Adrenergic (sympathomimetic) bronchodilators
After reading this chapter, the reader will be able to:
3. List all currently available β-adrenergic agents used in respiratory therapy
4. Differentiate between the specific adrenergic agents and formulations
5. Describe the mode of action for each specific adrenergic agent and formulation
6. Describe the route of administration available for β agonists
Agent that stimulates sympathetic nervous fibers, which allow relaxation of smooth muscle in the airway. Also known as sympathomimetic bronchodilator or β2 agonist.
Causes vasoconstriction and vasopressor effect; in the upper airway (nasal passages), this can provide decongestion.
Refers to the increasing incidence of asthma morbidity, and especially asthma mortality, despite advances in the understanding of asthma and availability of improved drugs to treat asthma.
Causes increased myocardial conductivity and increased heart rate as well as increased contractile force.
Causes relaxation of bronchial smooth muscle, with some inhibition of inflammatory mediator release and stimulation of mucociliary clearance.
Narrowing of the bronchial airways, caused by contraction of smooth muscle.
Group of similar compounds having sympathomimetic action; they mimic the actions of epinephrine.
Cyclic adenosine 3’, 5’-monophosphate (cAMP)
Nucleotide produced by β2-receptor stimulation; it affects many cells, but causes relaxation of bronchial smooth muscle.
Cyclic guanosine monophosphate (cGMP)
Nucleotide producing the opposite effect of cAMP; that is, it causes bronchoconstriction.
Long-term desensitization of β receptors to β2 agonists, caused by a reduction in the number of β receptors.
Drug that exhibits its pharmacologic activity when it is converted, inside the body, to its active form.
Producing effects similar to those of the sympathetic nervous system.
Clinical indications for adrenergic bronchodilators
Indication for short-acting agents
Short-acting β2 agonists such as albuterol, levalbuterol, or pirbuterol are indicated for relief of acute reversible airflow obstruction in asthma or other obstructive airway diseases. Short-acting agents are termed “rescue” agents in the 2007 National Asthma Education and Prevention Program Expert Panel Report 3 (NAEPP EPR 3) guidelines.1 Short-term acting agents may also be termed “relievers” as discussed in the Global Initiative for Asthma (GINA) guidelines.2
Specific adrenergic agents and formulations
Table 6-1 lists adrenergic bronchodilators currently approved for general clinical use in the United States as of the writing of this edition. Practitioners are urged to read package inserts on a drug before administration. These inserts give details of dosage strengths and frequencies, adverse effects, shelf life, and storage requirements, all of which are needed for safe application. Table 6-1 is not intended to replace more detailed information supplied by the manufacturer on each of the bronchodilator agents. There are three subgroups of adrenergic bronchodilators based on distinct differences in duration of action:
TABLE 6-1
Inhaled Adrenergic Bronchodilator Agents Currently Available in the United States
| DRUG | BRAND NAME | RECEPTOR PREFERENCE | ADULT DOSAGE | TIME COURSE (ONSET, PEAK, DURATION) |
| Ultra-Short-Acting Adrenergic Bronchodilator Agents | ||||
| Epinephrine | Adrenalin Chloride, Primatene Mist | α, β |
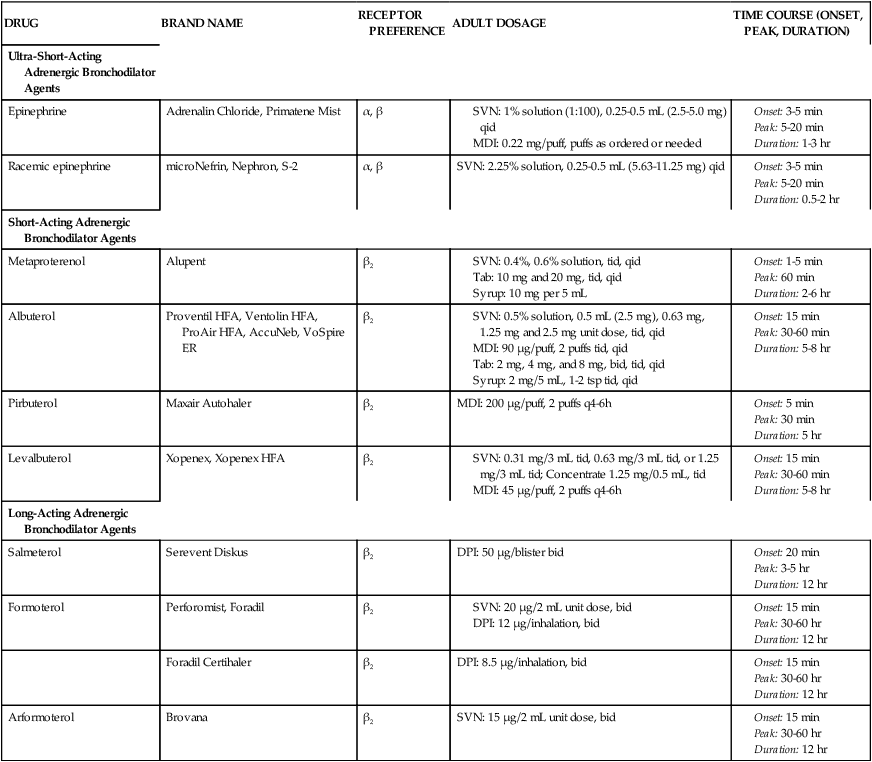
DPI, Dry powder inhaler; MDI, metered dose inhaler; SVN, small volume nebulizer.
• Ultra-short-acting (duration less than 3 hours): Epinephrine and racemic epinephrine
• Short-acting (duration 4 to 6 hours): Albuterol, levalbuterol, metaproterenol, and pirbuterol
• Long-acting (duration 12 hours): Salmeterol, formoterol, and arformeterol
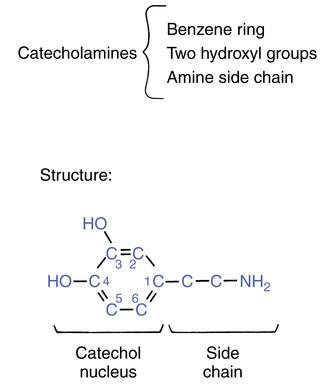
Catecholamines
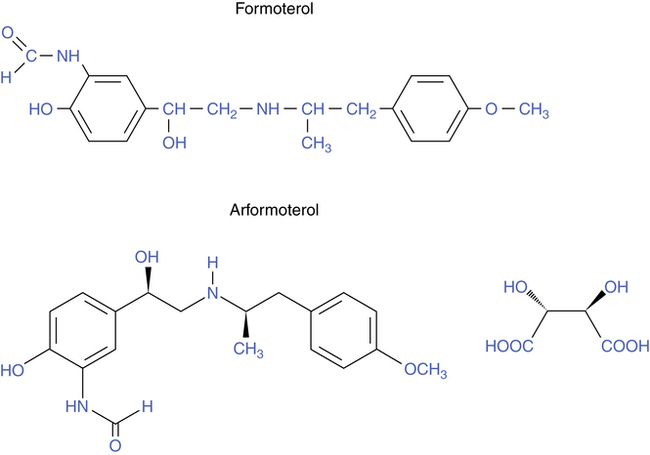
The sympathomimetic bronchodilators are all either catecholamines or derivatives of catecholamines. A catecholamine is a chemical structure consisting of an aromatic catechol nucleus and a dialiphatic amine side chain. In Figure 6-1, the basic catecholamine structure is seen to be composed of a benzene ring with hydroxyl groups at the third and fourth carbon sites and an amine side chain attached at the first carbon position.
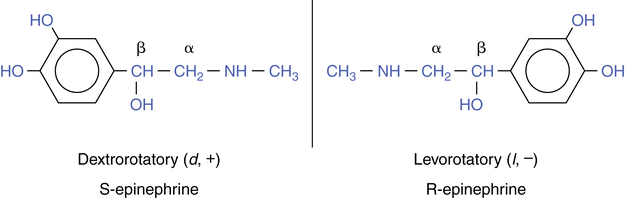
Adrenergic bronchodilators as stereoisomers
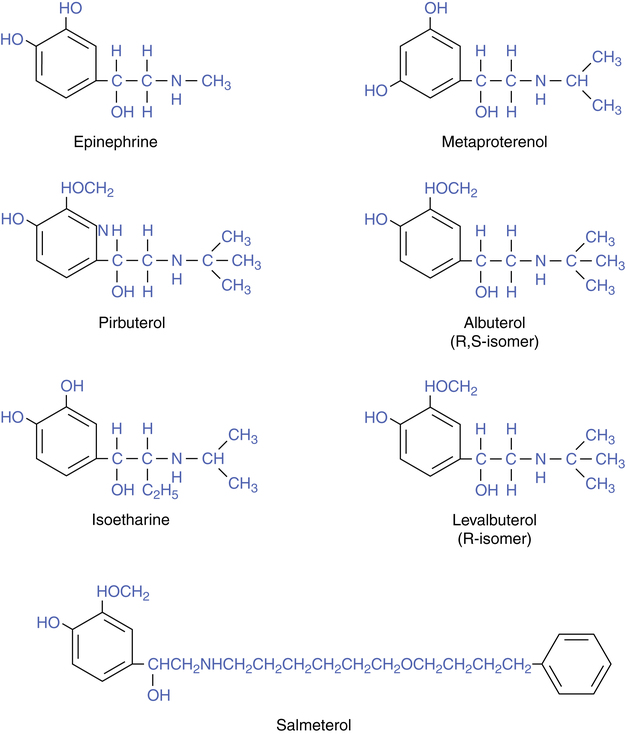
Adrenergic bronchodilators can exist in two different spatial arrangements, producing isomers. Rotation around the β carbon on the ethylamine side chain of the basic molecular structure seen in Figure 6-1 produces two nonsuperimposable mirror images, termed enantiomers or simply isomers. Figure 6-2 illustrates epinephrine as a stereoisomer, showing the (R)-isomers and (S)-isomers as the mirror image of each other. Enantiomers have similar physical and chemical properties but different physiologic effects. The (R)-isomer, or levo isomer, is active on airway β receptors, producing bronchodilation, and on extrapulmonary adrenergic receptors. The (S)-isomer, or dextro isomer, is not active on adrenergic receptors such as β receptors, and until more recently the (S)-isomer was considered physiologically inert. The two mirror images of the isomers rotate light in opposite directions, and this is the basis for designating them as dextrorotatory (d, +) or levorotatory (l, −). Using their actual spatial configuration, the levo isomer and dextro isomer are referred to as the (R)-isomer (for rectus, right) and (S)-isomer (for sinister, left), respectively. Adrenergic bronchodilators such as epinephrine, albuterol, and salmeterol have been produced synthetically as racemic mixtures, or 50:50 equimolar mixes of the (R)-isomers and (S)-isomers. Natural epinephrine found in the adrenal gland occurs as the (R)-isomer, or levo isomer, only. Levalbuterol, released in 1999, represents the first synthetic inhaled solution available as the single (R)-isomer of racemic albuterol. Structures of the currently available inhaled β agonists to be discussed are shown in Figure 6-3. Only a single isomer form is shown, for simplification and clarity.
Keyhole theory of β2 specificity
The theory that explains the shift from α activity to β2 specificity has been termed the keyhole theory of β sympathomimetic receptors: The larger the side chain attachment to a catechol base, the greater the β2 specificity. If the catecholamine structural pattern is seen as a keylike shape, then the larger the “key” (side chain), the more β2 specific the drug. The increase in side chain substitutions can be seen in the drug structures presented in Figure 6-3, for the three catecholamines described and subsequent β2-selective agents to be discussed.
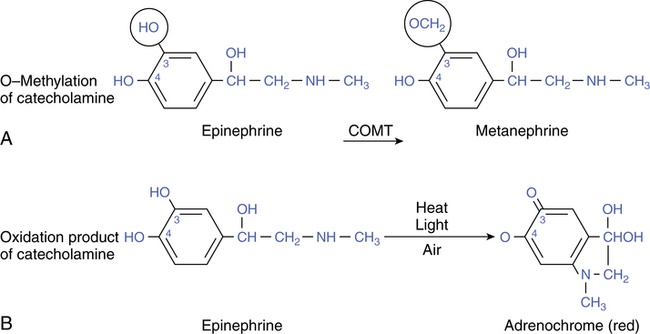
Metabolism of catecholamines
Despite the increase in β2 specificity with increased side chain bulk, all of the previously mentioned catecholamines are rapidly inactivated by the cytoplasmic enzyme COMT. This enzyme is found in the liver and kidneys and throughout the rest of the body. Figure 6-4, A, illustrates the action of COMT as it transfers a methyl group to the carbon-3 position on the catechol nucleus. The resulting compound, metanephrine, is inactive on adrenergic receptors. Because the action of COMT on circulating catecholamines is very efficient, the duration of action of these drugs is severely limited, with a range of 1.5 hours to at most 3 hours.
Catecholamines are also unsuitable for oral administration because they are inactivated in the gut and liver by conjugation with sulfate or glucuronide at the carbon-4 site. Because of this action, they have no effect when taken by mouth, limiting their route of administration to inhalation or injection. Catecholamines are also readily inactivated to inert adrenochromes by heat, light, or air (Figure 6-4, B). For this reason, racemic epinephrine is stored in an amber-colored bottle or a foil-protected wrapper. Nebulizer rainout (i.e., nebulized particles that condense and fall, under the influence of gravity) in the tubing may appear pinkish after treatment, and a patient’s sputum may even appear pink-tinged after using aerosols of catecholamines.
Resorcinol agents
Because the limited duration of action with catecholamines is unsuitable for maintenance therapy of bronchospastic airways, drug researchers sought to modify the catechol nucleus, which is so vulnerable to inactivation by COMT. As a result, the hydroxyl attachment at the carbon-4 site was shifted to the carbon-5 position, producing a resorcinol nucleus (see Figure 6-3). This change resulted in metaproterenol (named for the 3,5-attachments in the meta position). Because metaproterenol is not inactivated by COMT, it has a significantly longer duration of action of 4 to 6 hours compared with the short-acting catecholamine bronchodilators. Metaproterenol can be taken orally because it resists inactivation by sulfatase enzymes in the gastrointestinal tract and liver. For these reasons, the newer generation of resorcinols and other catecholamine derivatives is much better suited for maintenance therapy than the older catecholamine agents. Metaproterenol is slower to reach a peak effect (30 to 60 minutes) than epinephrine. The chlorofluorocarbon (CFC) version of metaproterenol was removed from the market on June 14, 2010.
Saligenin agents
A different modification of the catechol nucleus at the carbon-3 site resulted in the saligenin albuterol, referred to as salbutamol in Europe (see Figure 6-3). Albuterol is available in various pharmaceutical vehicles in the United States, including oral extended-release tablets, syrup, nebulizer solution, and MDI. As with the resorcinol bronchodilators, this drug has a β2-preferential effect; is effective via oral administration; and has a duration of up to 6 hours, with a peak effect in 30 to 60 minutes.
Pirbuterol
Pirbuterol is another noncatecholamine adrenergic agent currently available as pirbuterol acetate (Maxair) in an MDI formulation with a breath-actuated inhaler delivery device. The strength is 0.2 mg per puff, and the usual dose is 2 puffs. Pirbuterol is structurally similar to albuterol except for a pyridine ring in place of the benzene ring (see Figure 6-3). The onset of activity by aerosol is 5 to 8 minutes, with a peak effect at 30 minutes and a duration of action of approximately 5 hours. Pirbuterol is said to be less potent on a weight basis than albuterol and similar in efficacy and toxicity to metaproterenol.4 The side-effect profile is the same as with other β2 agonists. The CFC version of pirbuterol will not be able to be manufactured or sold after December 31, 2013.
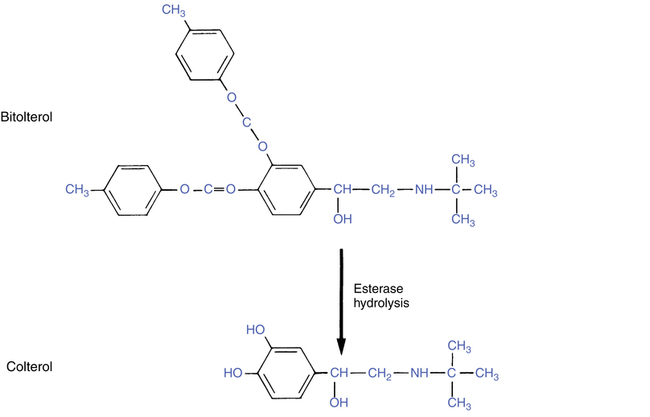
Prodrug: bitolterol
Bitolterol (Tornalate) differs from the previous agents discussed in that the administered form must be converted in the body to the active drug. Because of this, bitolterol is referred to as a prodrug. The sequence of activation is shown in Figure 6-5.
The bitolterol molecule consists of two toluate ester groups on the aromatic ring at the carbon-3 and carbon-4 positions. These attachments protect the molecule from degradation by COMT. The large N-tertiary butyl substituent on the amine side chain prevents oxidation by monoamine oxidase (MAO). Bitolterol is administered as an inhalation solution, and once in the body the bitolterol molecule is hydrolyzed by esterase enzymes in the tissue and blood to the active bronchodilator colterol. The process of activation begins when the drug is administered and gradually continues over time, resulting in a prolonged duration or sustained-release effect of up to 8 hours. Onset and peak effect are similar to those of the noncatecholamine agent metaproterenol, administered by inhalation. The active form, colterol, is a catecholamine and is inactivated by COMT similar to any other catecholamine. The speed of this inactivation is offset by the gradual hydrolysis of bitolterol, to provide a prolonged duration of activity. The bulky side chain gives a preferential β2 effect to the active form, colterol.5
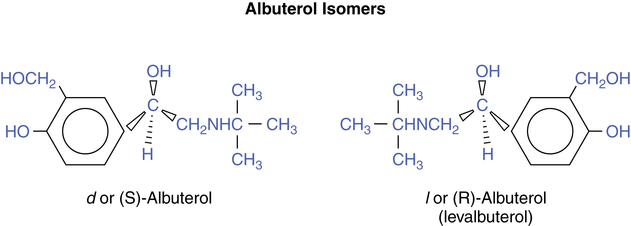
Levalbuterol: (R)-isomer of albuterol
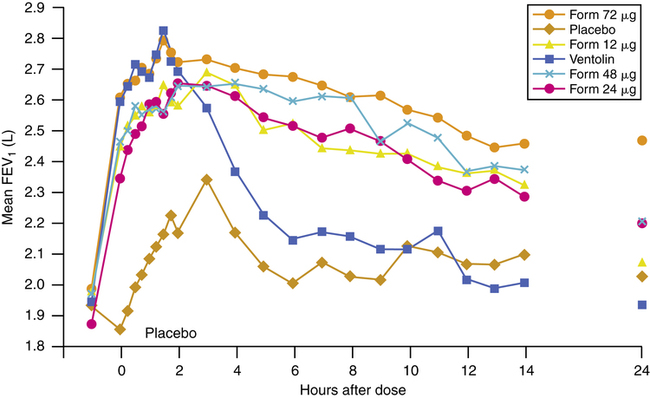
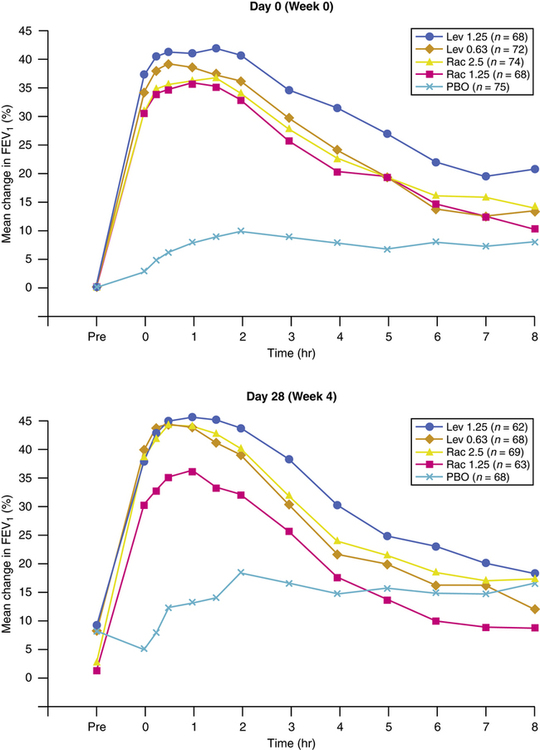
Previous inhaled formulations of adrenergic bronchodilators were all synthetic racemic mixtures, containing the (R)-isomer and the (S)-isomer in equal amounts. Levalbuterol is the pure (R)-isomer of racemic albuterol. Both stereoisomers of albuterol are shown in Figure 6-6. Although the (S)-isomer is physiologically inactive on adrenergic receptors, there is accumulating evidence that the (S)-isomer is not completely inactive. Barnes6 suggested, however, there is no difference between single isomer levalbuterol and racemic albuterol. Box 6-1 lists some of the physiologic effects of (S)-isomer of albuterol noted in the literature.7–13 The effects noted would antagonize the bronchodilating effects of the (R)-isomer of an adrenergic drug and promote bronchoconstriction. In addition, the (S)-isomer is more slowly metabolized than the (R)-isomer. Levalbuterol is the single (R)-isomer form of racemic albuterol and is available in an HFA-propelled MDI, with nebulization solution in three strengths: 0.31-mg, 0.63-mg, and 1.25-mg unit doses. Levalbuterol is also available as a concentrate, 1.25 mg in 0.5 mL. In a study by Nelson and associates,14 the 0.63-mg dose was found to be comparable to the 2.5-mg racemic albuterol dose in onset and duration (Figure 6-7).
Long-acting β-adrenergic agents
Salmeterol
Salmeterol, a β2-selective receptor agonist, is available in a dry powder formulation in the Diskus inhaler. Salmeterol xinafoate is a racemic mixture of two enantiomers, with the (R)-isomer containing the predominant β2 activity.15
Bronchodilator effect.
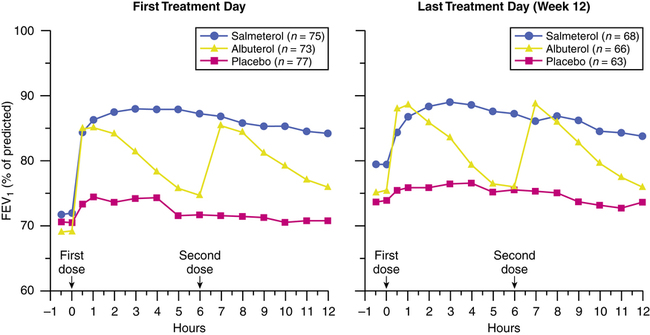
Salmeterol represents a new generation of long-acting β2-specific bronchodilating agents, whose bronchodilation profile differs from the agents previously discussed. The median time to reach a 15% increase in FEV1 above baseline (considered the onset of bronchodilation) in asthmatic subjects is longer with salmeterol than albuterol; it has been reported as between 14 and 22 minutes16,17 and generally is longer than 10 minutes.18 The slower onset of action with salmeterol is significant for its clinical application (discussed subsequently). The time to peak bronchodilating effect is generally 3 to 5 hours, and its duration of action in maintaining an FEV1 15% above pretreatment baseline is 12 hours or longer. At each point (onset, peak effect, and duration), salmeterol exhibits slower, longer times for effect compared with shorter acting bronchodilators such as albuterol.
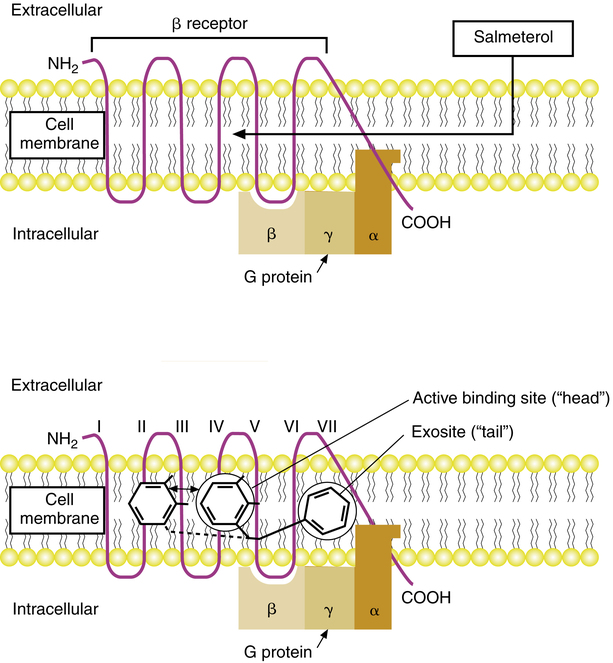
With inhaled salmeterol xinafoate, an initial peak plasma concentration of 1 to 2 μg/L is seen 5 minutes after inhalation, with a second peak of 0.07 to 0.2 μg/L at 45 minutes; the second peak is probably due to absorption of swallowed dose. The drug is metabolized by hydroxylation, with elimination primarily in the feces.19 The increased duration of action of salmeterol is due to its increased lipophilicity, conferred by the long side chain. The “tail” of the molecule anchors at an exosite in the cell membrane, allowing continual activation of the β receptor. The mode of action is discussed more fully subsequently.
Formoterol
The chemical structure of formoterol is shown in Figure 6-8. As with salmeterol, the extensive side chain, or “tail,” makes formoterol more lipophilic than the shorter acting bronchodilators and is the basis for its longer duration of effect. The increased lipophilicity of salmeterol and formoterol allows the drugs to remain in the lipid cell membrane. Even if a tissue preparation containing the drugs is perfused or washed, the drug activity persists. Salmeterol is more lipophilic than formoterol, and this along with its anchoring capability may explain why salmeterol is less prone to being “washed away” than formoterol.18
Bronchodilator effect.
Similar to salmeterol, formoterol has a prolonged duration of bronchodilating effect of up to 12 hours. In contrast to salmeterol, the onset of action for formoterol is significantly faster. The time from inhalation to significant bronchodilation is similar to that of albuterol. It has been reported that 1 minute after inhalation of formoterol there is a significant increase in specific airway conductance (SGAW).20 The onset of bronchodilation is generally considered to be 2 to 3 minutes with formoterol compared with 10 minutes or longer with salmeterol. Figure 6-9 shows the dose-proportional response to inhaled (R,R)-formoterol, the single isomer isolated from the racemic mixture of (R,R)-formoterol and (S,S)-formoterol, compared with inhaled racemic albuterol.21 In a study by van Noord and colleagues22 comparing racemic formoterol, 24 μg; salmeterol, 50 μg; and albuterol, 200 μg, the increase in SGAW (airway conductance) after 1 minute was 44%, less than 16%, and 44%. The time to maximal increase in airway conductance was 2 hours, 2 to 4 hours, and 30 minutes for the three drugs. The maximal increase was 135%, 111%, and 100%.20,22
The efficacy of formoterol in relaxing airway smooth muscle (its maximal effect) is greater than that of albuterol, which is greater than that of salmeterol. The lower intrinsic efficacy of salmeterol would make it a better agent than formoterol for patients with cardiovascular disease.18
Antiinflammatory effects
Both the short-acting and the long-acting β agonists show antiinflammatory effects in vitro. Salmeterol, formoterol, and arformoterol inhibit human mast cell activation and degranulation in vitro, prevent an increase in vascular permeability with inflammatory mediators, and generally diminish the attraction and accumulation of airway inflammatory cells.18 Despite these in vitro antiinflammatory effects, salmeterol and formoterol have not been shown to inhibit accumulation of inflammatory cells in the airway or the increase in inflammatory markers in vivo. Neither drug is considered to have a sufficient effect on airway inflammation in patients with asthma to replace antiinflammatory drugs such as corticosteroids.
Clinical use
Long-acting β agonists are indicated for maintenance therapy of asthma that is not controlled by regular low-dose inhaled corticosteroids and for COPD needing daily inhaled bronchodilator therapy for reversible airway obstruction. National guidelines recommend the introduction of a long-acting β agonist in step 3 care of asthma (asthma not controlled by lower doses of antiinflammatory medications)1 and use as deemed necessary for COPD.23 Use of long-acting β agonists may prevent the need to increase the inhaled dose of corticosteroid. Several points should be noted in the clinical use of long-acting agents because of their differences from shorter acting β agonists.
• Long-acting β2 agonists are not recommended for rescue bronchodilation because repeated administration with their longer duration and increased lipophilic property risk accumulation and toxicity.18
• A shorter acting β2 agonist, such as albuterol or other agents previously discussed, should be prescribed and available for asthmatics for treatment of breakthrough symptoms if additional bronchodilator therapy is needed between scheduled doses of a long-acting β2 agonist; asthmatics must be well educated in the appropriate use of the two types of β agonists (shorter acting versus long-acting).
• Although they have antiinflammatory effects, short-acting or long-acting β agonists are not a substitute for inhaled corticosteroids in asthma maintenance or for other antiinflammatory medications if such are required.
• The difference in rate of onset between salmeterol and formoterol may require classifying β2 agonists as “fast” and “slow” in addition to “short” and “long” acting, with salmeterol classified as a slow and long-acting bronchodilator versus formoterol as a fast and long-acting bronchodilator.24
The addition of a long-acting β2 agonist to inhaled corticosteroids can lead to improved lung function and a decrease in symptoms.25 A combination product of salmeterol and fluticasone in a Diskus inhaler (Advair Diskus) showed superior asthma control and better lung function than either drug taken alone.26–29 Because of their prolonged bronchodilation, long-acting β2 agonists taken twice daily have a greater area under the FEV1 curve compared with short-acting agents taken four times daily. Figure 6-10 illustrates dose-response curves for albuterol and salmeterol. In contrast to albuterol, which tends to return to baseline in 4 to 6 hours, salmeterol provides a more sustained level of bronchodilation, giving a higher baseline of lung function.30 The same effect has been found in comparing twice-daily salmeterol with four-times-daily inhaled ipratropium bromide, a shorter acting anticholinergic bronchodilator that is discussed in Chapter 7.31
Salpeter and associates,32 in a meta-analysis, reported that long-acting β2 agonists increased the risk of asthma hospitalizations and deaths compared with placebo. Nelson and colleagues33 also described an increase in death rate among patients using salmeterol; their findings reported the highest death rate among African Americans. These studies did not take into account the severity of asthma or whether the participants used other medications. Asthma in some participants may have been worse than in others. Concerning cotreatments, the studies could not account for other medications participants may have been taking or with what regularity they were taking them. The latter could have serious consequences if, for example, a participant stopped using inhaled corticosteroids that were prescribed to treat asthma. Any of these variables—asthma severity, presence of cotreatments, and patient adherence—could affect interpretation of data. Nevertheless, the labeling of these agents has been changed to warn that death can occur.
• Long-acting β2 agonists are not to be used without a controller medication (i.e., corticosteroid).
• Long-acting β2 agonists should not be used by patients who are controlled on low-dose or medium-dose inhaled corticosteroids.
• Long-acting β2 agonists should be used only if patients are not controlled with agents such as inhaled corticosteroids.
• Long-acting β2 agonists should be for short-term use only. Once asthma is controlled, the long-acting β2 agonist should be discontinued.
• Children should use a long-acting β2 agonist only in conjunction with a corticosteroid. The use of a combination product is needed to increase adherence.
Mode of action
• a-receptor stimulation: Causes vasoconstriction (i.e., a vasopressor effect); in the upper airway (nasal passages), this can provide decongestion
• b1-receptor stimulation: Causes increased myocardial conductivity and increased heart rate as well as increased contractile force
• b2-receptor stimulation: Causes relaxation of bronchial smooth muscle, with some inhibition of inflammatory mediator release and stimulation of mucociliary clearance
Both α and β receptors are examples of G protein–linked receptors. Table 6-2 lists each of the adrenergic receptor types, along with its particular type of G protein, effector system, and second messenger and an example of cell response in the lungs. As described in Chapter 2, the G protein is a heterotrimer whose α subunit differentiates the type of G protein. The G protein couples the adrenergic receptor to the effector enzyme, which initiates the cell response by means of a particular intracellular second messenger. The mode of action with β-receptor, α2-receptor, and α1-receptor stimulation is described for each.
TABLE 6-2
| RECEPTOR | G PROTEIN | EFFECTOR | SECOND MESSENGER | RESPONSE |
| α1 | Gq | Phospholipase C (PLC) | Inositol triphosphate (IP3), diacylglycerol (DAG) | Vasoconstriction |
| α2 | Gi | Adenylyl cyclase (inhibits) | cAMP (inhibits) | Inhibition of neurotransmitter release |
| β (β1, β2, β3) | Gs | Adenylyl cyclase (stimulates) | cAMP (increases) | Smooth muscle relaxation |

β-receptor and α2-receptor activation
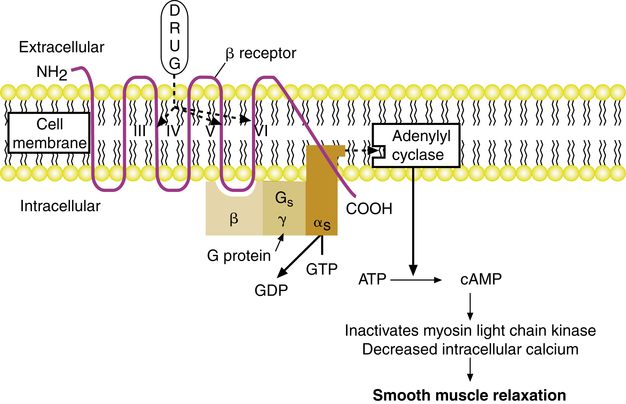
The mode of action of β agonists and the β receptors has been well characterized, although the activity of α receptors is not as well understood. The mode of action for relaxation of airway smooth muscle when a β2 receptor is stimulated is illustrated in Figure 6-11. Adrenergic agonists such as albuterol or epinephrine attach to β receptors, which are polypeptide chains that traverse the cell membrane seven times and have an extracellular NH2 terminus and an intracellular carboxy (COOH) terminus. This attachment causes activation of the stimulatory G protein, designated Gs. The actual binding site of a β agonist is within the cell membrane, inside the “barrel” or circle formed by the transmembrane loops of the receptor chain. The β agonist forms bonds with elements of the third, fifth, and sixth transmembrane loops. When stimulated by a β agonist, the receptor undergoes a conformational change, which reduces the affinity of the α subunit of the G protein for guanosine diphosphate (GDP). The GDP is replaced by GTP, and the α subunit dissociates from the receptor and the β-γ portion of the G protein to link with the effector system. The effector system for the β receptor is adenylyl cyclase, a membrane-bound enzyme. Activation of adenylyl cyclase by the α subunit of the Gs protein causes increased synthesis of the second messenger, cyclic adenosine 3’,5’-monophosphate (cAMP). cAMP may cause smooth muscle relaxation by increasing the inactivation of myosin light chain kinase, an enzyme that initiates myosin-actin interaction and subsequent smooth muscle contraction. An increase in cAMP also leads to a decrease in intracellular calcium.
A similar sequence of events is responsible for the action of α2-receptor stimulation, which can inhibit further neurotransmitter release from the presynaptic neuron when stimulated by norepinephrine in a feedback, autoregulatory fashion (see Chapter 5). However, stimulation of α2 receptors (not shown in Figure 6-11) results in activation of an inhibitory G protein, designated Gi, whose α subunit serves to inhibit the enzyme adenylyl cyclase, lowering the rate of synthesis for intracellular cAMP.
α1-receptor activation
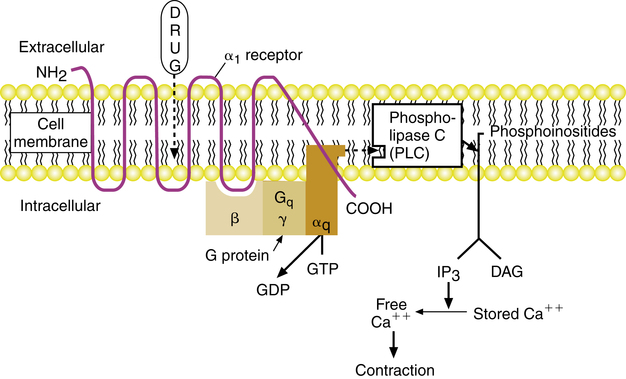
Stimulation of an α1 receptor by an agonist such as phenylephrine or epinephrine (which has affinity for both α and β receptors) results in vasoconstriction of peripheral blood vessels, including vessels in the airway. The mode of action for this effect as mediated by the G protein–linked α1 receptor is illustrated in Figure 6-12. Stimulation of the α1 receptor causes a conformational change in the receptor, which activates the G protein designated Gq. With activation, GDP dissociates from the G protein; GTP binds to the α subunit of the G protein; and the α subunit dissociates from the β-γ dimer, to activate the effector phospholipase C (PLC). Activation of the effector, PLC, leads to the conversion of membrane phosphoinositides into inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG). IP3 stimulates release of intracellular stores of calcium into the cytoplasm of the cell, and DAG activates protein kinase C. Contraction of vascular smooth muscle results.
Salmeterol, formoterol, and arformoterol: mechanism of action
The structure of salmeterol is shown in Figure 6-3, along with that of other β agonists for comparison. The drug is a modification of the saligenin albuterol, with a long nonpolar (i.e., lipophilic) N-substituted side chain. Salmeterol consists of a polar, or hydrophilic, phenyl ethanolamine “head,” with a large lipophilic “tail” or side chain. As a result of this structure, salmeterol is lipophilic, in contrast to most β agonists, which are hydrophilic and approach the β receptor directly from the aqueous extracellular space. In contrast, salmeterol, as a lipophilic molecule, diffuses into the cell membrane phospholipid bilayer and approaches the β receptor laterally, as shown in Figure 6-13. The lipophilic nonpolar side chain binds to an area of the β receptor referred to as the exosite, a hydrophobic region. With the side chain (“tail”) anchored in the exosite, the active saligenin “head” binds to and activates the β receptor at the same location as albuterol.
The binding properties of salmeterol, formoterol, and arformoterol differ from those of albuterol and other β agonists. Because the side chain of salmeterol is anchored at the exosite, the active “head” portion continually attaches to and detaches from the receptor site. This activity provides ongoing stimulation of the β receptor and is the basis for the persistent duration of action of salmeterol. This model of activity is supported by studies of the effect of β antagonists on the β-agonist action of salmeterol and molecular binding studies. If albuterol is attached to the β receptor, the smooth muscle relaxation can be fully reversed by a β-blocking agent such as propranolol or sotalol, indicating a competitive blockade. When the β-blocking agent is removed, there is no further relaxation of smooth muscle. The albuterol has been displaced, and the action of the drug is terminated. If salmeterol stimulates a receptor, a β antagonist such as propranolol would also reverse the effect of relaxation. However, when the propranolol is removed from the tissue, the relaxant effect of salmeterol is reestablished. This indicates that the salmeterol remains anchored in the receptor and is available to stimulate the β receptor continually after the blocking agent is removed.15
The prolonged activity of formoterol is also thought to be due to its lipophilicity, although formoterol is less lipophilic than salmeterol. Formoterol and arformoterol, which is moderately lipophilic, enter the bilipid cell membrane, where they are retained, with the lipid layer acting as a depot and giving a long-acting effect. At the same time, formoterol and arformoterol can approach the β receptor from the aqueous phase, giving it a rapid onset of action.20
Routes of administration
Inhalation route
• Smaller doses are needed compared with doses for oral use.
• Side effects such as tremor and tachycardia are reduced.
• Drug is delivered directly to the target organ (i.e., lung).
The use of aerosol delivery during an acute attack of airway obstruction has been questioned. However, several studies have failed to show substantial differences between inhaled and parenteral β-adrenergic agents in acute severe asthma.34,35 There is no reason to avoid these bronchodilators as inhaled aerosols during acute episodes.36 The inhalation route targets the lung directly. Combining oral delivery with additional inhalation has been shown to produce good additive effects with albuterol.37
Continuous nebulization
Administration of inhaled adrenergic agents by continuous nebulization has been used to manage severe asthma, in an effort to avoid respiratory failure, intubation, and mechanical ventilation. The Guidelines for the Diagnosis and Management of Asthma released by NAEPP EPR 3 recommend 2.5 to 5 mg of albuterol by nebulizer every 20 minutes for three doses and 10 to 15 mg/hr by continuous nebulization.1 Because a nebulizer treatment takes approximately 10 minutes, giving three treatments every 20 minutes requires repeated therapist attendance. Continuous administration by nebulizer may simplify such frequent treatments. The use of continuous nebulization of β-agonist bronchodilators was reviewed by Fink and Dhand,38 who presented a summary of studies, including dosages used. With continuous nebulization, there are no general standards for doses other than the recommendation from NAEPP EPR 3; in the studies cited by Fink and Dhand,38 dosages vary from 2.5 to 15 mg/hr and include schedules based on milligrams per kilogram per hour.
The impact and optimal use of continuous nebulization versus intermittent nebulization are unclear. In the five randomized controlled trials cited by Fink and Dhand,38 there was similar improvement between continuous versus intermittent nebulization. One study by Lin and associates39 showed faster improvement using continuous nebulization in patients with FEV1 less than 50% of predicted. A study by Shrestha and colleagues40 compared a high dose (7.5 mg) and low dose (2.5 mg) of albuterol with both continuous and intermittent nebulization. FEV1 improved more with continuous than intermittent nebulization, and the low dose of 2.5 mg was as effective as the higher dose of 7.5 mg with continuous administration. These and other results suggest that there is a benefit to continuous nebulization in severe airflow obstruction, but a dose less than 10 to 15 mg/hr may be effective, with less toxicity. Less clinician time is required for the administration of continuous nebulization. Fink and Dhand38 suggested that for emergency department patients with severe airway obstruction, who do not respond sufficiently after 1 hour of intermittent nebulization of β agonists, continuous nebulization offers a practical approach to optimal dosing in a cost-effective manner.
Toxicity and monitoring.
Continuous nebulization of β2 agonists is not standard therapy, and patients receiving this treatment have serious airflow obstruction. Potential complications include cardiac arrhythmias, hypokalemia, and hyperglycemia. Unifocal premature ventricular contractions were reported in one patient by Portnoy and associates.42 Significant tremor may also occur. Subsensitivity to continuous therapy was not observed by Portnoy and associates. Close monitoring of patients receiving continuous β agonists is necessary and includes observation and cardiac and electrolyte monitoring. Selective β2 agonists, such as albuterol, should be used to reduce side effects.
Oral route
The oral route has the advantages of ease, simplicity, short time required for administration, and exact reproducibility and control of dosage. However, In terms of clinical effects, this is not the preferred route. The time course of oral β agonists differs from that of inhaled β agonists. The onset of action begins in about 1.5 hours, with a peak effect reached after 1 to 2 hours and a duration of action between 3 and 6 hours.43 Larger doses are required than with inhalation, and the frequency and degree of unwanted side effects increase substantially. Catecholamines are ineffective by mouth, as previously discussed. Noncatecholamine bronchodilators in the adrenergic group seem to lose their β2 specificity with oral use, possibly because of the reduction of the side chain bulk in a first pass through the liver.44 Patient compliance on a three- or four-times-daily schedule may be better than with a nebulizer. If this is the case with an individual patient and the side effects are tolerable, oral use may be indicated for bronchodilator therapy. The introduction of an oral tablet of albuterol with extended action properties (Repetab, Volmax) offers the possibility of protection from bronchoconstriction for longer than 8 hours. However, inhaled salmeterol and formoterol now offer a 12-hour duration. Either the extended-release tablet or a long-acting β agonist is advantageous in preventing nocturnal asthma and deterioration of flow rates in the morning.
Parenteral route
β-adrenergic bronchodilators have been given subcutaneously and intravenously, usually in the emergency management of acute asthma. Subcutaneously, epinephrine 0.3 mg (0.3 mL of 1:1000 strength), every 15 to 20 minutes up to 1 mg in 2 hours, and terbutaline, 0.25 mg (0.25 mL of a 1-mg/mL solution) repeated in 15 to 30 minutes, not exceeding 0.5 mg in 4 hours, have been used. Shim45 suggested that for practical purposes both aerosolized and subcutaneous routes should be used to manage acute obstruction, although there may be little difference in effect with the two routes. No difference in effect between epinephrine and terbutaline has been found when given subcutaneously.
The intravenous route has been used most commonly with isoproterenol and with albuterol. Intravenous administration of these agents was thought to be useful during severe obstruction because these agents would be distributed throughout the lungs, whereas aerosol delivery would not allow them to penetrate the periphery. This assumption is questionable for both subcutaneous and intravenous bronchodilator therapy because aerosols do exert an effect with obstruction. Intravenous isoproterenol is not clearly advantageous as a bronchodilator, although this route is used for cardiac stimulation in shock and bradycardia. The dose-limiting factor is tachycardia. Intravenous therapy is a last resort and requires an infusion pump, cardiac monitor, and close attention. Pediatric dosages range from 0.1 to 0.8 μg/kg/min, and adult dosages range from 0.03 to 0.2 μg/kg/min, until bronchial relaxation or side effects occur.45 The combination of myocardial stimulation and hypoxia can cause serious arrhythmias; intravenous isoproterenol should be avoided in acute asthma, in favor of β2-specific agents. Albuterol has been given intravenously as a bolus of 100 to 500 μg or by infusion of 4 to 25 μg/min.46 Although albuterol is more β2 specific by aerosol than isoproterenol, the usefulness of intravenous administration compared with oral, aerosol, or subcutaneous administration is not clearly established.
Adverse side effects
Just as adrenergic bronchodilators exert a therapeutic effect by stimulation of α-adrenergic, β1-adrenergic, or β2-adrenergic receptors, they can likewise cause unwanted effects as a result of stimulation of these receptors. Generally, the term side effect indicates any effect other than the intended therapeutic effect. The most common clinically observed side effects of adrenergic bronchodilators are listed in Box 6-2 and are briefly discussed. The number and severity of these side effects vary among patients; not every side effect is seen with each patient. The later adrenergic agents (albuterol, bitolterol, pirbuterol, levalbuterol, and salmeterol) are much more β2 specific than previous agents such as ephedrine, epinephrine, or isoproterenol, and because of this, there is a greater likelihood of cardiac stimulation causing tachycardia and blood pressure increases with the last three agents than with the newer drugs. The more recent agents are safe, and the side effects listed are more of a nuisance than a danger and are easily monitored by clinicians. The introduction of single-isomer β agonists such as levalbuterol may show a further specificity and decrease in side effects, which are potentially caused by the detrimental effects of the (S)-isomer of β agonists.
Tremor
The annoying effect of muscle tremor with β agonists is due to stimulation of β2 receptors in skeletal muscle. It is dose related and is the dose-limiting side effect of the β2-specific agents, especially with oral administration. The adrenergic receptors mediating muscle tremor have been shown to be of the β2 type.47,48 As shown previously, this side effect is much more noticeable with oral delivery, which provides a rationale for aerosol administration of these agents. Tolerance to the side effect of tremor usually develops after days to weeks with the oral route, and patients should be reassured of this when beginning to use these drugs.
Cardiac effects
The older adrenergic agents with strong β1-stimulating and α-stimulating effects were considered dangerous in the presence of congestive heart failure. The dose-limiting side effect with these agents is tachycardia. They increase cardiac output and oxygen consumption by stimulating β1 receptors, leading to a decrease in cardiac efficiency, which is the work relative to oxygen consumption. Newer agents have a preferential β2 effect to minimize cardiac stimulation. However, tachycardia may also follow use of the newer agents, and there is evidence that this is due to the presence of β2 receptors even in the heart.49 β2 agonists cause vasodilation, and this can cause a reflex tachycardia. Despite this effect, agents such as terbutaline or albuterol can actually improve cardiac performance. Albuterol and terbutaline can cause peripheral vasodilation and increase myocardial contractility without increasing oxygen demand by the heart.34 The net effect is to reduce afterload and improve cardiac output with no oxygen cost. These agents are therefore attractive for use with airway obstruction combined with congestive heart failure.
Seider and colleagues50 reported that neither heart rate nor frequency of premature beats was significantly affected by inhaled terbutaline or ipratropium bromide (an anticholinergic bronchodilator) in 14 patients with COPD and ischemic heart disease. Although there are no written standards, most health care practitioners accept no more than a 20% change in pretreatment pulse after bronchodilator therapy has been initiated. This is why it is important to check the pulse rate before, during, and after bronchodilator therapy to evaluate cardiac response. If the pulse rate has increased more than 20% relative to the pretreatment pulse, stopping treatment with referral to the prescribing health care practitioner may be warranted to prevent unwanted cardiac effects.
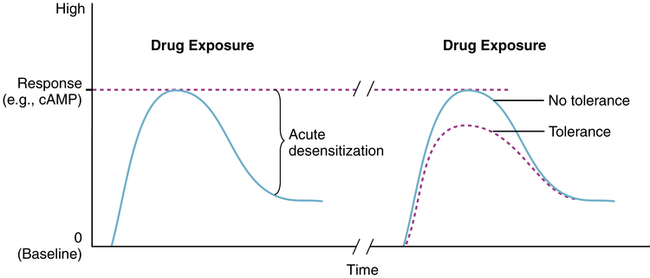
Tolerance to bronchodilator effect
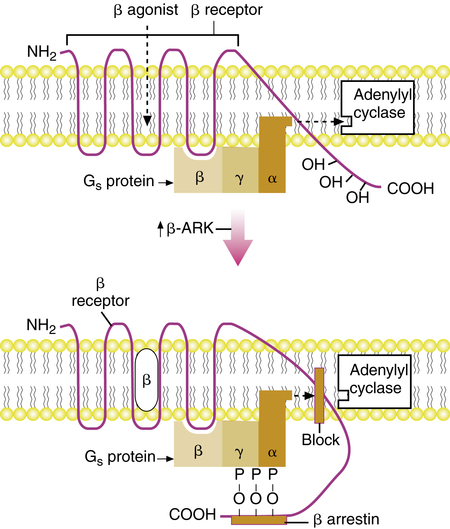
Adaptation to a drug with repeated use is a concern because use of the drug is actually reducing its effectiveness. With β agonists, there is in vitro evidence of an acute desensitization of the β receptor within minutes of exposure to a β agonist as well as a longer term desensitization. Figure 6-14 shows both an acute decrease in response during sustained exposure of the receptor to the agonist and a long-term decrease in maximal response with subsequent drug exposure. This decrease in bronchodilator response has been observed with short-acting and long-acting β agonists.
Exposure of cells with β receptors to isoproterenol causes a short-term, acute reduction in adenylyl cyclase activity and production of cAMP. The immediate desensitization is caused by an “uncoupling” of the receptor and the effector enzyme adenylyl cyclase.51 A model for desensitization of the β receptor is diagrammed in Figure 6-15. When stimulated by a β agonist, the β receptor goes into a low-affinity binding state (i.e., has reduced affinity for binding with a β agonist). Simultaneously, the β agonist causes an increase in cAMP, which increases protein kinase A, also referred to as β-adrenergic receptor kinase (β-ARK). β-ARK causes phosphorylation (transfer of phosphate groups [P]) of the hydroxyl groups (OH) on the carboxy-terminal portion of the β receptor. This phosphorylation induces binding of a protein named β-arrestin, which prevents the receptor from interacting with Gs and disrupts the coupling of Gs with the effector enzyme adenylyl cyclase. Removal of the β agonist allows the dissociation of β-arrestin and the phosphate groups from the receptor, and the receptor returns to a fully active state.
Long-term desensitization is considered to be caused by a reduction in the number of β receptors; this is termed downregulation. Both norepinephrine and albuterol have caused a reduction of almost 50% in vitro in the number of β-adrenergic receptors in airway smooth muscle of guinea pigs.51 Exposure of isolated human bronchus to isoproterenol or terbutaline produces similar desensitization.52 Long-term desensitization is also illustrated in Figure 6-14, as indicated by the lower peak response to subsequent administration of an adrenergic agonist.
Although use of an inhaled β agonist does cause a reduction in peak effect, the bronchodilator response is still significant and stabilizes within several weeks with continued use.18 Such tolerance is not generally considered clinically important and does not contraindicate the use of these agents. The same phenomenon of tolerance is also responsible for diminished side effects, such as muscle tremor, among patients regularly using inhaled β-agonist bronchodilators.
Corticosteroids can reverse the desensitization of β receptors and are said to be able to potentiate the response to β agonists.53 Corticosteroids have the following effects, in relation to β-agonist and β-receptor function:
• Corticosteroids increase the proportion of β receptors expressed on the cell membrane (upregulation).
• Corticosteroids increase the proportion of β receptors in the high-affinity binding state.
• Corticosteroids inhibit the release and action of inflammatory mediators such as PLA2, cytokines, and PAF.
β agonists may have a positive effect on corticosteroid function and activity. A review by Anderson25 explored possible mechanisms for the beneficial interaction of β agonists and corticosteroids.
Loss of bronchoprotection
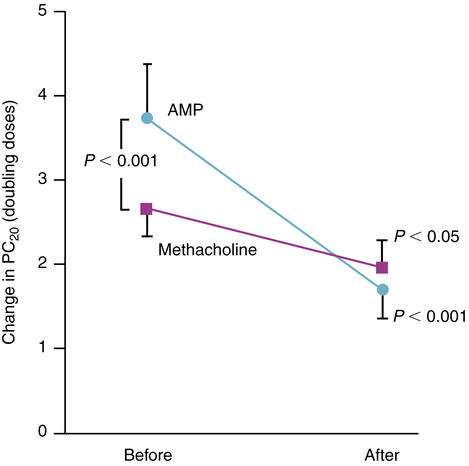
A distinction was found by Ahrens and colleagues54 to exist between the bronchodilating effect and the bronchoprotective effect of β agonists. The bronchodilating effect of a β agonist can be measured on the basis of airflow change, as indicated by a change in FEV1 or peak expiratory flow rate (PEFR). The bronchoprotective effect refers to the reaction of the airways to challenge by provocative stimuli such as allergens or irritants and is measured with doses of histamine, methacholine, or cold air. Ahrens and colleagues54 found that the protective effect with agonists such as metaproterenol or albuterol declines more rapidly than the bronchodilating effect. Not only is there a difference in time between these effects, but also it was found that tolerance occurs with the bronchoprotective effect of a β agonist, just as with the bronchodilating effect. Results from a study by O’Connor and associates55 are shown in Figure 6-16. The difference in dose of adenosine (AMP) and methacholine required to induce a 20% decline in FEV1 (PC20) after the inhalation of terbutaline compared with placebo is seen before and after 7 days of steady treatment with terbutaline. Airway response to challenge is seen to occur with a significantly lower dose of either AMP or methacholine in subjects with mild asthma, after 7 days of β-agonist exposure, showing tolerance to the protective effect of terbutaline. The development of tolerance to the bronchoprotective effect of the long-acting β agonist salmeterol was shown to occur both in the absence of corticosteroid therapy (by Bhagat and colleagues56) and with concomitant inhaled corticosteroid treatment (by Kalra and associates57). In a study by Rosenthal and associates,58 after the first 4 weeks of regular treatment with salmeterol, there was no increase in bronchial hyperresponsiveness or loss of bronchoprotection. Sustained improvements were seen in pulmonary function and asthma symptom control.58
The mechanism underlying the increase in bronchial hyperresponsiveness with use of β agonists is unclear. Evidence accumulating on the effects of the (S)-isomer of β agonists suggests a possible cause.59 The same effects could conceivably be implicated in the reduction in maximal bronchodilator effect with repeated use.
Fall in arterial oxygen pressure
A decrease in arterial oxygen pressure (Pao2) has been noted with isoproterenol administration during asthmatic bronchospasm, as ventilation improves and the exacerbation is relieved. The same effect has subsequently been noted with newer β agonists such as albuterol and salmeterol.60 The mechanism seems to be an increase in perfusion (i.e., blood flow) of poorly ventilated portions of the lung. It is known that regional alveolar hypoxia produces regional pulmonary vasoconstriction in an effort to shunt perfusion to lung areas of higher oxygen tension. This vasoconstriction is probably accomplished by α-sympathetic receptors.61
Administration of inhaled β agonists may reverse hypoxic pulmonary vasoconstriction by β2 stimulation, increasing perfusion to underventilated lung regions.62 Preferential delivery of the inhaled aerosol to better ventilated lung regions increases the ventilation-perfusion mismatch. It has been noted that such decreases in Pao2 are statistically significant but physiologically may be negligible.60,63 Oxygen tension decreases most in subjects with the highest initial Pao2. Decreases in Pao2 rarely exceed 10 mm Hg, and the Pao2 values tend to be on the flat portion of the oxyhemoglobin curve so that decreases in arterial oxygen saturation (Sao2) are minimized. Oxygen tensions usually return to baseline within 30 minutes.
Metabolic disturbances
Adrenergic bronchodilators can increase blood glucose and insulin levels and decrease serum potassium levels. This is a normal effect of sympathomimetics. In diabetic patients, clinicians should be aware of a possible effect on glucose and insulin levels. Hypokalemia has also been reported after parenteral administration of albuterol and epinephrine.64 The clinical importance of this side effect is controversial, and it would be of concern mainly for patients with cardiac disease or in interpreting serum potassium levels obtained shortly after use of adrenergic bronchodilators. The mechanism of the effect on potassium is probably activation of the sodium-potassium pump by the β receptor, with enhanced transport of potassium from the extracellular to the intracellular compartment. Such metabolic effects are minimized with inhaled aerosols of β-adrenergic agents because plasma levels of the drug remain low.
Propellant toxicity and paradoxic bronchospasm
The use of MDIs powered by CFC (e.g., Freon) can cause bronchospasm of hyperreactive airways. This reaction to the propellant was shown by Yarbrough and colleagues.65 They found that 7% of 175 subjects who used an MDI with placebo and propellant experienced a decrease of 10% or more in FEV1. The incidence was about 4% when using an MDI with metaproterenol and propellant, probably because the bronchodilating effect overcame the propellant effect. In most cases, bronchospasm lasts less than 3 minutes. There is apparently no noticeable difference in adverse reactions among patients using a CFC MDI and patients using an HFA MDI.66 A dry powder formulation is an ideal alternative formulation to an MDI if sensitivity to propellants exists, assuming drug availability and adequate inspiratory flow rate. Use of a nebulizer instead of an MDI may also be considered if bronchospasm occurs in a patient. Finally, the oral route offers an alternative to the inhalation route of administration. Cocchetto and associates67 reviewed the literature on paradoxic bronchospasm with use of inhalation aerosols.
Sensitivity to additives
Other additives and preservatives that can potentially have an effect on airway smooth muscle include benzalkonium chloride (BAC), ethylenediamine tetraacetic acid (EDTA), and hydrochloric or sulfuric acid to adjust pH of the solution. Asmus and colleagues68 recommended that only additive-free, sterile-filled unit-dose bronchodilator solutions be used for nebulizer treatment of acute airflow obstruction, especially if doses are given hourly or continuously. Clinicians should check aerosol formulations for BAC or EDTA, and if symptoms of bronchoconstriction occur, consider these as a possible cause.
Compatibility of other agents with bronchodilators
Several studies have tested the compatibility of common bronchodilators with other agents used in respiratory care. Kamin and associates69 found no chemical changes when they mixed albuterol with ipratropium, cromolyn, budesonide, tobramycin, or colistin. However, when antibiotics are to be administered in a nebulizer, it is best to separate the antibiotics and administer in the nebulizer individually. Bonasia and colleagues70 reported that levalbuterol is compatible with ipratropium, cromolyn, acetylcysteine, and budesonide for at least 30 minutes at room temperature. Akapo and coworkers71 discovered that formoterol nebulizer solution was compatible with ipratropium, cromolyn, acetylcysteine, and budesonide.
β-agonist controversy
The asthma paradox is a descriptive phrase for the increasing incidence of asthma morbidity, and especially asthma mortality, despite advances in the understanding of asthma and availability of improved drugs to treat asthma. Many studies have implicated the use of short-acting β agonists in asthma near-death emergencies and deaths,72,73 worsening clinical outcomes,74,75 and increased hyperreactivity.76,77 The events described in these studies may have been related to lack of corticosteroid use, leaving uncontrolled asthma symptoms to be treated only with short-acting β agonists.
Short-acting drugs, such as albuterol, and long-acting agents, such as salmeterol, have not in general been associated with a significant worsening of asthma.78 However, more recent evidence has indicated that long-acting β agonists may have a potential to increase asthma hospitalization or cause death.32,33 It has been noted that regular use of fenoterol and isoproterenol, but not other β agonists, may lead to worsening asthma control.59 Tolerance to the bronchodilator effect does occur with the use of β agonists, although this stabilizes and does not progress. There is an increase in bronchial hyperreactivity after institution of regular β-agonist therapy, which is not well explained. Evidence suggests that this effect may be caused or enhanced by the (S)-enantiomer of β agonists, which has a range of proinflammatory effects.
Asthma morbidity and mortality
A complete analysis of the relationship between β agonists and worsening asthma based on the literature seems to indicate that there is not a class effect of these drugs causing deterioration of asthma.59 Although it is unclear that β-agonist use increases risk of morbidity or death from asthma, asthma mortality is reported to be increasing in the United States and worldwide, despite more available treatment options, including β2-specific and longer acting adrenergic bronchodilators.79 There are several causes, not all involving β-agonist therapy, that may potentially lead to worsening asthma severity:
• Use of β agonists may allow allergic individuals to expose themselves to allergens and stimuli, with no immediate symptoms to warn them but with development of progressive airway inflammation and increasing bronchial hyperresponsiveness.
• Repeated self-administration of β agonists gives temporary relief of asthma symptoms through bronchodilation, which may cause underestimation of severity and delay in seeking medical help. β agonists do not block progressive airway inflammation, which can lead to death from lethal airway obstruction and hypoxia.
• Use of β agonists to alleviate symptoms of wheezing and resistance may lead to insufficient use, through poor patient education, poor patient compliance, or both, of antiinflammatory therapy to control the basic inflammatory nature of asthma.
• Accumulation of the (S)-isomer with racemic β agonists could exert a detrimental effect on asthma control.
• There is increased airway irritation with environmental pollution and lifestyle changes.80