Vascular surgery
A Abdominal aortic aneurysm
Surgical treatment of abdominal aortic aneurysm (AAA) may be required for atherosclerotic occlusive disease or aneurysmal dilation. These processes can involve the aorta and any of its major branches, leading to ischemia or rupture and exsanguination.
The primary event in aortic dissection is a tear in the intimal wall through which blood surges and creates a false lumen. The adventitia then separates up or down (or both) the aorta for various distances. Associated conditions include atherosclerosis and hypertension (which is present in 80% of these patients), Marfan syndrome, blunt chest trauma, pregnancy, and iatrogenic surgical injury (e.g., resulting from aortic cannulation during cardiopulmonary bypass [CPB]).
Aortic dissections involving the ascending aorta are considered type A. Surgical repair is through a median sternotomy using profound hypothermia and total circulatory arrest or CPB with moderate hypothermia. Aortic dissections involving the descending aorta (i.e., beyond the origin of the left subclavian artery) are considered type B. Aneurysms can also be classified as saccular, fusiform, or dissecting. Surgical repair involves proximal and distal clamping of the aorta, opening of the aneurysm, evacuation of the thrombus, and placement of a graft. A midline transabdominal surgical approach or retroperitoneal left thoracoabdominal approach may be used.
The incidence of AAAs has increased over the last 5 decades from 12.2 to 36.2 per 100,000 surgical procedures. This increase may partially be the result of the detection of asymptomatic aneurysms by noninvasive diagnostic modalities, such as computed tomography (CT), magnetic resonance imaging (MRI), and ultrasonography. The occurrence of AAAs has increased because of the increased age of the general population and the vascular changes that occur as a result of aging. Aortic aneurysms can be identified in approximately 1% to 4% of the population older than 50 years and in approximately 5% of the population older than 60 years. Aneurysms are more common in men than in women and in whites than in African Americans.
The present mortality rate ranges from 1% to 11% (although most commonly estimated at 5%) compared with the mortality rates in the 1950s of 18% to 30%. Advanced detection capabilities, earlier surgical intervention, extensive preoperative preparation, refined surgical techniques, better hemodynamic monitoring, improved anesthetic techniques, and aggressive postoperative management have all contributed to this improvement in surgical outcomes. Surgical intervention is recommended for AAAs 5.5 cm or larger in diameter. Estimates of mortality resulting from ruptured AAAs vary from 35% to 94%. The 5-year mortality rate for individuals with untreated AAAs is 81%, and the 10-year mortality rate is 100% Early detection and elective surgical intervention are advisable because rupture leads to an increased incidence of mortality.
a) Frequently, asymptomatic aneurysms are detected incidentally during routine examination or abdominal radiography. Smaller aneurysms are often undetected on routine physical examination.
b) Diagnostic techniques, such as ultrasonography, CT scan, and MRI, may identify vascular abnormalities in these patients. Such noninvasive techniques not only reveal the presence of aneurysms but also provide information about aneurysm size, vessel wall integrity, and adjacent anatomic definition.
c) Invasive techniques, including contrast-enhanced CT scan, contrast angiography, and digital subtraction angiography (DSA), can provide additional information and more detailed representations of arterial anatomy. DSA is the best method of evaluating suprarenal aneurysms because this method provides superior definition of the aneurysmal relationship to the renal arteries.
Abdominal aortic reconstruction
a) Most patients with abdominal aneurysms, including octogenarians, are considered surgical candidates. Although advancing age contributes to an increased incidence of morbidity and mortality, age alone is not a contraindication to elective aneurysmectomies.
b) Physiologic age is more indicative than chronologic age of increased surgical risk.
c) Contraindications to elective repair include intractable angina pectoris, recent myocardial infarction (MI), severe pulmonary dysfunction, and chronic renal insufficiency.
d) Patients with stable coronary artery disease (CAD) with coronary artery stenosis of greater than 70% requiring nonemergent AAA repair do not benefit from revascularization if beta blockade has been established.
a) Preoperative fluid loading and restoration of intravascular volume are perhaps the most important techniques used to enhance cardiac function during abdominal aortic aneurysmectomies.
b) Reliable venous access should be secured if volume replacement is to be accomplished. Large-bore intravenous (IV) lines and central lines can be used to infuse fluids or blood.
c) Massive hemorrhage is an ever-present threat; therefore, the availability of blood and blood products should be ensured. Provisions for rapid transfusion and intraoperative blood salvage should be confirmed.
a) Standard monitoring methods include electrocardiography (ECG) with display of lead II for detection of dysrhythmias and the precordial V5 lead for analysis of ischemic ST segment changes, pulse oximetry, and capnography.
b) An esophageal stethoscope allows for continuous auscultation of heart and breath sounds as well as temperature determination.
c) Placement of an indwelling urinary catheter is necessary for the continuous measurement of urinary output and renal function. Neuromuscular function is also routinely monitored.
d) Invasive blood pressure monitoring permits beat-to-beat analysis of the blood pressure, immediate identification of hemodynamic alterations related to aortic clamping, and access for blood sampling.
e) Pulmonary artery catheters can be used in abdominal aortic reconstruction for monitoring left-sided filling pressures as a guide for fluid replacement.
f) Pulmonary artery catheterization not only provides clinical indexes that reflect intravascular volume but also facilitates calculations of stroke volume, cardiac index, and left ventricular stroke work index.
g) Myocardial ischemia can be detected by analysis of pulmonary artery catheter tracings. Some pulmonary artery catheters allow for measurement of mixed venous oxygen saturation.
h) By detecting changes in ventricular wall motion, two-dimensional transesophageal echocardiography provides a sensitive method for assessing regional myocardial perfusion. Wall motion abnormalities also occur much sooner than ECG changes during periods of reduced coronary blood flow.
i) Myocardial ischemia poses the greatest risk of mortality after abdominal aortic reconstruction. Intraoperative monitoring may enable earlier detection and intervention during ischemic cardiac events.
4. Application of aortic cross-clamp: Hemodynamic alterations
a) The hemodynamic effects of aortic cross-clamping depend on the application site along the aorta, the patient’s preoperative cardiac reserve, and the patient’s intravascular volume.
b) The most common site for cross-clamping is infrarenal because most aneurysms appear below the level of the renal arteries. Less common sites of aneurysm development are the juxtarenal and suprarenal areas.
c) When aortic cross-clamping is used, hypertension occurs above the cross-clamp, and hypotension occurs below the cross-clamp. Organs proximal to the aortic occlusion may experience a redistribution of blood volume.
d) There is an absence of blood flow distal to the clamp in the pelvis and lower extremities.
e) Increases in afterload cause myocardial wall tension to increase. Mean arterial pressure (MAP) and systemic vascular resistance (SVR) also increase.
f) Cardiac output may decrease or remain unchanged. Pulmonary artery occlusion pressure (PAOP) may increase or display no change.
g) The percentages of change in cardiovascular indexes at different levels of aortic occlusion are listed in the following table.
Change in Cardiovascular Variables at Different Levels of Aortic Occlusion as Assessed by Two-Dimensional Transesophageal Echocardiography

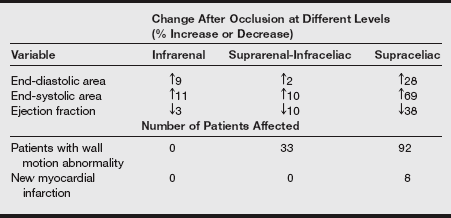
Modified from Roizen MF, Beaupre PN, Alpert RA, et al. Monitoring with two-dimensional transesophageal echocardiography: comparison of myocardial function in patients undergoing supraceliac, suprarenal-infraceliac, or infrarenal aortic occlusion. J Vasc Surg 1984;1(2):300-305.
h) Patients with ischemic heart disease or ventricular dysfunction are unable to fully compensate as a result of the hemodynamic alterations. The increased wall stress attributed to aortic cross-clamp application may contribute to decreased global ventricular function and myocardial ischemia. Clinically, these patients experience increases in PAOP in response to aortic cross-clamping. Aggressive pharmacologic intervention is required for restoration of cardiac function during this time.
a) After the application of an aortic cross-clamp, the lack of blood flow to distal structures makes these tissues prone to developing hypoxia. In response to hypoxia, metabolites (e.g., lactate) accumulate.
b) The release of arachidonic acid derivatives may also contribute to the cardiac instability that is observed during aortic cross-clamping. Thromboxane A2 synthesis, which is accelerated by the application of an aortic cross-clamp, may be responsible for the decrease in myocardial contractility and cardiac output that occurs.
c) Traction on the mesentery is a surgical maneuver used for exposing the aorta. Decreases in blood pressure and SVR, tachycardia, increased cardiac output, and facial flushing are common responses to mesenteric traction.
d) The neuroendocrine response to major surgical stress is believed to be mediated by cytokines such as interleukin 1-beta (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α), as well as plasma catecholamines and cortisol. These mediators are thought to be responsible for triggering the inflammatory response that results in increased body temperature, leukocytosis, tachycardia, tachypnea, and fluid sequestration.
6. Effects on regional circulation
a) Structures distal to the aortic clamp are underperfused during aortic cross-clamping. Renal insufficiency and renal failure have been reported to occur after abdominal aortic reconstruction.
b) Suprarenal and juxtarenal cross-clamping may be associated with a higher incidence of altered renal dynamics; however, reductions in renal blood flow can occur with any level of clamp application.
c) Infrarenal aortic cross-clamping is associated with a 38% decrease in renal blood flow and a 75% increase in renal vascular resistance. These effects may lead to acute renal failure, which is fatal in 50% to 90% of patients who have undergone aneurysmectomies.
d) Preoperative evaluation of renal function is one of the most significant predictors of postoperative renal dysfunction. Therefore, a complete evaluation of renal function is required in the preoperative period.
e) Spinal cord damage is associated with aortic occlusion. Interruption of blood flow to the greater radicular artery (artery of Adamkiewicz) in the absence of collateral blood flow has been identified as a causative factor in paraplegia.
f) The incidence of neurologic complications increases as the aortic cross-clamp is positioned in a higher or more proximal area.
g) Ischemic colon injury is a well-documented complication that is associated with abdominal aortic resections. Ischemia of the colon is most frequently attributed to manipulation of the inferior mesenteric artery, which supplies the primary blood supply to the left colon. This vessel is often sacrificed during surgery, and blood flow to the descending and sigmoid colon depends on the presence and the adequacy of the collateral vessels. Mucosal ischemia occurs in 10% of patients who undergo AAA repair. In fewer than 1% of these patients, infarction of the left colon necessitates surgical intervention.
a) While the aorta is occluded, metabolites that are liberated as a result of anaerobic metabolism, such as serum lactate, accumulate below the aortic cross-clamp and induce vasodilation and vasomotor paralysis.
b) As the cross-clamp is released, SVR decreases, and blood is sequestered into previously dilated veins, which decreases venous return.
c) Reactive hyperemia causes transient vasodilation secondary to the presence of tissue hypoxia, the release of adenine nucleotides, and the liberation of an unnamed vasodepressor substance that acts as a myocardial depressant and a peripheral vasodilator.
d) This combination of events results in decreased preload and afterload. The hemodynamic instability that may ensue after the release of an aortic cross-clamp is called declamping shock syndrome.
e) Evidence demonstrates that venous endothelin (ET)-1 may be partially responsible for the hemodynamic alterations that accompany declamping shock syndrome. Venous ET-1 has a positive inotropic effect on the heart as well as a vasoconstricting and vasodilating action on blood vessels.
f) The most frequently observed hemodynamic responses to aortic declamping are listed in the table below.
Hemodynamic Responses to Aortic Declamping
| Clinical Index | Response to Clamp Release |
| Mean arterial pressure | Decrease |
| Systemic vascular resistance | Decrease |
| Cardiac output | No change or increase |
| Pulmonary artery occlusion pressure | Decrease |
g) The magnitude of the response to unclamping the aorta may be manipulated. Although SVR and MAP decrease, intravascular volume may influence the direction and the magnitude of change in cardiac output.
h) Restoration of circulating blood volume is paramount in the provision of circulatory stability before release of the aortic clamp.
i) The site and the duration of cross-clamp application, as well as the gradual release of the clamp, influence the magnitude of circulatory instability. Partial release of the aortic cross clamp over time frequently results in less severe hypotension.
a) The standard approach for elective abdominal aortic reconstruction is the transperitoneal incision. The advantages of this route include exposure of infrarenal and iliac vessels, ability to inspect intraabdominal organs, and rapid closure. Unfavorable consequences associated with this approach include increased fluid losses, prolonged ileus, postoperative incisional pain, and pulmonary complications.
b) The retroperitoneal approach has gained popularity as an alternative to the standard route. Its advantages include excellent exposure (especially for juxtarenal and suprarenal aneurysms), decreased fluid losses, less incisional pain, and fewer postoperative pulmonary and intestinal complications. After implantation with a synthetic graft, the aortic adventitia is closed. In addition, the retroperitoneal approach does not elicit mesenteric traction syndrome. The reported limitations of this approach are unfamiliarity of surgeons with this technique, poor right distal renal artery exposure, and inability to inspect the integrity of the abdominal contents.
9. Management of fluid and blood loss
a) Extreme loss of extracellular fluid and blood should be expected with abdominal aortic aneurysmectomies. Evaporative losses and third spacing occur, with the magnitude of loss depending on the surgical approach, the duration of the surgery, and the experience of the surgeon.
b) Most blood loss occurs because of back bleeding from the lumbar and inferior mesenteric arteries after the vessels have been clamped and the aneurysm is opened.
c) The use of heparin also contributes to blood loss. Excessive bleeding, however, can occur at any point during surgery, and blood replacement is commonly administered during abdominal aortic resections.
d) Because of the heightened awareness of transfusion-related morbidity, the use of autologous blood has generated increasing interest. Presently, three options are available for the use of autologous transfusions: preoperative deposit, intraoperative phlebotomy and hemodilution, and intraoperative blood salvage.
a) The presence of underlying CAD in patients with vascular disease has been well documented. CAD is reported to occur in more than 50% of patients who require abdominal aortic reconstruction and is the single most significant risk factor influencing long-term survivability. MIs are responsible for 40% to 70% of all fatalities that occur after aneurysm reconstruction. In the presence of such threatening mortality rates, the extent of CAD and the subsequent functional limitations should be clearly defined and cardiac function optimized preoperatively before elective aortic vascular reconstruction is performed.
b) Advanced age, cardiac history, aberrations on physical examination, ECG abnormalities, and previous surgical procedures are identifiable factors in the cardiac risk index that contribute to cardiac complications.
c) Patients with unremarkable medical histories and normal physical examinations, exercise testing, ECG, and laboratory studies have a decreased surgical risk. Currently, investigators advocate the use of coronary angiography in selected patients who have positive findings on the initial cardiac evaluation.
d) Patients with symptomatic CAD require more extensive cardiac evaluation. Dipyridamole thallium testing is perhaps one of the most reliable methods for evaluating the extent of myocardial dysfunction associated with CAD and for predicting coronary events after vascular surgery.
e) Techniques capable of evaluating left ventricular performance, such as echocardiography, are of some value in the prediction of adverse cardiac events. Ambulatory ECG monitoring has also been very successful in the identification of postoperative cardiac complications. Coronary angiography provides the most reliable definition of coronary anatomy and the extent of CAD.
f) The end point of any method of preoperative cardiac evaluation for aneurysmectomy is identification of functional cardiac limitations. Depending on the degree of cardiac dysfunction, preoperative optimization of cardiac function may range from simple pharmacologic manipulation to surgical intervention.
g) Hypertension, chronic obstructive pulmonary disease, diabetes mellitus, renal impairment, and carotid artery disease are frequently observed in patients with AAAs.
h) Measures should be taken to optimize organ function because each of these disease states contributes to postoperative complications. Preoperative renal dysfunction deserves special consideration because aortic cross-clamping produces alterations in renal dynamics. The degree of preoperative renal insufficiency contributes to the extent of any postoperative renal damage.
(1) The anesthetic selection should be based on the following objectives: provision of analgesia and amnesia, facilitation of relaxation, maintenance of hemodynamic stability, preservation of renal blood flow, and minimization of morbidity and mortality.
(a) All inhalation anesthetics may depress the myocardium and cause hemodynamic instability. Therefore, high concentrations of inhalation agents in patients with moderate to severe decreased ejection fraction should not be used.
(b) Potential organ toxicity and lack of postoperative analgesia may be additional limitations to the use of these agents.
(c) Beneficial effects attributed to the use of inhalation agents include the ability to alter autonomic responses, reversibility, rapid emergence, and potentially earlier extubation.
(a) A balanced technique using a combination of high-dose narcotics with nitrous oxide can be used as the anesthetic for major vascular surgery.
(b) The cardiovascular stability provided by opioids has been well documented, and this feature is especially attractive for patients with ischemic heart disease and ventricular dysfunction.
(c) Provision of intense analgesia for the initial postoperative period after major abdominal vascular surgery, via the administration of a neuraxial opioid, does not alter the combined incidence of major cardiovascular, respiratory, and renal complications.
(a) The use of epidural anesthesia for abdominal aneurysmectomies has gained renewed interest. Several benefits of epidural use include decreased preload and afterload, preserved myocardial oxygenation, reduced stress response, excellent muscle relaxation, decreased incidence of postoperative thromboembolism, and increased graft flow to the lower extremities.
(b) Hypotension may also be a significant unfavorable result of an epidural technique. In fact, this technique requires the administration of approximately 1600 to 2000 mL more IV fluid than is usual with general anesthetic.
(c) The controversy regarding hematoma formation after heparinization during epidural techniques is still noteworthy. Studies have shown that the simultaneous use of epidural anesthesia and low-dose heparinization rarely produces complications.
(d) Postoperative pain control is vital to maintain hemodynamic stability and to alleviate patient suffering. Epidural narcotics have been shown to decrease pain after major surgery.
(e) Because of the high incidence of CAD in patients presenting for abdominal aortic reconstruction, severe postoperative pain can result in an increased heart rate and blood pressure, which may contribute to cardiac-related morbidity and mortality. Pain relief may decrease respiratory splinting and decrease the likelihood of hypoxemia.
(a) Combining anesthetic techniques for major vascular surgery is more popular than using them alone because the advantages of each technique contribute to a smoother anesthetic.
(b) A balanced technique supplemented by low-dose inhalation agents maintains cardiovascular hemodynamics and controls momentary autonomic responses to surgical stimulation.
(c) Another choice is to use epidural anesthesia combined with light general anesthesia. This provides the benefits of epidural anesthesia and the ability to provide amnesia and controlled ventilation.
(1) The maintenance of intravascular volume may be an extreme challenge during abdominal aortic resections. Controversy exists regarding whether the administration of crystalloids or colloids affects the overall incidence of morbidity and mortality.
(2) Crystalloids may be used for replacing basal and third-space losses at an approximate rate of 10 mL/kg/hr.
(3) Blood losses initially can be replaced with crystalloids at a ratio of three to one. The combination of crystalloid and colloid administration is also acceptable.
(4) Regardless of the choice of fluid, volume replacement should be dictated by physiologic parameters. Fluid replacement should be sufficient for the maintenance of normal cardiac filling pressures, cardiac output, and urine output of 1 mL/kg/hr.
(5) Patients with limited cardiac reserve can develop congestive heart failure if hypervolemia occurs.
(1) Hemodynamic changes are likely to occur throughout the anesthesia process.
(2) Momentary fluctuations in heart rate and blood pressure should be anticipated during induction and intubation.
(3) Preoperative replacement of fluid deficits prevents exaggerated responses to vasodilating induction agents.
(4) For patients with adequate left ventricular function, hemodynamic stability can be preserved with a “slow” induction using opioids and β-adrenergic blocking agents.
(5) Etomidate has minimal myocardial depressant effects and may be most suitable for patients with limited cardiac reserve.
(6) The response to mesenteric traction is also associated with momentary hemodynamic changes.
(7) Application of the aortic cross-clamp produces various hemodynamic responses. Patients without underlying ischemic heart disease usually demonstrate slight changes in PAOP when the aorta is occluded, requiring minimal intervention. However, patients with a history of CAD may experience an increase in PAOP and a decrease in cardiac output, indicating left ventricular decompensation.
(8) Although several different pharmacologic agents may be used, nitroglycerin appears to be the drug of choice because of its primary pharmacologic effect of decreasing preload and thereby decreasing myocardial oxygen demand.
(9) Whereas inotropic agents, such as dopamine and dobutamine, may improve cardiac output, pharmacologic agents that decrease afterload, such as sodium nitroprusside and isoflurane, may decrease SVR.
(10) The more proximal the application of the aortic cross-clamp, the greater the magnitude and the severity of these responses. Vasoactive medications should be readily available throughout the surgery.
(11) When the aortic cross-clamp is released, declamping shock syndrome may occur. Severe hypotension and reduction in cardiac output may ensue. These conditions can be prevented by volume loading and raising of the central venous pressure 3 to 5 mmHg or raising the PAOP 3 to 4 mmHg just before the clamp is released.
(12) If severe acidosis is present, sodium bicarbonate may be administered. Temporarily increasing minute ventilation may also be useful for the control of acidosis.
(1) The incidence of acute renal failure after infrarenal cross-clamping is 5%, and this value increases to 13% after suprarenal cross-clamping.
(2) The mortality rate is four to five times greater in patients who develop acute renal failure postoperatively.
(3) Alterations in renal dynamics during intrarenal cross-clamping may continue up to 1 hour after the clamp is released. Such alterations can be profound and can extend into the postoperative period.
(4) Mechanisms for the preservation of renal function during aortic cross-clamping include improving renal and glomerular blood flow.
(5) Maintenance of cardiac output and intravascular volume is vital. Prevention of hypovolemia is the best prophylaxis against renal failure.
(6) Administration of mannitol 20 to 30 minutes before aortic clamping may help preserve renal function because of hydroxyl free-radical scavenging properties.
(7) Further intervention includes IV administration of low-dose dopamine at 3 to 5 mcg/kg/min, fenoldopam, and use of loop diuretics. Renal dose dopamine has not been proven to decrease the risk of postoperative renal dysfunction.
12. Postoperative implications
a) Cardiac, respiratory, and renal failure are the most common complications observed postoperatively in patients recovering from abdominal aortic reconstruction.
b) Cardiovascular function should be closely monitored in the intensive care unit for at least 24 hours after surgery. Maintenance of adequate blood pressure, intravascular fluid volume, and myocardial oxygenation is paramount during this period.
c) MI frequently contributes to postoperative morbidity and mortality. Serial cardiac enzyme analysis may be monitored.
d) Pharmacologic agents used in the treatment of hypertension should also be available.
e) Most patients require ventilatory assistance during the postoperative period. Vigilant monitoring of respiratory function is mandatory, especially when epidural catheters are used for postoperative analgesia.
f) Renal function should be continuously evaluated in the postoperative phase. Urine output should be maintained at 1 mL/kg/hr. Administration of fluid, maintenance of physiologic hemodynamics, and concurrent administration of pharmacologic agents should be considered to improve urine output.
Juxtarenal and suprarenal aortic aneurysms
a) Although most AAAs occur below the level of the renal arteries, 2% extend proximally and involve the renal or visceral arteries.
b) Juxtarenal aneurysms are located at the level of the renal arteries, but they spare the renal artery orifice. More proximal suprarenal aneurysms include at least one of the renal arteries and may involve visceral vessels.
c) The effects of aortic cross-clamping for juxtarenal or suprarenal aneurysms are similar to those for infrarenal aortic occlusions; however, the magnitude of hemodynamic alterations increases as the aorta is clamped more proximally.
a) Preoperative preparation includes a thorough evaluation of coexisting disease, with an emphasis on cardiac function.
b) As the aorta is clamped more proximally, left ventricular afterload increases; consequently, myocardial ischemia is more likely to occur.
c) Diligent cardiac monitoring is necessary, and direct intraarterial blood pressure assessment, cardiac filling pressure monitoring, and transesophageal echocardiography are advocated to detect cardiac dysfunction and allow for immediate pharmacologic intervention.
d) Renal failure, although possible during infrarenal aortic cross-clamping, occurs more frequently as a result of suprarenal aortic occlusion. Maintaining adequate intravascular volume and administering osmotic and loop diuretics may minimize renal ischemia and dysfunction.
e) If the ischemic episode persists for longer than 45 minutes, renal cooling is suggested. Renal cooling consists of flushing the kidney with an iced electrolyte perfusate that contains heparin and glucose.
f) Paraplegia is possible when the blood supply to the spinal cord is interrupted by aortic cross-clamping at or above the level of the diaphragm.
g) Increasing the MAP or decreasing the cerebrospinal fluid (CSF) pressure may be used as a means to increase spinal cord perfusion pressure.
Ruptured abdominal aortic aneurysm
a) A high mortality rate of up to 94% is associated with a ruptured AAA.
b) The most common symptoms of ruptured AAAs are abdominal discomfort with a pulsatile mass, back pain, decreased peripheral pulses, and hypotension.
c) Hypotension and a history of cardiac disease are two factors associated with the poorest prognosis.
d) Patients with these symptoms should be immediately transferred to the operating room for surgical exploration. When hypotension is absent, more time is available for a comprehensive CT scan to search for other causes of abdominal discomfort.
e) When the patient arrives in the operative suite, a brief preoperative evaluation, establishing of venous access, and provisions for fluid and blood product administration can be completed.
f) Induction of anesthesia should follow the principles of trauma anesthesia. Hemodynamic stability should be the primary objective, and anesthetic induction and maintenance agents should be selected on a case-by-case basis.
g) Cardiovascular resuscitation is the anesthesia provider’s primary focus until blood loss from the proximal aorta is controlled by surgical intervention. Fluid resuscitation can begin with crystalloids, and blood products can be administered as they become available. Intraoperative blood salvage provisions should be secured.
h) If large amounts of blood products are given, coagulation studies and ionized calcium values should be calculated. The use of fresh-frozen plasma has been shown to decrease the total transfusion requirements and the incidence of coagulopathies. The ability to administer platelets may also be necessary.
i) After initial fluid resuscitation has been performed and hemodynamic stability has been ensured, direct arterial blood pressure monitoring should be instituted. A central venous or pulmonary artery catheter may be inserted.
j) The hemodynamic effects of aortic cross-clamping and release are similar to those for elective surgery; however, responses may be extreme, especially if hypotension exists when the clamp is released.
k) Measures for ensuring the adequacy of renal circulation, such as administering mannitol, should be incorporated. Because mannitol is an osmotic diuretic, decreased vascular volume resulting in hypotension can occur. Because most patients require large amounts of fluid and blood replacement, postoperative mechanical ventilation is recommended.
B Aortobifemoral bypass grafting
Aortobifemoral bypass grafting is commonly performed to correct symptomatic unilateral iliac occlusive disease, which generally occurs in men older than 55 years.
2. Preoperative assessment and patient preparation
a) History and physical examination
(1) Cardiovascular: 30% to 50% of patients have coexisting CAD. Other common risk factors are MI, hypertension, angina, valvular disease, congestive heart failure, and arrhythmias.
(2) Respiratory: Most patients have a significant history of smoking and possibly chronic obstructive pulmonary disease.
(3) Neurologic: Check for coexisting cerebrovascular disease.
(4) Renal: Chronic renal insufficiency is common.
(5) Endocrine: Many patients have diabetes and its associated complications.
(1) Laboratory tests: Complete blood count, prothrombin time, partial thromboplastin time, bleeding time, electrolytes, blood urea nitrogen, creatinine, creatinine clearance, and urinalysis are obtained.
(2) Diagnostic tests: 12-lead ECG, pulmonary function tests, arterial blood gases, chest radiography, MRI, CT, and arteriography are obtained.
(3) Preoperative medications: Knowledge of daily medications is essential. Cardiac medications are continued, and anticoagulant therapy is sometimes held for 4 hours before surgery. Anxiolytics, sedatives, and analgesics are used as indicated.
(4) IV therapy: Have a central line with two 14- to 16-gauge IV lines. The estimated blood loss is 500 mL.
a) Monitoring equipment: Standard with arterial line and central venous pressure catheter, pulmonary arterial catheter, or both. ST segment analysis and transesophageal echocardiography are beneficial.
b) Additional equipment: This includes a fluid warmer; consider a cell saver.
(1) Miscellaneous pharmacologic agents: Osmotic and loop diuretics, local anesthetics, antibiotics, adrenergic antagonists, inotropic agents, vasodilators or constrictors, and heparin are used.
(2) IV fluids: Calculate for major blood loss. Consider rapid infusion of crystalloids, colloids, or both to treat hypovolemic states.
(3) Blood: Type and cross-match for 4 units of packed red blood cells.
General anesthesia, epidural anesthesia, or a combination of general and regional anesthesia is used.
a) Induction: Use smooth induction to preserve cerebral perfusion and to maintain hemodynamic stability. For general anesthesia, consider etomidate, fentanyl, lidocaine, and muscle relaxants to decrease episodes of tachycardia and hypotension. For regional anesthesia, consider placing an epidural catheter before beginning anticoagulation.
b) Maintenance: For general anesthesia, consider oxygen and a volatile agent or narcotic. For regional anesthesia, use a local anesthetic, narcotic, or anxiolytic. Maintain blood pressure within the high-normal range.
c) Emergence: Maintain hemodynamic stability; prevent hypertension and tachycardia. For general anesthesia, use full reversal of muscle relaxants and smooth extubation.
Complications include hemodynamic instability, myocardial ischemia, hemorrhage, respiratory failure, renal failure, and neurologic changes.
C Carotid endarterectomy
Cerebrovascular accidents, or strokes, are the third leading cause of death in the United States and account for a yearly cost of $14 billion in medical expenses and lost productivity. Most strokes are caused by cerebral ischemia. In carotid atherosclerotic disease, subintimal fatty plaques can increase in size over time and incrementally occlude the vascular lumen, which results in decreased cerebral blood flow (CBF). The plaque may rupture and release fibrin, calcium, cholesterol, and inflammatory cells. This phenomenon can lead to abrupt occlusion of the lumen from thrombosis from platelet activation, or an embolus may form and decrease CBF distal to the carotid artery. In each scenario, an abrupt decrease in CBF leads to transient ischemic attacks (TIAs) or strokes.
More than half of all strokes are preceded by a TIA. The Framingham study reported that the risk of a stroke was 30% 2 years after a TIA and approximately 55% 12 years after a TIA had occurred. It is this increased risk of stroke associated with TIA that provides the rationale for use of carotid endarterectomy (CEA), the surgical procedure in which the internal carotid artery is incised, the carotid arterial lumen is opened, and the plaque within the lumen is removed to improve CBF.
The initial indication for CEA is symptomatic stenosis but not complete occlusive carotid disease. This presentation occurs in most patients who undergo carotid surgery. Some centers have extended the indications to include evolved (nondense), nonhemorrhagic strokes and asymptomatic severe stenosis or lesser stenosis associated with contralateral occlusive disease.
The surgical outcomes reported for CEA remain inconclusive because of differences in patient populations and varying degrees of surgical expertise. Other variables that cannot be stratified in studies but that may affect outcome include the state of collateral flow through the circle of Willis, the presence of concurrent atherosclerotic disease in the cerebral vasculature, the size and morphology of the offending plaque, the specific presenting symptoms, and the presence of concurrent cardiovascular disease. The perioperative mortality rate for CEA is approximately 0.5% to 2.5%, and the long-term postoperative stroke incidence ranges from 1% to 3% per year.
Criteria for the best candidates for carotid artery surgery remain unclear. The risks associated with having surgery and the possibility of a stroke should be measured against the risks associated with not having surgery and undergoing medical management. Several conditions that can increase the risk of perioperative complications include severe preoperative hypertension, CEA performed in preparation for coronary artery bypass, angina, internal carotid artery stenosis near the carotid siphon, age older than 75 years, and diabetes mellitus. Because CEA is performed prophylactically, it would seem prudent that patient selection be based on the risks associated with the neurologic and myocardial ischemia of surgery as opposed to the risks associated with the neurologic sequelae of nonsurgical management.
The neurologic symptoms of cerebrovascular dysfunction (e.g., TIAs and strokes) are most frequently related to a decrease in CBF. Asymptomatic carotid bruits may be a sign of the possibility of carotid artery disease. Amaurosis fugax or monocular blindness occurs in 25% of patients with high-grade carotid artery stenosis. This syndrome is believed to be caused by microthrombi that travel into the internal carotid artery and that decrease the blood supply of the optic nerve via the ophthalmic artery. Duplex ultrasonography, a noninvasive diagnostic modality that combines ultrasonography and Doppler analysis, is currently one of the most sensitive noninvasive techniques capable of evaluating extracranial occlusive disease. Arteriography may be performed if surgery is being contemplated and can provide anatomic details of arterial vessels. CT scan or MRI may be useful in patients with a neurologic deficit, in whom an alternative diagnosis may be discovered.
a) The presence of concurrent CAD and carotid stenosis is well documented. Although stroke is a devastating consequence of CEA, MI contributes more frequently to poor surgical outcomes than stroke. Although coronary angiography may not be justified in all patients undergoing CEA, a systematic approach to the identification of CAD and its subsequent risks should be performed before elective surgery.
b) Patients with no significant medical history, normal physical examination, and normal ECG should proceed directly to surgery because these patients have low surgical risks.
c) When abnormal cardiac information is obtained, further evaluation should be performed. Radionuclide imaging is highly sensitive in diagnosing CAD. Redistribution demonstrated on dipyridamole-thallium imaging is very suggestive of increased risk of adverse cardiac events. In these patients, coronary angiography is suggested as a means of quantifying CAD and selecting an appropriate therapeutic intervention.
d) The progression of surgical intervention when CAD is present with carotid artery disease is controversial. Most agree that in cases of mild CAD, patients may undergo CEA with a low degree of risk. However, in cases of moderate to severe CAD, the direction of surgical intervention is unclear. One option is the simultaneous performance of CEA and coronary revascularization. Decisions should be guided by the patient’s symptoms, the associated risk factors, and the center’s experience.
7. Perioperative considerations
(1) CBF can remain relatively constant at different cerebral perfusion pressures as a result of cerebrovascular autoregulation. Cerebral perfusion pressure can be expressed as the difference between MAP and intracranial pressure (ICP). During CEA, ICP is usually not elevated; therefore, MAP plays the predominant role in determining cerebral perfusion pressure.
(2) When MAP is maintained between 60 and 160 mmHg, CBF remains constant. However, the adverse effects of chronic systemic hypertension shift the patient’s cerebral autoregulatory curve to the right, and therefore a higher than normal MAP may be required to ensure adequate cerebral perfusion. CBF is also influenced by arterial carbon dioxide and oxygen levels as well as by inhalation agents.
(3) Carotid occlusive disease jeopardizes the cerebral perfusion pressure in the ipsilateral artery. Ischemia leads to the disruption of autoregulation and compensatory vasodilation, and thus blood flow becomes pressure dependent. During CEA, the anesthetic goals should focus on improvement and protection of CBF and diligent monitoring of brain function.
(1) In addition to standard monitoring, direct intraarterial pressure is continuously assessed to evaluate near–real-time values. During the administration of anesthetic agents, blood pressure fluctuation commonly occurs in patients who have a history of hypertension.
(2) Because of the high incidence of CAD and neurovascular disease in this patient population, prompt treatment of blood pressure values below 20% of the preoperative MAP value is imperative.
(3) Pulmonary artery catheterization is not warranted in most individuals unless the presence of concurrent cardiac disease justifies its use.
(4) Carbon dioxide has a potent effect on cerebrovascular tone. Both hypocapnia and hypercapnia directly affect CBF; therefore, maintenance of normocapnia is paramount.
(5) During repair, the carotid artery cross-clamp is applied.
(6) Various monitoring techniques have been proposed for the assessment of the adequacy of CBF during this maneuver.
(7) A summary of cerebral monitoring techniques is listed below. Each of these monitoring modalities has limitations, and the most sensitive and specific measure of adequate CBF is an awake patient.
(8) Electroencephalographic (EEG) monitoring constitutes the gold standard in the identification of neurologic deficits related to carotid artery cross-clamping. EEG has demonstrated reliability in the monitoring of cortical electrical function. Loss of β-wave activity, loss of amplitude, and emergence of slow-wave activity all are indicative of neurologic dysfunction.
(9) Cerebral monitoring modalities during general anesthesia during CEA are listed in the following box.
c) Cerebral protection: The major objective during carotid artery revascularization is to maintain CBF and decrease cerebral ischemia. Prevention of cerebral ischemia can be accomplished in one of two ways: by increasing the collateral flow (placement of intraluminal shunt) or by decreasing the cerebral metabolic requirements (pharmacologic adjunct).
(a) When the carotid artery is clamped, CBF is compromised. Therefore, maintenance of coronary perfusion pressure (CPP) is dependent on collateral blood flow for adequate cerebral perfusion. The EEG changes that are associated with cerebral ischemia can be reversed when an intraluminal shunt is inserted.
(b) The shunt acts as a temporary conduit that allows for arterial blood flow during the time the surgeon is dissecting plaque from the intima of the carotid artery. Although some surgeons routinely insert shunts before plaque removal, others do not use shunts or use shunts only in a select group of patients.
(c) The application of a shunt imposes the risk of embolic complications and intimal dissections. Cerebral ischemic events are most often the result of embolic complications. Stump pressures and EEG measurements are the intraoperative monitoring modalities that can be used to determine the need for shunt placement.
(1) Barbiturates and propofol have the capability of decreasing cerebral metabolism to 40% below normal values.
(2) During transient focal ischemia, barbiturates and propofol decrease the cerebral metabolic rate of oxygen consumption, which results in cerebral protection.
(3) The disadvantages of administering barbiturates and propofol during CEA surgery include myocardial depression and delayed emergence.
(4) The surgeon may request that one of these cerebral depressants be administered before the carotid artery is cross-clamped.
(5) Hypothermia is also associated with decreases in cerebral metabolic rates and oxygen consumption. A decrease in core temperature of 1° C decreases cerebral metabolic rate for oxygen (CMRO2) by 7%. When core temperature has been reduced to 12° to 20° C, the safe duration of ischemia is 30 to 60 minutes.
(6) Hypothermic techniques were initially advocated; however, the risks associated with these techniques outweigh the clinical usefulness.
(1) The presence of hypertension in patients with cerebrovascular disease is well known. Therefore, one of the most challenging aspects of care associated with anesthesia for CEA is blood pressure control. Patients with cerebral insufficiency are vulnerable to perioperative blood pressure instability.
(2) Hypotension occurs in 10% to 50% of patients who undergo CEA and is believed to be the result of carotid sinus baroreceptor stimulation. Conversely, 10% to 66% of patients experience hypertension, which is attributed to surgical manipulation of the carotid sinus.
(3) Preoperative blood pressure control, volume status, and depth of anesthesia can also contribute to intraoperative hemodynamic instability.
(4) All patients should continue taking their antihypertensive medications until the time of surgery.
(5) Additional pharmacologic adjuncts may be required in the preoperative period, especially during the insertion of IV and intraarterial catheters, to reduce increases in heart rate and blood pressure.
(6) The induction of anesthesia, initial incision, dissection, manipulation of the carotid sinus, and emergence from anesthesia are all events that precipitate blood pressure fluctuations. The use of pharmacologic adjuncts, such as short-acting β-adrenergic blockers, may stabilize blood pressure during induction and emergence.
(7) Continuous IV use of nitroglycerin or sodium nitroprusside should be available to treat hypertension. Patients with chronic hypertension are predisposed to dramatic decreases in blood pressure after the induction of general anesthesia. This condition should be treated promptly and can be successfully managed through provision of IV fluids or administration of a vasoconstrictor, such as phenylephrine hydrochloride.
(8) Hypotension and bradycardia, which result from carotid sinus baroreceptor manipulation, can be inhibited by infiltration with local anesthetic.
a) The anesthetic objectives for vascular surgery are similar to those for any type of elective procedure; they are to provide analgesia and amnesia, facilitate surgical intervention, and minimize operative morbidity and mortality.
b) Specific objectives for CEA include maintaining cerebral and myocardial perfusion and oxygenation, minimizing the stress response, and facilitating a smooth and rapid emergence.
c) Overall the anesthetic goal is to optimize perfusion to the brain, minimize myocardial workload, ensure cardiovascular stability, and allow for rapid emergence.
(1) There is no consensus that a particular technique is more effective in decreasing overall perioperative morbidity and mortality. Studies have shown that regional anesthesia is associated with improved postoperative outcomes such as lower mortality rate; lower perioperative stroke rate; less intraoperative hemodynamic variability; fewer major adverse cardiac events, including MI 30 days after endarterectomy; a 10% decreased rate of unnecessary shunting; and decreased length of hospital stay.
(2) Anesthetic selection should be based on the anesthesia provider’s familiarity and competence with a specific technique as well as the patient’s condition and the surgeon’s preference.
(a) A regional anesthetic technique during CEA can be accomplished by local infiltration or by superficial and deep cervical plexus block.
(b) The greatest advantage of regional anesthesia is the anesthesia provider’s ability to directly assess neurologic function in an awake patient. Assessing the level of consciousness is the most effective method assessing the adequacy of CBF and detecting cerebral ischemia.
(c) The use of regional anesthesia has been associated with shorter operative times, less frequent cardiopulmonary complications, and shorter postoperative hospitalization.
(d) The limiting factor for use of a regional technique is patient acceptance. Because these individuals are awake, preoperative patient education is essential, and their cooperation during surgery is vital.
(e) Anxiety, fear, and apprehension can initiate sympathetic stimulation and, as a result, extreme hemodynamic responses can occur. Deep sedation that can be required in an apprehensive patient may confound the neurologic assessment, which negates the advantages of a regional technique.
(f) Hypercarbia can result from hypoventilation, and dysphoria is most likely to occur. Converting to a general anesthetic technique after surgery has begun can be problematic.
(g) If adequate cerebral perfusion is compromised, symptoms include dizziness, contralateral weakness, decreased mentation, and loss of consciousness. In the event this scenario occurs, immediate shunt placement is warranted. Emergent airway management may be necessary.
(a) General anesthesia is commonly used during CEA. Perhaps the greatest benefit of this technique is that it counters the most cited disadvantage of regional anesthesia: lack of patient cooperation.
(b) General anesthesia promotes a motionless field during surgery. Inhalation agents may provide hemodynamic stability and may have beneficial effects on cerebral circulation.
(c) By decreasing cerebral and cardiac metabolism, inhalation agents provide a degree of protection against ischemia, an effect called anesthetic preconditioning.
(d) Comparison of inhaled agents with narcotic-based techniques yields no scientific evidence to suggest that patient outcome is improved.
(e) Remifentanil can be used and, because of rapid metabolism, neurologic recovery is improved.
(f) The inhalation agents may alter the monitoring methods used for detecting cerebral ischemia, such as EEG and somatosensory evoked potential (SSEP) monitoring.
(g) In these cases, general anesthetic techniques may require modification, and direct communication is required between the anesthesia provider and the surgical team.
(h) The use of nitrous oxide during CEA can potentially increase the incidence of a clinically significant pneumocephalus. During shunt placement and carotid artery cross-clamp release, microbubbles can be entrained into carotid artery blood flow. For this reason, if nitrous oxide is used, it should be discontinued before removal of the carotid artery cross-clamp.
a) The most common problem experienced in the postoperative period is hypertension. Although the specific cause remains unclear, postoperative hypertension may be related to events or conditions that alter cerebral autoregulation, such as use of halogenated hydrocarbons, diabetes mellitus, and cerebral hypoperfusion.
b) A systolic blood pressure greater than 180 mmHg is associated with an increased incidence of TIA, stroke, or MI.
c) Patients with systolic blood pressures of 145 mmHg or less have fewer postoperative complications.
d) Although an uncommon complication, carotid artery hemorrhage can occur in the postoperative phase. Hemorrhage is a devastating event that requires immediate surgical intervention. Initial manifestations of hemorrhage may be those of upper airway obstruction, which may make reintubation difficult or impossible because of tracheal deviation.
e) The complications associated with carotid artery stenting (CAS) are listed in the following box.
Carotid artery stenting
A less invasive surgical approach for treatment of carotid artery stenosis is carotid artery angioplasty and stenting. The safety and efficacy of CAS have been in question. Early stents were not equipped with distal protection devices, and a high number of patients developed CVAs as a result of embolization. Controversy exists regarding the degree of success that this procedure affords as an alternative to CEA. The long-term results of CAS appear to be comparable to CEA in terms of prevention from stroke, freedom from new neurologic events, and patency rates.
Carotid artery stenting should be used instead of CEA in the presence of specific patient factors or severe vascular or cardiac comorbidities, which are listed in the following box.
Before CAS procedure, patients receive an aortic arch, carotid, and cerebral angiogram or a high-resolution MRI. This allows the physicians to evaluate the individual anatomy and angiopathology of the aortic arch, brachiocephalic artery (for right carotid artery stent), or left common carotid artery. Determination of the type of sheaths, stents, and cerebral embolic protection device can then be planned. Femoral artery access is obtained, and then a sheath is threaded through the aortic arch and into the operative carotid artery. The guidewire or embolic protection device is advanced through the sheath and positioned across the stenotic region. An embolic protection device sequesters emboli during angioplasty and stenting to avoid distal occlusion in cerebral arteries. In elderly patients, adjunctive distal embolic protection lowers the risk of intraoperative and postoperative adverse events. Angioplasty with a 5.0-mm balloon dilates the carotid artery, and then the stent is deployed. The guidewire or device wire is removed after angiographic confirmation that carotid artery dissection or occlusion has not occurred.
a) The anesthesia that is routinely used for CAS is local anesthesia at the femoral insertion site and minimal sedation.
b) Fluoroscopy is used throughout the surgery; therefore, it is important that all operating room personnel are protected with lead.
c) Anticoagulation is attained with a heparin bolus 50 to 100 units/kg to maintain an activated clotting time (ACT) greater than 250 seconds.
d) Balloon inflation in the internal carotid artery can stimulate the baroreceptor response, resulting in prolonged bradycardia and hypotension. Glycopyrrolate or atropine can be administered before inflation to offset this vagal response.
e) The most common complication is stroke caused by thromboembolism. Interventions for a patient with an acute stroke include airway and hemodynamic management. Currently, the only treatment approved for acute ischemic stroke is IV recombinant tissue plasminogen activator (rt-PA). Mechanical devices such as snares and balloons are being developed so that the physician will be able to physically remove thromboembolic material and restore blood flow.
f) Patients typically remain in the postanesthesia care unit for 30 minutes after carotid stent placement and then are transferred to a monitored floor. A carotid duplex scan is performed before discharge, and scans are then routinely obtained at 6 weeks, 6 months, 1 year, and then yearly. Patients remain on aspirin therapy for anticoagulation for life.
D Endovascular aortic aneurysm repair
In 1991, the first endovascular stent was performed to repair an infrarenal aortic aneurysm. The development of this technique has created a less invasive approach to aortic aneurysm repair. Because of severe cardiac and respiratory pathology, it is believed that as many as 30% of patients with aortic aneurysms are not surgical candidates. Endovascular AAA repair (EVAR) was initially intended for patients with severe coexisting disease; however, its popularity has increased as the success of the procedure has improved. It is estimated that 20,000 EVAR procedures take place in the United States per year, which comprises 36% of aortic aneurysm repair.
Endovascular aortic aneurysm repair is also being used to treat patients with thoracic aortic aneurysms. The mortality rate for EVAR for elective descending thoracic aneurysm repairs range from 3.5% to 12.5% compared with an open approach, which is approximately 10%. There is a low incidence of spinal cord ischemia and paraplegia, which is reported from 0% to 6%. Potential reasons for the lack of spinal cord complications are no thoracic aortic cross-clamping and no prolonged periods of extreme hypotension. Perioperative hypotension (MAP <70 mmHg) was a significant predictor of spinal cord ischemia in patients having EVAR for thoracic aneurysm repair. Endograft therapy has also been used with success and may eventually become the treatment of choice in patients older than 75 years of age for thoracic aneurysm repair.
The mortality rate for patients with a ruptured AAA who are alive when diagnosed in emergency departments is 40% to 70%. The EVAR approach has been used successfully to repair ruptured AAAs. The number of patients treated and the quality of randomized controlled data on this subject are limited. Medical centers that consider EVAR for ruptured AAA repair should have emergent CT imaging capabilities, a trained endovascular team, adequate endovascular supplies available, and a surgical suite.
Endovascular aortic aneurysm repair involves deployment of an endovascular stent graft within the aortic lumen. The graft restricts blood flow to the portion or the aorta where the aneurysm exists. This procedure can be performed for patients who have descending thoracic aortic aneurysms or AAAs. Cannulation of both femoral arteries is performed. A guidewire is threaded through the iliac artery to the level of the aneurysm. Next, a sheath is inserted over the guidewire and positioned at the aneurysm location through the use of fluoroscopy. The proximal end of the sheath should extend beyond the aneurysm. After the sheath is deployed, migration is prevented by the use of radial force or fixation mechanisms such as hooks, barbs on the stent become embedded into the aortic wall.
The procedure frequently takes place in an interventional radiology suite. Advantages of the endovascular approach compared with the conventional surgical method include improved hemodynamic stability, decreased incidence of embolic events, decreased blood loss, reduced stress response, decreased incidence of renal dysfunction, and decreased postoperative discomfort.
a) Systemic anticoagulation with heparin 50 to 100 units/kg is administered before catheter manipulation.
b) A first-generation cephalosporin is recommended at the beginning of surgery.
c) The anesthetic techniques that can be used for EVAR include general anesthesia neuraxial blockade or local anesthesia with sedation.
d) There is presently a lack of data suggesting that one anesthetic technique is superior for patients having EVAR.
e) The goals for intraoperative management for EVAR include maintaining hemodynamic stability, providing analgesia and anxiolysis, and being prepared to rapidly convert to an open procedure.
f) With infrarenal or suprarenal EVAR, creatinine clearance values decrease by 10% in the first year. However, proximal endovascular graft migration can occur, causing renal artery occlusion and postoperative renal failure.
g) Other complications that can arise as a result of EVAR include endograft thrombosis, migration or rupture, graft infection, iliac artery rupture, and lower extremity ischemia. Fatal cerebral embolism resulting in sudden respiratory arrest has occurred during EVAR.
h) A serious complication that can occur as a result of this procedure is termed endoleak, defined as persistent blood flow and pressure (endotension) between the endovascular graft and the aortic aneurysm. This complication has been reported to occur in 15% to 52% of patients as diagnosed by postoperative CT scan. Most endoleaks are type II, and 70% spontaneously close within the first month postimplantation.
i) Type II endoleaks are caused by collateral retrograde perfusion. Type I and type III endoleaks are caused by device-related problems. The most frequent intervention used to correct these complications includes implantation of a second endograft or open repair.
j) Long-term results of endovascular aortic aneurysm repair have demonstrated that this procedure yields good results, but the overall durability of conventional surgical techniques is superior.
E Peripheral vascular procedures
Peripheral vascular procedures include femoral–femoral, femoral–popliteal, femoral–tibial, iliofemoral, axillofemoral, and embolectomies. Obstruction most often is in the superficial femoral artery followed by common iliac claudication in the gastrocnemius muscle, but pain and ischemic ulceration or gangrene occur with severe occlusion.
Procedures are classified as inflow or outflow vascular reconstruction. The inflow reconstruction procedures bypass the obstruction in the aortoiliac segment (aortoiliac endarterectomy or aortofemoral bypass). These are more stressful procedures requiring cross-clamping of the aorta. Outflow procedures are performed distal to the inguinal ligament to bypass the femoropopliteal or distal obstruction. The stages of aortic clamping and anesthetic considerations are shown in the table on pg. 587.
Stages of Aortic Cross-Clamping and Anesthestic Considerations
| Clamping Stage | Goals | Drug to Prepare |
| Preclamping | Maintain blood pressure 20% baseline (low normal) | Volatile anesthetic Nitroglycerin, nitroprusside (Nipride) If PCWP is increased and cardiac output is decreased, inotropic support (dopamine, epinephrine) may be needed. |
| Maximize urinary output | Mannitol, furosemide (Lasix) | |
| Minimize fluids | Monitor crystalloid administration | |
| Prevent thrombosis | Heparin: monitor activated clotting times | |
| Cross-clamping | Prevent myocardial infarction Maintain oxygenation Maintain urinary output (0.5 mL/kg/hr) |
Decrease afterload (nitroglycerin and nitroprusside) Monitor electrocardiogram for ischemia Monitor cardiac output, and expect a decline Oxygen/air or 100% oxygen Monitor oxygen saturation and arterial blood gases Anuria is rare Dopamine (1-5 mcg/kg) |
| Prerelease | Prevent MI from declamping, hypotension | Monitor ECG Ask surgeon for a 10-min warning before aortic clamp is removed Lighten anesthesia depth Discontinue vasodilating agents (nitroglycerin and nitroprusside) Increase CVP and PCWP 4 to 6 mmHg with fluids (and blood if needed) Have vasopressors ready |
| Postrelease | Maintain blood pressure and vital signsCorrect acidosis | Use vasopressors (dopamine, epinephrine, phenylephrine) Mechanical ventilation Bicarbonate administration (pH <7.25) Calcium chloride administration |
| Correct coagulation profile | Protamine | |
| Maintain urine output | Volume: Crystalloids and colloids, dopamine |
CVP, Central venous pressure; ECG, electrocardiography; MI, myocardial infarction; PCWP, pulmonary capillary wedge pressure.
a) History and physical examination
(1) See the discussion of AAA earlier in this section.
(2) Musculoskeletal: This includes decreased or absent popliteal and pedal pulses, delayed capillary refill, blanching on elevation of the leg followed by dependent edema after lowering it, and pain with walking relieved by rest.
(1) See the discussion of AAA earlier in this section.
(a) Determinations of systolic blood pressure at the level of the ankle are compared with brachial determinations.
(b) Assess the severity of ischemia, urgency of revascularization, and baseline values for evaluation of operative results.
(3) Angiography: Determine the precise site of the actual lesion.
c) Preoperative medications and IV therapy
(1) For elderly patients with coexisting medical disease requiring pharmacologic support (i.e., antihypertensives, antianginals, antiarrhythmics, digoxin), these medications may be continued up to the day of the operation.
(2) Sedatives and narcotics are used.
(a) Use with caution in patients with poor respiratory reserve.
(b) The onset of IV medications may be delayed because of potentially low cardiac output.
(3) Two peripheral large-bore (16- to 18-gauge) IV lines with moderate fluid management are used.
(4) Epidural catheter: Test the dose on the awake patient; there is a risk of epidural hematoma from anticoagulation during surgery.
(1) ECG leads V5 and II to detect myocardial ischemia and diagnose tachyarrhythmias.
(4) Arterial line and central venous pressure monitoring is possibly necessary.
a) Regional block, general anesthesia, or a combination. The technique of choice is regional anesthesia; it avoids airway problems and sequelae, provides greater hemodynamic stability with coexisting diseases, provides sympathetic block that increases circulation in the lower extremity, reduces the incidence of intravascular clotting, facilitates postoperative pain relief, suppresses the endocrine stress response, and decreases blood loss in selected cases.
b) Regional block: Analgesia to T10; epidural or spinal
(1) The elderly population is more sensitive to local anesthetics; reduce the dose by 50%.
(2) Some practitioners administer ephedrine prophylactically to prevent hypotension.
(3) The level is slightly above the skin dermatome necessary for the usual incisions (T12), but sympathetic innervation of lower extremities, which contain visceral afferent fibers, is believed to occur at T10 to L2.
(a) The goals are a smooth transition from awake state to surgical anesthesia and the maintenance of cardiovascular stability.
(b) A slow, “controlled” induction is preferred with an opioid and nondepolarizing muscle relaxant.
(c) Muscle relaxation may be chosen on the basis of cardiovascular effect.
(d) The onset of drugs may be delayed with low cardiac output.
(e) Omit thiopental and other cardiac depressors in patients with poor left ventricular function.
(f) Anticipate exaggerated blood pressure changes; maintain within 20% of baseline.
(g) Minimize pressor response during intubation of trachea by limiting the duration of laryngoscopy to less than 15 seconds.
(a) Review the principles of sympathetic block.
(b) Consider administering block with the operative side down so that the onset of sympathetic and sensory blockade is faster on the dependent site. Theoretically, the level will be higher on the dependent side. The total volume of anesthetic requirements may be decreased.
(1) The surgeon will ask that heparin be administered by IV push; ensure the patency of the port before injection.
(2) If intraoperative angiography is used:
(a) Allergic reactions may occur with dye.
(b) The degree of surgical stimulation changes; blood pressure decreases when surgical activity stops in preparation for angiography. Blood pressure and heart rate may increase when dye is injected.
(c) Repeated injection of contrast dye during multiple attempts at angiography may cause osmotic diuresis.
(3) Hyperkalemia and acidosis resulting from ischemic extremities are possible, and myoglobin can be released into the circulation.
(4) Maintain the hematocrit at more than 30 to maximize oxygen-carrying capacity.
(5) Unclamping of the femoral artery rarely affects hemodynamics significantly. The lower extremity receives arterial blood through collateral vessels, even when the femoral artery is occluded.
F Portosystemic shunts
Portosystemic shunt procedures are performed to prevent or cease variceal hemorrhage resulting from portal hypertension in patients with liver disease, cirrhosis, ascites, and hypersplenism. The redistribution of blood from the portal vein to the inferior vena cava causes variations in flow and resistance of the liver, intestine, and spleen. This hemodynamic alteration aids portal perfusion and oxygenation with the net effects of increased venous return and cardiac output. Variations in procedures include portocaval, end-to-end, end-to-side, mesocaval, mesorenal, and splenorenal shunts.
2. Preoperative assessment and patient preparation
a) History and physical examination
(1) Cardiac: Associated conditions include increased heart rate, circulating blood volume, and intrathoracic pressure. Variations of cardiac output, cardiomyopathy, congestive heart failure, CAD, and decreased response to catecholamines and SVR may be present.
(2) Respiratory: Hypoxemia may be related to ventilation/perfusion mismatch, increased closing volume, decreased functional residual capacity, atelectasis, right-to-left pulmonary shunting, increased diphosphoglycerate, pulmonary infections, and impaired hypoxic pulmonary vasoconstriction.
(3) Neurologic: Manifestations may include hepatic encephalopathy with associated confusion and obtundation.
(4) Renal: Renal impairment and failure with electrolyte imbalance are frequently observed.
(5) Gastrointestinal: Gastric or esophageal varices with gastrointestinal bleeding are common.
(6) Endocrine: Abnormal glucose utilization, increased growth hormone, intolerance to carbohydrates, and irregular sex hormone metabolism may be observed.
(1) Laboratory tests: Arterial blood gases, complete blood count, prothrombin time, partial thromboplastin time, bleeding time, electrolytes, blood urea nitrogen, creatinine, creatinine clearance, urinalysis, diffuse intravascular coagulation profile, albumin, bilirubin, serum glutamic-oxaloacetic transaminase, serum glutamic-pyruvic transaminase, ammonia, alkaline phosphatase, and lactate are obtained.
(2) Diagnostic tests: ECG, echocardiography, pulmonary function tests, and chest radiography are obtained.
(3) Preoperative medications: Avoid intramuscular injections. Anxiolytics are administered in small doses as indicated. Consider metoclopramide (10 mg) and ranitidine (50 mg).
(4) IV therapy: This involves a central line and two 14- to 16-gauge IV lines. Consider a pulmonary arterial catheter.
a) Monitoring equipment: Standard with arterial line, central venous pressure catheter, and urinary catheter
b) Additional equipment: Fluid warmer, cell saver, Bair Hugger, and rapid infuser
(1) Miscellaneous pharmacologic agents: Opioid (fentanyl), midazolam, vasodilators and vasoconstrictors, inotropes, nondepolarizing muscle relaxants, and antibiotics are used.
(2) IV fluids: Calculate for major blood loss. Estimated blood loss is 1000 to 2000 mL.
(3) Blood: Type and cross-match for 8 to 10 units of packed red blood cells, platelets, fresh-frozen plasma, and cryoprecipitate.
4. Perioperative management and anesthetic technique
a) General anesthesia, epidural anesthesia, or a combination of general and regional anesthesia is used.
b) General anesthetic is the technique of choice.
c) Induction: Use rapid-sequence induction with thiopental (3-5 mg/kg) and succinylcholine (1-2 mg/kg). Consider etomidate (0.2 mg/kg) or ketamine (1 mg/kg).
d) Maintenance: Inhalational agent, oxygen, fentanyl, midazolam and nondepolarizing muscle relaxant. Position is supine.
e) Emergence: The patient generally is transported to the intensive care unit. Patients may remain intubated while hemodynamic stability is achieved.
G Thoracic aortic aneurysm
The mortality rate associated with thoracic aneurysms is well established. Patients with aortic dissections have only a 3-month survival time if they do not undergo surgical repair because the incidence of rupture is high. The refinement of synthetic grafts, surgical and perfusion techniques, and intraoperative management has contributed to improved surgical outcomes. Today the early mortality rate is thought to be less than 10%, demonstrating that elective surgical intervention is an acceptable means of treating thoracic aortic aneurysms.
Aneurysms of the thoracic aorta may be classified with respect to type, shape, and location. Typically, aneurysms involving all three layers of the arterial wall, tunica adventitia, tunica media, and tunica intima are considered to be true aneurysms. In comparison, aneurysms that solely involve the adventitia are termed false aneurysms. The shape of the lesion can also serve as a means of characterizing aneurysms. Fusiform aneurysms have a spindle shape and result in dilation of the aorta. Saccular aneurysms are spherical dilations and are generally limited to only one segment of the vessel wall. Aortic dissection is the result of a spontaneous tear within the intima that permits the flow of blood through a false passage along the longitudinal axis of the aorta. Aneurysms can also be classified according to their location within the aortic arch. In addition, thoracoabdominal aneurysms can be classified into four types on the basis of their location.
Atherosclerosis is the most common cause of aneurysmal pathology. Atherosclerotic lesions occur most often in the descending and distal thoracic aorta and are most often classified as fusiform. Less common causes include the histologic contributions of cystic medial necrosis observed in patients with Marfan’s syndrome, infective and inflammatory processes within the vessel wall, and Takayasu’s arteritis. A genetic predisposition is thought to contribute to the development of aortic aneurysms.
The symptomatology of thoracic aneurysms is often related to the site of the lesion and its compression on adjacent structures. Pain, stridor, and cough may result from compression of thoracic structures. Symptoms related to aortic insufficiency may be observed in aneurysms of the ascending aorta. An upper mediastinal mass may be an incidental finding on conventional chest radiography in an asymptomatic patient. Further investigation with noninvasive methods (e.g., CT and MRI) can show the configuration and location of the aneurysm. Invasive aortography, although associated with a higher risk of complications, provides the most information because it allows evaluation of the coronary vessels and branches of the aortic arch.
Early detection and surgical intervention have made significant contributions to long-term survival. The surgical approach and mode of resection vary according to the location of the lesion within the thoracic aorta. Resection of the ascending aorta and graft replacement necessitate the use of CPB. The aortic valve may also require replacement. Surgical resection of lesions in the transverse arch compromises cerebral perfusion, although various bypass techniques combined with profound hypothermia and circulatory arrest have been used. Aneurysms of the descending aorta may be resected by application of an aortic cross-clamp. However, perfusion to distal organs can be compromised during this procedure. Endovascular thoracic aortic repair is also an option.
Aortic dissection
1. Aortic dissection is characterized by a spontaneous tear of the vessel wall intima, which permits the passage of blood along a false lumen. Although the cause of the dissection is unclear, lesions that were thought to be related to cystic necrotic processes may actually be caused by variations in wall integrity.
2. Hypertension is the most common factor that contributes to the progression of the lesion. Manipulation of the ascending aorta during cardiac surgery may be associated with aortic dissection.
3. The symptoms of aortic dissection are the result of the interruption of blood supply to vital organs. The most serious complication is aneurysm rupture.
4. Diagnosis can be accomplished by the previously mentioned noninvasive techniques; however, aortography appears to be most reliable.
5. Treatment of dissecting aortic lesions depends on their location within the thoracic aorta. Type A lesions have the highest incidence of rupture and require immediate surgical intervention. Type B lesions may initially be managed medically, with the administration of arterial dilating and β-adrenergic blocking agents. Surgical intervention contributes to an improved long-term survival rate. Studies have shown a survival rate of 94% to 95% for repair of dissecting aortic aneurysm by investigators using the newer techniques.
6. The surgical method used is dependent on the location of the aortic lesion. Anesthesia for aneurysms of the ascending and transverse aorta requires CPB.
Descending thoracic and thoracoabdominal aneurysms
Patients who undergo major vascular surgery are frequently elderly and have varying degrees of concurrent disease. The incidence of thoracic aortic aneurysm is increasing, and the 5-year survival rate after diagnosis without intervention is 9% to 13%. The preoperative evaluation focuses on cardiac, renal, and neurologic function. Although most fatalities related to thoracic aortic surgery are cardiac in origin, renal and neurologic dysfunction contribute to poor surgical outcomes.
(1) Intraoperative monitoring devices used for thoracoabdominal aneurysm resection are the same as those used for abdominal aneurysmectomies.
(2) Direct intraarterial blood pressure and pulmonary artery pressure monitoring is mandatory. If the aneurysm involves the thoracic region or the distal aortic arch, right radial arterial line monitoring is preferred because left subclavian arterial blood flow may be compromised during surgery.
(3) Use of two-dimensional transesophageal echocardiography is suggested for cardiac monitoring in patients with myocardial dysfunction.
(4) An indwelling urinary catheter is used for assessing renal function.
(5) To facilitate exposing the descending thoracic aorta, a double-lumen endotracheal tube (ETT) is inserted to allow for one-lung ventilation. As a result, careful monitoring of oxygenation is mandatory. Pulmonary artery catheters equipped with fiberoptics are useful for the measurement of mixed venous oxygen.
(6) Routine use of pulse oximetry may be limited if the left subclavian artery is manipulated; therefore, the right hand, the ear, or the nasal passages should be used for monitoring oxygen saturation.
(7) A lumbar intrathecal catheter is inserted to access CSF. SSEPs or motor evoked potentials (MEPs) may be used to detect neurologic dysfunction; however, their clinical usefulness remains uncertain.
(1) Simple aortic cross-clamping and graft replacement is an acceptable method for surgical repair of descending thoracic or thoracoabdominal aortic aneurysms.
(2) Application of a simple cross-clamp has eliminated the need for shunts and extracorporeal circulation; however, the consequences of this maneuver include myocardial compromise and occlusion of blood flow to distal structures.
(3) The hemodynamic alterations produced by the application of an aortic clamp are discussed earlier in the aortic aneurysms section. Similar responses are observed during proximal aortic occlusion; however, the magnitude of these responses is extreme.
(4) When the aorta is occluded, afterload and therefore myocardial workload increase. As a result, increases occur in left ventricular end-diastolic pressure, left ventricular stroke work index, and myocardial oxygen consumption.
(5) When oxygen demands are not accompanied by an increase in oxygen supply, ischemia results, and myocardial failure can occur.
(6) Although methods have been instituted to decrease myocardial afterload (e.g., partial bypass and shunts), the use of sodium nitroprusside appears to be the most effective means of decreasing afterload during cross-clamp application. Administration of nitroglycerin may be required to decrease preload during aortic occlusion.
c) Hemodynamic alterations: Unclamping
(1) The hemodynamic consequences of releasing the aortic cross-clamp are similar to those of abdominal aortic occlusion, but they are of greater magnitude.
(2) Metabolites that have accumulated during aortic cross-clamping are released into circulation. The combination of reactive hyperemia and sequestration of blood volume within hypoperfused areas can lead to severe hypotension.
(3) Restoration of blood volume, guided by left-sided filling pressures, before the gradual release of the clamp can minimize hemodynamic alterations.
(4) Increasing ventilation and the administration of sodium bicarbonate may control carbon dioxide increases at the time of declamping.
(1) Neurologic dysfunction is a serious complication of thoracic aortic reconstruction. The incidence of paraplegia is reported to be approximately 20% after elective surgery and as high as 40% after surgery for dissecting and ruptured aneurysms.
(2) Neurologic deficits are the result of hypoperfusion to the spinal cord during thoracic aortic reconstruction. The artery of Adamkiewicz, which is also known as the greater radicular artery, originates from the aorta branch between T8 and L2 and provides the majority of the blood flow to the anterior spinal artery. The anterior spinal artery perfuses the ventral aspect of the spinal cord, which is responsible for motor control.
(3) Although attempts have been made to reimplant the intercostal branches that contribute blood flow to the spinal cord, these efforts do not always decrease the incidence of paraplegia.
(4) The duration of aortic occlusion also contributes to spinal cord ischemia.
(5) Systemic hypothermia and selective cooling of the spinal cord may lengthen ischemic time intervals; however, the clinical benefits of these methods are unclear.
(6) The use of various bypass mechanisms and distal shunts may minimize the length of aortic occlusion time; however, the risks associated with the implementation of these techniques could be greater than the potential benefits.
(7) One method that has been successful is CSF drainage. CSF drainage involves manipulation of spinal cord perfusion pressure during aortic clamping. Spinal cord perfusion pressure can be defined as the arterial pressure minus the CSF pressure. During aortic clamping, CSF pressure increases, and arterial pressure decreases distal to the clamp. The spinal cord perfusion pressure can therefore be manipulated by alteration of arterial blood pressure and draining of CSF through the intrathecal catheter.
(8) The intraoperative use of SSEPs and MEPs can provide early identification of neurologic dysfunction; however, these monitoring modalities do not ensure spinal cord integrity.
(9) Factors that contribute to the development of neurologic deficit include level of aortic clamp application, ischemic time, embolization or thrombosis of a critical intercostal artery, failure to revascularize intercostal arteries, and urgency of surgical intervention.
(10) Neurologic dysfunction has also been reported to occur in the postoperative phase. Delayed paraplegia may be the result of reperfusion injury, although the exact mechanism of injury has not been proved.
(1) The incidence of renal dysfunction after thoracoabdominal aortic resection is estimated to be between 30% and 50%; the possibility of permanent renal failure requiring hemodialysis is estimated to be between 2% and 12%.
(2) The cause of renal insufficiency is ischemia, which is related to aortic occlusion. Intraoperative hypotension has been identified as an independent predictor of postoperative renal dysfunction.
(3) Maintenance of intravascular volume and stable circulatory status appears to be the most reliable method of minimizing renal dysfunction.
(4) Preoperatively, volume status should be corrected with non–glucose-containing crystalloid solutions.
(5) Intraoperative volume replacement should be guided by invasive monitoring.
(6) Pharmacologic adjuncts to produce diuresis, such as mannitol and furosemide, should be administered approximately 20 to 30 minutes before clamp application.
(7) Low-dose dopamine is also advocated as a means of increasing renal perfusion but has not been shown to protect renal function.
a) The principles of perioperative management of thoracic or thoracoabdominal aneurysms are similar to those previously discussed for abdominal aortic aneurysmectomies.
b) Anesthetic selection should be based on the presence of concomitant disease processes, with the objective of maintenance of cardiovascular stability and the minimization of morbidity and mortality.
c) Intraoperative monitoring should focus on detection of myocardial, neurologic, and renal ischemia. The hemodynamic consequences of aortic cross-clamping should be attenuated by the use of pharmacologic adjuncts.
d) Restoration of circulating blood volume, as guided by left-sided filling pressure, minimizes the hemodynamic alterations caused by the release of the aortic clamp.
e) Unique to thoracic aortic surgery is the use of one-lung ventilation. The highest degree of vigilance should be used to detect potential inadequacies in ventilation and oxygenation.
f) Extreme blood loss should be anticipated. Venous access and blood product availability should be confirmed during the preoperative phase. Methods of minimizing the use of homologous blood products, such as perioperative blood salvage, can be used.
g) Coagulopathy is a constant threat that results from the administration of blood products. The close monitoring of coagulation parameters and the administration of fresh-frozen plasma, platelets, or specific coagulation factors can minimize the incidence and severity of coagulopathies.
a) After surgery is completed, if a double-lumen ETT was used, it should be replaced with a standard ETT to provide a secure airway because postoperative ventilatory assistance is usually required. Anatomic landmarks may have become edematous during surgery, causing difficulty with reintubation. Under these circumstances, the double-lumen ETT may be left in place. Replacement, guided by fiberoptic evaluation, can proceed in the postoperative period after the airway edema has dissipated.
b) Close observation of circulatory and pulmonary status is warranted in the postoperative phase. Hemodynamic control is vital to maintain perfusion to vital organs without creating excessive demands on the heart or the aortic graft. Administration of dopamine may be continued during this time.
c) Careful monitoring of respiratory status aided by arterial blood gas analysis is important. Epidural analgesia using local anesthetics, narcotics, or both can be administered for pain relief.