Chapter 41 Surgery for Juvenile Idiopathic (Rheumatoid) Arthritis
Background/aetiology
In 1993 a taskforce was set up by the International League of Associations for Rheumatology (ILAR) to reclassify inflammatory joint disease of childhood and adolescents. The ILAR classification1 was proposed and, although this is now widely used, it is not universally accepted by rheumatologists. The requirement for diagnosis of this condition is persistent arthritis for 6 weeks or more, affecting individuals under the age of 16 years, with the classification being based on clinical and serological factors as well as on the onset and course of the disease.
The ILAR classification avoided using terms such as juvenile chronic arthritis,2 used by the European League Against Rheumatism (EULAR), and juvenile rheumatoid arthritis,2,3 used both by EULAR and by the American College of Rheumatology (ACR). The ILAR introduced the term juvenile idiopathic arthritis (JIA) to encompass the heterogeneous forms of arthritis that begin before the age of 16 years. At the same time ILAR identified seven different categories:
JIA is therefore not a single disease but a group of heterogeneous conditions.5 It is firstly an autoimmune disease with T-cell abnormalities and chronic, probably cell mediated, synovitis. Multiple autoantibodies, immune complexes and complement activation may all play a role as humoral agents. Secondly there is a genetic element with various forms of JIA displaying non-mendelian inheritance with interactions between multiple genes. With this genetic predisposition it has been postulated that there are a number of aetiological triggering events including infection, trauma, hormonal abnormalities, psychological stress and an abnormal immune response.
Presentation, investigation and treatment options
The incidence of JIA varies from 2 to 20 per 100 000 population, while the prevalence has been quoted from 16 to 150 per 100 000.5 JIA is often self-limiting with about 60% of patients reaching adulthood with no active ongoing synovitis or functional limitation.6
Presentation
Girls are affected more than boys, although the ratio varies from 2 : 1 to over 6 : 1 depending on the category. The only exception to this is the systemic type that affects both sexes equally.6 The overall average age of onset is 6 years with two peaks between 1 and 4 and between 9 and 14 years.7 This variation is related to the disease category except again for the systemic onset type, which shows no increased frequency at any particular age.
Involvement of the elbow in JIA has been reported to be as high as 66%,8 although the number ultimately requiring surgery is very small. This is reflected in the small number of surgical procedures recorded in the literature.
Investigations
The diagnosis of JIA is essentially clinical, although a variety of laboratory tests can be used to provide evidence of inflammation or disease activity, and to support the diagnosis. In addition they can also be useful as a research tool to understand the pathogenesis of the condition.5 Haematological tests include the haemoglobin level (which can sometimes be significantly lowered), leucocyte count (that can be very high) and platelet count (which may be high in systemic or polyarticular disease). The erythrocyte sedimentation rate and, more reliably, C-reactive protein can be used to monitor inflammatory response. Serum immunoglobulins are a marker of disease activity, with IgA and IgM levels also correlating with the type of onset and sex.5 Rheumatoid factor is more commonly present in the older age onset with polyarticular disease and is unusual in disease onset before 7 years. The detection of rheumatoid factor, however, rises with age and duration of the disease. Antinuclear antibodies are more useful in the diagnosis of JIA than rheumatoid factor.
Summary Box 41.1
| Investigation | Finding |
|---|---|
| Haemoglobin | May be significantly reduced in systemic onset |
| Leucocyte count | May be very raised in systemic onset |
| Platelet count | May be high in systemic and polyarticular disease |
| Rheumatoid factor | More commonly raised in older age onset with polyarticular disease |
Radiological examination
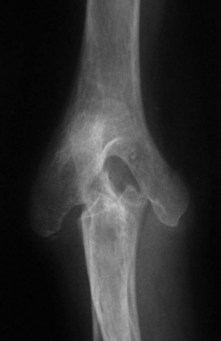
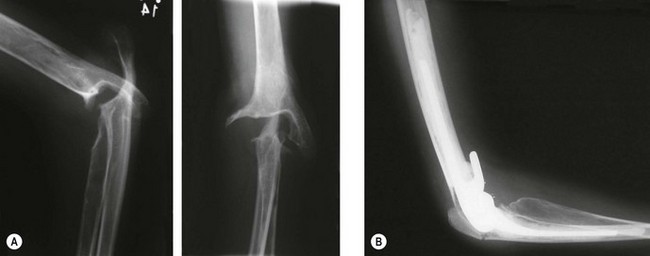
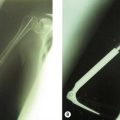
X-ray changes of the elbow in JIA are similar to those described by Larsen et al in adult rheumatoid disease9 (Fig. 41.1), but in addition bony ankylosis may occur as a result of the inflammatory disease being active during skeletal growth. This complication has resulted in the original five-type Larsen classification being modified to include a sixth type (Fig. 41.2).
Surgical techniques
Outcome including literature review
Synovectomy
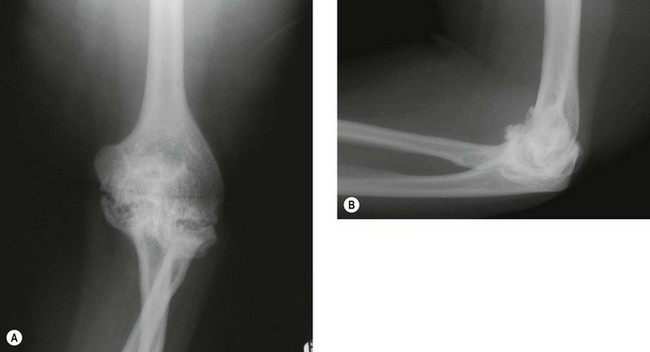
This procedure can be undertaken in isolation or in combination with radial head excision. Wilson et al10 noted that like other bones, for example the greater trochanter of the femur, the radial head may be overgrown as a result of JIA (Fig. 41.3). Similar results to those obtained by synovectomy in adult rheumatoid patients were originally reported in JIA.10–12 However, in 2003 slightly inferior results were noted by Maenpaa et al in juvenile rheumatoid patients at an average follow-up of 5 years.13 They performed 24 elbow synovectomies in patients with JIA with 96% having elbow disease classified as Larsen 0–2. Complete pain relief occurred in 44%, although there was no gain in functional ability, and no overall improvement in range of movement, including forearm prono-supination. This compares to the outcome of this procedure in adult rheumatoid patients, in whom postoperative pain has been reported to reduce to 70–84% at 6–12 months but reducing to 45–54% at 5–6 years.14–16 The operation has a greater chance of long-term success when preoperative forearm rotation is reduced to below 50% of normal and when flexion and extension are not severely limited. In this subgroup, gains of 50° in forearm rotation and 11° in flexion arc have been reported16 in contrast to that reported for JIA.13
Excision/interposition arthroplasty
Although excision arthroplasty was originally described by Ollier17 in 1882 for ankylosis of the elbow secondary to tuberculosis, it was Schuller in 1893 who recommended the operation for the treatment of the rheumatoid elbow.18 Various materials were inserted to interpose between the resected humerus and ulna to prevent reankylosis and in 1960 Gschwend and Spirig advocated the use of dermis as the interposition material.19 This then became the primary method of treating the destroyed rheumatoid elbow until the advent of modern total elbow arthroplasties. Indeed even after this time some authors continued to advise that interposition arthroplasty is the better first choice operation especially in JIA, with prosthetic replacement reserved as a salvage procedure.20
The use of interposition arthroplasty has been reported in mixed populations of adult rheumatoid patients and children with JIA. Kimura and Vainio published their results of 208 interposition arthroplasties in patients with adult and juvenile rheumatoid patients but made no distinction between the two groups.21 The average age of the patients was only 39 years with a range from 14 to 73 years. They performed 53 Ollier-type excision arthroplasties, which involved complete excision of the distal end of the humerus, olecranon and radial head, and 155 Hass-type excisions,22 in which the distal humerus was fashioned and a more conservative partial resection of the olecranon performed. In 152 cases the distal humerus was covered with a full thickness skin graft. Pain relief was good with complete relief in 81% and 67%, respectively, while a virtual 100° mean arc of flexion was obtained. The main reported complication was instability causing ulnar nerve paraesthesia and significant bone resorption in the region of the olecranon fossa. Unfortunately the follow-up period was not recorded and therefore the speed at which this bone resorption occurs is unknown. A surgical modification involving bone grafting the olecranon fossa was suggested when incidence of forking of the humerus was seen in 20% of cases followed for at least 1 year.
Further reports of interposition arthroplasty in which small numbers of patients with JIA are included have shown good functional ranges of movement.23,24 Only one paper specifically details the results in JIA patients.24 This reported four out of 12 interposition arthroplasties in patients aged 11–15 years at the time of surgery. All achieved an improvement in range of movement, which varied between a 60° and 130° flexion arc, at a long-term follow-up of between 25 and 32 years. This study, however, merely reports the flexion arc outcome and makes no mention of forearm rotation, pain, or the presence or absence of bony erosions or disease progression. Indeed it is the bone loss and instability complications of interposition arthroplasty together with the improved results obtained by elbow arthroplasty that has made the latter the first choice in the surgical treatment of the majority of patients with rheumatoid elbow disease.25 Interposition arthroplasty may, however, still be a useful option in some young patients with ankylosing disease.26
Arthroplasty
Hip and knee arthroplasties are well reported in JIA, but there is little in the literature on shoulder and elbow replacements. Only two series of shoulder replacements have been reported27,28 with a similarly small number of publications that specifically document the outcome of total elbow arthroplasty in JIA.29,30 Other series report a range of pathologies including some patients with JIA but extracting the JIA results from these is difficult or impossible since the number of such patients is usually small.
In 1972 Dee was the first to recognize, in the context of arthroplasty, that the bony architecture in burnt-out Still’s disease was grossly abnormal and advised that implant stems may need to be shortened to facilitate insertion. Unfortunately in his original series of 12 replacements using his hinged prosthesis he did not specify either the results or indeed the number of juveniles in the group.31
In 1990 Dennis et al reported modest gains in movement using the capitellocondylar prosthesis in 21 adult and juvenile rheumatoid patients.29 Less improvement was noted in the juvenile group.29 Ewald et al also reported the results of the capitellocondylar arthroplasty in patients with rheumatoid arthritis.32 Out of 202 arthroplasties in 179 individuals, 13 were in patients with JIA, representing 8% of the patients treated. Unfortunately again no distinction was made between the results for the adult and juvenile groups. In 1994 Kraay et al reported their experience of using the Osteonics prosthesis including 86 cases of inflammatory arthritis (rheumatoid arthritis, juvenile rheumatoid arthritis (JRA), systemic lupus erythematosus and psoriatic arthropathy) but once more no detail of either numbers or results is given for the patients with JRA.33
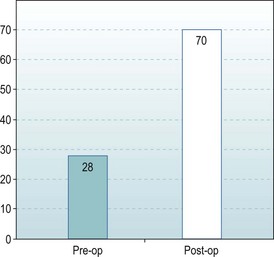
Connor and Morrey30 reported the results of 24 elbow replacements in 19 patients with juvenile rheumatoid arthritis at a mean follow-up of 7.4 years. Eighteen linked (Coonrad–Morrey) and six unlinked (capitellocondylar) implants were used over a 12-year period (1982–94). The authors recognized the difficulty of performing total elbow replacements in these patients because of the contracture of the soft tissues and the extremely small bones and intramedullary cavities. Unlike in my practice, however, they did not find it necessary to use custom-made prostheses. Nine of their patients had undergone previous surgery to the elbow, seven having synovectomy and radial head excision and two having interposition arthroplasty. They noted little or no pain at follow-up but achieved only a moderate increase in movement, with the flexion arc increasing from 63° to 90° postoperatively. Despite this, the average Mayo elbow performance score (MEPS)34 rose from 31 points (range 5 to 55 points) preoperatively to 90 points (range 55–100 points) postoperatively. These results are consistent with the same author’s experience of using the Coonrad–Morrey total elbow replacement in adult rheumatoid patients when at a minimum 10-year follow-up an identical postoperative MEPS of 90 was reported.35 The main difference in the use of total elbow arthroplasty for the treatment of adult versus juvenile rheumatoid patients is the quoted complication rate of 14% in the former and 50% in the latter.
The only other reference specific to elbow arthroplasty in JIA is an abstract from the closed meeting of the American Shoulder and Elbow Surgeons in November 1998 published in 1999.36 This abstract reports the largest series of total elbow arthroplasties for JIA. Forty-five patients were treated using three types of linked implants. The average age of the patients at the time of surgery was 28.5 years (range 14–53). Nineteen customized implants were used because of the small size of the humerus and ulna. An overall improvement in the flexion arc from 38° to 101° was achieved and this is particularly noteworthy since 16 of the elbows were ankylosed preoperatively. In the ankylosed group the flexion arc improved from 0° to 83° whereas in the non-ankylosed group it increased from 59° to 110°. The Hospital for Special Surgery Score improved from a mean preoperative value of 32 (range 6–52) to a mean postoperative score of 91 (range 0–100) at an average follow-up of 10.5 years (range 2–23). The reported complication rate was 29%, the most common complications being fractures to either the epicondyle or olecranon. It is unclear, however, whether these occurred intraoperatively or postoperatively. Only two arthroplasties loosened despite the use of nine uncemented implants. Although published as an abstract, the definitive paper has not subsequently appeared in the literature and therefore no further details on this group of patients are available.
The only other literature on total elbow arthroplasties in this group of patients is either confined to case reports,37 or includes small numbers of JIA patients with adult rheumatoid cases and makes no distinction regarding the outcome in the two groups.38 There are two reports published in this century dealing with total elbow replacement for ankylosed or stiff elbows, and, although these contain some patients with JIA, the results of this subgroup are not separated from the whole group.39,40
My experience
Patients and methods
Eight elbows had undergone surgery prior to the elbow replacement. There had been six interposition arthroplasties using fascia lata, and two synovectomies with radial head excision. No patients were lost to follow-up. All were evaluated at a mean follow-up of 64 months (range 18–120) using MEPS.34
Preoperative assessment
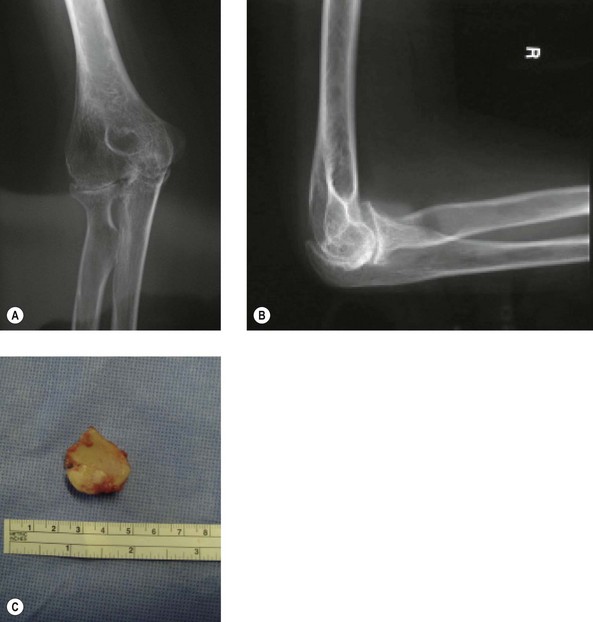
For this reason it is essential that whenever a total elbow arthroplasty is being undertaken in a patient with JIA, appropriate preoperative radiographs are performed in order to allow templating of the prosthesis (Fig. 41.4). This will enable identification of the correct size of the implant and permit customization if extra-small prostheses are required. Of the six Coonrad–Morrey prostheses inserted in this series, all of the humeral and four of the ulnar components were small, with the remaining two ulnar implants being extra-small because of the small size of the intramedullary canal. Of the 13 titanium Kudo implants inserted nine were small components, two extra-small humeral and ulnar components were customized preoperatively because of the small intramedullary canal, and two ulnar components were customized intraoperatively.
The Kudo prosthesis was originally made for the Japanese population and as such the small components are very small. It was for this reason that this prosthesis was chosen in these patients. It was my view that it gave the best option for dealing with the significant challenges presented by the small intramedullary canal size of patients with JIA.30,41
In addition to appropriate preoperative surgical preparation it is essential to have an experienced anaesthetist with skills in endoscopic intubation. Patients with JIA often have temporomandibular joint involvement and in childhood that can result in micrognathia and retrognathia, with an anterior open underbite, which significantly limits the ability to open the jaw.5 This combined with cervical spine disease and a reduction in neck extension can make intubation very difficult.
Rehabilitation
Results
Pain relief
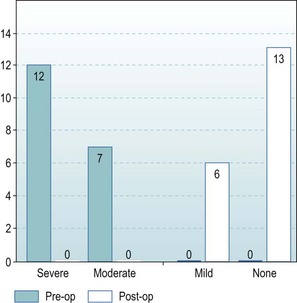
Preoperatively seven patients had moderate pain and 12 had severe pain. Postoperatively the pain was rated as none by 13 and mild by six (Fig. 41.6).
Motion
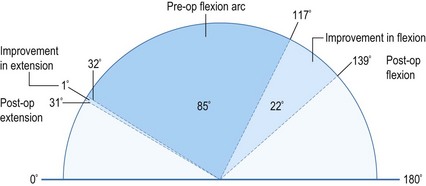
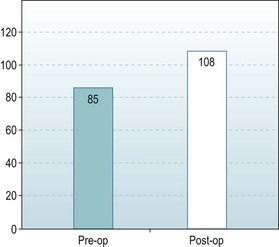
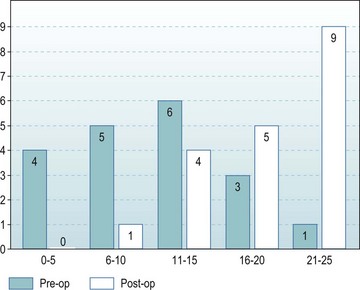
The preoperative range of movement was 32–117°. Postoperatively the range improved to 31–139° (Fig. 41.7). There was therefore no improvement in extension but the mean arc of flexion/extension improved from 85° to a functional 108° (Fig. 41.8).
Activities of daily living
The measured activities within the Mayo elbow performance score improved considerably indicating a marked improvement in function following elbow replacement. This was a consequence of the abolition of pain and the restoration of elbow stability. It was most marked in the five cases where preoperative bone loss had caused significant joint instability (Fig. 41.9).
Complications of treatment
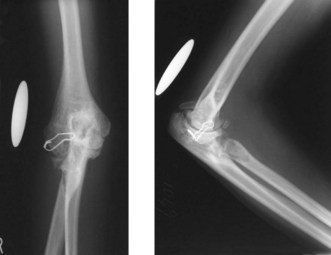
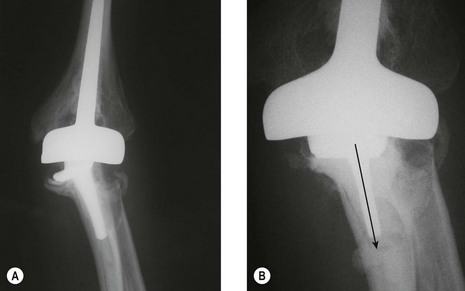
Two patients had transient ulnar neuritis that resolved spontaneously, with another patient having persistent symptoms that were alleviated after the nerve was explored and released 9 months following the joint replacement. One patient had a triceps rupture 1 year postoperatively which required surgical repair. Six years later, the same elbow was revised for a failed Coonrad–Morrey implant (Figs 41.10, 41.11).
Complications and reoperation
There were 11 complications affecting nine out of the 19 elbows (47%). This compares well to the 50% reported by Gill and Morrey.35 All but one of the six complications and four of the five revisions occurred in patients who had undergone previous surgery to the elbow. Four out of the five revisions were in patients whose first operation had been an interposition arthroplasty.
Both previous interposition arthroplasty42 and synovectomy with radial head excision43 have been shown to be associated with an increased complication rate following elbow arthroplasty. The complication rates quoted for elbow arthroplasty in adult rheumatoid patients varies considerably. Gschwend44 reported an overall rate of 43% on reviewing the literature from 1986 to 1992. Little et al45 in a review of the literature to 2003 noted complication rates of between 14% and 80% with a median of 33%. A complication rate of between 47% and 50% is, therefore, not excessive given this highly complex group of patients, many of whom had already undergone previous surgery.
Conclusions/personal view
Patients with JIA represent a group of severely handicapped individuals who, because of the age of onset of their disease, frequently require multiple joint procedures to both their lower and upper limbs. At the elbow synovectomy, interposition arthroplasty and elbow replacement are all surgical options that can be used to reduce pain and improve range of movement and function. The development of the newer generation disease-modifying drugs, however, together with better overall medical management should reduce severe joint destruction, limit soft tissue contractures, and prevent the abnormalities of growth that have historically characterized the juvenile rheumatoid patient. It remains to be seen whether this more modern management will affect the presentation of such cases to the orthopaedic surgeon. At the present time I assess a patient for radial head excision and synovectomy along the lines suggested by Gendi et al.16 I consider the important part of the operation to be the restoration of forearm rotation which is achieved by removal of the frequently overgrown and impinging radial head.
1 Fink CW, Task Force for Classification Criteria. Proposal for the development of classification for idiopathic arthritides of childhood. J Rheumatol. 1995;22:1566-1569.
2 European League Against Rheumatism. EULAR Bulletin No. 4: Nomenclature and Classification of Arthritis in Children. Basel: National Zeitung AG; 1977.
3 Brewer EJJr, Bass J, Baum J, et al. Current proposed revision of JRA criteria. Arthritis Rheum. 1977;20(Suppl.):195.
4 Still GF. On a form of chronic joint disease in children. Med Chirurg Trans. 1897;80:47.
5 Cassidy JT, Petty RE. Juvenile rheumatoid arthritis. In: Cassidy JT, Petty RE, editors. Textbook of Paediatric Rheumatology. 4th ed. W.B. Saunders; 2001:218-321.
6 Packham JC, Hall MA. Long-term follow up of 246 adults with juvenile idiopathic arthritis: functional outcome. Rheumatology. 2002;41(12):1428-1435.
7 Glueck D, Gellman H. Management of the upper extremity in juvenile rheumatoid arthritis. J Am Acad Orthop Surg. 2005;13(4):254-266.
8 Mozziconacci P, Prieur AM, Hayem F, et al. Joint prognosis in systemic chronic juvenile arthritis (100 cases). Sem Hop. 1983;59(48):3357-3360.
9 Larsen A, Dale K, Eek M. Radiographic evaluation of rheumatoid arthritis and related conditions by standard reference films. Acta Radiol. 1977;18:481-491.
10 Wilson DW, Arden GP, Ansell BM. Synovectomy of the elbow in Rheumatoid arthritis. J Bone Joint Surg (Br). 1973;55(1):106-111.
11 Arden GP. Surgical treatment of Still’s disease. Ann R Coll Surg Engl. 1973;53(5):288-299.
12 Arden GP. Surgical treatment of Still’s disease (juvenile chronic arthritis). Ann Acad Med Singapore. 1983;12(2):174-184.
13 Maenpaa H, Kuusela P, Lehtinen J, et al. Elbow synovectomy on patients with juvenile rheumatoid arthritis. Clin Orthop Relat Res. 2003;412:65-70.
14 Rymaszewski LA, Mackay I, Amis AA, et al. Long-term effects of excision of the radial head in rheumatoid arthritis. J Bone Joint Surg (Br). 1984;66(1):109-113.
15 Taylor AR, Mukerjea SK, Rana NA. Excision of the head of the radius in rheumatoid arthritis. J Bone Joint Surg (Br). 1976;58:485-487.
16 Gendi NS, Axon JMC, Carr AJ, et al. Synovectomy of the elbow and radial head excision in rheumatoid arthritis. J Bone Joint Surg (Br). 1997;79(6):918-923.
17 Ollier L. Demonstration anatomique de la reconstitution du coude apres la resection sousperiostee. Examen d’une serie de 106 cas de cette operations. Cbl Chir. 1882;33:548-549.
18 Schuller M. Chirungische mittheilungen uber die chronish rheumatischen gelenkentzundungen. Arch Klin Chir. 1893;45:153.
19 Gschwend N, Spirig B. Vorlaufige mitteilung uber die Anwendungsmoglichkeiten von in cialit Kanzervierter Haut. Vertrag Jahresvers Schweiz Ges Orthop Zurich. 1960.
20 Ruther W, Tillmann K. Resection interposition arthroplasty of the elbow of the elbow in rheumatoid arthritis. In: Ruther W, editor. Replacement and Non Endoprostheic Procedures. Berlin: Springer Verlag; 1996:57-67.
21 Kimura Ch, Vainio K. Arthroplasty of the elbow in rheumatoid arthritis. Arch Orthop Unfall-Chir. 1976;84:339-348.
22 Hass J. Die mobilisierung ankylotischer ellenbogen – und kniegelenke mittels arthroplastik. Arch Klin Chir. 1930;160:693-715.
23 Uuspaa V. Anatomical interposition arthroplasty with dermal graft. A study of 51 elbow arthroplasties on 48 rheumatoid patients. Z Rgeumatol. 1987;46(3):132-135.
24 Fernandez-Pallazzi F, Rodriguez J, Oliver G. Elbow interposition arthroplasty in children and adolescents: long-term follow up. Int Orthop. 2008;32(2):247-250.
25 Ljung P, Jonsson K, Larsson K, et al. Interposition arthroplasty of the elbow with rheumatoid arthritis. J Shoulder Elbow Surg. 1996;5:81-85.
26 Hamalainen MMJ, Kitaoka Y. Late radiographic results after resection skin interposition arthroplasty of the elbow in rheumatoid arthritis. In: Hamalainen M, Hagena FW, editors. Rheumatoid Arthritis Surgery of the Elbow. Rheumatology, vol. 15. Basel: Karger; 1991:42-46.
27 Thomas S, Price AJ, Sankey RA, Thomas M. Shoulder hemiarthroplasty in patients with juvenile idiopathic arthritis. J Bone Joint Surg (Br). 2005;87(5):672-676.
28 Jolles BM, Grosso P, Bogoch ER. Shoulder arthroplasty for patients with juvenile idiopathic arthritis. J Arthroplasty. 2007;22(6):876-883.
29 Dennis DA, Clayton ML, Ferlic DC, et al. Capitello-Condylar total elbow arthroplasty for rheumatoid arthritis. J Arthroplasty. 1990;5(Suppl):S83-S88.
30 Connor PM, Morrey BF. Total elbow arthroplasty in patients who have juvenile rheumatoid arthritis. J Bone Joint Surg (Am). 1998;80(5):678-688.
31 Dee R. Total replacement arthroplasty of the elbow for rheumatoid arthritis. J Bone Joint Surg (Br). 1972;54(1):88-95.
32 Ewald FC, Simmons EDJr, Sullivan JA, et al. Capitellocondylar total elbow replacement in rheumatoid arthritis. J Bone Joint Surg (Am). 1993;75:498-507.
33 Kraay MJ, Figgie MP, Inglis AE, et al. Primary semiconstrained total elbow arthroplasty. Survival analysis of 113 consecutive cases. J Bone Joint Surg (Br). 1994;76:636-640.
34 Morrey BF, Adams RA. Semiconstrained arthroplasty for the treatment of rheumatoid arthritis of the elbow. J Bone Joint Surg (Am). 1992;74:479-490.
35 Gill DR, Morrey BF. The Coonrad-Morrey total elbow arthroplasty in patients who have rheumatoid arthritis. A ten to fifteen year follow up. J Bone Joint Surg (Am). 1998;80(9):1327-1335.
36 Figgie MP, Dunbar RL, Inglis AE. Total elbow replacement for juvenile arthritis. ASES abstracts. J Shoulder Elbow Surg. 1999;8(6):662.
37 Kocialkowski A, Wallace WA. One stage arthroplasty of the ipsilateral shoulder and elbow. J Bone Joint Surg (Br). 1990;72(3):520.
38 Gschwend N, Scheier NH, Baehler AR. Long-term results of the GSB III elbow arthroplasty. J Bone Joint Surg (Br). 1999;81(6):1005-1012.
39 Mansat P, Morrey BF. Semiconstrained total elbow arthroplasty for ankylosed and stiff elbows. J Bone Joint Surg (Am). 2000;82:1260-1268.
40 Peden JP, Morrey BF. Total elbow replacement for the management of the ankylosed or fused elbow. J Bone Joint Surg (Br). 2008;90(9):1198-1204.
41 Dee R. Total replacement of the elbow joint. Orthop Clin North Am. 1973;4:415-433.
42 Krey D, Schmielau G, Tilman K, et al. Results of elbow endoprostheses in patients with rheumatoid arthritis in correlation with previous operations. J Shoulder Elbow Surg. 2002;11(4):360-367.
43 Whaley A, Morrey BF, Adams R. Total elbow arthroplasty after previous resection of the radial head and synovectomy. J Bone Joint Surg (Br). 2005;87(1):47-53.
44 Gschwend N, Simmen BR, Matejovsky Z. Late complications in elbow arthroplasty. J Shoulder Elbow Surg. 1996;5(2 Pt 1):86-96.
45 Little CP, Graham AJ, Carr AJ. Total elbow arthroplasty: a systemic review of the literature in the English language until the end of 2003. J Bone Joint Surg (Br). 2005;87(4):437-444.