmias, there are no studies that have compared the efficacy of this source with other ablation platforms in this patient population. On the basis of the surgical VT ablation experience at the authors’ center, cryothermy is certainly capable of creating effective lesions.10 However, to create large lesions, the probe needs to be able to cool to a temperature of −150° C. This is best achieved in cold and immobile hearts. Thus, cold cardioplegia is essential for effective cryothermy during surgical ablation of ventricular arrhythmias.
Acute End Points of Ablation and Postsurgical Arrhythmia Management
The acute end points of surgical VT ablation are determined to a large extent by the mapping strategy used. In cases where the arrhythmia is sustained and mapped during surgical ablation, VT termination and noninducibility should be the end point(s) for procedural success. However, as was pointed out earlier, this usually requires a beating heart setup or euthermic cardiac temperatures. In situations where cryothermy is used to create lesions under cold cardioplegia, VT may be impossible to induce or maintain. In these instances, after the lesion set has been completed, the heart is allowed to rewarm and VT induction can then be attempted by programmed electrical stimulation (PES). Whenever possible, the authors have used this strategy in their surgical VT experience.10 In patients with ICDs, PES can be easily performed in the OR through the device. In subjects without ICDs, this can be accomplished by temporarily suturing an electrode to the cardiac surface. However, PES in the OR can be challenging for several reasons: (1) In patients undergoing additional cardiac procedures (valve or bypass surgery) this may not be possible to do immediately after the surgical VT ablation; (2) in situations where there is acute decompensation during the surgical procedure, VT induction may not be clinically advisable; (3) other variables (e.g., anesthetic agents, autonomic tone, cardiac filling) can effect arrhythmia induction; (4) lack of standard 12-lead ECG recording may make it difficult to compare arrhythmia induced in the OR with the clinical arrhythmia; and (5) arrhythmias induced in the OR may be nonspecific. An alternative method to assess ablation efficacy may be to evaluate physical properties of the targeted tissue. As previously discussed, lesions created in the OR by cryothermy are transmural and cause marked induration, which is palpable from both the endocardial and epicardial surfaces. Effective lesions should also result in alteration in the electrical properties of the tissue. Thus, inability to capture the ablated site at high pacing outputs can serve as an end point for effective lesion creation. In some situations in which lesions are delivered across reentrant channels, one can test for the presence of postablation conduction block across the targeted channel.22 Although whenever possible, arrhythmia induction in the OR should be attempted, the authors also found PES beyond the acute phase to be useful for predicting long-term success. In patients with ICDs who underwent VT ablation at their center, despite acute procedural success (inability to induce VT at the end of the procedure), they performed noninvasive programmed stimulation (NIPS; through the ICD) 48 to 72 hours after surgery. They found that lack of VT induction 48 to 72 hours after the ablation procedure is a strong predictor of long-term VT-free survival.30
Although surgical ablation is successful for treating refractory clinical VT, patients may experience other arrhythmias (e.g., AF, premature ventricular complexes, nonsustained VT) early after the procedure.31 Thus it is not uncommon for these patients to be maintained on AAD therapy immediately after surgery. In the authors’ surgical VT ablation series (eight patients), only one patient who had idiopathic VT originating from the superior basal IVS region was discharged without AADs after successful ablation. The remaining subjects were all prescribed at least one AAD initially, which were subsequently tapered and discontinued.10 Attempts should be made to modify AAD regimen acutely after the procedure, either by changing patients who are on multiple agents to a single drug or reducing the dose if the patients are only taking one AAD. For patients who have been on extended amiodarone therapy, the authors’ practice after successful surgical VT ablation is to switch to another AAD. In patients who have sustained refractory ventricular arrhythmias (not the original clinical VT) early after the surgical ablation, consideration should be given to catheter mapping and ablation. In some instances after surgical ablation, a different VT (previously not seen) can become manifest and this may be amenable to conventional percutaneous ablation. In the authors’ experience, early after the surgical VT ablation (≤1 month), the pericardial space is accessible, and so if indicated, percutaneous epicardial mapping and ablation are feasible. However, beyond this period, pericardial adhesions may form and then epicardial access can be challenging.
Complications
Surgical VT ablation is a highly invasive procedure. Furthermore, it is usually performed on subjects who are experiencing VT that is refractory to multiple AADs and percutaneous catheter ablation attempts. Not infrequently, these patients require additional cardiac interventions (e.g., valve replacement or repair, coronary artery bypass grafting) at the time of the surgical ablation. These patients also have concomitant heart failure. For all of these reasons, procedure-related complications are not uncommon in this group of patients. The majority of adverse events seen in patients undergoing surgical VT ablation are similar to those observed in patients undergoing open-heart surgery for other indications. These include infection, altered mentation, worsening heart failure, prolonged inotropic support, persistent hypoxemia, and arrhythmias.31 Complications that are specific to the surgical ablation itself are few and include:
• Ventricular arrhythmias—as previously discussed, surgical VT ablation can sometimes be proarrhythmic and, in rare instances, patients may have worsening ventricular arrhythmias immediately after.10,32 If these are nonsustained and do not cause hemodynamic compromise, then a combination of AADs is sufficient to control them. However, if these arrhythmias become sustained then patients may need extra corporeal membrane oxygenation support or a ventricular assist device to maintain end-organ perfusion. In these instances, consideration should also be given to percutaneous catheter ablation. For sustained ventricular arrhythmias that remain refractory to all of these interventions, cardiac transplantation may be the only remaining option. Patients with acute sustained ventricular arrhythmias after surgical VT ablation have the worst prognosis, and early mortality in this group can be as high as approximately 50%.2,5,10
• Worsening heart failure—this is not uncommon after surgical VT ablation for several reasons: A majority of patients undergoing the procedure have baseline LV dysfunction, surgical ablation lesions can worsen LV function, myocardial contractility may be suppressed after cold cardioplegia, etc. In this context, precise lesion creation is critical to minimizing ablation-related worsening of LV function. This is best achieved by targeting critical components of the VT circuit identified by either mapping in the OR or mapping a priori in the EP laboratory. The latter is an effective strategy in patients with nonischemic cardiomyopathy in whom the scar is patchy and not easily identifiable visually during open heart surgery.10,13,33 The overall occurrence of complications in our recent surgical VT ablation experience is 25%.10 Two patients died during the index hospitalization (at 6 and 10 weeks after the surgical ablation): one from progressive heart failure and second from sepsis. In the remaining six patients, the median time from surgery to discharge was 7 days (range, 5 to 11 days).
Long-Term Expectations
The most extensive long-term outcome experience of surgical VT ablation is in patients who underwent the procedure 20 to 30 years earlier for refractory VT in the setting of healed myocardial infarcts.2,3,7,34,35 The ablation strategy in the majority of these cases comprised aneurysmectomy and subendocardial resection. More recently, surgical ablation has been used to successfully treat refractory VT in patients with nonischemic cardiomyopathy.10 However, this constitutes a relatively small group of patients with an average postprocedure follow-up duration of approximately 24 months. Despite this limitation, the outcome data in these patients are encouraging. Of the six patients from a cohort of eight subjects, all of whom had nonischemic cardiomyopathy and underwent surgical VT ablation (two patients died during the index hospitalization after the ablation) over a mean follow-up of 23 ± 6 months (range, 15 to 34 months), four patients remained free of VT, one patient had a single episode of VT resulting in one ICD shock, and one patient had three episodes of VT, all occurring during the first 3 postoperative months, but none over the remaining 12 months of follow-up (without modification of medical therapy).10 Overall, there was a significant reduction in the burden of VT and ICD therapies. The number of ICD shocks per patient declined from 6.6 shocks in the preceding 3 months before surgery to 0.6 shocks during the first 3 postsurgical months (P < .01). In another series, 1-year survival after surgical VT ablation was 80%. However, over a 5-year period, there was a steady decline in survival so that at the fifth year, 25% of the subjects had died.35 Although these survival data are sobering, they are comparable with 5-year survival data in patients with impaired LV function and heart failure who have undergone open-heart surgery for other indications. Thus, if it is successful, surgical VT ablation can improve long-term mortality in an otherwise sick group of patients who may not have survived acutely without this intervention.7,35
Future Developments
Despite the overall success of surgical ablation in treating refractory ventricular arrhythmias, the procedure can be challenging. A major limitation of surgical VT ablation remains the ability to induce and map clinical arrhythmias during the procedure. Thus any development that can facilitate ventricular arrhythmia mapping in the OR is highly desirable. It is possible that hybrid rooms, which are capable of supporting percutaneous interventions and open-heart surgical procedures, may help accomplish this. In such a setup, it is conceivable to induce and map the arrhythmia using the same tools as in the EP laboratory (intracardiac multipolar electrode recordings and EAM), albeit with an open heart.36 Using this approach, the critical parts of the VT circuit and/or site of origin may be better identified. However, targeting these critical locations in the OR effectively would still require cold cardioplegia, particularly when using cryothermy. Development of energy sources and platforms that can create effective lesions without the need for cold cardioplegia is also highly desirable because it will enhance the ability to ablate during an ongoing arrhythmia. This can provide a more rigorous and easily reproducibly end point of arrhythmia termination during lesion creation and inability to induce the targeted arrhythmia afterward. Another improvement in the surgical ablation technique would be the ability to map and ablate ventricular arrhythmias using a less invasive approach (without median sternotomy). This has been accomplished for AF ablation, which can now be performed by minimally invasive surgery. However, the challenge during ablation of refractory VA remains the need to target areas of the heart that typically require full sternotomy for the best visualization. However, certain areas of the heart (inferior IVS regions, anterior LV wall, RV) can be accessed by partial sternotomy. Additional innovations that can allow us to map and successfully ablate VA arising from other cardiac locations using minimally invasive surgical techniques are also desirable because they may help reduce procedure-related morbidity.
References
1. Chen, WY, Lai, ST, Shih, CC. Endoaneurysmorrhaphy and cryoablation for post infarction left ventricular aneurysm with ventricular tachycardia. J Chin Med Assoc. 2007; 70(3):117–120.
2. Sami, M, Chaitman, BR, Bourassa, MG, et al. Long term follow-up of aneurysmectomy for recurrent ventricular tachycardia or fibrillation. Am Heart J. 1978; 96:3030–3308.
3. Josephson, ME, Harken, AH, Horowitz, LN. Endocardial excision: a new surgical technique for the treatment of recurrent ventricular tachycardia. Circulation. 1979; 60:1430–1439.
4. Horowitz, LN, Harken, AH, Kastor, JA, et al. Ventricular resection guided by epicardial and endocardial mapping for treatment of recurrent ventricular tachycardia. N Engl J Med. 1980; 302:589–593.
5. Wellens, F, Geelen, P, Demirsoy, E, et al. Surgical treatment of tachyarrhythmias due to post infarction left ventricular aneurysm with endoaneurysmorrhaphy and cryoablation. Eur J Cardiothorac Surg. 2002; 22:771–776.
6. Haines, DE, Lerman, BB, Kron, IL, et al. Surgical ablation of ventricular tachycardia with sequential map-guided subendocardial resection: electrophysiologic assessment and long-term follow-up. Circulation. 1988; 77:131–141.
7. Matthias Bechtel, JF, Tolg, R, Graf, B, et al. High incidence of sudden death late after anterior aneurysm repair. Eur J Cardiothorac Surg. 2004; 25:807–811.
8. Aliot, EM, Stevenson, WG, Almendral-Garrote, JM, et al. EHRA/HRS Expert Consensus on Catheter Ablation of Ventricular Arrhythmias: developed in a partnership with the European Heart Rhythm Association (EHRA), a Registered Branch of the European Society of Cardiology (ESC), and the Heart Rhythm Society (HRS); in collaboration with the American College of Cardiology (ACC) and the American Heart Association (AHA). Heart Rhythm. 2009; 6(6):886–933.
9. Zipes, DP, Camm, AJ, Borggrefe, M, et al. ACC/AHA/ESC 2006 Guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines (writing committee to develop Guidelines for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death): developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation. 2006; 114:e385–e484.
10. Anter, E, Hutchinson, MD, Deo, R, et al. Surgical ablation of refractory ventricular tachycardia in patients with non-ischemic cardiomyopathy. Circ Arrhythm Electrophysiol. 2011; 4:494–500.
11. Bhavani, SS, Tchou, P, Chung, M, et al. Intra-operative electro-anatomic al mapping and beating heart ablation or ventricular tachycardia. Ann Thorac Surg. 2006; 82:1091–1093.
12. Bajan, V, Gerstenfeld, E, Garcia, F, et al. Site specific twelve lead ECG features to identify an epicardial origin for left ventricular tachycardia in the absence of myocardial infarction. Heart Rhythm. 2007; 4(11):1403–1410.
13. Haqqani, HM, Tschabrunn, CM, Tzou, WS, et al. Isolated septal substrate for ventricular tachycardia in nonischemic dilated cardiomyopathy: incidence, characterization and implications. Heart Rhythm. 2011; 8(8):1169–1176.
14. Mill, MR, Wilcox, BR, Anderson, RH. Surgical anatomy of the heart. In: Cohn LH, ed. Cardiac Surgery in the Adult. New York: McGraw-Hill; 2008:29–50.
15. Mihaljevic, T, Sayeed, MR, Stamou, SC, et al. Pathophysiology of aortic valve disease. In: Cohn LH, ed. Cardiac Surgery in the Adult. New York: McGraw-Hill; 2008:825–840.
16. Fann, JI, Ingels, NB, Jr., Miller, DC. Pathophysiology of mitral valve disease. In: Cohn LH, ed. Cardiac Surgery in the Adult. New York: McGraw-Hill; 2008:973–1012.
17. Rubino, AS, Onorati, F, Serraino, GF, et al. Transmitral approach to monopolar radiofrequency ablation of inferior papillary muscle for refractory ischemic ventricular tachycardia. Tex Heart Inst J. 2010; 37(3):371–372.
18. Bhawani, SS, Tchou, P, Saliba, W, et al. Surgical options for refractory ventricular tachycardia. J Card Surg. 2007; 22:533–534.
19. Hammon, JW. Extracorporeal Circulation: organ damage. In: Cohn LH, ed. Cardiac Surgery in the Adult. New York: McGraw-Hill; 2008:389–414.
20. Chitwood, WRJ, Rodriguez, E. Minimally invasive and robotic mitral valve surgery. In: Cohn LH, ed. Cardiac Surgery in the Adult. New York: McGraw-Hill; 2008:1079–1100.
21. Michowitz, Y, Mathuria, N, Tung, R, et al. Hybrid procedures for epicardial catheter ablation of ventricular tachycardia: value of surgical access. Heart Rhythm. 2010; 7:1635–1643.
22. Mavroudis, C, Deal, BJ, Backer, CL, et al. Arrhythmia surgery in patients with and without congenital heart disease. Ann Thorac Surg. 2008; 86:857–868.
23. Van Dessel, PF, Van Hemel, NM, Van Swieten, HA, et al. Successful surgical ablation of sustained ventricular tachycardia associated with mitral valve prolapse guided by a multielectrode basket catheter. PACE. 2001; 24:1029–1031.
24. Dixit, S, Callans, DJ. Role of contact and non-contact mapping systems in the treatment of cardiac arrhythmias. Curr Opin Cardiol. 2002; 17(1):65–72.
25. Krul, SP, Driessen, AH, van Bowen, WJ, et al. Thoracoscopic video assisted pulmonary vein antrum isolation, ganglionated plexus ablation, and periprocedural confirmation of ablation lesions. First results of hybrid surgical-electrophysiological approach for atrial fibrillation. Circ Arrhythm Electrophysiol. 2011; 4:262–270.
26. Mesnildrey, P, Laborde, F, Bruneval, P, et al. Therapeutic and prophylactic surgical treatment of ventricular tachycardia by Nd-YAG laser irradiation. In: Fontaine G, Schienman MM, eds. Ablation in Cardiac Arrhythmias. Mount Kisco, New York: Futura Publishing; 1987:441–448.
27. Saksena, S, Gielchinsky, I, Tullo, NG. Argon laser ablation of malignant ventricular tachycardia associated with coronary artery disease. Am J Cardiol. 1989; 64:1298–1304.
28. Thomas, SP, Clout, R, Deery, C, et al. Microwave ablation of myocardial tissue: the effect of element design, tissue coupling, blood flow, power and duration or exposure on lesion size. J Cardiovasc Electrophysiol. 1999; 10:72–78.
29. Braun, MU, Knaut, M, Rauwolf, T, et al. Microwave ablation of an ischemic sustained ventricular tachycardia during aortocoronary bypass, mitral valve and tricuspid valve surgery guided by a three- dimensional nonfluoroscopic mapping system (CARTO). J Interv Cardiac Electrophysiol. 2005; 13:243–247.
30. Frankel, DS, Mountantonakis, SE, Zado, ES, et al. Noninvasive programmed ventricular stimulation early after ventricular tachycardia ablation to predict risk of late recurrence. J Am Coll Cardiol. 2012; 59:1529–1535.
31. Khalpey, ZI, Ganim, RBi, Rawn, JDi. Postoperative care of cardiac surgery patients. In: Cohn LH, ed. Cardiac Surgery in the Adult. New York: McGraw-Hill; 2008:465–486.
32. Eckart, RE, Epstein, LMi. Interventional therapy for atrial and ventricular arrhythmias. In: Cohn LH, ed. Cardiac Surgery in the Adult. New York: McGraw-Hill; 2008:1357–1374.
33. Hutchinson, MD, Gerstenfeld, EP, Desjardins, B, et al. Endocardial unipolar voltage mapping to detect epicardial ventricular tachycardia substrate in patients with nonischemic left ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2011; 4:49–55.
34. Lindblom, D, Albage, A, Sartipy, U. Surgery for ventricular tachycardia in patients undergoing surgical ventricular restoration. Multimed Man Cardiothorac Surg. 2007; 1–5.
35. Pirk, J, Bytesnik, J, Kautzner, J, et al. Surgical ablation of ventricular tachycardia guided by mapping in sinus rhythm: long term results. Eur J Cardiothorac Surg. 2004; 26:323–329.
36. Mathuria, N, Michowitz, Y, Shivkumar, K. Hybrid surgical procedures for epicardial ventricular tachycardia ablation: an update. J Innovations in Card Rhythm Management. 2010; 1:38–41.