Chapter 51 Surgery for Temporal Lobe Epilepsy
• The most common medically intractable epilepsy appropriate for epilepsy surgery has a temporal lobe origin.
• Mesial temporal lobe epilepsy (MTLE) represents a large percentage of all localization-related epilepsy and has a strong association with an early injury before the age of 4 years. The seizures arise in the hippocampal and parahippocampal areas and in the amygdala. MTLE also presents common diagnostic features including unilateral interictal and ictal electroencephalography (EEG) and magnetic resonance imaging (MRI) features showing sclerosis of the mesial structures, resistance to medical therapy, and responsiveness to selective resection. The pathogenesis of this syndrome represents a special substrate: the so-called mesial temporal sclerosis (MTS).
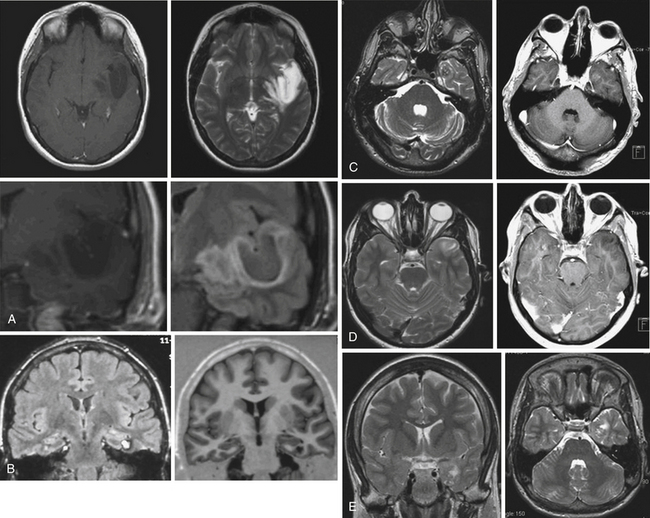
• Lesional temporal lobe epilepsy may be seen in the presence of many different histopathological entities such as tumors (astrocytoma, dysembryoplastic neuroepithelial tumor, ganglioglioma) (see Fig. 51.1), vascular malformations, and developmental lesions (cortical dysplasia, neuronal heterotopias, and others).
• Cognitive impairment in cases of pharmacoresistant epilepsy of early onset is not uncommon, and is related to different overlapping factors: the electrophysiological abnormalities of continuous seizures on a developing brain, the constant need for anticonvulsant drugs, and the presence of multiple medication regimens.
General Features
When epilepsy is uncontrolled by traditional anticonvulsant therapy, surgical resection of the epileptogenic region may be performed. The most common uncontrolled epilepsy eligible for epilepsy surgery has temporal lobe origin. In fact, the temporal lobe neocortex and the amygdalohippocampal complex are highly prone to seizure-induced brain injury. The efficacy of temporal lobe resection for the treatment of epilepsy in children was reported over 40 years ago by Davidson and Falconer.1
Natural History
The natural history of medically intractable epilepsy has demonstrated the prognosis to be poor,2 especially as children with temporal lobe epilepsy of early onset can be affected in all areas of cognitive functions and intelligence as well as behavior and psychosocial skills. The cognitive impairment of pharmacoresistant epilepsy of early onset is postulated to be related not only to the continuous seizures and their electrophysiological abnormalities on a developing brain but also to different overlapping factors. These factors include the constant need for anticonvulsant drugs, the presence of multiple medication regimens, and the use of near-toxic doses in case of status epilepticus, as demonstrated in animals models.3,4 An onset of uncontrolled seizures under the age of 3 carries the worst prognosis, with a low IQ and motor delays with tonic and myoclonic features and spasms.5 Other adverse prognostic factors are the presence of daily complex partial seizures, at least five episodes of grand mal, at least one episode of status epilepticus, and a long duration of seizures prior to control.6
Classification of Temporal Lobe Epilepsy
The most recent classification of the International League Against Epilepsy reports mesial temporal lobe epilepsy (MTLE) with hippocampal sclerosis as a specific electrophysiological syndrome.7 MTLE represents a large percentage of all localization-related epilepsy and has a strong association with an early injury before the age of 4 years, particularly febrile seizure, which may be present in 40% of cases. The seizures arise in the hippocampal and parahippocampal areas and in the amygdala. MTLE also presents common diagnostic features including unilateral interictal and ictal electroencephalography (EEG) and magnetic resonance imaging (MRI) features showing sclerosis of the mesial structures, resistance to medical therapy, and responsiveness to selective resection.
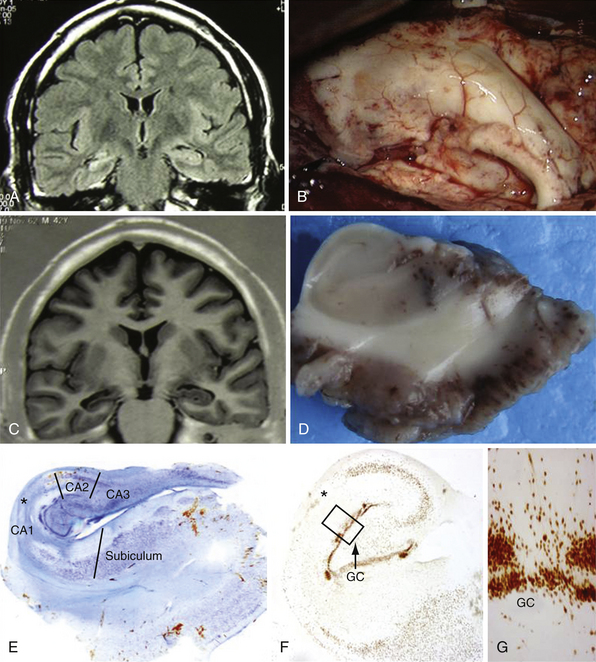
The pathogenesis of this syndrome represents a special substrate: the so-called mesial temporal sclerosis (MTS). The cause of MTS is unclear. An association with a history of febrile seizures has been proposed8 and it is suggested to be associated with complex features of febrile seizures.9 However, this connection has been questioned.10 Instead of being the consequence of recurrent temporal lobe seizures the MTS is frequently associated with a history of unusual febrile seizures in childhood with a later development of complex partial seizures. From a histopathological point of view the mesial structures show a loss of neurons with gliosis in the hippocampus associated with a modification of the normal gray matter architecture. Especially there is an involvement of the Sommer’s sector, the end folium, and the CA3 regions.11 MTS tends to be more common in adults than children but occurs frequently in association with cortical dysplasia in these patients, accounting for the early onset of epilepsy in this group.12,13 Despite the multitude of clinical and basic science studies on MTS the cause and maintenance mechanisms of epileptogenicity are not fully understood.
Lesional temporal lobe epilepsy may be seen in the presence of many different histopathological entities such as tumors (astrocytoma, dysembryoplastic neuroepithelial tumor, ganglioglioma) (Fig. 51.1), vascular malformations, and developmental lesions (cortical dysplasia, neuronal heterotopias and others). Even in patients with long-lasting histories of seizures when the hippocampus has been resected, the neuronal loss is small (up to 25%) and there is no architectural reorganization (see Fig. 51.1).14,15
Seizure Semiology
Temporal lobe seizures may originate in hippocampal and immediately adjacent structures or arise from extrahippocampal neocortical regions. The clinical differentiation of hippocampal and extrahippocampal temporal lobe epilepsy may help guide the extent of resection.16 The presence of an aura with experiential phenomena, such as a feeling of depersonalization or familiarity, or visual or auditory illusions is associated with a neocortical temporal ictal origin. In 70% of cases the epigastric aura occurs in the setting of mesial temporal lobe epilepsy; however, this rate significantly increases (98% of cases) if the abdominal aura evolves into an automotor seizure.17 Fearful and olfactory auras are commonly associated with involvement of the amygdala, whereas gustatory auras may occur in the hippocampal region or arise from extrahippocampal neocortical regions.18 The “déjà vu” onset needs a concomitant activation of hippocampus and temporal neocortex and it is not specific for the activation of hippocampus or neocortex.
Usually the ictal behavior shows different patterns including behavioral arrest and motionless staring that are described as typical symptoms of mesial temporal origin. Oral automatisms (chewing, lip smacking, tongue protruding, and lip pursing) and manual automatisms (manual exploratory behavior, grabbing, and rubbing) are both associated with temporal lobe epilepsy (TLE).19 The early onset of automatisms is frequently associated with a primary involvement of the mesial temporal lobe; moreover, the association of ipsilateral hand automatism with simultaneous contralateral dystonic posturing allows a correct lateralization. Contralateral head rotation just before seizures and secondarily generalized and unilateral tonic and dystonic posturing can be of value in localization, but they are not always accurate.20 A variety of autonomic symptoms, including elevated blood pressure, tachycardia, skin pallor, pilomotor erection, and mydriasis, have been reported in the course of MTLE seizures.21 Ictal speech and postictal dysphasia has been demonstrated to be consistent with seizure onset in the dominant hemisphere; in addition, active testing of postictal reading ability indicated a seizure focus in the language-dominant hemisphere. It has been reported that early postictal nose wiping is a reliable lateralizing sign pointing to the ipsilateral hemisphere.22
Temporal lobe seizures are more likely to occur during wakefulness, whereas frontal lobe seizures have a greater chance of occurring during sleep, implying that sleep has distinct effects on seizure threshold in different brain regions.23 Furthermore, sleep can influence the extent of seizure spread, such that seizures in temporal lobe epilepsy are more likely to secondarily generalize during sleep than during wakefulness.24,25
Evaluation for Surgery
Presence of mesial temporal lobe sclerosis resistant to pharmacotherapy is the most common indication for surgery in adults, accounting for 75% in adult surgical series.26,27 The major indication for TLE surgery in children is a lateral neocortical alteration, frequently cortical dysplasia alone, or eventually in combination with MTS and lesion-related TLE, especially gangliogliomas and dysembryoplastic neuroepithelial tumors (DNET).
The correlation between electroclinical data and hippocampal sclerosis in MTLE has been known for over half a century.8,28–30 To examine the evidence base for surgery for uncontrolled seizures, the Quality Standards Subcommittee of the AAN (American Academy of Neurology) performed a systematic review of the efficacy and safety of anterior temporal lobe resections.31 Based on one randomized trial and observational studies, they found that anterior temporal lobe resection reduced the occurrence of disabling seizures and improved patients’ quality of life. Furthermore, they speculated that the “greater potential for achieving freedom from disabling seizures offered by surgical treatment, as opposed to continuing pharmacotherapy, may reduce the risks of long-term mortality.” Moreover, pharmacoresistant epilepsy has been associated with decreased survival,32,33 and in the appropriate candidate, temporal lobectomy has actuarial benefit on life expectancy.34
Noninvasive Evaluation
The semiology, interictal and ictal scalp video-EEG, and imaging (anatomical, metabolic, and functional) as well as neurocognitive evaluation, represent the armamentarium of evaluation for surgical candidacy in the patient with pharmacoresistant epilepsy. Most evaluations would include interictal scalp recordings and inpatient video-EEG to capture typical seizures. However, the best outcomes are found in patients with concordant MRI and interictal scalp EEG concordant for unilateral MTLE.35 In fact, lack of concordance with interictal EEG and other studies is a worse predictor of surgical outcome than ictal disconcordance. However, in all but the most straightforward of cases, several typical clinical spells are usually of value to be recorded by inpatient EEG.
Imaging of MTLE by MRI has made the diagnosis of hippocampal sclerosis much more straightforward, in addition to a variety of evolving imaging tools.36 Fine-cut coronal sequences perpendicular to the axis of the hippocampus are particularly useful. Coronal fluid-attenuated inversion recovery (FLAIR) sequences suppress the cerebrospinal fluid signal and enhance the increased tissue free-water signal characteristic of mesial temporal sclerosis. Volumetric hippocampal MRI studies through serial thin-cut coronal images permit a comparison of the volumes of both hippocampi, unmasking eventual differences in volume between the normal and the affected mesial structure (Fig. 51.2A and B).37 Recent advances in high-field MRI have been particularly helpful in identifying subtle abnormalities in hippocampal or neighboring mesial temporal structures.38,39
Magnetic resonance spectroscopy (MRS) can also detect abnormalities in various metabolites using the proton signal.40,41 Nuclear medicine studies are also employed, primarily interictal positron emission tomography (PET) and ictal/interictal single-photon emission computed tomography (SPECT). Fluorodeoxyglucose (FDG) PET looks for interical hypometabolism. It can be helpful in identifying surgical candidates in the setting of normal MRI scans, and also may detect those with bilateral disease with poorer outlook for seizure freedom.42 However, the area of hypometabolism demonstrated by FDG PET often shows greater extent than foci demonstrated by EEG or MRI. Although PET is a sensitive diagnostic method, it provides only approximate localization of the epileptic zone and may not be adequate for precise localization, though it may be useful for differentiating between TLE of mesial or lateral origin.43
SPECT studies require injection very soon after seizure onset for maximal accuracy. The use of SPECT image registration to subtract an ictal from interictal injection, when coregistered with the anatomical MRI, can result in useful ictal localization information, even with a normal MRI scan.44
Invasive Evaluation
When noninvasive methods do not indicate the seizure focus, invasive diagnostic recordings can be used. When bilateral MTLE is of concern, bitemporal electrodes can be used. The two strategies most often employed are depth electrodes that penetrate the brain structures, or subdural electrodes that are passed subtemporally and medially toward mesial structures. Though these methods are typically used on a less favorable group of patients, good outcomes can be achieved in this subset and are generally well tolerated.45 Invasive monitoring indications and interpretation can be more challenging in the pediatric population because of the frequent temporal and extratemporal neocortex involvement.46
Neuropsychology
A thorough neuropsychological evaluation is part of the presurgical assessment for medically intractable seizures.47 Neuropsychological testing can help lateralize the seizure focus48,49 because dominant temporal lobe epilepsy is typically associated with verbal memory deficits as a predominant factor. Excessive speech difficulties, global memory problems, or extensive difficulties in other domains should raise concerns as to the localization of medial temporal lobe epilepsy.
Neuropsychological testing is also critical to assess for the emotional, behavioral, and psychiatric disorders that are very common in this population.50 Depression in particular can be quite significant, and many preoperative factors and expectations can impact surgical decision making, perceived benefit from surgery, and quality of life outcomes.51
Testing is also important to establish language dominance and define memory risks preoperatively. The cerebral amytal test has long been used to determine dominance and assess the effect on memory of anesthetizing one hemisphere through intracarotid injection.52 The test is invasive and there is variability in its use. Because right-handed patients with right-sided lesions have a very small chance of being dominant,53 some of these patients, for example, may not require extensive study of dominance through intracarotid amytal testing. Functional MRI can make assessment of dominance and memory, but the exact tests and their reliability are still a subject of debate.54 The precise language test to be used in functional MRI is unknown, but some batteries have been quite successful in establishing dominance.55,56 Memory in particular is difficult to assess by either method.57
Surgical Techniques
The “classic” or “standard” anterior temporal lobectomy includes resection of anterior neocortex and the mesial temporal structures. This technique is used historically in case of MTS, and also addresses seizures of lateral neocortical origin (in those cases when mesial structures are to be included in the resection). The resection would typically involve the anterior 4 cm of dominant, and 6 cm of nondominant temporal lobe.58
A variant of this approach involves alternative entries to the ventricle, including a stereotactic entry through the middle temporal gyrus to the ventricle, subtemporal entry through the basal temporal lobe, and the sylvian fissure approach that requires entry of the hippocampal structures medially. Selective approaches may have advantages in cognitive outcome over the larger “standard” approaches.59
The selective amygdalohippocampectomy has been advocated by Yasargil and associates for nonlesional temporal lobe epilepsy and for MTS without lateral neocortex contribution.60,61 The amygdalohippocampal complex is resected, avoiding passage through the lateral neocortex by a trans-sylvian arachnoid splitting approach. Specifically, the surgeon exposes the middle cerebral artery branches by splitting the sylvian fissure arachnoid and identifies the limen insulae. The ventricle is entered through the superior aspect of the temporal stem that is mesial to the superior temporal gyrus and posterior to the limen insulae, by a transcortical approach. A longitudinal corticectomy is made in the depth of the first temporal sulcus reaching the lateral aspects of the temporal horn. Alternatively, a subtemporal approach is performed reaching the inferolateral aspects of the temporal horn through a small corticectomy in the depth of the collateral sulcus between the third and the fourth temporal gyri.62,63
None of these techniques is truly selective owing to the fact that each of them violates the gray matter of the lateral/ventral temporal lobe cortex or the white matter fibers of the temporal stem as the fasciculus uncinatus that harbor important associative functions. However, the subtemporal amygdalohippocampectomy has a direct trajectory through the collateral sulcus to the temporal horn. As an alternative strategy, the degree of resection can be guided by patient-specific factors, including intraoperative interictal electrocorticography (ECoG). Intraoperative ECoG permits the surgeon to map the electrical abnormalities. The language is mapped in the dominant hemisphere of an awake patient by direct electrocortical stimulation administering language tasks to the patient during surgery. The hippocampus is resected on the basis of the intraoperative ECoG findings by the use of a strip electrode that is introduced in the temporal horn and slides posteriorly. The extent of the posterior margin of resection of the hippocampus tail can be guided based on the intraoperative ECoG.64,65 Resection of the lateral temporal lobe on the dominant side can also be tailored to the subject’s own language anatomy66 in attempts to minimize cognitive deficits. Comparisons of “selective” anatomical studies have not been directly compared to the tailored methods in epilepsy outcome studies.
Radiosurgery
Despite this high success rate of resective epilepsy surgery, novel surgical therapies for MTLE are being evaluated,64 and radiosurgery poses an attractive alternative in this respect. The use of radiosurgery to successfully treat various types of epilepsy as arteriovenous malformation or cavernomas related to epilepsy has been reported.67,68
A multicenter study on the use of radiosurgery for the treatment of MTLE has shown promising initial results with 85% of patients who received 24 Gy to the 50% isodose line becoming seizure-free.69 Moreover the data coming from the Marseilles group is encouraging in terms of neuropsychological improvements and outcome. From the authors’ perspective the long-term safety and efficacy of radiosurgery for MTLE is comparable to surgical resection, but radiosurgery has the advantage of sparing verbal memory in patients treated by Gamma Knife (GK) on the dominant side.70
However, other authors have reported less positive outcomes in the treatment of MTLE with radiosurgery. In these papers radiosurgery did not lead consistently to seizure control and sometimes led to transient seizure worsening associated with the risk of brain edema and intracranial hypertension.71–73 All the authors agree with the strategy of surgery resection of the amygdalohippocampus complex in patients who demonstrate radiosurgery treatment failure.
Deep Brain Stimulation and Neuromodulation
Patients with complex partial seizures arising from the hippocampus who undergo resective surgery of the epileptic focus have a good outcome. Nevertheless, there are a number of candidates in whom it is not advisable to perform temporal lobectomy or hippocampectomy because of the risk of memory deficit,74 or even severe amnesia.75 This appears to be more likely to occur in patients with bilateral hippocampal surgery.76 Therefore, patients with bilateral mesial temporal epilepsy are either excluded from resective procedures, or surgery is performed after extensive counseling in which the risks include incomplete epileptic foci resections and residual seizures. Consequently, there is a current need to develop nonresective alternative therapies. Neuromodulation has been proposed in these patients. Several neuroanatomical targets have been suggested and stimulated, including the centromedian thalamic nucleus,77 vagal nerve,78 and a variety of cerebellar targets.79
All these targets have improved secondary seizure generalization, but have shown inconsistent results regarding complex partial seizures. The neuromodulation of the hippocampal epileptic foci has been proposed by Velasco and co-workers. In surgical series of patients with bilateral mesial temporal epilepsy and normal MRI, the chronic stimulation of bilateral or unilateral hippocampus at long-term follow-up has demonstrated a seizure reduction of between 95% and 50%. No patient had neuropsychological deterioration, nor did any patient show side effects.80 Similar results were collected by Boon and co-workers, who reported a series of 12 patients requiring invasive monitoring to exclude bitemporal epilepsy. After a follow-up of more than 12 months, 9 out of 10 patients receiving stimulation of both hippocampi had a seizure frequency reduction higher than 50%, and 1 patient was seizure-free.81
Results
Postoperative Outcome
After temporal lobe resection, usually including uncus, amygdala, sclerotic hippocampus, and the lateral temporal lobe, 48% to 84% of patients are seizure-free (Table 51.1). The short-term (<5 years) rate of seizure-free patients is around 70%.31,51,82,83
TABLE 51.1 Evidence-Based Medicine Evidence Class for Preoperative Features, Postoperative Factors, and Surgical Techniques as Predictors of Seizure-Free Outcome in Drug-Resistant Temporal Lobe Epilepsy
| Evidence Factor | Class |
|---|---|
| Febrile seizure | IIb |
| Effectiveness of surgery versus medication | Ia |
| Effectiveness of temporal lobectomy versus selective amygdalohippocampectomy | IIIa |
| MTLE versus MTLE with neocortical dysplasia | IIIa |
| Postoperative result after 6 months | IIb |
MTLE, mesial temporal lobe epilepsy.
Positive predictive factors for seizure-free outcome after temporal lobe resection include preoperative hippocampal sclerosis (unilateral), focal localization of interictal epileptiform discharges, absence of preoperative generalized seizures, tumor etiology, and complete resection of the lesion with or without medial structures.84–86
Younger age at surgery or at onset of epilepsy and absence of seizures in the first postoperative week often predict good seizure outcome after temporal lobe resection.87,88 Long-term follow-up (>5 years) studies show that 41% to 79% of patients remain seizure-free after temporal lobe resection89–92 and that 15% to 20% of patients have relapses after initial seizure freedom at 5 to 10 years after surgery. Although potential explanations for this phenomenon, such as contralateral mesial temporal pathology or dual pathology, have been suggested, the only uniform predictor for late relapse is failure to enter remission immediately after surgery.93,94
Longitudinal studies of patients with anterior temporal lobe resections have shown that the seizure status at 1 year after surgery does not remain stable over subsequent follow-up. Some studies provide long-term data of seizure control (up to 10 years) status after anterior temporal lobe resection.95–99 Among patients seizure-free at the end of year 1 following surgery, the annual probability of seizure relapse between years 1 and 5 is 5.6%, and beyond year 5 the probability is 4.2%.97–99 In contrast, Kelley and Theodore found that the likelihood of further relapse for patients seizure-free at year 10 was 0%.97 Among patients with persistent seizures at the end of year 1 after surgery, the annual probability of becoming seizure-free between years 1 and 5 is only 5.9% and the probability of becoming seizure-free after year 5 is a mere 2.0%.95,97 So far Choi and colleagues, applying the same relative risk reduction used by other authors, predicted that the probability for seizure-free outcome falls to 1.6% beyond year 5.34
Factors that predict good seizure outcome include presence of a discrete lesion on MRI, complete resection of the lesion, localized scalp EEG ictal onset, concordant hypometabolism on FDG-PET, longer duration of epilepsy, and lack of febrile seizures.26,31,100
Some studies include both adult and pediatric patients and a mixture of seizure etiology, to include mesial temporal sclerosis (dual pathology). In recent surgical series, 1% to 11% of patients had reoperation for surgical failure, which is most common after neocortical resections. Causes of surgical failures include incomplete resection of lesions, erroneous identification of epileptogenic regions, generation of new epileptogenic zones, and limited resections due to the risk of functional impairment. Surgical resections are usually done in the same region as the previous surgery. After reoperation, 39% to 57% of patients are seizure-free. Siegel and colleagues100 found that a duration of epilepsy of 5 years or less and preoperative focal interictal epileptiform discharges predicted good outcome.
Complications, Cognitive, and Psychosocial Outcomes
Any surgical procedure, including temporal lobectomy, carries both general surgical and specific inherent risks. Although the overall risk of morbidity is quite low, there are significant complications associated with this operation.101 Reported complications include infection, cerebrospinal fluid leak, and damage to the perforating arteries that can lead to hemiparesis, hemianopia, sensory loss, and aphasia on the dominant side.
Varying degrees of deficits have to be expected in mesial temporal lobe surgery. Visual field deficits from damage to Meyer’s loop can be present even in selective approaches.102 Advances in the application of techniques of MRI tractography may help reduce this risk;103 however, the vulnerable location of these visual field fibers is an anatomical reality.
Cognitive deficits can be found after basal temporal resection. Memory deficits can be potentially seen in the presurgical setting, and these can worsen postoperatively, especially in the setting of normal MRI and better presurgical memory.104 Word-finding deficits, especially of specific items such as faces and landmarks, are common after dominant TLE. Recognition deficits can also follow lobectomy for nondominant TLE.105 Favorable neuropsychological and social outcomes are seen in temporal lobectomy, especially when seizure freedom is achieved.101
Future Perspectives
The surgical treatment of temporal lobe epilepsy is frequently performed when psychological and social rehabilitation for intractable epilepsy is unlikely. New insights into the role of MTLE in humans and in animal models have unravelled specific events related to seizures.106 Recent pathophysiology and technology advances contributed to our understanding of mesial temporal lobe epilepsy and promoted early identification of surgically remediable epilepsy as soon as the seizure disorder becomes progressive and pharmacoresistant.
The high-frequency (250-500 Hz) interictal epileptiform EEG activity is recorded in the hippocampus of patients with mesial temporal lobe or neocortical epilepsy. This EEG abnormality, named fast ripples, could identify the epileptogenic region, and even predict the development of epilepsy.107–109 So far the possibility to record fast ripples by magnetoencephalography or by specific spike-associated blood flow patterns of scalp EEG coupled with functional MRI could be practically useful to define the epileptogenesis noninvasively.110
Neuroimaging studies offer an interesting perspective in the presurgical stage. MRI mapping of structural patterns of the hippocampi could predict the development of epilepsy after prolonged febrile seizures in children with hippocampal abnormalities, and may predict disease progression and pharmacoresistance.111,112 Moreover, there is evidence that α-methyltryptophan (AMT) uptake is identified with the epileptogenic tuber in patients with tuberous sclerosis, and in other forms of epilepsy.113,114 Other radionuclide tracers, such as flumazenil PET, may also provide additional information on localization for temporal lobe epilepsy 11C-flumazenil PET in temporal lobe epilepsy with unilateral and bilateral hippocampal sclerosis.115 Finally, magnetic resonance spectroscopy studies allow the detection of neuroprogenitor cells and is able to measure neurogenesis in vivo in mesial temporal lobe epilepsy with hippocampal sclerosis.116
It is possible that advances of transcranial magnetic stimulation (TMS), particularly with the development of TMS-EEG,117 will identify changes associated with epileptogenesis in neocortical areas accessible to this technique.
Conclusion
Temporal lobectomy is a highly effective treatment for epilepsy in the appropriate population. Advances in diagnosis through novel radiological/anatomical and physiological techniques may expand the population who may benefit from surgical treatment strategies. The only caveat is that outcomes as favorable as those seen in mesial temporal sclerosis with concordant electrophysiology are not to be expected. Cognitive, neuropsychological, and social outcomes are not perfect, and the ideal surgical approaches to mitigate the deleterious effects of epilepsy surgery are still evolving.
Berg A.T., Berkovic S.F., Brodie J.M., et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE commission on classification and terminology, 2005-2009. Epilepsia. 2010;51(4):676-685. (Epub 2010 Feb 26)
Bien C.G., Kurthern M., Baron K., et al. Long-term seizure outcome and antiepileptic drug treatment in surgically treated temporal lobe epilepsy patients: a controlled study. Epilepsia. 2001;42:1416-1421.
González-Martínez J.A., Srikijvilaikul T., Nair D., Bingaman W.E. Long-term seizure outcome in reoperation after failure of epilepsy surgery. Neurosurgery. 2007;60:873-880.
Marks W.J.Jr., Laxer K.D. Semiology of temporal lobe seizures: value in lateralizing the seizure focus. Epilepsia. 1998;39:721-772.
Schramm J. Temporal lobe epilepsy and the quest for optimal extent of resection: a review. Epilepsia. 2008;49:1296-1307.
Uijl S.G., Leijten F.S., Arends J.B., et al. Decision-making in temporal lobe epilepsy surgery: the contribution of basic non-invasive tests. Seizure. 2008;17:364-373.
Wiebe S., Blume W.T., Girvin J.P., et al. For the effectiveness and efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized controlled trial of surgery for temporal lobe epilepsy. N Engl J Med. 2001;345:311-318.
Please go to expertconsult.com to view the complete list of references.
1. Davidson S., Falconer M.A. Outcome of surgery in 40 children with temporal lobe epilepsy. Lancet. 1973;1:1260-1263.
2. Holmes G.L. The long-term effects of seizures on the developing brain: clinical and laboratory issues. Brain Dev. 1991;13:393-409.
3. Diaz J., Schain R.J. Phenobarbital: effects of long-term administration on behavior and brain of artificially reared rats. Science. 1978;199:90-91.
4. Gilliam F., Kuzniecky R., Meador K., et al. Do seizures cause brain damage? Epilepsia. 1991;32:514-528.
5. Fogarasi A., Jokeit H., Faveret E., et al. The effect of age on seizure semiology in childhood temporal lobe epilepsy. Epilepsia. 2002;43(6):638-643.
6. Holmes M.D., Born D.E., Kutsy R.L., et al. Outcome after surgery in patients with refractory temporal lobe epilepsy and normal MRI. Seizure. 2000;9:407-411.
7. Berg A.T., Berkovic S.F., Brodie J.M., et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE commission on classification and terminology, 2005-2009. Epilepsia. 2010;51(4):676-685. (Epub 2010 Feb 26)
8. Falconer M.A., Serafetinides E.A., Corsellis J. Etiologies and pathogenesis of temporal lobe epilepsy. Arch Neurol. 1964;10:233-248.
9. Lewis D.V. Febrile convulsions and mesial temporal sclerosis. Curr Opin Neurol. 1999;12:197-201.
10. Tarkka R., Paakko E., Phytinen J., et al. Febrile seizures and mesial temporal sclerosis: no association in a long-term follow-up study. Neurology. 2003;60:215-218.
11. Babb T.L., Brown W.J. Pathological findings in epilepsy. In: Engel J.E., editor. Surgical Treatment of the Epilepsies. New York: Raven; 1987:511-540.
12. Duchowny M., Levin B., Jayakar P., et al. Temporal lobectomy in early childhood. Epilepsia. 1992;33(2):298-303.
13. Wyllie E., Chee M., Granström M.L., et al. Temporal lobe epilepsy in early childhood. Epilepsia. 1993;34:859-868.
14. Fried I., Kim J.H., Spencer D.D. Hippocampal pathology in patients with intractable seizures and temporal lobe masses. J Neurol Neurosurg. 1992;76(5):735-740.
15. Kim J.H., Guimaraes P.O., Shen M.Y., et al. Hippocampal neuronal density in temporal lobe epilepsy with and without gliomas. Acta Neuropathol. 1990;80:41-45.
16. Gil-Nagel A., Risinger M.W. Ictal semiology in hippocampal versus extrahippocampal temporal lobe epilepsy. Brain. 1997;120:183-192.
17. Henkel A., Noachtar S., Pfander M., et al. The localizing value of the abdominal aura and its evolution: a study in focal epilepsies. Neurology. 2002;58:271-276.
18. Binder D.K., Garcia P., Elangovan G.K., et al. Characteristics of auras in patients undergoing temporal lobectomy. J Neurosurg. 2009;111:1283-1289.
19. Uijl S.G., Leijten F.S., Arends J.B., et al. Decision-making in temporal lobe epilepsy surgery: the contribution of basic non-invasive tests. Seizure. 2008;17:364-373.
20. Marks W.J.Jr., Laxer K.D. Semiology of temporal lobe seizures: value in lateralizing the seizure focus. Epilepsia. 1998;39:721-772.
21. Kotagal P. Seizures: symptomatology of temporal lobe seizures. In: Luders H.O., editor. Epilepsy Surgery. New York: Raven Press; 1992:143-156.
22. Geyer J.D., Payne T.A., Faught E., et al. Postictal nose-rubbing in the diagnosis, lateralization and localization of seizures. Neurology. 1999;52:743-745.
23. Sinha S., Brady M., Scott C.A., et al. Do seizures in patients with refractory epilepsy vary between wakefulness and sleep? J Neurol Neurosurg Psychiatry. 2006;77:1076-1078.
24. Bazil C.W., Walczak T.S. Effects of sleep and sleep stage on epileptic and nonepileptic seizures. Epilepsia. 1997;38:56-62.
25. Herman S.T., Walczak T.S., Bazil C.W. Distribution of partial seizures during the sleep-wake cycle: differences by seizure onset site. Neurology. 2001;56:1453-1459.
26. Harkness W. Temporal lobe resections. Childs Nerv Syst. 2006;22:936-944.
27. Wiebe S., Blume W.T., Girvin J.P., et al. For the effectiveness and efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized controlled trial of surgery for temporal lobe epilepsy. N Engl J Med. 2001;345:311-318.
28. Corsellis J.H. Epilepsy and the temporal lobes: a clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1966;89:499-530.
29. Earle K.A., Baldwin M.A., Penfield W. Incisural sclerosis and temporal lobe seizures produced by hippocampal herniation at birth. Arch Neurol Psychiatry. 1953;69:27-42.
30. Morris A.A. Temporal lobectomy with removal of uncus, hippocampus, and amygdala. Arch Neurol Psychiatry. 1956;79:479-496.
31. Engel J.Jr., Wiebe S., French J., et al. Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Neurology. 2003;60(4):538-547.
32. Ryvlin P., Kahane P. Does epilepsy surgery lower the mortality of drug-resistant epilepsy? Epilepsy Res. 2003;56:105-120.
33. Sperling M.R. The consequences of uncontrolled epilepsy. CNS Spectr. 2004;9:98-101.
34. Choi H., Sell R.L., Lenert L., et al. Epilepsy surgery for pharmacoresistant temporal lobe epilepsy: a decision analysis. JAMA. 2008;300(21):2497-2505.
35. Gilliam F., Boling S., Bilir E., et al. Association of combined MRI, interictal EEG, and ictal EEG results with outcome and pathology after temporal lobectomy. Epilepsia. 1997;38:1315-1320.
36. Duncan J.S. Imaging in the surgical treatement of epilepsy. Nat Rev Neurol. 2010;6:537-550.
37. Keller S.S., Wieshmann U.C., Mackay C.E., et al. Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry. 2002;73:648-655.
38. Huppertz J.H. Enhanced visualization of blurred gray/white matter junction by voxel-based 3D-MRI appearance. Epilepsy Res. 2005;67:35-50.
39. Iwasaki M., Nakasato N., Suzuki H., et al. Endofolium sclerosis in temporal lobe epilepsy diagnosed preoperatively by 3-tesla imaging. J Neurosurg. 2009;110:1124-1126.
40. Hubesch B., Marinier D.S., Hetherington H.P., et al. Clinical MRS studies of the brain. Invest Radiol. 1989;24:1039-1042.
41. Willmann O., Wennberg R., May T. The role of 1H magnetic resonance spectroscopy in pre-operative evaluation for epilepsy surgery. A meta-analysis. Epilepsy Res. 2009;71:149-156.
42. Bolling W.W., Lancaster M., Krazpulski, et al. Fluorodeoxyglucose-positron emission tomographic imaging for the diagnosis of mesial temporal lobe epilepsy. Neurosurgery. 2008;63:1130-1138.
43. Willmann O., Wennberg R., May T., et al. The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy. A meta-analysis. Seizure. 2007;16:509-520.
44. Bell M.L., Rao S., So E.L., et al. Epilepsy surgery outcomes in temporal lobe epilepsy with a normal MRI. Epilepsia. 2008;50:2053-2060.
45. Placantonakis D.G., Shariff S., Lafaille F., et al. Bilateral intracranial electrodes for lateralizing intractable epilepsy: efficacy, risk, and outcome. Neurosurgery. 2010;66:274-283.
46. Hauser W.A. Seizure disorders: the changes with age. Epilepsia. 1992;33(Suppl 4):6-14.
47. National Institutes of Health Consensus development conference statement. surgery for epilepsy. Epilepsy Res Suppl. 1992;5:241-246.
48. Dodrill C.B. The use of neuropsychological testing to locate the epileptogenic zone. In: Miller J.W., Silbergeld D.L., editors. Epilepsy Surgery: Principles and Controversies. New York: Taylor & Francis; 2006:200-209.
49. Jones-Gotman M. Presurgical neuropsychological evaluation for localization and lateralization of seizure. In: Luders H., editor. Epilepsy Surgery. New York: Raven Press; 1991:469-475.
50. Dodrill C.B., Batzel L.W., Queisser H.R., et al. An objective method for the assessment of psychological and social problems among epileptics. Epilepsia. 1980;21:123-135.
51. Gilliam F., Kuzniecky R., Meador K., et al. Patient-oriented outcome assessment after temporal lobectomy for refractory epilepsy. Neurology. 1999;53:687-694.
52. Spencer D.C., Morrell M.K., Risinger M.W. The role of the intracarotid amobarbital in evaluation of patients for epilepsy surgery. Epilepsia. 2000;41:320-325.
53. Miller J.W., Dodrill C.B., Born D.E., et al. Atypical speech is rare in individuals with normal developmental histories. Neurology. 2005;60:1042-1044.
54. Binder J.R., Sabsevitz D.S., Swanson S.J., et al. Use of functional MRI to predict verbal memory decline after temporal lobe epilepsy surgery. Epilepesia. 2008;49:1377-1394.
55. Binder J.R., Swanson S.J., Hammeke T.A., et al. A comparison of five fMRI protocols for mapping speech comprehension systems. Epilepsia. 2008;49:1980-1997.
56. Gaillard W.D., Balsamo L., Xu B., et al. Language dominance in parital epilepsy patients identified with an fMRI reading task. Neurology. 2002;59:256-265.
57. Dodrill C.B., Ojemann G.A. An exploratory comparison of three methods of memory assessment with the intracarotid amytal procedure. Brain Cognition. 1997;33:210-223.
58. Spencer D.D., Spencer S.S., Mattson R.H., et al. Access to the posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurgery. 1984;15:667-671.
59. Schramm J. Temporal lobe epilepsy and the quest for optimal extent of resection: a review. Epilepsia. 2008;49:1296-1307.
60. Yaşargil M.G., Krayenbühl N., Roth P., et al. The selective amygdalohippocampectomy for intractable temporal limbic seizures. J Neurosurg. 2010;112:168-185.
61. Yasargil M.G., Wieser H.G., Valavanis A., et al. Surgery and results of selective amygdalohippocampectomy in one hundred patients with nonlesional epilepsy. Neurosurg Clin North Am. 1993;4(2):243-261.
62. Park T.S., Bourgeois B.F., Silbergeld D.L., et al. Subtemporal transparahippocampal amygdalohippocampectomy for surgical treatment of mesial temporal lobe epilepsy. Technical note. J Neurosurg. 1996;85:1172-1176.
63. Wheatley B.M. Selective amygdalohippocampectomy: the trans-middle temporal gyrus approach. Neurosurg Focus. 2008;25:E4.
64. McKhann G.M. Novel surgical treatments for epilepsy. Curr Neurol Neurosci. 2004;4:335-339.
65. Silbergeld D.L., Ojemann G.A. The tailored temporal lobectomy. In: Silbergeld D.L., Ojemann G.A., editors. Epilepsy Surgery. Neurosurgery Clinics of North America. Philadelphia: WB Saunders; 1993:273-282.
66. Ojemann G., Ojemann J., Lettich E., et al. Cortical language localization in left, dominant hemisphere. J Neurosurg. 1989;71:316-326.
67. Bartolomei F., Régis J., Kida Y., et al. Gamma knife radiosurgery for epilepsy associated with cavernous hemangiomas: a retrospective study of 49 cases. Stereotact Funct Neurosurg. 1999;72(Suppl 1):22-28.
68. Gerszten P.C., Adelson P.D., Kondziolka D., et al. Seizure outcome in children treated for arteriovenous malformations using gamma knife radiosurgery. Pediatr Neurosurg. 1996;24:139-144.
69. Barbaro N.M., Quigg M., Broshek D.K., et al. A multicenter, prospective pilot study of gamma knife radiosurgery for mesial temporal lobe epilepsy: seizure response, adverse events, and verbal memory. Ann Neurol. 2009;65:167-175.
70. Regis J., Arkha Y., Yomo S., et al. Radiosurgery for drug-resistant epilepsies: state of the art, results and perspectives. Neurochirurgie. 2008;54:320-331.
71. Prayson R.A., Yoder B.J. Clinicopathologic findings in mesial temporal sclerosis treated with gamma knife radiotherapy. Ann Diagn Pathol. 2007;11:22-26.
72. Srikijvilaikul T., Najm I., Foldvary-Schaefer N., et al. Failure of gamma knife radiosurgery for mesial temporal lobe epilepsy: report of five cases. Neurosurgery. 2004;54:1395-1402.
73. Vojtech Z., Vladyka V., Kalina M., et al. The use of radiosurgery for the treatment of mesial temporal lobe epilepsy and long-term results. Epilepsia. 2009;50:2061-2071.
74. Helmstaedter C., Kurthen M., Lux S., et al. Chronic epilepsy and cognition: a longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003;54:425-432.
75. Kapur N., Prevett M. Unexpected amnesia: are there lessons to be learned from cases of amnesia following unilateral temporal lobe surgery? Brain. 2003;26:2573-2585.
76. Scoville W.B., Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol. 1957;20:11-21.
77. Velasco F., Velasco M., Jimenez F. Predictors in the treatment of difficult to control seizures by electrical stimulation of the centromedian thalamic nucleus. Neurosurgery. 2000;47:295-305.
78. Amar A.P., Apuzzo M.L.J., Liu C.Y. Vagus nerve stimulation therapy alters failed cranial surgery for intractable epilepsy: results from the vagus nerve stimulation therapy patient outcome registry. Neurosurgery. 2004;55:1086-1093.
79. Velasco F., Carrillo-Ruiz J.D., Brito F., et al. Double-blind randomized controlled pilot study of bilateral cerebellar stimulation for treatment of intractable motor seizures. Epilepsia. 2005;46:1-11.
80. Velasco A.L., Velasco F., Velasco M., et al. The role of neuromodulation of the hippocampus in the treatment of intractable complex partial seizures of the temporal lobe. Acta Neurochir Suppl. 2007;97:329-332.
81. Boon P., Vonck K., De Herdt V., et al. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48(8):1551-1560.
82. González-Martínez J.A., Srikijvilaikul T., Nair D., Bingaman W.E. Long-term seizure outcome in reoperation after failure of epilepsy surgery. Neurosurgery. 2007;60:873-880.
83. Tellez-Zenteno J.F., Dhar R., Wiebe S. Long-term seizure outcomes following epilepsy surgery: a systematic review and meta-analysis. Brain. 2005;128:1188-1198.
84. Berg A., Shinnar S., Levy S.R., et al. Early development of intractable epilepsy in children. Neurology. 2001;6:1445-1452.
85. Jutila L., Immonen A., Mervaala E., et al. Long term outcome of temporal lobe epilepsy surgery: analyses of 140 consecutive patients. J Neurol Neurosurg Psychiatry. 2002;73:486-494.
86. Radhakrishnan K., So E.L., Silbert P.L., et al. Predictors of outcome of anterior temporal lobectomy for intractable epilepsy. Neurology. 1998;51:465-471.
87. Jeong S.W., Lee S.K., Kim K.K., et al. Prognostic factors in anterior temporal lobe resections for mesial temporal lobe epilepsy: multivariant analysis. Epilepsia. 1999;40:1735-1739.
88. Tonini C., Beghi E., Berg A.T., et al. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy Res. 2004;62:75-87.
89. Al-kaylani M., Konrad P., Lazenby B., et al. Seizure freedom of antiepileptic drugs after temporal lobe epilepsy surgery. Seizure. 2007;16:95-98.
90. Bien C.G., Kurthern M., Baron K., et al. Long-term seizure outcome and antiepileptic drug treatment in surgically treated temporal lobe epilepsy patients: a controlled study. Epilepsia. 2001;42:1416-1421.
91. So E.L., Radhakrishnan K., Silbert P.L., et al. Assessing changes over time in temporal lobectomy; outcome by scoring seizure frequency. Epilepsy Res. 1997;27:119-125.
92. Yoon H.H., Kwon H.L., Mattson R.H., et al. Long- term seizure outcome in patients initially seizure-free after resective epilepsy surgery. Neurology. 2003;61:445-450.
93. Berg A.T., Vickrey B.G., Shinnar S., et al. Relapse in post-surgical seizure-free patients: the role of AED reduction. Epilepsia. 2004;45(Suppl 7):189-190.
94. Spencer S.S., Berg A.T., Vickrey B.G., et al. For the multicenter study of epilepsy surgery. predicting long-term seizure outcome after resective epilepsy surgery. Neurology. 2005;65:912-1018.
95. Foldvary N., Nashold B., Mascha E., et al. Seizure outcome after temporal lobectomy for temporal lobe epilepsy. Neurology. 2000;54(3):630-634.
96. Kelemen A., Barsi P., Eross L., et al. Long-term outcome after temporal lobe surgery—prediction of late worsening of seizure control. Seizure. 2006;15:49-55.
97. Kelley K., Theodore W.H. Prognosis 30 years after temporal lobectomy. Neurology. 2005;64:1974-1976.
98. McIntosh A.M., Kalnins R.M., Mitchell L.A., et al. Temporal lobectomy: long term seizure outcome, late recurrence, and risks for seizure recurrence. Brain. 2004;127:2018-2030.
99. Schwartz T.H., Jeha L., Tanner A., et al. Late seizures in patients initially seizure free after epilepsy surgery. Epilepsia. 2006;47:567-573.
100. Siegel A.M., Jobst B.C., Thadani V.M., et al. Medically intractable, localization related epilepsy with normal MRI: pre-surgical evaluation and surgical outcome in 43 patients. Epilepsia. 2001;42:883-888.
101. Tanriverdi T., Ajlan A., Poulin N., et al. Morbidity in epilepsy surgery: an experience based on 2449 epilepsy surgery procedures from a single institution. J Neurosurg. 2009;110:1111-1123.
102. Yeni S.N., Tanriover N., Uyanik O., et al. Visual field defects in selective amygdalohippocampectomy for hippocampal sclerosis: The fate of Meyer’s loop during the transsylvian approach to the temporal horn. Neurosurgery. 2008;63:507-515.
103. Thudium M.O., Campos A.R., Urbach H., et al. The basal temporal approach for mesial temporal surgery; sparing the Meyer loop with navigated diffusion tensor tractography. Neurosurgery. 2010;67:385-390.
104. Martin R.C., Sawrie S.M., Roth D.L., et al. Individual memory change after anterior temporal lobectomy; a base rate analysis using regression-based outcome methodology. Epilepsia. 1998;39:1075-1082.
105. Drane D., Ojemann G., Aylward E., et al. Category-specific naming and recognition deficits in temporal lobe epilepsy surgical patients. Neuropsycholgia. 2008;46:1242-1255.
106. Dalby N.O., Mody I. The process of epileptogenesis: a pathophysiological approach. Curr Opin Neurol. 2001;14:187-192.
107. Mormann F., Andrzejak R.G., Elger C.E., et al. Seizure prediction: the long and winding road. Brain. 2007;130:314-333.
108. Staba R.J., Wilson C.L., Bragin A., et al. Quantitative analysis of high frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743-1752.
109. Urrestarazu E., Chandler R., Dubeau F., et al. Interictal high-frequency oscillations (100-500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130:2354-2366.
110. Laufs H., Duncan J.S. Electroencephalography functional MRI in human epilepsy: what it currently can and cannot do. Curr Opin Neurol. 2007;20:417-423.
111. Lin J.J., Salamon N., Dutton R.A., et al. Three-dimensional preoperative maps of hippocampal atrophy predict surgical outcomes in temporal lobe epilepsy. Neurology. 2005;65:1094-1097.
112. Lin J.J., Salamon N., Lee A.D., et al. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cerebral Cortex. 2007;17:2007-2018.
113. Fedi M., Reutens D., Okazawa H., et al. Localizing value of alpha-methyl-l-tryptophan PET in intractable epilepsy of neocortical origin. Neurology. 2001;57:1629-1636.
114. Natsume J., Kumakura Y., Bernasconi N., et al. Alpha-[11C]methyl-l-tryptophan and glucose metabolism in patients with temporal lobe epilepsy. Neurology. 2003;60:756-761.
115. Koepp M.J., Labbe C., Richardson M.P., et al. 11C-flumazenil PET in temporal lobe epilepsy with unilateral and bilateral hippocampal sclerosis. Brain. 1997;120:1865-1876.
116. Parent J.M., Coulter D.A., Bertram E.H. The effect of seizures on the brain. In: Engel J.Jr., Pedley T.A., editors. Epilepsy: A Comprehensive Text Book. 2nd ed. Philadelphia: Lippincott-Raven; 2008:481-493.
117. Valentin A., Arunachalam R., Mesquita-Rodrigues A., et al. Late EEG responses triggered by transcranial magnetic stimulation (TMS) in the evaluation of focal epilepsy. Epilepsia. 2008;49(3):470-480.