Surfactant agents
After reading this chapter, the reader will be able to:
1. Define key terms that pertain to surfactant agents
2. List all available exogenous surfactant agents used in respiratory therapy
3. Describe the mode of action for exogenous surfactant agents
4. Discuss the route of administration for exogenous surfactant agents
5. Recognize hazards and complications of exogenous surfactant therapy
Chapter 10 reviews pharmacologic agents termed surfactants, which are intended to alter the surface tension of alveoli and the resulting pressures needed for alveolar inflation. The physical principles of surfactants and surface tension forces are reviewed as a basis for introducing agents that have been used or are currently used in respiratory care. The use of current exogenous surfactant agents in the treatment of respiratory distress syndrome (RDS) of the newborn is presented.
Physical principles
Surface tension
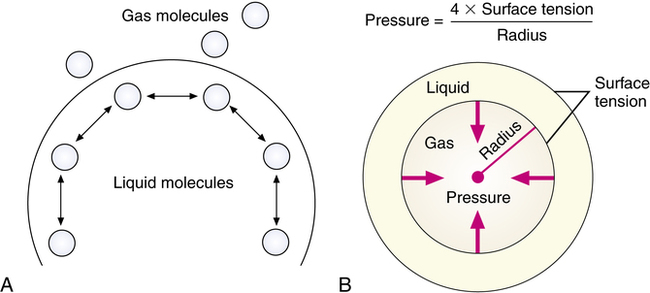
Surface tension is the force caused by attraction between like molecules that occurs at liquid-gas interfaces and holds the liquid surface intact. The units of measure for surface tension are usually dynes per centimeter (dyn/cm), indicating the force required to cause a 1-cm rupture in the surface film. Because the molecules in a liquid are more attracted to each other than to the surrounding gas, a droplet or spherical shape usually results (Figure 10-1, A).
Laplace’s law
LaPlace’s law is the physical principle describing and quantifying the relationship between the internal pressure of a drop or bubble, the amount of surface tension, and the radius of the drop or bubble (Figure 10-1, B). For a bubble, which is a liquid film with gas inside and out, LaPlace’s law is as follows:
< ?xml:namespace prefix = "mml" />
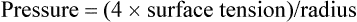
In alveoli, there is only a single air-liquid interface, and LaPlace’s law is as follows:
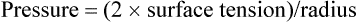
Clinical indications for exogenous surfactants
• Prophylactic treatment: Prevention of RDS in infants with very low birth weight and in infants with higher birth weight but with evidence of immature lungs, who are at risk for developing RDS
• Rescue treatment: Retroactive, or “rescue,” treatment of infants who have developed RDS
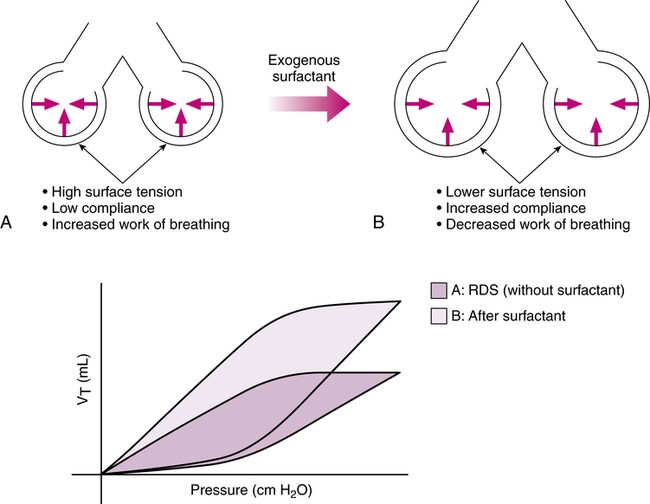
The basic problem in RDS is lack of pulmonary surfactant as a result of lung immaturity. This lack of pulmonary surfactant results in high surface tensions in the liquid-lined, gas-filled alveoli. Increased ventilating pressure is required to expand the alveoli during inspiration, which leads to ventilatory and respiratory failure in an infant without ventilatory support. This concept and the effect of exogenous surfactant are shown in Figure 10-2. Exogenous surfactants are also being investigated for efficacy in the treatment of acute respiratory distress syndrome (ARDS), acute lung injury (ALI), and meconium aspiration syndrome (MAS), although MAS is not an approved clinical application at this time.1
Identification of surfactant preparations
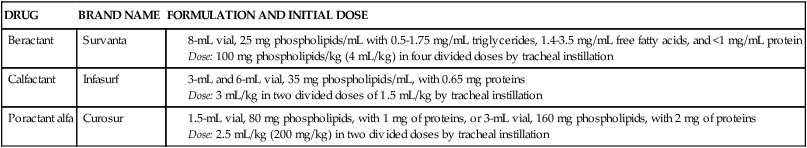
Table 10-1 lists surfactant formulations that currently have U.S. Food and Drug Administration (FDA) approval for general clinical use in the United States. Detailed differences between these formulations and details of their dosing and administration are discussed subsequently for each agent in separate sections.
TABLE 10-1
Exogenous Surfactant Preparations Currently Approved for Use in the United States*
| DRUG | BRAND NAME | FORMULATION AND INITIAL DOSE |
| Beractant | Survanta |
*Individual agents are discussed subsequently in a separate section. Detailed information on each agent should be obtained from the manufacturer’s drug insert.
The term exogenous, used to describe this class of drugs, refers to the fact that these are surfactant preparations from outside the patient’s own body. These preparations may be obtained from other humans, from animals, or by laboratory synthesis. The clinical use of exogenous surfactants has been to replace the missing pulmonary surfactant of the premature or immature lung in RDS of the newborn. These agents have also been investigated for use in ARDS and have been beneficial in improving oxygenation, although results have been inconsistent.2,3
Composition of pulmonary surfactant
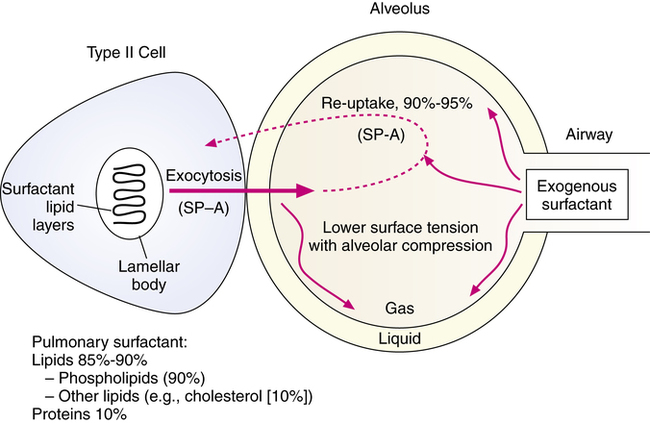
Pulmonary surfactant is a complex mixture of lipids and proteins (Box 10-1). The surfactant mixture is produced by alveolar type II cells. Their primary function, although not their only function, is to regulate the surface tension forces of the liquid alveolar lining. Surfactant regulates surface tension by forming a film at the air-liquid interface. Surfactant reduces surface tension as it is compressed during expiration, reducing the amount of pressure and inspiratory effort required to reexpand the alveoli during a succeeding inspiration. The amount of extracellular (i.e., outside the type II cell) surfactant in animals is 10 to 15 mg/kg body weight in adults and 5 to 10 times that in mature newborns.4 Figure 10-3 illustrates the source, basic composition, and regulation of pulmonary surfactant in the alveolus. Each of the major components is described in the following sections.
Lipids
Lipids make up about 85% to 90% of surfactant by weight. The lipid component of surfactant is approximately 90% phospholipids, such as phosphatidylcholine, phosphatidylglycerol, sphingomyelin, and others, and 10% other lipids, most of which is cholesterol.5 Phospholipids have lipophilic and hydrophilic properties and are able to achieve low surface tensions at air-liquid interfaces. Phosphatidylcholine constitutes about 75% to 80% of the phospholipids in surfactant, and about half of this is dipalmitoylphosphatidylcholine (DPPC), which is also known as lecithin. DPPC is the surfactant component predominantly responsible for the reduction of alveolar surface tension. The hydrophilic choline residue of DPPC is associated with the liquid phase in alveoli, whereas the hydrophobic palmitic acid residue projects into the air phase.6
Proteins
The total protein portion of surfactant is about 10% by weight. Approximately 80% of this portion is contaminating serum proteins, and 20% is surfactant-specific proteins. Four surfactant-specific proteins (SPs) have been identified so far: SP-A, SP-B, SP-C, and SP-D.4 Proteins of the surfactant mixture are reviewed by Johansson and associates.7
All of the components of surfactant are synthesized by the alveolar type II cell. The type I cell, which is the basic alveolar epithelial cell on 95% of the alveolar surface, has no known role in surfactant synthesis or metabolism. The type II cell also secretes other proteins, such as cytokines, growth factors, and antibacterial proteins, into the alveolar space. The role of the surfactant-associated proteins is well reviewed by Hawgood and Poulain8 and Possmayer.9
Surfactant protein A (SP-A).
SP-A is a high-molecular-weight, water-soluble glycoprotein. This protein is specific to surfactant; it has also been denoted as SP-35, apoprotein A, and SAP-35.4 SP-A seems to regulate secretion and exocytosis of surfactant from the type II cell and the reuptake of surfactant for recycling and reuse.
Surfactant protein D (SP-D).
SP-D is the fourth protein identified in natural endogenous surfactant. SP-D has similarities to SP-A as a large, water-soluble protein, although differences exist in their molecular configuration.8 There is no clear role for SP-D in surfactant function at this time, calling into question whether SP-D is correctly designated as a surfactant-associated protein.
Production and regulation of surfactant secretion
The surfactant lipids are synthesized in the alveolar type II cells and stored in vesicles termed lamellar bodies (see Figure 10-3). Surfactant in the lamellar bodies is secreted by exocytosis out of the type II cell and into the alveolus. The major stimulus for secretion of lamellar bodies into the alveolar space seems to be inflation of the lung, with a chemically coupled stretch response.10 SP-A and SP-B facilitate the formation of an intermediate lattice form of surfactant, termed tubular myelin, before it reaches the air-liquid interface. SP-C also helps to “break” the lipid layers of surfactant so that adsorption and spreading of the compound as a monolayer proceeds quickly through the air-liquid interface. Surfactant is converted to small vesicles, which can be taken back into the type II cell or taken in alveolar macrophages. The two major alveolar forms of surfactant are large surfactant aggregates (lamellar bodies and tubular myelin–like structures) and small vesicles or aggregates.11 The secretion of surfactant material from the type II cell is estimated as 10% of the intracellular pool every hour,12 with an alveolar half-life between 15 and 30 hours.5 The constant secretion of surfactant is balanced by two clearance mechanisms: endocytosis back into the type II cells and clearance via degradation by alveolar macrophages.8 In addition, clearance can occur by degradation within the alveoli and by mucociliary removal and transport.5
A key feature of surfactant production, which is the basis for the success of replacement therapy with exogenous compounds, is the recycling activity in surfactant production. Most surfactant (90% to 95%) is taken back into the alveolar type II cell, reprocessed, and secreted again. For this reason, exogenously administered surfactant is successful in replacing missing surfactant with one or two doses. The exogenous surfactant is taken into the type II cells and becomes the surfactant pool, through the reuptake and recycling mechanism. The reuptake is regulated, at least partly, by SP-A. Surfactant-specific proteins, or apoproteins, are critical for the surface-active functioning of surfactant and the metabolic regulation of the surfactant pool. This process is well described by Wright and Clements.6 The normal function of endogenous surfactant also depends on the structural organization of the compound. Smaller surfactant aggregates have less SP-A and are less surface active than larger aggregates.4
Pulmonary surfactant has also been found to contribute to host defense by increasing bacterial killing, modifying macrophage function, and downregulating the inflammatory response through decreased mediator release from inflammatory cells.13 Surfactant also enhances ciliary beat frequency and maintains patency of conducting airways.11
Types of exogenous surfactant preparations
Exogenous surfactant preparations can be placed into three categories. These categories and examples of each are listed in Table 10-2 and described in the following sections. A more complete technical description is given by Jobe and Ikegami.4
TABLE 10-2
Types of Surfactant Preparations and Examples
| CATEGORY | DESCRIPTION | EXAMPLES |
| Natural | Surfactant from natural sources (human or animal) with addition or removal of substances | Survanta (bovine) Curosurf (porcine) Infasurf (bovine) |
| Synthetic | Surfactant that is prepared by mixing in vitro–synthesized substances that may or may not be in natural surfactant | Lucinactant (experimental only at the time of this edition) |
| Synthetic natural | Surfactant prepared in vitro by genetic engineering | None at present |
Natural and modified natural surfactant
Natural surfactant is an apt descriptive term for the category of surfactants obtained from animals or humans by alveolar wash or from amniotic fluid. The large surface-active aggregates of natural surfactant are recovered from the fluid by centrifugation or simple filtration. Because these are natural surfactants, the ingredients necessary for effective function to regulate surface tension are present. Specifically, this includes the surface proteins needed for adsorption and spreading. Depending on the source, natural surfactants can be expensive and time-consuming to obtain and prepare. In addition, there is concern over contamination with viral infectious agents or immunologic stimulation and antibody production in response to foreign proteins. Natural surfactant preparations are usually modified by the addition or removal of certain components. Examples of natural surfactants are given in Table 10-2.
Another example of a modified natural surfactant is surfactant TA (Surfacten), prepared by Mitsubishi Pharma Corporation in Osaka, Japan, and used by Fujiwara and colleagues in their work.14 Surfactant TA is a reconstituted chloroform-methanol extract from minced cow lungs, with DPPC and other lipids added. Poractant alfa (Curosurf) is another modified natural surfactant, obtained as a pig lung extract. Calfactant (Infasurf) is a chloroform-methanol extract of fluid lavaged from calf lung; similar to Survanta, it contains only the surfactant proteins SP-B and SP-C but not SP-A.15 Bovactant (Alveofact) is an organic solvent extract of cow lung lavage containing 99% phospholipids and neutral lipids and 1% SP-B and SP-C.16
Synthetic surfactant
At present, no synthetic surfactant is available. Colfosceril palmitate (Exosurf), which was the only synthetic surfactant on the market, has been removed.1 A new synthetic surfactant, lucinactant (Surfaxin), is currently being considered by the FDA for the treatment of RDS. Moya and colleagues17 found that Surfaxin was better at reducing RDS and bronchopulmonary dysplasia than Exosurf but was not better than Survanta. Another study by Sinha and coworkers18 found no difference between Surfaxin and Curosurf. Surfaxin has been given orphan designation by the FDA for treatment of bronchopulmonary dysplasia. Additionally, lucinactant (Surfaxin) is being studied to replace surfactant in an aerosolized form.
Synthetic natural surfactant
An ideal solution to the problems of natural and artificial surfactants would be genetically engineered surfactant produced by recombinant DNA technology. In such a preparation, the phospholipid, lipid, and protein ingredients of the natural surfactant aggregate would be produced by characterizing, by in vitro cloning, the gene or genes responsible for human surfactant. No products are available for general use at this time, but work progresses on their development. The genes and amino acid sequence for each of the surfactant proteins have been characterized.19–21 A genetically engineered surfactant that closely resembles the structure and effect of natural human surfactant would be the ideal preparation.
Specific exogenous surfactant preparations
Beractant (survanta)
Beractant (Survanta) is considered a modified natural surfactant. It is a natural bovine lung extract mixed with colfosceril palmitate (DPPC), palmitic acid, and tripalmitin. These three ingredients are used to standardize the composition of the drug preparation and to reproduce the surface tension–lowering properties of natural surfactant. The ingredients are suspended in 0.9% saline. The composition of beractant, given in the product literature, is described in Table 10-1. The extract from minced bovine lung contains natural phospholipids; neutral lipids; fatty acids; and the low-molecular-weight, hydrophobic surfactant proteins SP-B and SP-C. The hydrophilic, high-molecular-weight protein SP-A is not contained in beractant. SP-A helps regulate surfactant reuptake and secretion by alveolar type II cells. However, the addition of SP-A to beractant by Yamada and colleagues22 did not improve the biophysical activity (spreading and absorption) or the physiologic activity (lung compliance change) of the mixture.
Indications for use
Specific guidelines for use of beractant are as follows:
• Prophylactic therapy of premature infants less than 1250 g birth weight or with evidence of surfactant deficiency and risk of RDS: The agent should be given within 15 minutes of birth or as soon as possible.
• Rescue treatment of infants with evidence of RDS: The agent should be given within 8 hours of age.
Calfactant (infasurf)
Calfactant (Infasurf) is another modified natural surfactant preparation from calf lung (bovine). It is an organic solvent extract of calf lung surfactant obtained by cell-free bronchoalveolar lavage. The extract contains phospholipids, neutral lipids, and hydrophobic proteins SP-B and SP-C. The preparation is a suspension, which does not require reconstitution. Its composition is given in Table 10-1. Each milliliter contains 35 mg of total phospholipids, including 26 mg of phosphatidylcholine, of which 16 mg is disaturated phosphatidylcholine, and 0.65 mg of proteins, which includes 0.26 mg of SP-B. The protein SP-A is not contained in the preparation. The formulation is heat sterilized and contains no preservatives.
Clinical outcome
There can be a dramatic improvement in oxygenation after surfactant administration, but Davis and associates23 did not report a corresponding increase in compliance. Although exogenous surfactant should reduce surface tension and increase lung compliance, there is disagreement in the literature over whether the primary clinical effect of exogenous surfactant is one of increasing lung compliance.24 Fujiwara and colleagues14 noted that there was clearing of the chest radiograph associated with a good clinical response to surfactant, suggesting that surfactant treatment increased the functional residual capacity (FRC). A study by Goldsmith and associates25 measured an increase in the FRC within 15 minutes of treatment with natural surfactant, correlating with the time of blood gas improvements. The surfactant stabilizes alveoli on expiration and prevents collapse, increasing residual volume and FRC. An increase in oxygenation would be seen with this effect.24 An increase in lung volume resulting from improved residual volume and FRC shifts the tidal ventilation to a new pressure-volume curve. At higher lung volumes, chest wall elastic recoil is higher. A shift to the flatter part of the pressure-volume curve could give the same tidal volume for a given pressure change, masking the actual increase in static compliance.21
Hazards and complications of surfactant therapy
Airway occlusion, desaturation, and bradycardia
Because the current method of administration is by direct tracheal instillation, a large volume of surfactant suspension may cause an acute obstruction of infant airways, with subsequent hypoxemia and bradycardia.26 Repetitive small additions of the dose and a transient increase in ventilating pressure may help distribute the surfactant to the periphery.
Future directions in surfactant therapy
In addition to RDS of the newborn, surfactant replacement therapy has been considered in MAS. In a meta-analysis, Soll and Dargaville27 reported that surfactant therapy may be beneficial in reducing respiratory illness severity and reducing an infant’s need for extracorporeal membrane oxygenation (ECMO).
Surfactant therapy had been studied for various adult respiratory disorders. This topic is comprehensively reviewed by Hamm and colleagues.5 ARDS in adults is a potential target for surfactant therapy. In RDS of the newborn, the primary abnormality of lung function is related to surfactant deficiency. In ARDS in adults, the surfactant deficiency is secondary to lung injury with complex inflammatory responses.5 This finding would suggest a difference in clinical response to surfactant therapy between premature infants and adults with ARDS. In addition, dose amount and administration techniques may need to be modified for therapy in adults, who have large lung volumes compared with infants. If lung injury in ARDS is nonuniform in distribution, exogenous surfactant, especially if aerosolized, may distribute unevenly to more compliant lung areas instead of to areas needing surfactant the most.11
The availability of newer surfactant preparations, specifically human recombinant surfactants, may offer better results to improve outcomes in adults with ARDS.21 Finer and colleagues28 found that lucinactant (Aerosurf) has been successful as an aerosol. The agent has been nebulized using a vibrating mesh nebulizer (see Chapter 2 for more information) for the possible treatment of ARDS in adults and neonates. Other adult respiratory disorders in which either surfactant replacement therapy or abnormal surfactant regulation may occur include pneumonia, lung transplants, sarcoidosis, hypersensitivity pneumonitis, idiopathic pulmonary fibrosis, alveolar proteinosis, obstructive lung disease including asthma, radiation pneumonitis, and drug-induced pulmonary disease.29