Chapter 192 Surgery for Metastatic Spine Disease
Roughly 500,000 deaths occur per year from complications of metastatic disease. It is estimated that among living cancer patients, 10% experience symptomatic secondary metastases,1 with the most common sites of distant metastases being the liver, lungs, and skeleton. Within the skeleton, the spinal column has the highest incidence of metastasis,2 with as many as 90% of cancer patients affected on autopsy studies.3 The most prevalent lesions arise from breast, lung, and prostate tumors because these are the most common systemic malignancies and because they each have a marked tendency to metastasize to bone.4 Brihaye and colleagues reported on the origin of spinal metastasis in a large series of patients and found the breakdown of origin to be 16.5% from breast cancer, 15.6% from lung cancer, and 9.2% from prostate cancer.5 Surgical intervention has been estimated to occur in approximately 5% to 10% of spinal metastases; however, up to 50% of spinal metastases necessitate at least some modality of treatment.6 At the moment, the highest incidence of spinal metastases is found in persons 40 to 64 years of age. Women are less prone than men to develop spinal metastases, possibly due to the overall higher prevalence of systemic prostate and lung cancer when compared to breast cancer.
Mechanism of Spread to the Spine
Metastatic tumors often exhibit characteristic biological behavior of the primary tumor, and thus the mechanism of spread is correlated to the type of primary tumor. Understanding the mechanism is crucial when surgically treating such patients because it can predict recurrence (local versus distant) and can change the goals of resection (debulking versus extensive local control). These mechanisms include hematogenous seeding,1 direct extension or invasion,2 and seeding via the cerebrospinal fluid.3 Hematogenous seeding occurs via arterial or venous pathways and is the most usual route of metastatic spread to the spine, presumably because of the highly vascular nature of the vertebral bodies.7 Venous spread occurs mainly via Batson’s plexus, which connects to multiple venous networks including renal, pulmonary, intercostal, azygous, portal, and caval systems. Owing to the valveless nature of these veins, tumor cells may deposit both antegrade and retrograde in the spine as physiologic pressure changes occur within the major body cavities.8
Classification of Spine Tumors
Classification is based on the anatomic location of the lesion: intramedullary, intradural-extramedullary, or extradural. The main reason for this is that once the anatomic location is determined, the differential diagnosis of the lesion can be quickly narrowed. Of these locations, the extradural compartment is the site at which spinal metastasis most commonly occurs. Extradurally, the most common site to which spinal metastases localize is the vertebral body, followed by the paravertebral regions and epidural space. Intramedullary and intradural metastases have a low incidence and are usually the result of CSF seeding. With regard to region, the thoracic spine is the most common site for tumor localization (45% to 70%), followed by the lumbar spine (20% to 35%) and finally by the cervical (10% to 20%) and sacral (10% to 20%) regions.9
Management
Proper treatment of metastatic spine disease requires a multidisciplinary approach necessitating the involvement of various types of therapy and medical specialties such as neurosurgery, orthopedic surgery, surgical oncology, medical oncology, radiation oncology, interventional radiology, pain specialists, and rehabilitation medicine. Classically, treatment goals have generally been considered to be palliative, centered on mechanical stabilization, pain relief, and preservation of neurologic function. However, preliminary data now suggest the aggressive resections of spinal lesions arising from indolent, well-controlled systemic disease (e.g., renal cell carcinoma, breast adenocarcinoma) might provide oncologic control and improve overall prognosis. All such goals can be successfully obtained via surgical intervention; however, before choosing surgical intervention as a treatment, patient variables such as age, life expectancy, functional status, and tumor burden must be carefully considered.
Selecting Patients for Surgical Intervention
Increased patient survival time due to advancements in systemic chemotherapy and radiation therapy has paralleled more aggressive surgical decompression and fixation for patients with metastatic spine disease.10 For example, since the 1990s, the combination of improvements in implant and instrumentation technology combined with reduction in approach-related morbidity have resulted in improved outcomes following tumor resection and spinal reconstruction. Selection of patients for surgical treatment remains challenging; however, in general most clinicians agree that a patient’s survival must be in excess of at least 3 months to be considered for surgical treatment.
To facilitate the selection process for surgical candidates, scoring systems have been created to evaluate the preoperative status and predict the postsurgical outcome of patients following large decompression and fixation procedures. Tokuhashi and colleagues11 created a scoring system that considers primary tumor histology, number of vertebral metastases, presence of extraspinal or visceral metastases, overall functional status, and neurologic function. They were able to correlate such factors with survival following tumor resection. Not surprisingly, presence of less-aggressive tumors, single vertebral metastases, lack of other metastases, good overall functional status, and normal neurologic function were associated with a higher point total and longer survival. As a result, they recommended that patients with a score greater than 9 of 12 should be considered for aggressive excisional surgery with reconstruction. Patients with scores lower than 5 of 12 had a poor prognosis, and they recommended either palliative surgery (limited decompression) or no surgery at all.
Tomita and colleagues12 also constructed a scoring system to assess surgical candidates. In Tomita’s system, unlike Tokuhashi’s, lower scores indicate a positive prognosis and vice versa. In addition, Tomita’s system is based on only three main parameters: primary tumor histology, presence of visceral metastases, and solitary versus multiple vertebral metastases.
More recently, significant attention has been paid to the concept of metastatic epidural spinal cord compression (MESCC). In the past, surgical interventions were restricted to decompressive laminectomies. However, decompressive laminectomy alone does not resolve anterior compression originating from the vertebral body, and it induces spinal instability by destabilizing the posterior elements, leading to increasing pain and potentially worsening neurologic function. For this reason, direct decompression and reconstruction approaches are now the standard for treating patients with a reasonably good prognosis. The first prospective randomized, controlled trial of direct decompressive surgical resection with radiation therapy versus radiation therapy alone in the treatment of patients with MESCC was published by Patchell and colleagues in 2005.13 Their data suggest that surgery with radiation is statistically superior to radiation alone as demonstrated by the greater post-treatment ambulatory rate, duration of ambulation, maintenance of ambulation after treatment, and return of ambulation after treatment. The study also demonstrated that patients who received both surgery and radiation had improved functional ability (Frankel scores), muscle strength (ASIA scores), continence, and survival time when compared to the group that received radiation alone. Important exclusion parameters within the selection criteria include patients with highly radiosensitive tumors such as small cell lung carcinoma, myeloma, and lymphoma. In such patients radiation alone may still be the preferred treatment for MESCC.
Determining the Surgical Approach
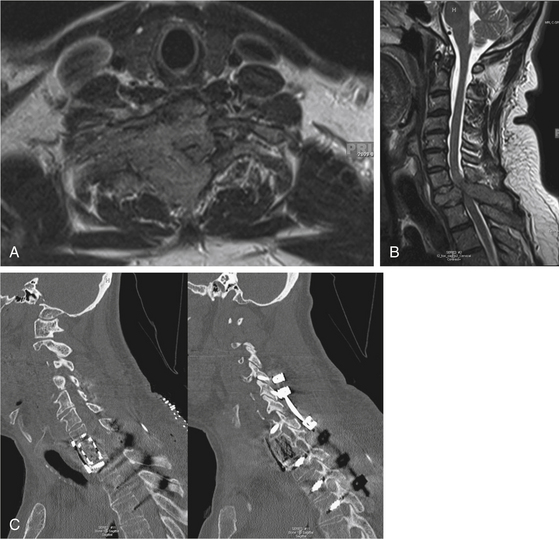
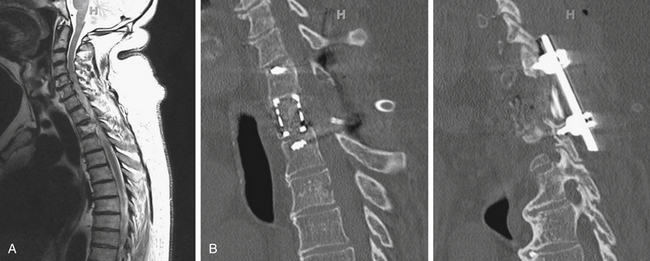
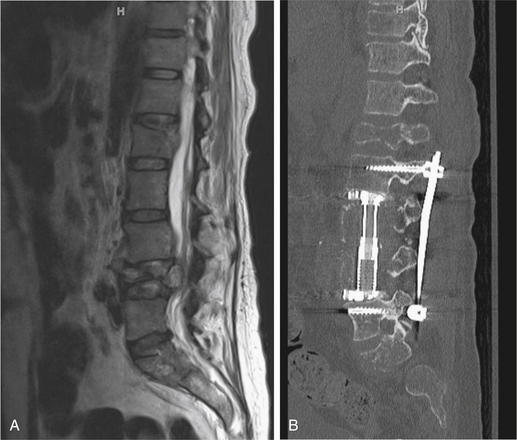
Determining the spinal segment involved is crucial for determining the surgical approach because not all segments are equally accessible. For example, most spinal metastatic disease localizes to the vertebral body and most spine surgeons are familiar with anterior exposures of the cervical spine, making lesions in this area readily accessible. However, the upper thoracic segment of the spine (T1–4) may be difficult to expose anteriorly (Fig. 192-1). In such an instance, an anterolateral cervical approach combined with sternotomy or thoracotomy could be required. Some cases require access to spinal segments that are occluded by the presence of great vessels, and in such instances a posterolateral or transpedicular approach is often used (Fig. 192-2). For the lower thoracic region (T5–12), a right-sided thoracotomy allows access to the spine while still avoiding the great vessels and aortic arch. However, if the majority of the tumor is located on the left, a left-sided thoracotomy is appropriate. Access for a thoracolumbar junction (T11–L1) decompression and resection often requires a combined thoracotomy and retroperitoneal approach. It is possible to approach the lumbar spine (L1–5) anteriorly; however, a posterior approach allowing posterior excision and stabilization is often indicated for managing metastatic spine disease (Fig. 192-3).
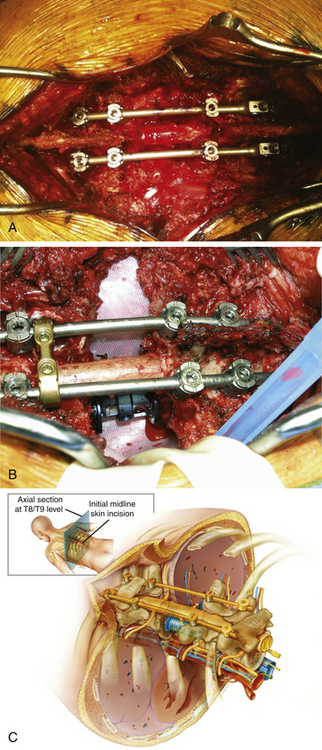
In general, patients with MESCC require direct decompression, which might require approaching the patient posteriorly, anteriorly, or via a combined approach. Many surgeons have opted for circumferential decompression from an entirely posterior approach. Circumferential spinal reconstruction can be accomplished from an entirely posterior approach as well.14 Specifically, the vertebral body can be removed via a bilateral costotransversectomy approach, and anterior struts (e.g., cage, bone) can be inserted around the cord. The procedure first involves an incision made over the index level, followed by exposure of multiple levels of the posterior spine to allow multiple anchor points of spinal instrumentation. Pedicle screws are placed early so that temporary rods can stabilize the spinal column during vertebrectomy. Bilateral laminectomies, facetectomies, and pediculectomies are then done. Rib resection near the affected pedicles is essential to allow a corridor to the most medial and dorsal aspects of the vertebral body (3 to 7 cm of rib). Vertebrectomy can then be accomplished piecemeal while a temporary rod is in place (Fig. 192-4). To obtain better exposure ventrally, segmental arteries can be sacrificed, and nerve roots can be ligated if within the thoracic spine. An anterior strut can then be used to reconstruct the ventral aspect of the spinal column while the posterior construct is compressed and tightened.
Following decompression and resection, the vertebral body commonly requires construction. This is usually accomplished by using structural implants that are static mesh cages or distractible cages. Most commonly used implants are titanium, but plastic implants made of polyethylethylketone (PEEK) can also be used. Alternatively, polymethyl methacrylate (PMMA) cement and anterolateral plating may be used. Structural femoral or tibial autograft may be used; however, these are generally not recommended because most patients with metastatic disease will undergo postoperative radiation treatment, which can later compromise the graft’s integrity. Pedicle screw instrumentation is used in cases where posterior stabilization is indicated, including posterior column damage (intrinsic or iatrogenic), significant kyphosis, resections in high-stress areas such as the occipitocervical, cervicothoracic, thoracolumbar, and lumbosacral junctions, and to supplement anterior reconstructions following two or more adjacent vertebrectomies. A lone posterior approach to achieve anterior decompression and reconstruction may be considered before transcavitary decompressions, because surgical morbidity is not significantly different, and the posterior approach may be more familiar to most spine surgeons.15
Numerous studies have suggested a bilateral transpedicular approach via costotransversectomy to expose the anterior elements and decompress the spinal cord.14 Anterior element reconstruction via this approach can be achieved with PMMA injected into a chest tube support, expandable or static titanium or PEEK cages, autologous bone graft, or titanium mesh cage.
Percutaneous Biopsy
About 10% to 20% of spinal metastases have no known source. Despite recent advances in medical imaging that can detect primary lesions elsewhere in the body, biopsies are still often required for the definite diagnostic identity of a tumor. Over time, biopsy techniques have significantly improved, yielding diagnostic accuracy rates as high as 90%.16 Performing biopsies has also become more practical, and they are now performed even in the outpatient setting using percutaneous placement of needles under computed tomography (CT) guidance. In circumstances where excisional biopsy or surgery is not immediately indicated, percutaneous biopsy can help formulate prognostic predictions based on the tumor type. This information is especially relevant when no other lesions exist elsewhere in the body, and it is important to make sure the lesion is a metastasis and not a primary spine tumor. In the latter case, the surgical goal may be very different, involving plans for radical resection with goals of oncologic cure rather than palliation.
Assessing Instability in Spinal Metastatic Disease
Biomechanical studies have demonstrated that vertebral bodies are responsible for the support for as much as 80% of the axial load of the spine.17 This loading capacity is significantly affected by osteolytic lesions, which significantly debilitate the vertebral body. The degree to which the vertebral body is affected by lesions is mainly determined by the size of the lesion, cross-sectional area of the intact vertebral body, overall bone mineral density, and whether the lesion is primarily lytic, blastic, or both.
Metastatic disease can ultimately lead to pathologic compression or burst fractures resulting from very minor trauma or loading. These types of fractures can project tumor debris and bone fragments into the neural foramina and spinal canal, thereby compressing neurologic structures and resulting in pain and/or neurologic dysfunction. Several studies have attempted to assess the probability of spinal instability following metastatic disease pathology. Windhagen and colleagues performed a study that found that the probability of pathologic fracture could be calculated by obtaining the product of the bone mineral density and the product of the cross-sectional area of the vertebral body.18 Taneichi and colleagues also found that both 35% to 45% lysis of the lower thoracolumbar spine and 50% to 60% lysis of the thoracic spine were strong predictors of collapse.19 From the latter studies, it has been concluded that the cervicothoracic and thoracolumbar junctions are subject to fracture despite less tumor burden compared to other regions of the spine. Interestingly, histology might also matter: Chaichana and colleagues found that vertebrae in patients with breast cancer were more likely to fracture compared to vertebrae in patients with tumors of other histology.20 It many cases, lesions also occur in the posterior element, either alone or more commonly in conjunction with anterior involvement. When destabilizing lesions occur in this area, the treating physician should understand that pathologic dislocation, spondylosis, or translational instability is more likely.
Principles of trauma-induced instability of the spine are not equal to the instability as a result of neoplastic processes, and thus injury mechanisms may be substantially different. To address the latter, Cybulski and colleagues designed a system based on the three-column model to assess spine stability in the setting of a spine tumor. The following are the criteria for spinal instability under Cybulski’s system21:
• Anterior and middle column destruction (greater than 50% collapse of vertebral body height)
• Collapse of two or more adjacent vertebral bodies
• Tumor involvement of middle and posterior columns (possible forward shearing deformity)
• Iatrogenic instability (laminectomy with failure to recognize anterior and middle column disease)
The Spinal Oncology Study Group (SOSG) has also created a system to assess instability in the setting of spine tumors. Factors associated with instability include location (junctional areas are more unstable), lytic versus blastic, clinical history of mechanical spine pain, amount of vertebral body involved, presence of posterior involvement, and associated deformity or lithesis.
Spinal Radiosurgery
Studies employing SRS to treat spinal column metastases resulted in cessation of tumor growth, along with pain relief and few undesirable side effects. Degen and colleagues performed a study assessing neurologic function outcomes following treatment with the CyberKnife.22 They found that in 78% of patients neurologic function was improved. Furthermore, they also found that treatment increased pain relief and quality of life was maintained. Follow-up more than 1 year after treatment demonstrated 100% tumor control and a low rate of treatment-associated morbidity. However, despite these positive results, long-range outcome assessments are still necessary to accurately evaluate efficacy.
Percutaneous Vertebroplasty and Kyphoplasty
Although both are minimally invasive, vertebroplasty and kyphoplasty differ in technical application of cement. With vertebroplasty, PMMA cement is directly injected into the vertebral body. Kyphoplasty, in contrast, involves a balloon that is first inflated within the collapsed vertebral body before the addition of PMMA. PMMA cement injections provide a degree of stabilization to fractures, thereby also providing the patient with pain relief. Studies have also found that in addition to providing mechanical stability, PMMA might have intrinsic analgesic characteristics that aid in pain relief. A study conducted by Lee and colleagues found that of 136 patients who underwent percutaneous vertebroplasty, 84% experienced short- or long-term symptomatic improvements.23
Complications in vertebroplasty and kyphoplasty are uncommon, and most often are caused by improperly placed or leaked PMMA, resulting in compression of neurologic structures and hematogenous embolization to the lungs. Although the main advantages of vertebroplasty and kypholasty are that they are minimally invasive pain-reduction procedures, they are not intended to relieve spinal cord compression, correct deformity, or overcome overt mechanical instability of the spine.
Aaron A.D. The management of cancer metastatic to bone. JAMA. 1994;272(15):1206-1209.
Arguello F., Baggs R.B., Duerst R.E., et al. Pathogenesis of vertebral metastasis and epidural spinal cord compression. Cancer. 1990;65(1):98-106.
Babu N.V., Titus V.T., Chittaranjan S., et al. Computed tomographically guided biopsy of the spine. Spine. 1994;19(21):2436-2442.
Bilsky M.H., Lis E., Raizer J., et al. The diagnosis and treatment of metastatic spinal tumor. Oncologist. 1999;4(6):459-469.
Brihaye J., Ectors P., Lemort M., Van Houtte P. The management of spinal epidural metastases. Adv Tech Stand Neurosurg. 1988;16:121-176.
Chaichana K.L., Pendleton C., Sciubba D.M., et al. Outcome following decompressive surgery for different histological types of metastatic tumors causing epidural spinal cord compression. J Neurosurg Spine. 2009;11(1):56-63.
Constans J.P., de Divitiis E., Donzelli R., et al. Spinal metastases with neurological manifestations. Review of 600 cases. J Neurosurg. 1983;59(1):111-118.
Cybulski G.R. Methods of surgical stabilization for metastatic disease of the spine. Neurosurgery. 1989;25(2):240-252.
Degen J.W., Gagnon G.J., Voyadzis J.M., et al. CyberKnife stereotactic radiosurgical treatment of spinal tumors for pain control and quality of life. J Neurosurg Spine. 2005;2(5):540-549.
DeWald R.L., Bridwell K.H., Prodromas C., Rodts M.F. Reconstructive spinal surgery as palliation for metastatic malignancies of the spine. Spine. 1985;10(1):21-26.
Ibrahim A., Crockard A., Antonietti P., et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. J Neurosurg Spine. 2008;8(3):271-278.
Lee B., Franklin I., Lewis J.S., et al. The efficacy of percutaneous vertebroplasty for vertebral metastases associated with solid malignancies. Eur J Cancer. 2009;45(9):1597-1602.
Lenz M., Freid J. Metastases to the skeleton, brain and spinal cord from cancer of the breast and effect of radiotherapy. Ann Surg. 1931;93:278-293.
Magerl F., Aebi M., Gertzbein S.D., et al. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3(4):184-201.
Perrin R.G., Livingston K.E., Aarabi B. Intradural extramedullary spinal metastasis. A report of 10 cases. J Neurosurg. 1982;56(6):835-837.
Sciubba D.M., Gallia G.L., McGirt M.J., et al. Thoracic kyphotic deformity reduction with a distractible titanium cage via an entirely posterior approach. Neurosurgery. 2007;60(4):223-230. discussion 230-231
Sundaresan N., Digiacinto G.V., Hughes J.E., et al. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery. 1992;29(5):645-650.
Taneichi H., Kaneda K., Takeda N., et al. Risk factors and probability of vertebral body collapse in metastases of the thoracic and lumbar spine. Spine. 1997;22(3):239-245.
Tokuhashi Y., Matsuzaki H., Toriyama S., et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine. 1990;15(11):1110-1113.
Tomita K., Kawahara N., Kobayashi T., et al. Surgical strategy for spinal metastases. Spine. 2001;26(3):298-306.
Windhagen H.J., Hipp J.A., Silva M.J., et al. Predicting failure of thoracic vertebrae with simulated and actual metastatic defects. Clin Orthop Relat, 1997;344:313-319
Xu R., Garces-Ambrossi G.L., McGirt M.J., et al. Thoracic vertebrectomy and spinal reconstruction via anterior, posterior, or combined approaches: clinical outcomes in 91 consecutive patients with metastatic spinal tumors. J Neurosurg. Spine. 2009;11(3):272-284.
1. Sundaresan N., Digiacinto G.V., Hughes J.E., et al. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery. 1992;29(5):645-650.
2. Aaron A.D. The management of cancer metastatic to bone. JAMA. 1994;272(15):1206-1209.
3. Lenz M., Freid J. Metastases to the skeleton, brain and spinal cord from cancer of the breast and effect of radiotherapy. Ann Surg. 1931;93:278-293.
4. Constans J.P., de Divitiis E., Donzelli R., et al. Spinal metastases with neurological manifestations. Review of 600 cases. J. Neurosurg. 1983;59(1):111-118.
5. Brihaye J., Ectors P., Lemort M., Van Houtte P. The management of spinal epidural metastases. Adv Tech Stand Neurosurg. 1988;16:121-176.
6. Bilsky M.H., Lis E., Raizer J., et al. The diagnosis and treatment of metastatic spinal tumor. Oncologist. 1999;4(6):459-469.
7. Arguello F., Baggs R.B., Duerst R.E., et al. Pathogenesis of vertebral metastasis and epidural spinal cord compression. Cancer. 1990;65(1):98-106.
8. Perrin R.G., Livingston K.E., Aarabi B. Intradural extramedullary spinal metastasis. A report of 10 cases. J Neurosurg. 1982;56(6):835-837.
9. Ibrahim A., Crockard A., Antonietti P., et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. J Neurosurg Spine. 2008;8(3):271-278.
10. DeWald R.L., Bridwell K.H., Prodromas C., Rodts M.F. Reconstructive spinal surgery as palliation for metastatic malignancies of the spine. Spine. 1985;10(1):21-26.
11. Tokuhashi Y., Matsuzaki H., Toriyama S., et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine. 1990;15(11):1110-1113.
12. Tomita K., Kawahara N., Kobayashi T., et al. Surgical strategy for spinal metastases. Spine. 2001;26(3):298-306.
13. Patchell R.A., Tibbs P.A., Regine W.F., et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366(9486):643-648.
14. Sciubba D.M., Gallia G.L., McGirt M.J., et al. Thoracic kyphotic deformity reduction with a distractible titanium cage via an entirely posterior approach. Neurosurgery. 2007;60(4):223-230. discussion 230-231
15. Xu R., Garces-Ambrossi G.L., McGirt M.J., et al. Thoracic vertebrectomy and spinal reconstruction via anterior, posterior, or combined approaches: clinical outcomes in 91 consecutive patients with metastatic spinal tumors. J Neurosurg. Spine. 2009;11(3):272-284.
16. Babu N.V., Titus V.T., Chittaranjan S., et al. Computed tomographically guided biopsy of the spine. Spine. 1994;19(21):2436-2442.
17. Magerl F., Aebi M., Gertzbein S.D., et al. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3(4):184-201.
18. Windhagen H.J., Hipp J.A., Silva M.J., et al. Predicting failure of thoracic vertebrae with simulated and actual metastatic defects. Clin Orthop Relat, 1997;344:313-319
19. Taneichi H., Kaneda K., Takeda N., et al. Risk factors and probability of vertebral body collapse in metastases of the thoracic and lumbar spine. Spine. 1997;22(3):239-245.
20. Chaichana K.L., Pendleton C., Sciubba D.M., et al. Outcome following decompressive surgery for different histological types of metastatic tumors causing epidural spinal cord compression. J Neurosurg Spine. 2009;11(1):56-63.
21. Cybulski G.R. Methods of surgical stabilization for metastatic disease of the spine. Neurosurgery. 1989;25(2):240-252.
22. Degen J.W., Gagnon G.J., Voyadzis J.M., et al. CyberKnife stereotactic radiosurgical treatment of spinal tumors for pain control and quality of life. J Neurosurg Spine. 2005;2(5):540-549.
23. Lee B., Franklin I., Lewis J.S., et al. The efficacy of percutaneous vertebroplasty for vertebral metastases associated with solid malignancies. Eur J Cancer. 2009;45(9):1597-1602.