CHAPTER 30 Surgery for Acquired Cardiac Disease
* The views expressed in this chapter are those of the author and do not necessarily reflect the official policy or position of the Department of the Navy, Army, Department of Defense, or the U.S. government.
We certify that all individuals who qualify as authors have been listed; each has participated in the conception and design of this work, the analysis of data (when applicable), the writing of the document, and the approval of the submission of this version; that the document represents valid work; that if we used information derived from another source, we obtained all necessary approvals to use it and made appropriate acknowledgments in the document; and that each takes public responsibility for it.
The history of cardiac surgery dates back to the late 19th century, with the repair of pericardial and cardiac trauma by Williams of Chicago in 1893. The modern era of cardiac surgery began in earnest with the development of the cardiopulmonary bypass machine by Gibbon of Boston in 1953. The modern concepts and techniques of extracorporeal circulation were refined further by Lillehei and Kirklin in the early 1950s. Extracorporeal circulation, along with improved techniques of myocardial protection, has allowed surgeons to perform the most complex procedures in the current realm of cardiothoracic surgical practice. The most commonly performed procedure since its inception in the 1960s is coronary artery bypass graft (CABG) surgery.
The development of coronary cine angiography in the early 1960s enabled the identification of stenoses of the coronary arteries in living patients (Fig. 30-1). This identification made it possible for the directed treatment of ischemic heart disease through CABG surgery and percutaneous interventions of obstructed arteries. The treatment for coronary artery disease was revolutionized, dramatically improving the therapeutic options for patients with this commonly occurring condition.
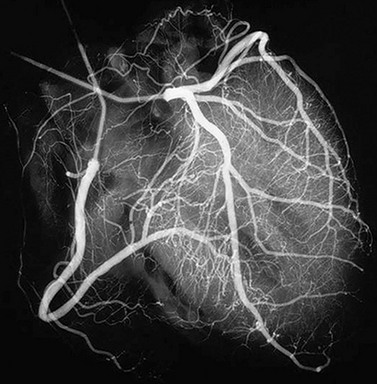
 FIGURE 30-1 Coronary angiogram of right and left coronary artery systems.
FIGURE 30-1 Coronary angiogram of right and left coronary artery systems.
(From Mayo Clinic Cardiothoracic Grand Rounds, picture used in slide presentation, 2002.)
SURGERY FOR CORONARY ARTERY DISEASE
Description and Special Anatomic Considerations
Myocardial blood flow is provided by the left and right coronary arteries, originating from the aortic root. The left main coronary artery bifurcates into the left anterior descending (LAD) and the left circumflex arteries. The left circumflex artery further branches into the obtuse marginal arteries, which together with the LAD artery provide most blood flow to the left ventricle. The right coronary artery provides blood flow to the right ventricle and terminates as the posterior descending artery, providing blood flow to the inferior wall of the left ventricle (Fig. 30-2).
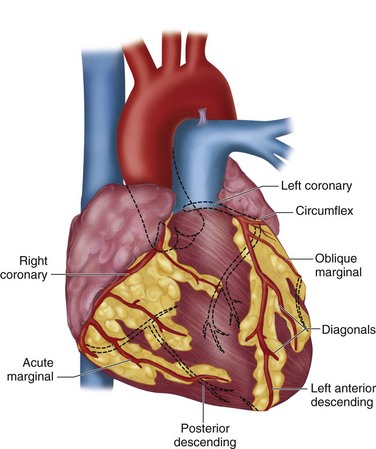
 FIGURE 30-2 Coronary arteries of the heart.
FIGURE 30-2 Coronary arteries of the heart.
(Redrawn from University of Utah Health Sciences Center. 2002. Available at: http://healthcare.utah.edu/healthinfo/adult/cardiac/arteries.htm.)
The basic goal of CABG surgery is to provide new blood flow beyond significantly stenotic epicardial vessels. Stenosis is considered hemodynamically significant when the diameter is reduced by greater than 50%, which equates to a reduction in the cross-sectional area of 75% (Fig. 30-3). The technique of CABG surgery involves four stages: (1) conduit harvest, (2) institution of extracorporeal circulation, (3) construction of vascular anastomoses, and (4) separation from cardiopulmonary bypass. Most commonly used conduits for bypass grafts include the left internal mammary artery and greater saphenous vein. Other possible conduits include the right internal mammary artery, the radial artery, the right gastroepiploic artery, and the lesser saphenous vein. Generally, arterial conduits have better long-term patency than venous grafts.
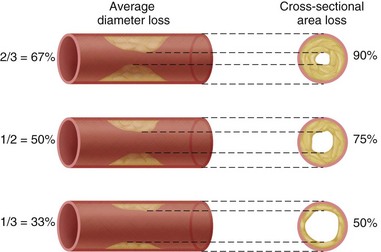
 FIGURE 30-3 Relationship of coronary artery stenosis in diameter and cross-sectional area.
FIGURE 30-3 Relationship of coronary artery stenosis in diameter and cross-sectional area.
(From Brandt PW, Partridge JB, Wattie WJ. Coronary arteriography: a method of presentation of the arteriogram report and a scoring system. Clin Radiol 1977; 28:361.)
CABG surgery is performed with the use of cardiopulmonary bypass, which provides circulatory support and gas exchange during the operation. Anticoagulation with heparin is typically used during the period of cardiopulmonary bypass. To establish a still and bloodless field on which to sew the anastomoses, the heart is arrested using hyperkalemic cardioplegia solution. The basic cardiopulmonary bypass circuit is shown in Figure 30-4. When the heart is arrested, and epicardial arteries are exposed (Fig. 30-5), the vascular anastomoses may be constructed.
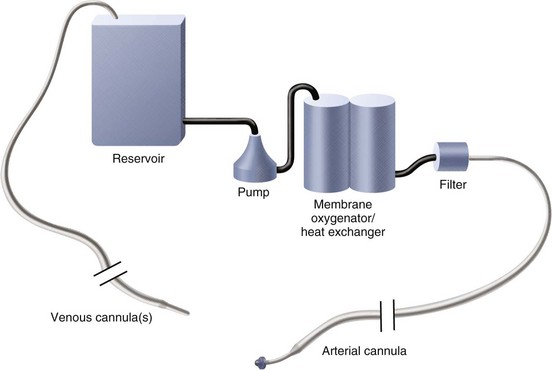
 FIGURE 30-4 Basic cardiopulmonary bypass circuit.
FIGURE 30-4 Basic cardiopulmonary bypass circuit.
(Redrawn from Mayo Clinic Cardiothoracic Grand Rounds, picture used in slide presentation, 2002.)
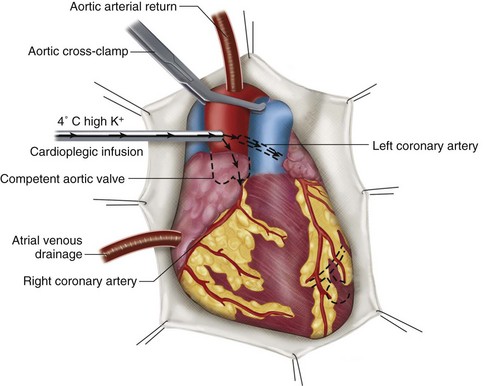
 FIGURE 30-5 Common cannulation and myocardial protection techniques used for cardiopulmonary bypass.
FIGURE 30-5 Common cannulation and myocardial protection techniques used for cardiopulmonary bypass.
(Redrawn from Roberts AJ. Efficacy of intraoperative myocardial protection in adult cardiac surgery. In Roberts AJ [ed]. Difficult Problems in Adult Cardiac Surgery. Chicago, Year Book Medical Publishers, 1985, p 386.)
The microvascular anastomoses are constructed using fine suture material, under loupe magnification, as shown in Figure 30-6. The left internal mammary artery is most commonly placed to the LAD artery as an in-situ graft (Fig. 30-7), whereas the greater saphenous vein is placed as a reversed graft from the ascending aorta to the coronary artery (Fig. 30-8).
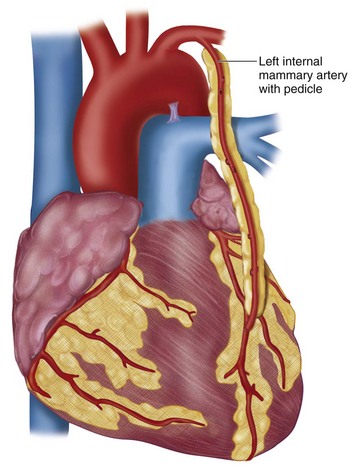
 FIGURE 30-7 In-situ left internal mammary artery graft to left anterior descending coronary artery.
FIGURE 30-7 In-situ left internal mammary artery graft to left anterior descending coronary artery.
(Redrawn from Rhead J, Sundt TM. What is coronary artery bypass grafting? Society of Thoracic Surgeons, 2008. Available at: www.sts.org/sections/patientinformation/adultcardiacsurgery/cabg.)
Indications
Over the years, accepted indications for CABG surgery have changed slightly, primarily as a result of the advent of catheter-based revascularization techniques, especially coronary artery stenting. The American Heart Association (AHA) has published comprehensive guidelines regarding the indications for CABG.1 These recommendations generally are classified into one of eight subsets of patients as shown in Table 30-1. Class I or II indications for surgery include patients with stable or unstable angina and three-vessel disease (including the main branches of the right coronary artery, LAD artery, and left circumflex artery), patients with two-vessel disease with proximal LAD artery stenosis, or asymptomatic patients with significant left main stenosis. CABG surgery after acute myocardial infarction is indicated in these same conditions and is usually delayed for 1 week, if possible, to allow myocardial recovery before surgery. Emergent CABG surgery may be indicated for cardiogenic shock secondary to acute myocardial infarction in patients younger than 75 years (which can salvage 50% of patients) or for post–percutaneous intervention complications.
TABLE 30-1 American Heart Association Recommended Indications for Coronary Artery Bypass Graft (CABG) Surgery: Clinical Subsets of Patients
From Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery. Circulation 2004; 110:e340-e437.
Contraindications
All patients are risk-stratified to predict the probability of operative morbidity and mortality. Factors that increase risk for surgery are presented in Table 30-2. The most important predictor of operative mortality is the preoperative left ventricular ejection fraction. There are several acceptable methods to calculate perioperative risk in cardiac surgery. The Society of Thoracic Surgeons (STS) Risk Calculator and the EUROSCORE are two of the most commonly used methods. When the calculated risks of surgery outweigh the potential benefits of the operation, surgery should not be performed.
TABLE 30-2 Preoperative Risk Factors for Coronary Artery Bypass Graft Surgery
Outcomes and Complications
Overall hospital mortality after CABG surgery has declined over the past 20 years and presently is 2% to 3%, according to the STS surgical database. Most in-hospital deaths after CABG surgery are due to cardiac failure. Overall 5-year survival is greater than 90%, and overall 10-year survival is 70% to 80%. The frequent use of the left internal mammary artery has favorably affected short-term and long-term survival. For patients with preoperative angina, resolution of symptoms is approximately 60% at 10 years. Recurrent angina is typically due to progression of atherosclerotic disease of the native coronary arteries or vein grafts. The aggressive use of statin therapy and β blockers can delay the progression of coronary artery or vein graft disease after CABG surgery.2
The results of three randomized prospective studies comparing CABG surgery with medical management conclusively showed survival advantages with CABG surgery, particularly among patients with three-vessel disease, left main disease, and decreased left ventricular ejection fraction (35% to 50%).3–5 In diabetic patients with multivessel disease, CABG surgery has been shown to be superior to percutaneous coronary interventions. In the current era of drug-eluting stents, CABG surgery has been shown to have a reduced rate of reintervention, particularly in the setting of multivessel disease.
Imaging Findings
Preoperative Planning
With the advent of selective coronary angiography by Mason Sones in 1967, and the CASS study,4 the gold standard for assessment of coronary artery anatomy and disease was established. Several projections obtained using fluoroscopic guidance allow the surgeon to obtain a three-dimensional assessment of the coronary arteries (Table 30-3). Figure 30-9 shows several commonly used projections of coronary artery catheterization. Additionally, by injecting contrast material with the catheter positioned in the left ventricle, an image of the left ventricle throughout the cardiac cycle may be obtained to assess left ventricular function.
TABLE 30-3 Standard Projectional Views for Cardiac Catheterization
The high-resolution, 64-detector CT scanner has emerged as a tool for performing coronary angiography quickly and less invasively.6 Data quality is high, and has improved with increasingly sophisticated technology. Sensitivity and specificity of CT angiography have been shown to be comparable to conventional coronary angiography in detecting stenoses in symptomatic patients.7 Positive and negative predicted values also are comparable. Current limitations of CT angiography that reduce the reliability for image interpretation include heavy vessel wall calcifications, persistent irregular heart rhythm (and the need for heart rates <70 beats/min), and existing coronary stents.8 The estimated radiation dose used for CT angiography is also higher than the dose used for conventional angiography. MR angiography is another modality used to assess coronary artery anatomy, particularly in patients with coronary anomalies.
SURGERY FOR AORTIC VALVULAR DISEASE
Description and Special Anatomic Considerations
Although the number of coronary artery bypass procedures has gradually declined in current cardiovascular surgical practice in the United States, the number of valve procedures has steadily increased over the last 10 years. In the executive summary of the STS Spring 2007 Report, the number of isolated aortic valve replacements (AVRs) and tricuspid valve procedures showed the greatest increase since 1997.9
Aortic Valve Anatomy
Situated centrally at the base of the heart, the aortic valve resides in a unique position because of its proximity to all chambers of the heart and the three other cardiac valves (Fig. 30-10). The aortic valve is trileaflet, consisting of three semilunar cusps and the fibrous aortic annulus to which the cusps are attached. Sinuses of Valsalva are areas of aortic dilation adjacent to the aortic valve cusps, and are identified by the respective coronary artery origination as left, right, and noncoronary (Fig. 30-11). The commissures are structures where adjacent aortic cusps abut each other and have special anatomic significance during surgery. The commissure between the noncoronary and right coronary cusps is directly cephalad to the penetration of the atrioventricular bundle and membranous septum (see Fig. 30-11). The commissure between the noncoronary and left coronary cusps bisects the aortic-mitral curtain and the anterior leaflet of the mitral valve (see Fig. 30-11). This area is of particular significance during aortic root enlargement and in cases of aortic valve endocarditis as an avenue of spread for infection to the mitral valve. The last commissure between the left and right cusps defines the adjacent pulmonary valve and right ventricular outflow tract (see Figs. 30-10 and 30-11).
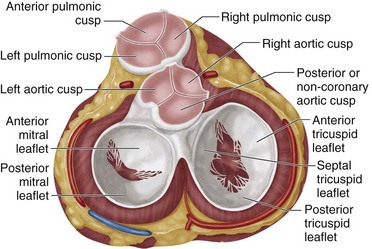
 FIGURE 30-10 Aortic valve position in base of the heart.
FIGURE 30-10 Aortic valve position in base of the heart.
(Redrawn from Miljevic T, Sayeed MR, Stamou SC, et al. Pathophysiology of aortic valve disease. In Cohn LH [ed]. Cardiac Surgery in the Adult. New York, McGraw-Hill, 2008, pp 825-840.)
Pathophysiology of Aortic Stenosis and Regurgitation
Acquired calcific aortic valvular stenosis is most commonly due to degeneration and calcification of the aortic valve leaflets; this disease primarily affects elderly patients. In contrast, significant aortic stenosis in younger patients is most commonly due to premature calcification and degeneration of a bicuspid aortic valve, and typically occurs in the fourth or fifth decade of life. In calcific aortic stenosis, the calcification and thickening of the aortic valve leaflets occurs through an active inflammatory process, partially related to hypercholesterolemia, similar to the process of coronary atherosclerosis.10–13 As the leaflets calcify and fuse together, the functional area of the valve decreases to cause a measurable obstruction to outflow. The progressive pressure overload to the left ventricle results in maladaptive left ventricular hypertrophy, and eventually results in clinically significant obstruction.
Over time, changes include an increase in chamber compliance to accommodate the increased volume state, with an increase in left ventricular end-diastolic and end-systolic dimensions. Eventually, left ventricular systolic dysfunction develops with progressive chamber enlargement as the left ventricular chamber conforms to a more spherical geometry from the normal ellipsoid shape. This change results in a decrease in left ventricular myocardial contractility and correlates with the onset of symptoms of heart failure.13 This process is gradual and can take many years until the development of clinically relevant aortic regurgitation. In contrast to chronic aortic regurgitation, acute severe aortic regurgitation is less well tolerated. The sudden volume overload to the ventricle creates marked hemodynamic changes frequently resulting in pulmonary edema and cardiogenic shock unless the volume overload is corrected. Infective endocarditis of the aortic valve and ascending aortic dissection are two common causes of acute severe aortic regurgitation.
Aortic Valve Replacement
The technique of AVR is similar for aortic stenosis and aortic regurgitation. Implementations of cardiopulmonary bypass and cardioplegic arrest of the heart are important steps in the successful conduct of the operation. Because of the location of the aortic valve, it is crucial to avoid injury to related structures (see Figs. 30-10 and 30-11). In all AVRs, the concept of adequate myocardial protection must be ensured. This is particularly true in operations on the aortic valve because the ventricle is hypertrophic and susceptible to injury during surgery. Delivery of cardioplegia should include antegrade cardioplegia either into the aortic root (aortic stenosis) or directly into the coronary ostia in cases of aortic regurgitation and retrograde cardioplegia into the coronary sinus for balanced myocardial protection.
The ascending aorta is opened, and the aortic valve is inspected and excised carefully (Fig. 30-12). In cases of aortic stenosis, the annulus requires débridement of calcium to seat the prosthesis and prevent paravalvular regurgitation. The aortic annulus is sized for an appropriate-sized valve. Sutures (supported with pledgets on either the ventricular or aortic side) are placed around the aortic annulus and then passed through the sewing cuff of the prosthetic valve, which is then seated (Figs. 30-13 and 30-14). After seating the prosthesis, the aortotomy is closed, the heart is deaired, and the cross-clamp is released. After confirmation of satisfactory hemodynamics and adequate prosthesis function by intraoperative transesophageal echocardiography (TEE), the patient is separated from extracorporeal circulation, and anticoagulation is reversed.
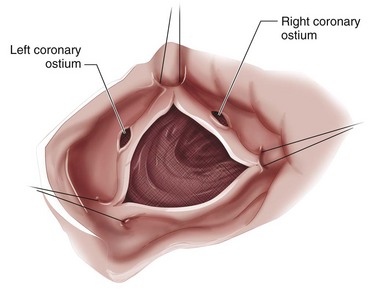
 FIGURE 30-12 Excised aortic valve and annular débridement.
FIGURE 30-12 Excised aortic valve and annular débridement.
(Redrawn from Desai ND, Christakis GT. Bioprosthetic aortic valve replacement: stented pericardial and porcine valves. In Cohn LH [ed]. Cardiac Surgery in the Adult. New York, McGraw-Hill, 2008, pp 857-894.)
Indications
The evaluation and degree of severity of aortic stenosis can be estimated using echocardiography with Doppler measurements and cardiac catheterization, and can be graded based on several parameters (Table 30-4).13 Hemodynamically, but not clinically significant obstruction occurs when the valve area decreases from the normal 3 to 4 cm2 to less than 1.5 to 2 cm2. The classic symptoms of aortic stenosis are angina; syncope; and symptoms of congestive heart failure, such as dyspnea. When symptoms occur, survival is dismal, unless the valve is replaced. About 75% of patients with symptomatic aortic stenosis die within 3 years after the onset of symptoms unless the aortic valve is replaced.10,11 Current indications for AVR as defined by the American College of Cardiology (ACC) and AHA are listed in Table 30-5.13 Asymptomatic patients with severe aortic stenosis can be observed until symptoms develop.
TABLE 30-5 Indications for Aortic Valve Replacement for Aortic Stenosis (AS)
| Indication | Class of Evidence* |
|---|---|
| Symptomatic | |
| Severe AS that is symptomatic | I |
| Asymptomatic | |
| Severe AS and undergoing coronary artery bypass graft surgery | I |
| Severe AS and undergoing surgery of the aorta | I |
| Severe AS and undergoing surgery for other heart valves | I |
| Severe AS and left ventricular systolic dysfunction (ejection fraction <50%) | I |
| Moderate AS and undergoing other heart surgery | IIa |
* Class I refers to conditions for which there is evidence or general agreement (or both) that the procedure or treatment is beneficial, useful, and effective. Class IIa refers to conditions for which there is conflicting evidence or a divergence of opinion (or both). Weight of evidence/opinion is in favor of usefulness or efficacy or both.
The severity of aortic regurgitation can be quantified by echocardiography or MRI as defined by Table 30-6. Operative therapy for chronic aortic regurgitation is controversial in asymptomatic patients. Because the natural history of severe asymptomatic aortic regurgitation is a gradual decline in ventricular function, patients require close follow-up to detect the onset of clinically evident heart failure. The rate of progression and the predictors of outcome are debatable and not well defined based on randomized trials. Observational data support surgery in asymptomatic patients with evidence of ventricular enlargement or decrease in ejection fraction. Extreme left ventricular dilation (left ventricular end-diastolic dimension >80 mm) may be a risk factor for sudden death.14 The use of vasodilators in delaying the rate of progression of aortic regurgitation is controversial. Afterload-reducing agents such as calcium channel blockers and angiotensin-converting enzyme inhibitors have been examined, and the results are equivocal.15 Similarly, the regulation of serum lipid levels to delay the progression of aortic stenosis has been studied with mixed results.16
Patients with aortic regurgitation and the presence of severe symptoms, categorized as New York Heart Association class III-IV, should have aortic valve surgery. The indications of AVR for aortic regurgitation are summarized in Table 30-7.13
TABLE 30-7 Indications for Aortic Valve Replacement in Severe Aortic Regurgitation (AR)
| Indication | Class of Evidence* |
|---|---|
| Symptomatic | |
| Severe AR with NYHA class III-IV | I |
| Asymptomatic | |
| Severe AR and evidence of LV dysfunction (EF <50%) | I |
| Severe AR and undergoing coronary bypass graft surgery | I |
| Severe AR and undergoing other valve or aortic surgery | I |
| Severe AR and normal EF and evidence of LV dilation (LVEDD >75 mm or LVESD >55 mm) | IIa |
EF, ejection fraction; LV, left ventricular; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; NYHA, New York Heart Association.
* Class I refers to conditions for which there is evidence or general agreement (or both) that the procedure or treatment is beneficial, useful, and effective. Class IIa refers to conditions for which there is conflicting evidence or a divergence of opinion (or both). Weight of evidence/opinion is in favor of usefulness or efficacy or both.
Contraindications
There is no effective medical therapy for severe aortic stenosis, and given the fatality of symptomatic aortic stenosis, there are no absolute contraindications for AVR. Three situations warrant special discussion when considering the risks of AVR. (1) AVR has been shown to be lifesaving in octogenarians or older individuals in the absence of major coexisting illnesses.17 Age by itself is not a contraindication. (2) Among patients with left ventricular dysfunction and substantial transvalvular gradients (mean >40 mm Hg), the result of surgery is excellent.13 Patients with a reduced ejection fraction and a low transvalvular gradient (mean <30 mm Hg) have high operative risk and reduced survival after surgery.18 Even these high-risk patients benefit from surgery, however, and such patients should be considered for operative therapy unless prohibitive risks are encountered. (3) Asymptomatic patients with severe aortic stenosis have an excellent prognosis without valve replacement; however, there is a risk of sudden death in 1% to 2% of asymptomatic patients. In addition, the onset of symptoms can be insidious and not clinically apparent. Exercise testing and echocardiography may help identify asymptomatic patients who may benefit from early valve surgery.
Otto and colleagues12 found patients whose transvalvular velocity exceeded 4 m/s had a 70% risk of becoming symptomatic and requiring AVR in 2 years. In patients with prohibitive risk of AVR, aortic balloon valvotomy has been used as a bridge to surgery in hemodynamically unstable adults with severe left ventricular dysfunction. The acute complication rate is greater than 10%, and restenosis is rapid in most patients. This form of therapy should be limited to centers with extensive experience in this procedure.13
Outcomes and Complications
To discuss the outcomes of AVR, we first briefly discuss the various types of prostheses because the prosthesis is one of the most important determinants of long-term results. Prosthetic aortic valves are divided into two major types: tissue and mechanical. A mechanical prosthesis requires lifelong anticoagulation to prevent thromboembolic complications and is associated with long-term bleeding risk, particularly in elderly patients. Structural valve degeneration does not occur with mechanical valves, and they can last the lifetime of the patient. The evolution of mechanical valves has progressed from the “ball in cage” valve (e.g., Starr-Edwards valve) through the tilting disk valve (e.g., Björk-Shiley valve) to the current design of a bileaflet valve (e.g., St. Jude valve) (Fig. 30-15). Bileaflet anatomy of the valve provides the best flow dynamics and the least trauma to red blood cells.

 FIGURE 30-15 Mechanical bileaflet prosthetic aortic valve.
FIGURE 30-15 Mechanical bileaflet prosthetic aortic valve.
(St. Jude Regent valve courtesy of St. Jude Medical, Minneapolis, MN.)
In contrast to mechanical valves, a tissue prosthesis provides the surgeon a variety of choices. Tissue valves can be separated into three major classes based on tissue origin: autograft, allograft, and xenograft. Xenograft tissue prosthesis (bovine or porcine) can be divided further into stent supported (Fig. 30-16A) and unsupported (see Fig. 30-16B). Nonstented valves are supported by the native aortic root and provide better hemodynamics than stented valves.
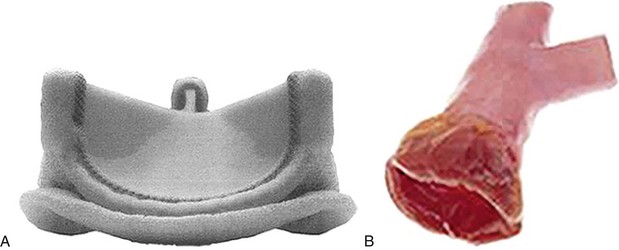
 FIGURE 30-16 A, Stented tissue prosthetic aortic valve. B, Unstented aortic valve (cadaver homograft).
FIGURE 30-16 A, Stented tissue prosthetic aortic valve. B, Unstented aortic valve (cadaver homograft).
(A, Carpentier-Edwards Perimount Magna valve courtesy of Edwards Lifesciences, Irvine, CA.)
A second important consideration to AVR outcome is the concept of patient-prosthesis mismatch as described in detail by Pibarot and Dumesnil.19 Patient-prosthesis mismatch is due to the inherent limitations in effective orifice area (EOA) obligatory in all stented tissue prostheses. To overcome this problem, much technology has been invested in designing stented tissue valves with larger EOA. Although it is unclear if a particular type of stented tissue valve offers an advantage compared with another, it is important to index the EOA to the patient’s body surface area (EOA index). Generally, a minimum EOA index greater than 0.75 is necessary, and an EOA index greater than 0.85 is optimal to prevent patient-prosthesis mismatch.19 Unless an adequate-sized valve is replaced at the aortic position, continued obstruction of left ventricular outflow can result in decreased functional improvement and progression of left ventricular hypertrophy with increased short-term and long-term mortality.19 To prevent the problems related to patient-prosthesis mismatch, two options are available: aortic root enlargement or placing an unstented tissue prosthesis. Aortic root enlargement usually allows the seating of a prosthesis one size larger and is required in 10% of patients undergoing AVR.17
The results of surgery for aortic stenosis are excellent compared with medical therapy (Fig. 30-17) with improvement in long-term survival. In the executive summary of the STS Spring 2007 Report, the unadjusted aortic valve operative mortality was 3% to 4% for the last 10 years.13 If CABG surgery is required in addition to AVR, the mortality is increased to 5% to 7%. If concomitant mitral valve surgery is required, the risk of death increases further to 7% to 11%.13
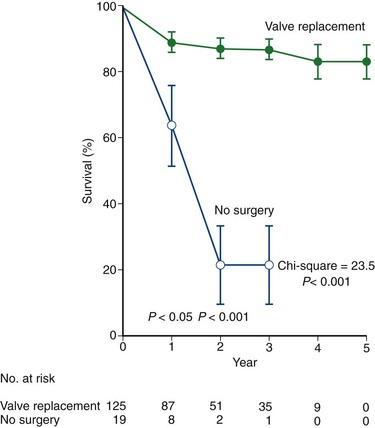
 FIGURE 30-17 Survival comparison between surgical and medical therapy for symptomatic aortic stenosis.
FIGURE 30-17 Survival comparison between surgical and medical therapy for symptomatic aortic stenosis.
(From Emery RW, Emery AM, Knutsen AI, et al. Aortic valve replacement with a mechanical cardiac valve prosthesis. In Cohn LH [ed]. Cardiac Surgery in the Adult. New York, McGraw-Hill, 2008, pp 841-856.)
Imaging Findings
Preoperative Planning
All patients older than 40 years should undergo selective left heart catheterization to exclude concomitant coronary artery disease that may require bypass grafting. Among patients with severe aortic stenosis and angina, the prevalence of coronary artery disease is 40% to 50%.13 In cases where noninvasive testing is equivocal to the severity of aortic stenosis, left heart catheterization can measure transvalvular pressure gradient and calculate the aortic valve area.
MRI may be used to characterize further left ventricular volume, function, and mass in aortic stenosis and aortic regurgitation. MRI can be used to determine the severity of valvular disease and to characterize specific valvular anatomy, such as a bicuspid valve (Fig. 30-18). MRI can quantify the severity of aortic stenosis by determining the valve area by direct planimetry, negating the need for TEE. Cardiac CT has the ability to detect and quantify aortic valve calcification, which may have prognostic significance or allow the monitoring of progression of aortic stenosis (Fig. 30-19). Imaging of the thoracic aorta with either CT or MRI should be obtained in patients at risk of aortic root or ascending aortic aneurysm (Figs. 30-20 and 30-21).
Postoperative Surveillance
The ACC/AHA guidelines provide some guidance regarding the follow-up of patients who have undergone valve replacement.13 For patients with prosthetic heart valves, a history and physical examination and Doppler transthoracic echocardiography (TTE) is obtained at 2 to 4 weeks. Routine follow-up should be conducted annually with earlier evaluation if there is a change in the clinical status. Education for patients who are to take oral warfarin (Coumadin) anticoagulation and antibiotic prophylaxis is essential for excellent long-term results.
SURGERY FOR MITRAL VALVULAR DISEASE
Description and Special Anatomic Considerations
Situated between the left atrium and left ventricle, the integrity of the mitral valve is paramount to the normal function of the heart. Mitral valve anatomy can be viewed as valvular and subvalvular components. The valvular components include the anterior and posterior leaflets, the leaflet scallops, the anterior and posterior commissures, and the left and right trigones (Fig. 30-22A). Subvalvular components include the chordal attachments to the free edges of the anterior and posterior leaflets, the anterolateral and posteromedial papillary muscles, and the left ventricle (see Fig. 30-22B). The mitral valve annulus is a ring of fibroconnective tissue on which each leaflet is attached and is a dynamic structure that varies with each cardiac cycle. Valve coaptation is enhanced by the fact that the annulus reduces the effective mitral valve orifice by 25% to 40% during systole.13
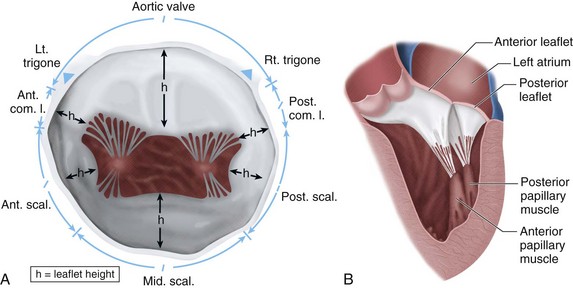
 FIGURE 30-22 A and B, Mitral valve en face and association with aortic valve.
FIGURE 30-22 A and B, Mitral valve en face and association with aortic valve.
(A, Redrawn from Fann JI, Ingels NB Jr, Miller DC. Pathophysiology of mitral valve disease. In Cohn LH [ed]. Cardiac Surgery in the Adult. New York, McGraw-Hill, 2008, pp 973-1012.)
The surface area of the valve together with the annulus comprises the total cross-sectional area, which ranges from 5 to 12 cm2. The mobile anterior leaflet accounts for two thirds of the valve area and one third of the anterior circumference, whereas the posterior leaflet accounts for one third of the area and two thirds of the circumference. The two leaflets are separated by commissures, which must be differentiated from the trigones during valve surgery (see Fig. 30-22A). The trigones are relatively fixed structures that are part of the fibrous skeleton of the heart.
Similar to the aortic valve, the mitral valve sits in proximity to several important cardiac structures (Fig. 30-23). Each leaflet is divided by three roughly equal scallops: left, middle, and right corresponding to the proximity with the left and right trigones. The left scallop closest to the left trigone of the posterior leaflet is designated as P1; the middle, as P2; and the right, as P3. The anterior leaflet is similarly designated as A1 to A3 (Fig. 30-24).
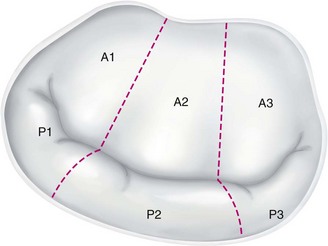
 FIGURE 30-24 Mitral valve scallops.
FIGURE 30-24 Mitral valve scallops.
(Redrawn from Chen FY, Cohn LH. Mitral valve repair. In Cohn LH [ed]. Cardiac Surgery in the Adult. New York, McGraw-Hill, 2008, pp 1013-1030.)
The papillary muscles constitute approximately 30% of the left ventricular mass,13 and are integral to normal mitral valve function. Ischemic injury to the either papillary muscle can result in significant mitral valve regurgitation.
Mitral Regurgitation
Mitral valve regurgitation is usually due to myxomatous degeneration. Other causes include collagen vascular and rheumatic heart disease, certain drugs, and infective endocarditis. Ischemic mitral regurgitation is differentiated from organic mitral regurgitation by the presence of coronary artery disease and ischemic injury to the left ventricle and papillary muscles. Severe chronic mitral regurgitation results from the inability of proper leaflet coaptation and can be due to valvular and subvalvular pathology. Carpentier identified three basic pathoanatomic lesions resulting in mitral regurgitation (Fig. 30-25). Type II lesions are most common and are due to myxomatous disease of the mitral valve with either elongated or ruptured chordae. Type I and III lesions are typically seen in ischemic mitral regurgitation. The severity of mitral regurgitation can be quantified using Doppler echocardiographic measurements. Severe mitral regurgitation is classified as a regurgitation volume greater than 60 mL or an effective regurgitant orifice greater than 40 mm.
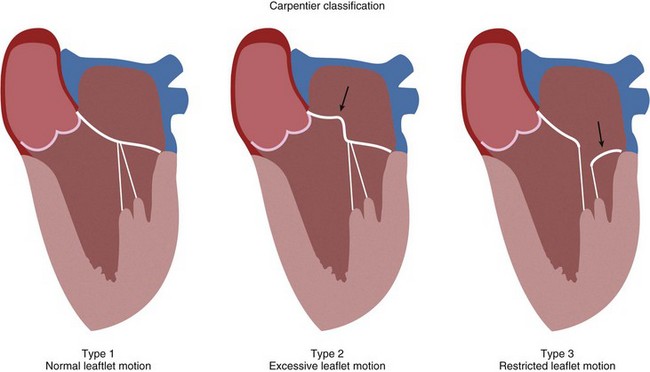
 FIGURE 30-25 Classification of mitral valve leaflet pathoanatomy.
FIGURE 30-25 Classification of mitral valve leaflet pathoanatomy.
(Redrawn from Chen FY, Cohn LH. Mitral valve repair. In Cohn LH [ed]. Cardiac Surgery in the Adult. New York, McGraw-Hill, 2008, pp 1013-1030.)
Severe mitral regurgitation is characterized initially by eccentric ventricular hypertrophy with increases in ventricular dimensions to maintain forward flow. This compensated phase is accompanied by increases in left atrial size. Eventually, the mitral regurgitation results in increased left ventricular dimensions indicating contractile dysfunction. As the left ventricle dilates, the annulus enlarges along the unsupported posterior circumference (Fig. 30-26). This annular dilation results in further worsening of mitral regurgitation.
Mitral Stenosis
The patient is placed on cardiopulmonary bypass, and the heart is arrested using cold cardioplegia. The left atrium is opened anterior to the right pulmonary veins longitudinally (Fig. 30-27). The mitral apparatus is brought into view being cognizant of surrounding important structures (Fig. 30-28A). If the patient has atrial fibrillation, the left atrial appendage may be sutured closed to reduce stroke risk. Valve competency is evaluated with cold saline injection into the left ventricular cavity, and a determination of either valve repair or valve replacement is made (see Fig. 30-28B). For replacement, excision of variable amounts of anterior leaflet and posterior leaflet is performed with the goal of preserving the papillary muscles and chordal attachments of the subvalvular apparatus (Fig. 30-29). After the proper sizing of the mitral valve orifice, pledget-supported sutures are placed around the mitral valve annulus and the sewing cuff of the prosthesis (Fig. 30-30). After seating the prosthesis, the left atriotomy is closed, the heart is deaired, and the patient is separated from cardiopulmonary bypass. Intraoperative TEE is essential, particularly in cases where valve repair is entertained.
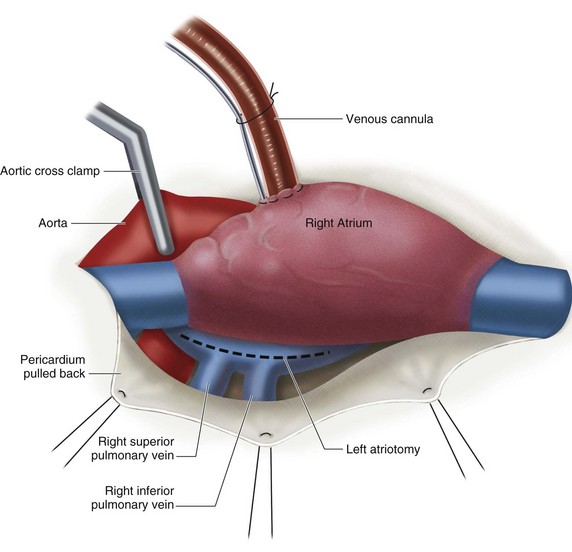
 FIGURE 30-27 Left atriotomy to view the mitral valve.
FIGURE 30-27 Left atriotomy to view the mitral valve.
(Redrawn from Mayo Clinic Cardiothoracic Grand Rounds, picture used in slide presentation, 2002.)
Indications
The indications for mitral valve replacement for mitral regurgitation were defined by the ACC/AHA guidelines in 2006 (Table 30-8). In addition to these indications, some authors have recommended early mitral valve surgery if the effective regurgitant orifice is greater than 40 mm, and there is a high probability of mitral valve repair.
TABLE 30-8 Indications for Mitral Valve Surgery in Patients with Mitral Regurgitation (MR)
| Indication | Class of Evidence* |
|---|---|
| Symptomatic | |
| Severe MR and symptoms | I |
| Severe MR and NYHA class II-IV | I |
| Asymptomatic | |
| Severe MR and decreased EF (normal >60%) and LVESD >40 mm | I |
| Severe MR and preserved EF and LVESD <40 mm† | IIa |
| Severe MR and new-onset atrial fibrillation | IIa |
| Severe MR and pulmonary hypertension‡ | IIa |
| Severe MR and NYHA class II-IV and severe LV dysfunction in which valve repair is likely | IIa |
EF, ejection fraction; LV, left ventricular; LVESD, left ventricular end-systolic dimension; NYHA, New York Heart Association.
* Class I refers to conditions for which there is evidence or general agreement (or both) that the procedure or treatment is beneficial, useful, and effective. Class IIa refers to conditions for which there is conflicting evidence or a divergence of opinion (or both). Weight of evidence/opinion is in favor of usefulness or efficacy or both.
† Only if valve repair is likely.
‡ Pulmonary artery systolic pressure at rest >50 mm Hg and >60 mm Hg with exercise.
Interventions for mitral stenosis are more complex because percutaneous balloon valvuloplasty and surgery have similar outcomes. Indications for percutaneous balloon valvuloplasty have been defined by the ACC/AHA (Table 30-9). Key determinants of successful percutaneous balloon valvuloplasty are based on valve leaflet mobility, leaflet and subvalvular thickening, and the degree of leaflet calcification. These parameters can be determined by echocardiography. Favorable anatomy includes little valvular calcification, minimal valvular and subvalvular fibrosis, and nominal thickening of the valve. Mitral valve replacement is indicated when percutaneous balloon valvuloplasty is impossible and in cases where percutaneous balloon valvuloplasty has failed or is contraindicated. Surgical indications for repair of mitral stenosis are listed in Table 30-10.
TABLE 30-9 Indications for Percutaneous Mitral Balloon Valvotomy in Moderate to Severe Mitral Stenosis
| Indication | Class of Evidence* |
|---|---|
| NYHA class II-IV failure | I |
| Asymptomatic with pulmonary hypertension† | I |
| NYHA class II- IV failure and not surgical candidate | IIa |
| Asymptomatic with new-onset atrial fibrillation | IIb |
| NYHA class II-IV and mitral valve area >1.5 cm 2 and pulmonary hypertension | IIb |
| Alternative to surgery | IIb |
NYHA, New York Heart Association.
* Class I refers to conditions for which there is evidence or general agreement (or both) that the procedure or treatment is beneficial, useful, and effective. Class IIa refers to conditions for which there is conflicting evidence or a divergence of opinion (or both). Weight of evidence/opinion is in favor of usefulness or efficacy or both. Class IIb refers to conditions for which usefulness/efficacy is less well established by evidence or opinion.
† Pulmonary artery systolic pressure >50 mm Hg at rest or >60 mm Hg with exercise.
TABLE 30-10 Indications for Surgery for Mitral Stenosis (MS)
| Indication | Class of Evidence* |
|---|---|
| Symptomatic NYHA class III-IV failure and PMBV is unavailable, contraindicated, or not favorable owing to valve morphology | I |
| Symptomatic with moderate to severe MS and moderate to severe MR | I |
| Severe pulmonary hypertension, NYHA class I-II, and not candidate for PMBV | IIa |
| Asymptomatic with moderate to severe MS and recurrent emboli while on anticoagulation if valve morphology is favorable to valve repair | IIb |
MR, mitral regurgitation; NYHA, New York Heart Association; PMBV, percutaneous mitral balloon valvotomy.
* Class I refers to conditions for which there is evidence or general agreement (or both) that the procedure or treatment is beneficial, useful, and effective. Class IIa refers to conditions for which there is conflicting evidence or a divergence of opinion (or both). Weight of evidence/opinion is in favor of usefulness or efficacy or both. Class IIb refers to conditions for which usefulness/efficacy is less well established by evidence or opinion.
Contraindications
Special caution should be exercised in elderly patients with mitral regurgitation, in particular patients 75 years old or older who require valve replacement. Operative mortality in this subgroup can be particularly high, exceeding 20% in low-volume centers.13 Among patients with asymptomatic or mildly symptomatic mitral regurgitation, medical therapy should be implemented in lieu of surgery. In cases of moderate to severe mitral regurgitation and mitral stenosis or in the presence of a left atrial thrombus, percutaneous balloon valvuloplasty is contraindicated, and surgery should be pursued.
Outcomes and Complications
When treated medically, severe mitral regurgitation secondary to myxomatous disease is associated with excess mortality and high morbidity. Survival rates ranging from 27% to 97% at 5 years have been reported. Surgery is almost unavoidable within 10 years of diagnosis and is associated with improved prognosis.20
The durability of mitral valve repair is excellent. The Mayo Clinic published the results of 917 patients who underwent isolated mitral valve procedures over a 15-year period.21 In this study, the authors found survival was superior after repair compared with replacement. The reoperation rate was higher after repair of the anterior leaflet compared with the posterior leaflet, and reoperation was similar after repair or replacement of the valve. A reoperation rate of 28% was noted at 15 years for anterior leaflet repair and 11% for posterior leaflet repair. The authors also noted that the reoperation rate decreased for both leaflets in patients treated more recently with a decrease to 5% for posterior leaflet repairs and 10% for anterior leaflet repairs at 10 years. In another study of 170 consecutive patients over a 14-year period who underwent mitral valve repair owing to nonrheumatic causes, the authors found the 20-year Kaplan-Meier survival rate was similar to the survival rate for a normal population with the same age.22 The primary determinant of operative mortality in mitral valve procedures is left ventricular function.20–23
Overall, the 10-year survival for untreated symptomatic mitral stenosis is 50% to 60%, whereas asymptomatic patients have a greater than 80% 10-year survival.13 When patients with symptomatic mitral stenosis undergo valve intervention (percutaneous balloon valvuloplasty or mitral valve replacement), survival is improved to near survivals of asymptomatic patients. Key determinants of long-term outcome in mitral stenosis include the degree of pulmonary hypertension, the presence of atrial fibrillation, and left ventricular function.12
The unadjusted mortality for mitral valve repair as noted in the STS Spring 2007 Report is 2% or less.9 Operative mortality for mitral valve replacement is higher at 5% and 6%. Combined procedures have a higher mortality as noted with AVR. Concomitant coronary artery surgery increases the risk of surgery, as do procedures involving multiple valves. Mitral valve replacement and CABG surgery has one of the highest unadjusted mortality rates (>10%). This high mortality is due to the fact that these patients have significant left ventricular impairment and ischemic mitral regurgitation.
Structures at risk for injury during mitral valve surgery are shown in Figures 30-23 and 30-27. These structures include the atrioventricular node or nodal artery; circumflex coronary artery, particularly in cases of left-sided dominance; aortic valve; and coronary sinus. Similar to AVR, there are patient-related and valve-related complications.
Imaging Findings
Postoperative Surveillance
Early close follow-up is important because most valve repairs fail early, typically within the first year of surgery. Annual echocardiographic evaluation and physical examination are recommended in the ACC/AHA guidelines.13 Endocarditis prophylaxis is important to implement regardless of repair or replacement. Long-term anticoagulation is unnecessary with tissue prosthesis unless associated atrial fibrillation is present.
INFECTIVE ENDOCARDITIS
Description and Special Anatomic Considerations
Endocarditis is a microbial infection of the endocardial surface of the heart. Such infections include most commonly the cardiac valves, but also the chambers of the heart and the great vessels. Preexisting valve lesions and indwelling cardiac devices, such as pacemakers, predispose patients to these infections. Endocardial vegetation is the characteristic lesion defining infective endocarditis and is a complex of fibrin, microbial organisms, and inflammatory cells. Native valve endocarditis is typically due to mitral valve prolapse, the most common cardiovascular diagnosis predisposing patients to endocarditis in North America. Prosthetic valve endocarditis is a serious infection typically involving the sewing cuff of the prosthesis, and has an equal frequency between mechanical and tissue prostheses in the aortic and mitral positions. Mechanical prostheses have a slightly higher incidence of endocarditis in the initial 3 months after surgery. Microbiologic features in most series include staphylococci and viridans streptococci as the most common pathogens. Streptococcus bovis is prevalent in elderly patients and is associated with colonic neoplasms.24
One of the most devastating manifestations of infective endocarditis is embolic injury to the brain (Fig. 30-31). More than 65% of embolic events in infective endocarditis involve the central nervous system, and neurologic complications develop in 20% to 40% of all patients with infective endocarditis.24 Different diagnostic criteria exist for the diagnosis of infective endocarditis with differing levels of sensitivity. The von Reyn and Duke criteria are less useful than the modified Duke criteria, which have a sensitivity near 90% (Table 30-11).25 Echocardiography is the most important initial diagnostic evaluation, and TTE is highly cost-effective. In cases of prosthetic valve endocarditis, TEE may provide better detail than TTE. Death from infective endocarditis is usually due to CNS complications or due to congestive heart failure, and the overall mortality rate is 20% to 25% for native valve endocarditis and prosthetic valve endocarditis. Right-sided endocarditis (tricuspid valve) has a lower mortality.24
TABLE 30-11 Modified Dukes Criteria for Infective Endocarditis
| Major Criteria |
|
A Positive blood culture for infective endocarditis (1 of the following 3)
|
* HACEK group—Haemophilus spp., Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella spp., and Kingella kingae.
From Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30:633-638.
Specific surgical techniques are similar to the techniques described in earlier discussions of the aortic and mitral valves. Goals of surgical therapy are to reverse the process of heart failure and remove the source of sepsis and systemic embolization. The degree of valve destruction and extravalvular extension dictates if valve repair is possible in this situation. Infective endocarditis of the aortic valve often results in extensive paravalvular destruction with aortic root abscess, which negates valve repair. Aortic root reconstruction with a cryopreserved allograft offers the patient the best chance of survival and decreases the risk of reinfection. If isolated leaflet perforation is found, a pericardial patch repair may be possible as may be seen in cases of mitral or healed aortic valve endocarditis. In situations where only the posterior leaflet is involved, repair may be more feasible than situations where the anterior or both leaflets are involved (Fig. 30-32). If the annulus is destroyed by infection, repair is unlikely.
Indications
Indications for surgery in infective endocarditis as recommended by the ACC/AHA are detailed in Tables 30-12 and 30-13. The most important indication for surgery is the development of congestive heart failure.
TABLE 30-12 Indications for Surgery in Native Valve Infective Endocarditis
| Indication | Class of Evidence* |
|---|---|
| Congestive heart failure | I |
| Elevated left ventricular end-diastolic pressure | I |
| Elevated left atrial pressure† | I |
| Endocarditis caused by highly resistant organism‡ | I |
| Complications of extravalvular extension§ | I |
| Recurrent emboli or persistent vegetations | IIa |
| Mobile vegetations >10 mm | IIb |
* Class I refers to conditions for which there is evidence or general agreement (or both) that the procedure or treatment is beneficial, useful, and effective. Class IIa refers to conditions for which there is conflicting evidence or a divergence of opinion (or both). Weight of evidence/opinion is in favor of usefulness or efficacy or both. Class IIb refers to conditions for which usefulness/efficacy is less well established by evidence or opinion.
† Evidence of premature closure of mitral valve with aortic regurgitation, rapid decelerating mitral regurgitation signal by continuous-wave Doppler, or moderate to severe pulmonary hypertension.
‡ Fungal, gram-negative bacilli (Pseudomonas); Staphylococcus spp. in some cases.
§ Evidence of heart block, annular abscess, or destructive penetrating lesions resulting in intracardiac or extracardiac fistulas or, in the case of atrioventricular endocarditis, mitral valve leaflet perforation.
TABLE 30-13 Indications for Surgery in Prosthetic Valve Endocarditis
| Indication | Class of Evidence* |
|---|---|
| Congestive heart failure | I |
| Evidence of prosthesis dehiscence† | I |
| Evidence of increasing obstruction or regurgitation | I |
| Extravalvular complications such as abscess | I |
| Evidence of persistent bacteremia despite antibiotics | IIa |
| Evidence of recurrent emboli despite antibiotics | IIa |
| Relapsing infection after initial therapy | IIa |
* Class I refers to conditions for which there is evidence or general agreement (or both) that the procedure or treatment is beneficial, useful, and effective. Class IIa refers to conditions for which there is conflicting evidence or a divergence of opinion (or both). Weight of evidence/opinion is in favor of usefulness or efficacy or both.
† Cine fluoroscopic examination showing “rocking” prosthesis.
Contraindications
Recent neurologic complications of infective endocarditis are considered a relative contraindication to surgery in infective endocarditis. Owing to the numerous patients who have cerebral emboli from infective endocarditis, the timing of operative therapy becomes critical because anticoagulation necessary for cardiopulmonary bypass can result in injury expansion. Although standardized recommendations are lacking, the longer the patient is allowed to recover from a recent cerebral injury, the better the outcome. Hemorrhagic infarcts portend a worse prognosis than purely ischemic injury. Hemodynamically unstable patients with multiorgan failure, particularly renal failure, are a subgroup with very high mortality. In such a situation, a multidisciplinary discussion involving the patient and family should be undertaken before embarking on operative therapy. It is always preferable to allow the initiation of antimicrobiologic therapy, even if only for a few days before surgery. Valve replacement in a patient with positive blood cultures increases the risk of relapse up to 10% to 15% and is highest if the offending organism is Staphylococcus, and should be avoided if the hemodynamic status of the patient is stable.13
Outcomes and Complications
A systematic review of the literature showed mitral valve repair to be superior to mitral valve replacement in infective endocarditis with lower in-hospital mortality, long-term mortality, recurrent endocarditis, and early and late cerebrovascular events.26 The choice of a tissue versus a mechanical prosthesis is debatable; prosthetic mechanical valves have a slightly higher rate of infection early after surgery.
The mortality rate for infective endocarditis varies depending on the causative organism and coexisting conditions at the time of operation. Staphylococci, gram-negative bacilli, and fungi have the highest reported rates (up to 50%). Patients with congestive heart failure treated with antibiotics alone have mortality rates greater than 56% to 86%, whereas patients treated with surgery and antibiotics have mortality rates of 11% to 35%.24 Shock, ventricular dysfunction, and concomitant coronary artery disease are predictors of poor outcome. The hemodynamic status of the patient is the principal determinant of operative mortality, and early intervention before the onset of ventricular dysfunction is preferred.
Imaging Findings
Preoperative Planning
In situations where a mechanical prosthesis is being considered, and concern for mycotic aneurysm exists, selective cerebral angiography may be indicated to rule out mycotic aneurysm (Fig. 30-33). Catheter-based selective cerebral artery embolization offers a less invasive form of therapy (Fig. 30-34).
SURGERY FOR CARDIAC MYXOMA
Description and Special Anatomic Considerations
Primary tumors of the heart are mostly benign and constitute more than 75% of such neoplasms. Cardiac myxoma is the most common primary tumor of the heart; it constitutes more than 50% of all benign cardiac tumors.27 Other benign neoplasms include lipomas, papillary fibroelastomas, and rhabdomyomas. Cardiac myxomas occur in all age groups, but are particularly common among women 30 to 60 years old. Myxomas typically originate from the endocardium of the interatrial septum and are found most commonly in the left atrium. The right atrium and very rarely the ventricular cavity are other sites.
Two distinct morphologic types of myxomas are identified: polypoid and pedunculated round tumors with a gelatinous consistency (Fig. 30-35A) and the less common, villous myxoma, which is characterized by multiple, fine villous extensions and has a propensity for embolism owing to fragility (Fig. 30-35B). Clinical presentation includes the triad of congestive heart failure owing to either inflow or outflow obstruction from tumor bulk; embolism; and constitutional symptoms such as fever, myalgia, and anorexia. Cardiac obstruction manifests with findings similar to either mitral or tricuspid valve stenosis. Embolism is found in 30% to 40% of patients and typically occurs to the central nervous system. Diagnosis is made by echocardiography and the findings of an intracavitary mass.
Indications
Resection of a benign intracardiac tumor is indicated in all situations because there is no effective alternative therapy. When an atrial myxoma is identified, surgery should not be delayed because there is a risk of embolization and death while awaiting surgery.27
Outcomes and Complications
The results are excellent in most cases. In most patients, myxomas are sporadic, and recurrence is rare. Risk of recurrence is higher in patients with familial or complex myxomas with rates of 12% to 22%, whereas patients with sporadic myxomas have a rate of recurrence of 1% to 3%.27 Specific complications related to resection depend on the location of the tumor. Surgical risks include conduction abnormalities, valve injury, atrial septal defect, and embolization.
Imaging Findings
Preoperative Planning
Imaging with either CT or MRI can differentiate tissue composition making it possible to identify solid, liquid, and fatty space-occupying tumors within the heart (Figs. 30-36 and 30-37). In situations of a right atrial myxoma, CT angiography to image the pulmonary vasculature is important to rule out synchronous tumor deposits.
SURGERY FOR THE ASCENDING THORACIC AORTA
Aortic Dissection
Aortic dissection occurs when blood enters between the medial layers of the aortic wall through an intimal tear. The blood propagates and creates a false lumen, which may eventually communicate across the dissection flap with the true lumen through another downstream intimal tear (Fig. 30-38). Causes of dissection are listed in Table 30-14, and include iatrogenic causes (as during cannulation for cardiopulmonary bypass or during catheterization) and numerous pathologic conditions, including hypertension, Marfan syndrome, or bicuspid aortic valve. Of patients with acute aortic dissection, 40% die before reaching a hospital, and 50% die within the initial 48 hours from presentation.28 Causes of death from ascending aortic dissection include rupture into the pericardial sac causing tamponade, severe acute aortic incompetence, occlusion of coronary arteries or branch vessels causing myocardial infarction or stroke, and free rupture into the pleural space with exsanguination.
TABLE 30-14 Risk Factors for Type A and B Thoracic Aortic Dissection
Two classification systems are used to characterize aortic dissection (Fig. 30-39). The Stanford classification is the simplest and most closely correlated with the clinical implications. Type A dissections are treated surgically and constitute a cardiovascular emergency. Type B dissections are treated medically and only rarely require surgery.
Aortic Aneurysm
Ascending aortic aneurysms are associated with many conditions, including degenerative conditions such as cystic medial degeneration and atherosclerosis, connective tissue disorders such as Marfan and Ehlers-Danlos syndrome, bicuspid aortic valve, infectious aortitis (mycotic), chronic dissection, coarctation, and trauma. The natural history of this entity has been studied extensively, and the indications for elective repair have become clearer over the past decade.29,30 The goal is to perform elective repair before potential complications of rupture (spontaneous or traumatic), or dissection may occur.
Description and Special Anatomic Considerations
Concomitant aortic valve repair or replacement along with CABG surgery may be performed as needed (Fig. 30-40). The complexity of the graft repair is determined by the extent of aorta that must be excised. The repair may involve anastomosis to or reconstruction of the branch vessels, or may require excision and replacement of the aortic root, with composite valve-conduit graft and reimplantation of the coronary arteries onto the graft. The goal of surgery is to excise and replace the entire pathologic area of the ascending aorta.
Indications
Emergent surgical repair is indicated in all cases of Stanford type A dissection, acute aortic syndrome, and spontaneous or traumatic aortic rupture. Elective surgical repair of thoracic aortic aneurysm is generally indicated if the ascending aorta diameter reaches 5 to 5.5 cm, the descending aorta reaches 6 cm, or the rate of growth is 1 cm/yr or greater (see Fig. 30-39). These criteria are based on extensive analysis of the natural history of thoracic aortic aneurysms by Coady and associates,29 which showed a fourfold increase in risk of rupture or dissection after the ascending aorta reaches a diameter of 6 cm. In patients with Marfan syndrome or other familial aneurysms, earlier intervention is recommended. Bicuspid or unicuspid aortic valve has been associated with an abnormality of elastin formation in the aortic wall, and earlier intervention is indicated in these patients. Finally, patients undergoing valve or CABG surgery as the primary indication for surgery may also have a moderately dilated ascending aorta. Based on several studies, including one by Prenger and colleagues,30 it is recommended that an aorta with a diameter of 4 to 5 cm be repaired concomitantly because of a 27% risk of future dissection without repair.
Outcomes and Complications
Operative mortality for elective repair of thoracic aortic aneurysm is very low, in most series 2% to 4%. The overall hospital mortality for repair of acute aortic dissection is higher, approximately 10% to 20%. Mortality is increased for patients presenting with hemodynamic instability, and is higher for patients requiring repair of the aortic arch, as shown in several series. Published 5-year survival for patients undergoing repair of acute type A aortic dissection is roughly 80%, and 60% at 10 years, whereas 5-year and 10-year postoperative survival for type B dissections is lower, 50% and 30%, respectively.28
Imaging Findings
Preoperative Planning
For evaluation of thoracic aortic aneurysm and acute aortic syndrome, the most common method of assessment is CT, or more specifically CT angiography. CT scanning provides a quick identification of size, location, and extent of dissection, ulcer, or intramural hematoma and calcification (Fig. 30-41). Three-dimensional reconstruction provides increased accuracy regarding the size of the aneurysm in patients whose aorta is tortuous. Serial CT scanning provides valuable information in documenting the rate of growth of ascending aneurysms, to determine timing of surgery for asymptomatic patients.
Postoperative Surveillance
All patients who have had surgery of the thoracic aorta should undergo periodic, long-term surveillance. Aortic tissue remaining after aneurysm repair is often abnormal, and patients should undergo periodic CT or MRI because of their risk of aneurysm, dissection, or pseudoaneurysm formation. This risk is elevated in patients who have undergone surgical repair of aortic dissection, and they should have a more vigilant follow-up program. Table 30-15 shows an acceptable surveillance schedule as reported by Borst and associates.31 Before hospital discharge, CT or MRI should be performed as a baseline postoperative evaluation, then repeated at the appropriate interval, depending on whether the patient has residual dissection, dilated aorta, or normal diameter. If valve reconstruction was performed, TTE should be performed before hospital discharge, then serially examined in follow-up. Patients with suspected malperfusion at any time should undergo aortography.
TABLE 30-15 Surveillance Studies after Repair of Acute Ascending Aortic Dissection
| Timing of Study | Study | Indication |
|---|---|---|
| Before hospital discharge | MRI or CT | All patients |
| TTE | Valve procedure | |
| Arteriography | Suspected malperfusion | |
| After hospital discharge | ||
| 3 mo | MRI or CT | Dilated aorta or residual dissection |
| TTE | Valve procedure | |
| 9 mo | MRI or CT | Dilated aorta or residual dissection |
| TTE | Valve procedure | |
| Subsequent examinations | ||
| Every 6 mo | MRI or CT | Progression of aortic disease, Marfan syndrome |
| TTE | Aortic incompetence | |
| Every 12 mo | MRI or CT | Aortic diameter ≥5 cm |
| Every 24 mo | MRI or CT | Aortic diameter <5 cm |
From Borst HG, Heinemann MK, Stone CD. Surgical Treatment of Aortic Dissection. New York, Churchill Livingstone, 1996, p 343.
DISORDERS OF THE PERICARDIUM
The pericardium is composed of a serous component and a fibrous, semicompliant membranous component that envelops the heart like a cocoon. The serous portion is bordered by the visceral pericardium (a layer on the epicardium) and parietal pericardium. The fibrous component is a membrane in continuity with the parietal pericardium. The function of the pericardium is to maintain the heart’s position in the mediastinum and preserve cardiac performance by preventing myocardial distention when volume overload occurs. The serous pericardium contains fluid that provides lubrication during cardiac motion, reducing friction with the pericardial sac. Two natural recesses called sinuses provide a pathway around the heart within the pericardium (Fig. 30-42). Common disorders of the pericardium include conditions of excessive fluid accumulation within the serous pericardium and involvement of the pericardium with disease (pericarditis). The end result of inflammatory pericarditis is constrictive pericarditis. These derangements cause hemodynamic effects that can be fatal. Table 30-16 lists conditions associated with these pericardial disorders.
From Little WC, Freeman GL. Pericardial disease. Circulation 2006; 113:1622-1632.
Constrictive Pericarditis
Constrictive pericarditis can occur with any condition that causes inflammation of the pericardium with subsequent fibrosis. Causes of constrictive pericarditis are listed in Table 30-16. Constrictive pericarditis is seen in 4% to 5% of patients who have undergone previous cardiac surgery.32 This insidious condition can manifest in a widely variable postoperative time interval (days to years after surgery). Patients may present with worsening symptoms of congestive heart failure with a normal left ventricular ejection fraction and in the absence of other cardiac pathology. Constrictive pericarditis can also be caused by radiation therapy to the mediastinum, malignancy, and infection. Tuberculous pericarditis is less common today than in the early 20th century.
The basic physiologic derangement in constrictive pericarditis is the inability of the heart to fill normally because the constricting membrane prevents chamber distention. The diagnosis of constrictive pericarditis is made using hemodynamic measurements as listed in Table 30-17. The most specific of these findings, ventricular interdependence, is seen as a decrease in left ventricular systolic pressure with an increase in right ventricular systolic pressure during inspiration, measured in the cardiac catheterization laboratory.
TABLE 30-17 Hemodynamic Findings with Constrictive Pericarditis
LV, left ventricular; RV, right ventricular.
Effusive Pericarditis
Acute and chronic effusive pericarditis with pericardial effusion may manifest with acute pericardial tamponade. Tamponade occurs as the semicompliant fibrous pericardium accommodates an increase in volume up to a point at which the compliance is exceeded, and intrapericardial pressure acutely increases. Figure 30-43 illustrates the hemodynamic derangement that only a small increase in the amount of pericardial fluid can result in a dramatic increase in intrapericardial pressure. A common finding on physical examination is pulsus paradoxus, which is a dissociation of cardiac and intrathoracic pressures, causing a decrease in systolic pressure of greater than 10 mm Hg with inspiration.
Description and Special Anatomic Considerations
The surgical treatment for constrictive pericarditis is complete pericardiectomy (Fig. 30-44). This may be performed through a median sternotomy or a left thoracotomy, and requires meticulous dissection of the visceral (off the epicardium) and parietal layers of the pericardium. The use of cardiopulmonary bypass may be necessary to complete the operation. The pericardiectomy includes removal of all encasing pericardial tissue (visceral, parietal, and fibrous). The extent of removal is limited laterally by the phrenic nerves, inferiorly by the diaphragm, and superiorly by the great vessels. The goal of the surgery is to free the left and right ventricles sufficiently to allow normal filling in diastole. Technically, this can be extremely challenging and bloody because of the dense pericardial adhesions and calcification that may be encountered.
Outcomes and Complications
The results of surgical procedures for pericardial disease vary with the etiology and severity of the disease. Total pericardiectomy for constrictive pericarditis has an operative mortality risk of 10% to 20%,33 depending on the degree of preoperative congestive heart failure, elevated right atrial pressure, and other significant comorbid conditions. Operative morbidity includes bleeding owing to the difficulty dissecting dense epicardial adhesions, injury to the phrenic nerve, and injury to epicardial vessels and bypass grafts. Patients usually respond well to surgery with a decrease in heart failure symptoms, and generally shed much of the accumulated preoperative water weight. Long-term survival is diminished, however, in patients with previous cardiac surgery, reduced preoperative ejection fraction, or radiation-induced disease. Results after postpericardiotomy open pericardial drainage are very good, with immediate improvement in hemodynamics and alleviation of symptoms, with low morbidity and mortality.34
Imaging Findings
Preoperative Planning
Chest radiograph and echocardiography often provide sufficient information in the diagnosis of acute pericardial tamponade. Figure 30-45 shows a typical chest radiograph of a patient with a large pericardial effusion and tamponade.
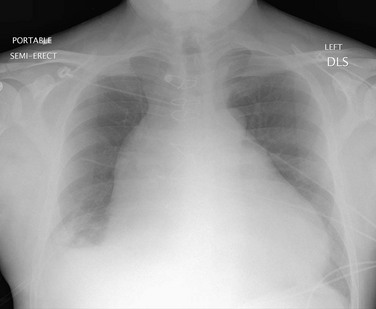
 FIGURE 30-45 Chest radiograph showing enlarged cardiac silhouette in a patient with a large pericardial effusion.
FIGURE 30-45 Chest radiograph showing enlarged cardiac silhouette in a patient with a large pericardial effusion.
In patients with constrictive pericarditis, echocardiography, CT, or MRI may show a thickened pericardium (Fig. 30-46); however, echocardiography provides much more useful information with the ability to assess hemodynamics. Echocardiography may reveal a respiratory variation in early mitral filling, along with increased diastolic flow reversal in the hepatic veins during expiration. Right and left cardiac catheterization provide the most accurate assessment of hemodynamic criteria to diagnose constrictive pericarditis, as stated previously.
Postoperative Surveillance
KEY POINTS
 Catheter coronary angiography remains the gold standard to assess for coronary disease, but coronary CT angiography has emerged as a promising technique for screening of patients with suspected coronary disease or surveillance of patients with known coronary disease.
Catheter coronary angiography remains the gold standard to assess for coronary disease, but coronary CT angiography has emerged as a promising technique for screening of patients with suspected coronary disease or surveillance of patients with known coronary disease. Patients with symptomatic aortic stenosis have increased risk for mortality (75% die by 3 years) and require AVR. Patients with severe aortic regurgitation are at risk for progressive heart failure and benefit from AVR.
Patients with symptomatic aortic stenosis have increased risk for mortality (75% die by 3 years) and require AVR. Patients with severe aortic regurgitation are at risk for progressive heart failure and benefit from AVR. Surgical options for mitral valve surgery include valve replacement, valve repair, and percutaneous balloon valvuloplasty.
Surgical options for mitral valve surgery include valve replacement, valve repair, and percutaneous balloon valvuloplasty. Infective endocarditis can occur especially in patients with mitral valve prolapse or in patients who have undergone prosthetic valve replacement.
Infective endocarditis can occur especially in patients with mitral valve prolapse or in patients who have undergone prosthetic valve replacement.1 Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation. 2004;110:e340-e437.
2 Post Coronary Artery Bypass Graft Trial Investigators. The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous vein coronary artery bypass grafts. N Engl J Med. 1997;336:153-162.
3 Veterans Administration Coronary Artery Bypass Surgery Cooperative Study Group. Eleven-year survival in the Veterans Administration randomized trial of coronary bypass surgery for stable angina. N Engl J Med. 1984;311:1333-1339.
4 Coronary Artery Surgery Study (CASS). A randomized trial of coronary artery bypass surgery: quality of life in patients randomly assigned to treatment groups. Circulation. 1983;68:951-960.
5 Varnauskas E. Twelve-year follow-up of survival in the randomized European Coronary Surgery Study. N Engl J Med. 1988;319:332-337.
6 Simon AR, Baraki H, Weidemann J, et al. High-resolution 64-slice helical-computer-assisted-tomographical-angiography as a diagnostic tool before CABG surgery: the dawn of a new era? Eur J Cardiothorac Surg. 2007;32:896-901.
7 Mollett NR, Cademartiri F, van Meighem CA, et al. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318-2323.
8 Plass A, Grunenfelder J, Leschka S, et al. Coronary artery imaging with 64-slice computed tomography from cardiac surgical perspective. Eur J Cardiothorac Surg. 2006;30:109-116.
9 Society of Thoracic Surgeons Executive Summary, STS Sprint 2007 Report. Duke Clinical Research Institute. Available at www.sts.org/documents/pdf/ndb/Fall_2007_Executive_Summary.pdf —2007-01-01 (accessed August 1, 2007)
10 Carabello BA. Aortic stenosis. N Engl J Med. 2002;346:677-682.
11 Carabello BA, Crawford FA. Valvular heart disease. N Engl J Med. 1997;337:32-41.
12 Otto CM, Burwash IG, Leggert ME, et al. Prospective study of asymptomatic valvular aortic stenosis: clinical, echocardiographic and exercise predictors of outcome. Circulation. 1997;95:2262-2270.
13 Bonow RO, Carabello BA, Chatterjee K, et al. ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the 1998 Guidelines for the Management of Patients with Valvular Heart Disease): developed in collaboration with the Society of Cardiovascular Anesthesiologists: endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. Circulation. 2006;114:e84-e231.
14 Enriquez-Sarano M, Tajik AJ. Aortic regurgitation. N Engl J Med. 2004;351:1539-1546.
15 Carabello BA. Vasodilators in aortic regurgitation—where is the evidence of their effectiveness? N Engl J Med. 2005;353:1400-1402.
16 Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352:2389-2397.
17 Mullany CJ. Aortic valve surgery in the elderly. Cardiol Rev. 2000;8:333-339.
18 Connolly HM, Oh JK, Schaff HV, et al. Severe aortic stenosis with low transvalvular gradient and severe left ventricular dysfunction: result of aortic valve replacement in 52 patients. Circulation. 2000;101:1940-1946.
19 Pibarot P, Dumesnil JG. Prosthesis-patient mismatch: definition, clinical impact and prevention. Heart. 2006;92:1022-1029.
20 Ling LH, Enriquez-Sarano M, Seward JB, et al. Clinical outcome of mitral valve regurgitation due to flail leaflet. N Engl J Med. 1996;335:1417-1423.
21 Mothy D, Orszulak TA, Schaff HV, et al. Very long-term survival and durability of mitral valve repair for mitral valve prolapse. Circulation. 2001;104:11-17.
22 Braunberger E, Deloche A, Berrebi A, et al. Very long-term results (more than 20 years) of valve repair with Carpentier’s techniques in nonrheumatic mitral valve insufficiency. Circulation. 2001;104:8-11.
23 Enriquez-Sarano M, Avierinos JF, Messika-Zeitoun D, et al. Quantitative determinants of the outcome of asymptomatic mitral regurgitation. N Engl J Med. 2005;352:875-883.
24 Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318-1330.
25 Li JS, Sexton DJ, Mick N, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633-638.
26 Feringa HH, Shaw LJ, Poldermans D, et al. Mitral valve repair and replacement in endocarditis: a systematic review of the literature. Ann Thorac Surg. 2007;83:564-570.
27 Reynen K. Cardiac myxomas. N Engl J Med. 1995;333:1610-1617.
28 Reece TB, Green GR, Kron IL. Aortic dissection. In: Cohn LH, editor. Cardiac Surgery in the Adult. New York: McGraw-Hill; 2008:1195-1222.
29 Coady MA, Rizzo JA, Elefteriades JA. Developing surgical intervention criteria for thoracic aortic aneurysms. Cardiol Clin. 1999;17:827.
30 Prenger K, Pieters F, Cheriex E. Aortic dissection after aortic valve replacement: incidence and consequences for strategy. J Card Surg. 1994;9:495.
31 Borst HG, Heinemann MK, Stone CD. Surgical Treatment of Aortic Dissection. New York: Churchill Livingstone; 1996.
32 Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100:1380.
33 McCaughan BC, Schaff HV, Piehier JM, et al. Early and late results of pericardiectomy for constrictive pericarditis. J Thorac Cardiovasc Surg. 1985;89:340.
34 Aksöyek A, Tütün U, Ulus T, et al. Surgical drainage of late cardiac tamponade following open heart surgery. Thorac Cardiovasc Surg. 2005;53:285-290.

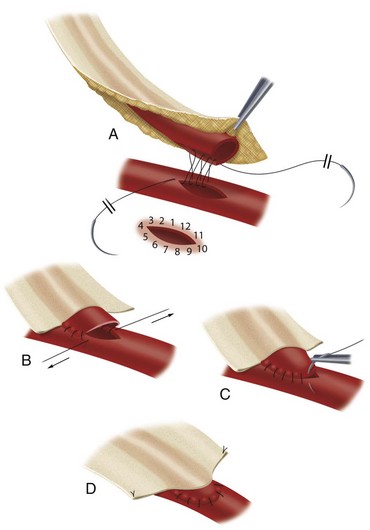
 FIGURE 30-6
FIGURE 30-6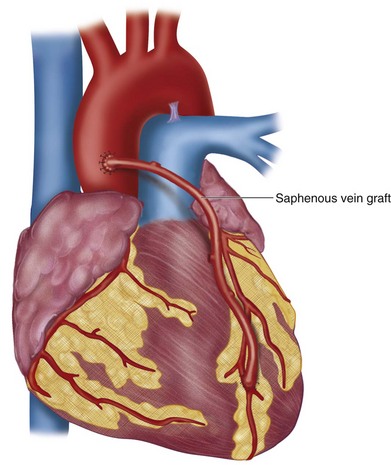
 FIGURE 30-8
FIGURE 30-8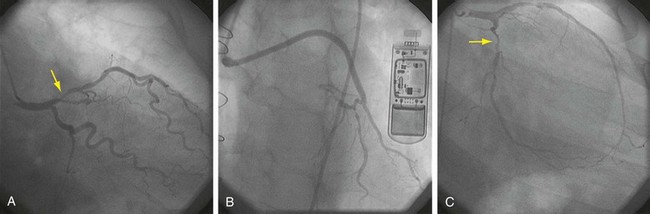
 FIGURE 30-9
FIGURE 30-9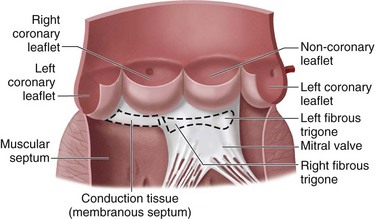
 FIGURE 30-11
FIGURE 30-11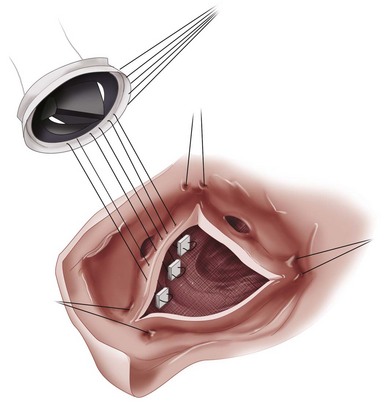
 FIGURE 30-13
FIGURE 30-13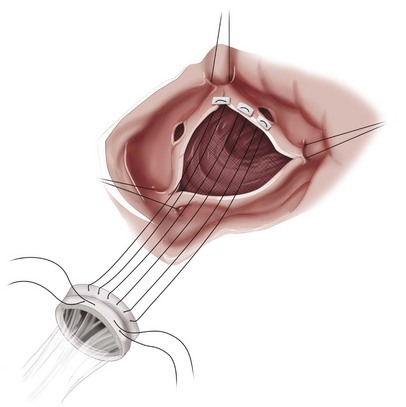
 FIGURE 30-14
FIGURE 30-14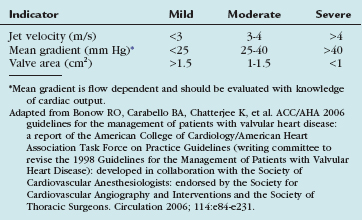
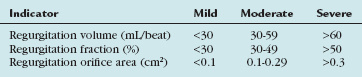
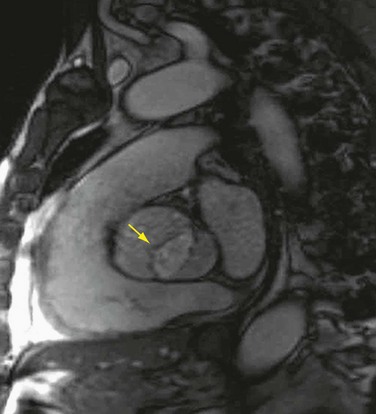
 FIGURE 30-18
FIGURE 30-18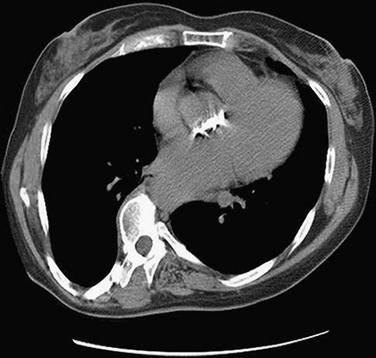
 FIGURE 30-19
FIGURE 30-19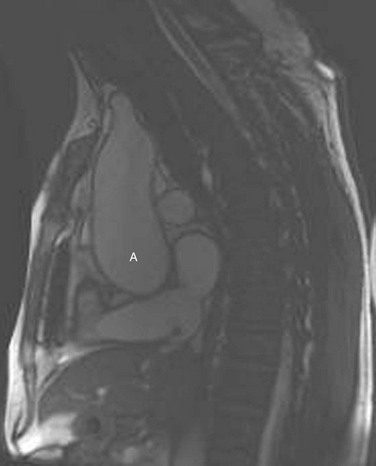
 FIGURE 30-20
FIGURE 30-20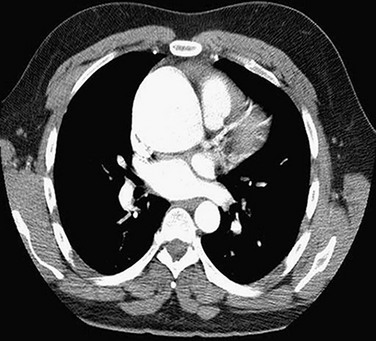
 FIGURE 30-21
FIGURE 30-21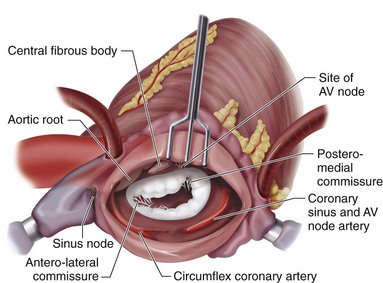
 FIGURE 30-23
FIGURE 30-23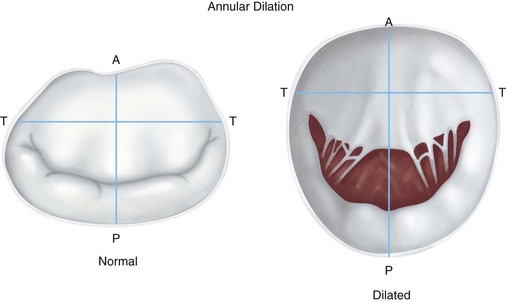
 FIGURE 30-26
FIGURE 30-26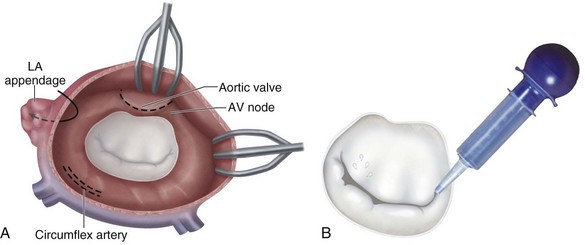
 FIGURE 30-28
FIGURE 30-28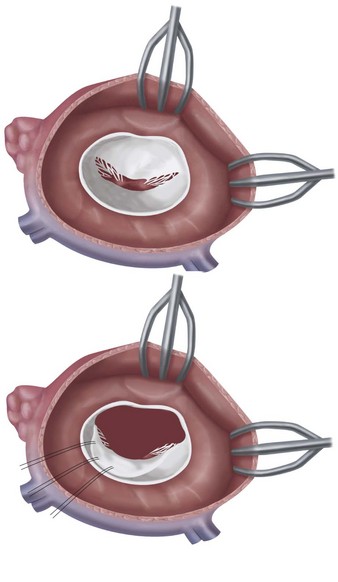
 FIGURE 30-29
FIGURE 30-29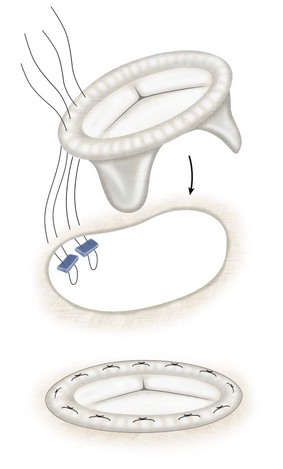
 FIGURE 30-30
FIGURE 30-30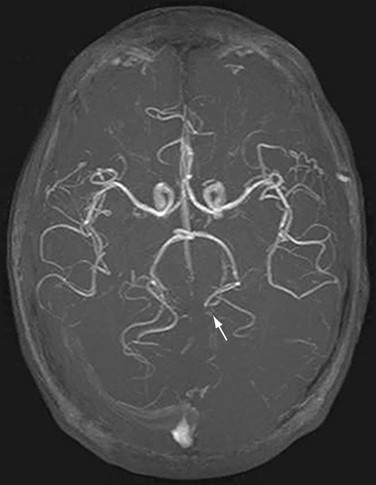
 FIGURE 30-31
FIGURE 30-31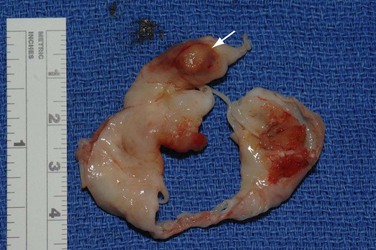
 FIGURE 30-32
FIGURE 30-32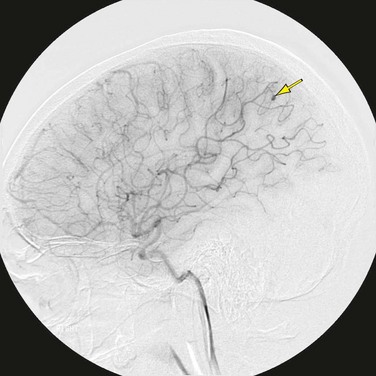
 FIGURE 30-33
FIGURE 30-33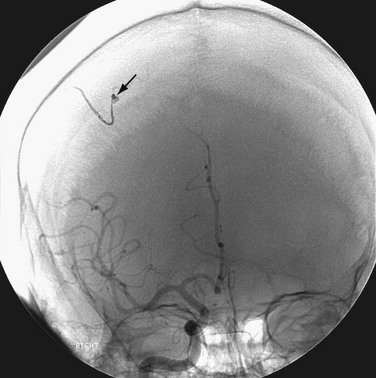
 FIGURE 30-34
FIGURE 30-34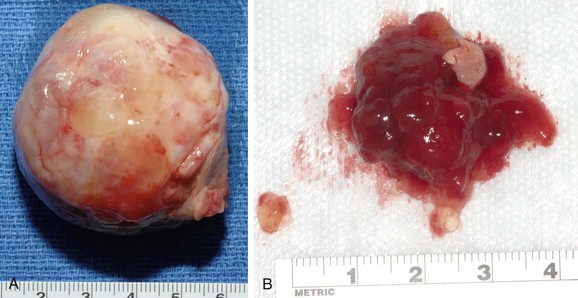
 FIGURE 30-35
FIGURE 30-35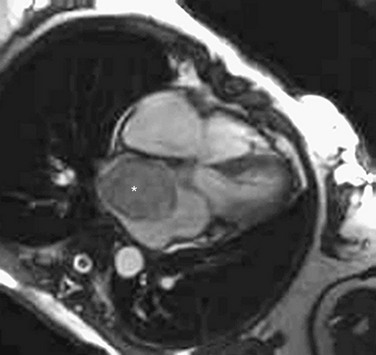
 FIGURE 30-36
FIGURE 30-36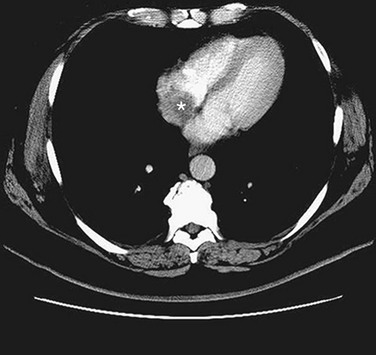
 FIGURE 30-37
FIGURE 30-37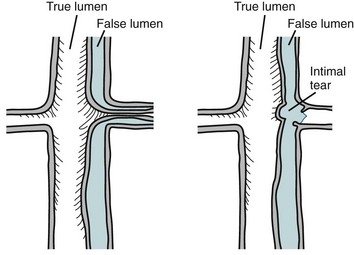
 FIGURE 30-38
FIGURE 30-38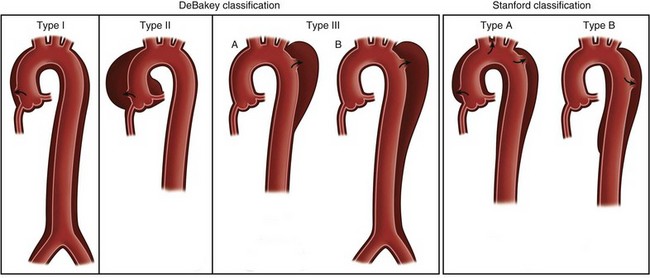
 FIGURE 30-39
FIGURE 30-39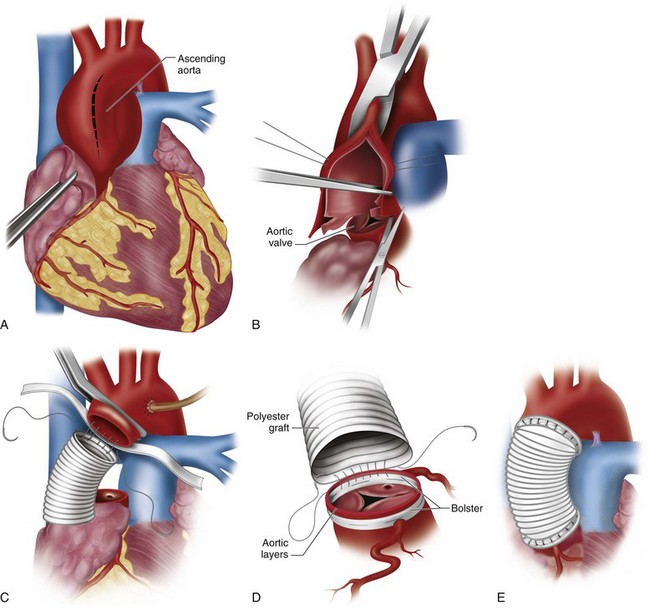
 FIGURE 30-40
FIGURE 30-40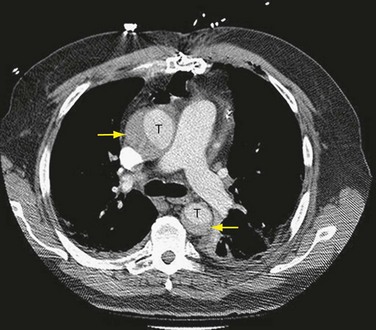
 FIGURE 30-41
FIGURE 30-41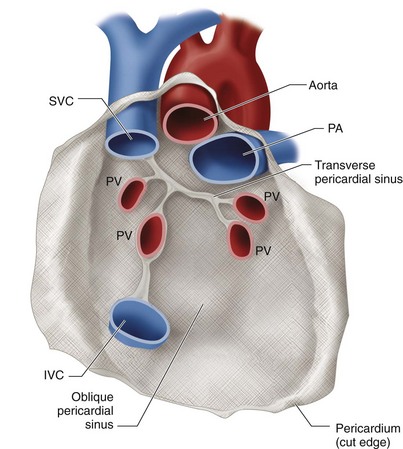
 FIGURE 30-42
FIGURE 30-42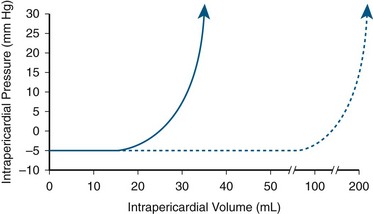
 FIGURE 30-43
FIGURE 30-43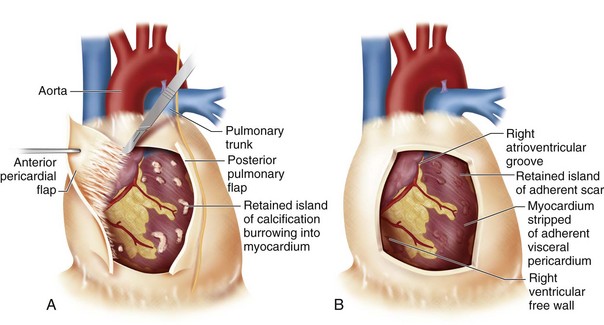
 FIGURE 30-44
FIGURE 30-44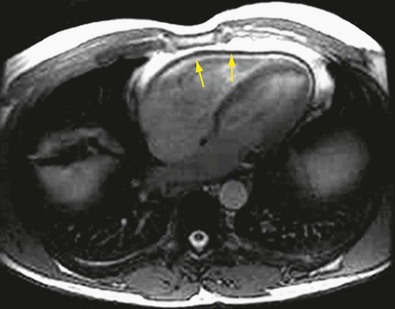
 FIGURE 30-46
FIGURE 30-46




