Chapter 64
Superior Vena Cava Occlusion
Endovascular Treatment
Carlos F. Bechara, Peter H. Lin
Superior vena cava (SVC) occlusion precludes normal blood venous return to the heart. This condition, also known as SVC syndrome, is an uncommon occurrence that affects approximately 15,000 patients each year in the United States. The SVC functions as the primary venous drainage system from the head, neck, upper extremities, and upper thorax; the occlusion can be due to an extraluminal compression or intraluminal obstruction in nature. In general, SVC is 6 to 8 cm long and extends from the junction of the right and left innominate veins to the right atrium. Located in the middle mediastinum, the SVC is surrounded by relatively rigid structures, such as the trachea, right bronchus, sternum, aorta, pulmonary artery, and paratracheal and perihilar lymph nodes. Because the SVC is a low-pressure and thin-walled venous structure, the venous wall of the SVC can be compressed easily as it traverses the right side of the mediastinum.
SVC syndrome was first reported in 1757, when Dr. William Hunter described it in a patient with syphilitic aortic aneurysm.1 Historically, infections were the most common cause of SVC syndrome. In a study reported in 1954 by Schechter,2 who analyzed 274 confirmed cases of SVC syndrome, 40% were due to either syphilitic aneurysms or tuberculous mediastinitis. In the past 5 decades, there has been a gradual decline of these infectious etiologies as primary causes of SVC syndrome, owing in part to improved antimicrobial therapy. In later reports, carcinoma of the lung resulting in extraluminal SVC compression is the predominant cause of SVC obstruction. Patients with SVC obstruction require immediate diagnostic evaluation and therapeutic intervention, because it is common in patients with undiagnosed malignancy within the thorax.
Pathophysiology
The majority of cases of SVC syndrome are caused by neoplastic progression into the venous wall with resultant tumor mass compression against the relatively fixed, thin-walled SVC. Alternatively, SVC obstruction can also be due to intravascular thrombosis caused by neoplastic involvement. Postmortem studies have demonstrated that complete SVC obstruction is the result of extrinsic tumor compression in conjunction with intravascular thrombosis. In contrast, incomplete SVC obstruction is more frequently caused by extrinsic compression without associated intravascular thrombosis.3,4 Figure 64-1 summarizes various causes of SVC syndrome.
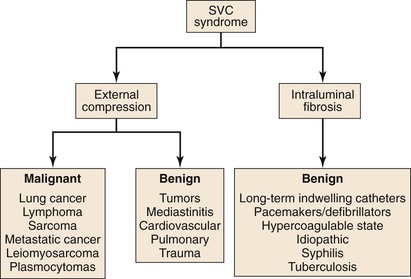
Figure 64-1 Causes of superior vena cava (SVC) syndrome.
Because of the SVC’s location, primary carcinoma of the lung located in the right upper lung lobe has a higher likelihood of causing SVC obstruction than pulmonary carcinomas located elsewhere. Among various neoplastic etiologies of SVC syndrome, mediastinal malignancy is responsible for 80% of cases. Specifically, bronchogenic carcinomas account for 75% to 80% of all cases, with the majority being small-cell carcinomas. Non-Hodgkin lymphoma is responsible for the remaining 10% to 15% of cases of SVC syndrome of malignant etiology. Other malignant causes of SVC syndrome, albeit less common, are Hodgkin disease, metastatic cancers, primary leiomyosarcomas of the mediastinal vessels, and plasmocytomas.
SVC obstruction can be caused by nonmalignant diseases, which account for less than 25% of all cases of SVC syndrome.3–5 Infectious conditions such as syphilis and tuberculosis accounted for the majority of cases of SVC syndrome around the turn of the twentieth century. The development of effective pharmacologic therapy in the past 4 decades has largely eradicated syphilitic and tuberculosis-related SVC syndrome. The most common nonmalignant cause of SVC syndrome is mediastinal fibrosis from an infectious or radiation-induced process.6 With an aging population on the rise, the frequency of placement of central venous devices, such as pacemakers, defibrillators, central venous infusion ports, and long-term hemodialysis catheters, is similarly increasing. As a result, data have shown that benign disease accounted for 35% to 40% of cases of SVC obstruction.7 These indwelling venous devices can cause SVC obstruction through mechanical trauma to the venous endothelium, which can result in intraluminal fibrosis.8–17
Collateral Venous Pathways
Venous collateral network formation can occur from chronic SVC obstruction. There are several important venous collaterals that return venous circulation from the upper half of the torso to the right atrium. The first and most important pathway is the azygos venous system, which includes the azygos vein, the hemiazygos vein, and the connecting intercostal veins. The second pathway involves the internal mammary venous system as well as tributaries and secondary communications to the superior and inferior epigastric veins. The long thoracic venous system, which represents the third collateral venous network, is connected to the femoral veins and vertebral veins. The development of these collateral venous pathways is influenced by the chronicity of SVC obstruction as well as underlying causative factors (Table 64-1).
Table 64-1
Stanford Classification for Superior Vena Cava (SVC) Obstruction
| Type | Description |
| I | Up to 90% stenosis with patency of the azygos vein |
| II | 90% stenosis to complete occlusion of SVC with patency of the azygos vein and antegrade flow through the azygos vein |
| III | 90% stenosis to complete occlusion of SVC with patency of the azygos vein and retrograde flow through the azygos vein |
| IV | Complete occlusion of SVC and one or more of its branches including the azygos vein |
Clinical Presentations and Diagnostic Evaluation
Clinical manifestations of SVC syndrome and diagnostic evaluation of patients in whom it is suspected are described in detail in Chapter 63.
Treatment Considerations
The traditional treatment for SVC syndrome associated with thoracic malignancy has been radiotherapy, chemotherapy, or both. The treatment response varies, depending on the invasiveness of thoracic malignancy, so typically resolution of symptoms may take several months. Although surgical construction of the SVC to bypass the venous obstruction along with removal of the underlying intrathoracic malignancy is an acceptable treatment option, this treatment approach requires a median sternotomy, which represents a major surgical challenge. The treatment benefits and operative morbidities must be weighed carefully against the patient’s life expectancy in those with malignant SVC syndrome. In patients with nonmalignant SVC syndrome, surgical reconstruction of the SVC creates a new venous conduit, which enables future dialysis access of pacemaker placement.
In patients who have overt SVC syndrome or whose disease does not respond to medical therapy, additional catheter-based treatment such as balloon angioplasty or stenting is necessary in order to maintain SVC luminal patency. In contrast to chemotherapy and radiation treatment for malignant SVC syndrome, endovascular stenting of the SVC establishes immediate luminal patency and provides rapid symptomatic relief. Endovascular stenting has been used prior to chemotherapy in neoplastic SVC syndrome with success.18 Similarly, endovascular treatment options can be equally applied to patients with nonmalignant SVC syndrome. In patients whose SVC obstruction is caused by intraluminal thrombosis, thrombolytic therapy to dissolve the thrombus is effective.
On the basis of an abundance of studies, endovascular therapy should be considered the first line of treatment in both malignant and nonmalignant SVC syndrome.19–22 The use of endovascular therapy to treat SVC syndrome has been extended to treat the pediatric and young adult populations.23 The only contraindication to endovascular therapy is inpatients with a contraindication to thrombolytic therapy or anticoagulation. Patients whose symptoms fail to respond or who are not candidates for endovascular therapy should be evaluated for surgical reconstruction.
Techniques of Endovascular Intervention
The endovascular procedure should be performed in either an interventional suite or operating room with a dedicated angiographic capability or a mobile C-arm fluoroscopic unit. Access for percutaneous stenting of the SVC is typically obtained via the femoral vein, but a brachial, basilic, or internal jugular vessel can also provide useful therapeutic access to the central veins. In the event of venous occlusion involving the SVC or brachiocephalic veins, bilateral upper extremity venous access via either a brachial or basilic vein should be considered because it facilitates the catheterization of the central vein occlusion. In addition to femoral access, additional upper extremity venous access is necessary in about 20% of the cases.22 This dual venous access not only enables antegrade and retrograde venograms but also facilitates crossing the SVC lesion.
Femoral venous access is typically established with a 7F introducer sheath (Boston Scientific, Natick, Mass). A 260-cm Bentson guide wire (Boston Scientific) followed by a pigtail catheter is placed in the SVC. Venography of the SVC and brachiocephalic veins is performed via a femoral approach to visualize the SVC lesion. Whenever possible, a hydrophilic guide wire is used to traverse the SVC lesion. Once the lesion is successfully cannulated with the hydrophilic guide wire, the guide wire is exchanged for a stiff Amplatz wire (Boston Scientific) or a Lunderquist wire (Cook Medical, Inc., Bloomington, Ind) for balloon or stent delivery. If the wire is unable to cross an SVC occlusion or a high-grade lesion, a thrombolytic infusion catheter is placed for delivery of a thrombolytic drug either as a bolus or continuously for a certain period.
Systemic anticoagulation with intravenous heparin (5000 U/kg) is given prior to any catheter-based intervention. We routinely perform an initial SVC balloon dilatation because this maneuver widens the lumen, facilitating subsequent stent deployment; other researchers have similarly supported this maneuver.24,25 In contrast, yet others have advocated avoiding this maneuver to reduce the risk of thrombus embolization.26 Treatment by means of balloon dilatation alone yields poor clinical results in the long term owing to a high rate of restenosis. Given the available clinical results, stenting of the SVC lesion is preferred over to SVC balloon angioplasty.
Balloon angioplasty to dilate the SVC or brachiocephalic venous lesion is performed with either a 10 mm × 40 mm or 12 mm × 40 mm balloon angioplasty catheter. Following balloon dilatation of the SVC lesion, either a balloon-expandable stent or a self-expanding stent is deployed across the lesion. Infrequently a covered stent-graft is used to treat the SVC lesion. Occasionally, more than one stent is needed to treat the underlying pathology. Post-stenting balloon dilatation is routinely performed when a self-expanding stent or a stent-graft is deployed. In our series, if the segment of SVC adjacent to the stricture was greater than 16 mm in diameter, a bilateral brachiocephalic “kissing stent” technique using either a 12-mm- or a 14-mm-diameter stent is performed.22 Completion venography is obtained to document treatment results (Fig. 64-2). Introducer sheaths and guide wires are next removed, and manual compression is applied to achieve hemostasis.
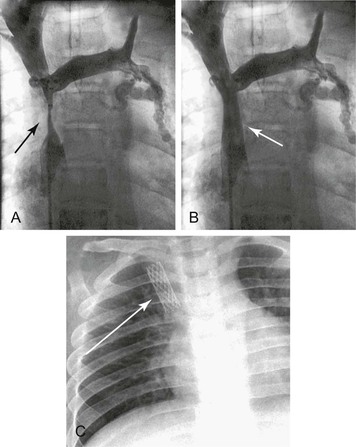
Figure 64-2 Images of a patient with symptomatic superior vena cava (SVC) syndrome. A, A high-grade SVC stenosis is depicted in the venogram (arrow). B, Luminal patency was established with the placement of a balloon-expandable Palmaz stent (arrow). C, Chest radiograph demonstrated the location of the Palmaz stent (arrow).
In the event of chronic venous occlusion whereby conventional catheter and guide wire techniques are not successful in crossing the venous occlusion, radiofrequency guide wire technology has been shown to be beneficial in establishing access across the venous occlusion. The PowerWire Radiofrequency Guidewire (Baylis Medical Company Inc, Montreal) can be used to facilitate crossing vessels with thrombotic occlusion. This 0.035-inch wire has a hot tip and low-friction insulation but is not steerable. The device monitor displays delivered radiofrequency power, impedance, and elapsed time to allow for continuous monitoring. The utility of this technology was highlighted in a series of three patients with malignant SVC syndrome whose SVC occlusions were successfully crossed with use of a radiofrequency guide wire, followed by stent placement.27
Thrombolytic Therapy
The first successful thrombolytic therapy in SVC syndrome caused by a pacemaker lead was described in 1974, in which streptokinase was delivered via catheter-directed infusion.28 This treatment modality with or without angioplasty or stenting has been well validated.28–31 Thrombolytic therapy could lead to complete clot resolution or could partially recanalize the thrombus, allowing further endovascular management such as balloon angioplasty or stenting once the lesion is crossed with a wire. In general, chronic venous occlusions tend to be easier to cross with a wire than chronic arterial occlusions. Catheter-directed thrombolysis utilizes a smaller thrombolytic dose than systemic thrombolysis. The earlier the administration of thrombolytic therapy after the onset of symptoms, the better the response and the thrombus resolution. Most nonmalignant SVC syndromes respond to thrombolytic therapy, because the patients present with subacute or acute-on-chronic thrombosis. The process of thrombotic occlusion in malignant SVC syndrome is more insidious in nature. By the time symptoms occur, sufficient venous collaterals have usually developed.
When thrombosis occurs and results in SVC occlusion, adjuvant thrombolytic therapy may be beneficial to dissolve the thrombus and unmask the underlying lesion prior to definitive endovascular treatment. Many physicians who performed endovascular stenting for SVC syndrome described the benefits of thrombolytic agents.32 Ariza et al24 administered urokinase in a 500,000 IU bolus followed by an infusion of 80,000 to 100,000 IU/hr for 24 to 48 hours after stent implantation. Gray et al33 described the safety and benefit of using streptokinase and urokinase for thrombolysis in SVC syndrome of both malignant and benign etiologies. They reported successful results when thrombolytic infusion was administered through an indwelling catheter within 5 days of the onset of symptoms. In SVC or brachiocephalic thrombotic occlusion, direct thrombolytic infusion via a catheter embedded in thrombus or through a preexisting indwelling catheter is an effective technique for treating SVC thrombosis. Rosenblum et al34 reported the utility of thrombolytic therapy in successfully treating patients with SVC syndrome due to long-term use of indwelling catheter. Sheikh et al25 reported the use of urokinase and recombinant tissue plasminogen activator (t-PA) prior to angioplasty and stent deployment in patients with SVC syndrome.25 Their study shows a high rate of treatment success and supports the use of adjuvant thrombolytic therapy in patients with SVC syndrome with underlying venous thrombosis and no contraindications to thrombolytic therapy. Heparin, oral anticoagulants, or antiplatelet therapy is typically given after IVC stenting, for a duration of at least 6 months and possibly indefinitely.24–26 Overall this anticoagulation is well tolerated and appears to decrease the risk of stent thrombosis, although fatal subdural hematoma was reported in one patient with nonmalignant SVC syndrome.25
Over the past decade, percutaneous mechanical thrombectomy has emerged as an effective treatment modality in patients with thrombotic venous occlusion.35 The clinical advantage of such a thrombectomy system is that it allows simultaneous catheter-directed infusion of thrombolytic agents, thereby creating a pharmacomechanical thrombectomy (PMT) strategy. It is accomplished with the initial administration of thrombolytic agents to exert the pharmacologic benefit of thrombus dissolution, followed by mechanical thrombectomy for thrombus extraction. The pharmacomechanical thrombectomy treatment strategy combines the advantages of pharmacologic thrombolysis and mechanical thrombectomy, in which the thrombolytic dosage can be reduced and the efficacy of mechanical thrombectomy is maximized. This treatment technique was adapted from our previously reported treatment strategy in DVT intervention.35 In our reported series, this pharmacomechanical thrombectomy with rheolytic thrombectomy and catheter-directed tissue plasminogen activator administration has yielded successful treatment outcome in patients with acute thrombosis of the SVC and brachiocephalic vein.22
Balloon Angioplasty and Stenting
Vessel wall recoil following balloon angioplasty is a well-known phenomenon that has diminished the attraction of this treatment modality for use in patients with SVC syndrome. In general, a short-segment venous disease may respond very well to plain balloon angioplasty. Because most patients with SVC syndrome have more extensive disease, additional interventions beyond balloon angioplasty are frequently necessary. Consequently, the role of balloon angioplasty has evolved from a primary intervention to an adjunctive procedure used prior to stenting.36 Advances in intraluminal stent placement in the past 2 decades have proven to be effective in treating venous outflow obstruction with low morbidity and mortality while maintaining a high treatment success rate. The therapeutic role of percutaneous stenting has been validated in both malignant and benign SVC syndrome.21–26 It establishes immediate luminal patency and provides rapid symptomatic relief with remarkable clinical success.
The therapeutic objective of SVC stenting is similar to that of open surgery, which is to restore luminal patency and relieve obstructive symptoms. The choice of plain balloon angioplasty, balloon-expandable stent, self-expanding stent, or stent-graft depends on the venous lesion pattern, length of stenosis, and presence of venous branches or collaterals adjacent to the SVC lesions. Among these various therapeutic options, the intraluminal stent is the most commonly used endovascular strategy. Although the therapeutic efficacy of percutaneous stenting in intra-arterial lesions is well proven,37 comparable evidence in venous pathology remains scarce, particularly with regard to vena cava obstruction. Since the first use of stenting in SVC syndrome was described, the use of a variety of metallic stents has been described for the palliative treatment of SVC syndrome.38–43
Several metallic stents can be used in the treatment of SVC obstruction. The four most commonly used are the Gianturco-Z stent (Cook Medical, Inc.), the Palmaz stent (Cordis Corporation, Bridgewater, NJ), SMART stent (Cordis Corporation), and the Wallstent (Boston Scientific). The Gianturco Z-stent was one of the very first stents used in treating SVC obstruction and is described in the earlier clinical series.19 It is a rigid self-expanding stainless steel stent with the greatest radial force in its middle segment. It comes in diameters ranging from 15 to 35 mm and a length of 5 cm. Because the Z-stent provides the greatest radial force in its middle segment, placement of multiple overlapping Z-stents is necessary for treatment of long venous lesions. Several studies have shown the benefit of using Z-stents in recanalized SVC occlusions.44,45 Despite the high radial force of the stent, it did not gain wide acceptance in clinical practice owing to metal fatigue with the possibility of fracture in long-term study.46
Another type of stent that has been utilized in SVC lesions is the Palmaz stent, a balloon-expandable stent with a high radial force. This stent is ideally suited for lesions with extrinsic luminal compression. Available in a variety of diameters and lengths, it can be precisely deployed with limited foreshortening and can dilate to nearly 80% larger than its intended diameter. This over-dilatation can be accomplished with the use of using an oversized balloon catheter while strong radial force is maintained. The beneficial role of the Palmaz stent in treating SVC syndrome has been documented.22,29 Its disadvantage relates to its compressibility, which may lead to stent deformation or occlusion due to extrinsic compression from an adjacent extraluminal mass.33
The SMART stent is a self-expanding stent composed of nitinol, a nickel-titanium metallic alloy. This stent possesses shape memory, which enables it to assume its predetermined shape once deployed and at body temperature. Although the SMART stent comes in a variety of lengths and diameters, the most useful size for SVC intervention has a length of 60 mm and a diameter of 14 mm, which can accommodate large vessels such as the vena cava.
The Wallstent is the most widely used stent for SVC syndrome treatment as reported in the literature.8,20–22 The device is a self-expanding stent with longitudinal flexibility, making it ideal for long tortuous lesions. The primary advantages of this stent are its flexibility and ease of deployment, which are ideally suited for long or tortuous lesions. However, a potential disadvantage is noteworthy; use of a large-diameter Wallstent in a patient with a critically stenotic SVC may result in low radial force owing to uneven stent conformability. Another disadvantage of the Wallstent relates to its considerable foreshortening during deployment, which can occur in up to 30% of the stent length. This foreshortening phenomenon can lead to migration of the stent if it is not deployed precisely. When this stent is used to treat a long-segment vascular lesion, significant stent foreshortening can occur which may lead to stent migration if the device is not centered on the stenosis.47,48 However, a beneficial feature of this device is that the stent can be re-sheathed and repositioned during the deployment process, minimizing potential stent misemployment. The Wallstent is available in a wide range of diameters (up to 18 mm) and lengths (up to 94 mm), making it versatile in treating the SVC or brachiocephalic veins.
Although researchers have proposed the use of stent-grafts in venous pathology, we do not believe that it has a significant advantage over the Wallstent because of potential coverage of important venous collaterals by a stent-graft in a patient with SVC syndrome, which might worsen the symptoms if the stent-graft becomes occluded. Clinical experience of the stent-graft in treating SVC syndrome remains scarce; there are only two reports of its use in patients in whom tumor ingrowth had developed in stents previously placed for malignant SVC syndrome.49,50 The stent-graft material prevents further tumor ingrowth, and in one case the stent-graft remained patent after 12 months.49 Stent-grafts are thought to have less predisposition to develop neointimal hyperplasia, but in-stent stenosis is a potential problem. Also, as previously mentioned, placing a stent-graft could potentially cover important venous collaterals in a patient with SVC syndrome, worsening the symptoms, especially when the stent-graft gets occluded. Whether stent-grafts should be used more in treating malignant SVC syndrome to prevent tumor or thrombus ingrowth warrants further study.
Clinical Results of Superior Vena Cava Stenting
The clinical efficacy of Wallstent in SVC obstruction has been reported by several studies, including our institutional experience.18,20–22,51 Dyet et al21 reported a primary patency rate of 90% and secondary patency rate of 100% in patients with symptomatic SVC syndrome.21 We and other researchers have reported 95% to 100% clinical success rates in the use of endovascular interventions to treat patients with malignant SVC syndrome.22,52 In our series, clinical success was achieved in 96% of cases. Primary patency rates in patients with malignant and benign causes of SVC syndrome were 64% and 76% at 1 year, respectively.22 Others have reported 95% technical success rates with primary and secondary clinical patency rates of 79% and 93%, respectively.29
Randomized studies comparing the different stents in treating SVC syndrome are lacking. Oudker et al53 reported the outcome of a comparative analysis between Wallstent and the Z-stent in palliative treatment of SVC syndrome. They reported early Wallstent thrombosis at 2 weeks and lower long-term patency (69%) in comparison with the Z-stent (100%). Because the majority of currently available self-expanding nitinol stents do not have a diameter greater than 12 mm, their role in the management of SVC syndrome remains limited. In contrast, the wide range of large-diameter (up to 18 mm) Wallstents provides versatile treatment options in structures such as the SVC and brachiocephalic vein.
Patients with benign SVC syndrome have better survival than those with SVC syndrome of malignant causes (Fig. 64-3).22 Open surgery is considered a better option than endovascular therapy in maintaining luminal patency for future catheters or devices. Later reports have shown endovascular intervention with stenting to be an accepted first line of therapy for benign SVC syndrome.54 In our series, benign disease was responsible for SVC syndrome in 29% of patients,22 a surprisingly greater proportion than in other contemporary series.* The clinical outcome of our series is comparable to that of a study by Ariza et al,24 whose clinical success rate was 85%. Petersen et al39 reported the long-term results of stenting in 19 patients with SVC syndrome caused by benign processes. With a mean follow-up period of 33 months, the primary patency rate was 83%, and the secondary patency was 100%. In a similar study, Sheikh et al25 performed SVC stenting in 19 patients with nonmalignant SVC syndrome. It is noteworthy that in 74% of them (16/19), SVC syndrome developed as a result of underlying indwelling intravascular catheters or devices. These patients had a remarkable clinical response after stenting, with a clinical success rate of 100%. Long-term primary and secondary patency rates of more than 70% and 100%, respectively, have been reported.22,24,25,54 As expected, patients with benign causes of SVC syndrome have a better long-term stent patency than those with malignant causes.
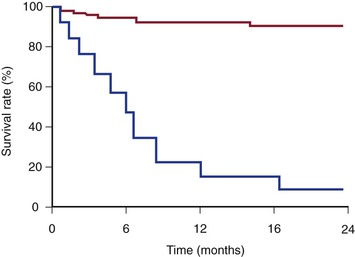
Figure 64-3 Kaplan-Meier survival curves of patients with malignant (lower line) and benign (upper line) superior vena cava syndrome. (From Barshes NR, et al: Percutaneous stenting of superior vena cava syndrome: treatment outcome in patients with benign and malignant etiology. Vascular 15:314-321, 2007.)
SVC or brachiocephalic vein occlusion in patients requiring chronic hemodialysis poses a particular clinical challenge. Owing in part to the high turbulent flow created during episodic hemodialysis treatment, the central venous lesion tends to exhibit a higher incidence of restenosis following stent placement as well as shorter treatment patency. Kovalik et al56 reported their experience of treating 20 patients requiring hemodialysis in whom symptomatic central vein stenosis developed. In those patients who showed nonelastic central venous stenosis (defined as residual lumen greater than 50%), the recurrence rate was 81% during a follow-up period of 7.6 months. In contrast, in patients with elastic stenosis, the average time from treatment to recurrence was 2.9 months, and the recurrence rate was 100%. The investigators observed that the placement of Wallstent prostheses in nonelastic lesions had a lower recurrence rate than in elastic stenotic lesions. Consequently, Kovalik et al56 postulated that it may be necessary to place stents in nonelastic stenoses in patients subjected to hemodialysis, but not in patients with stenoses that are elastic or have minimal elasticity. A similar finding was noted in our series, in which all of the four patients with long-term indwelling hemodialysis catheters had in-stent stenosis following SVC intervention.22 Secondary intervention was necessary in all these patients undergoing hemodialysis to restore the luminal patency of their stents.
A comparative study was reported in 73 patients with malignant SVC syndrome who were treated with either bare metal stents or covered stents. In this study, Gwon et al57 treated 37 patients with expanded polytetrafluoroethylene (ePTFE)–covered stents and retrospectively analyzed results in 36 patients treated with uncovered stents. There were no differences in technical success or complications between the two groups. Although the 1-year cumulative patency rate was significantly greater in the covered stent group (94% vs. 48%), there were no differences in overall clinical success or patient survival rate. Although the study noted a potential advantage of a high patency rate with covered stents, the investigators underscored two cautionary points with regard to the placement of covered stents in patients with malignant SVC syndrome. First, the risk of stent migration or dislodgement remains present whether the stent is covered or uncovered. To reduce the possibility of stent migration, they recommend oversizing the device by 10% to 20% in comparison with the SVC diameter. Second, placement of a covered stent across the confluence of brachiocephalic veins may occlude contralateral upper extremity venous drainage, resulting in contralateral upper extremity deep vein thrombosis. However, this study showed no evidence of contralateral upper extremity swelling or venous thrombosis in those patients in whom stents were placed in the SVC or brachiocephalic vein.57 This observation suggests that unilateral relief of obstruction using covered stents may allow contralateral venous return via mediastinal or cervical collateral venous circulation.
In a Cochrane review of treatment for malignant SVC obstruction, Rowell and Gleeson5 reported that stent insertion provided more rapid symptom relief in a higher proportion of patients than did radiation therapy or chemotherapy.5 Other researchers have reported similar success rates, of 95% to 100%, in patients with malignancy-associated SVC syndrome after endovascular interventions.52 Kee et al29 reported a technical success rate of 95%, a primary clinical patency rate of 79%, and a secondary clinical patency rate of 93%. Their mortality and morbidity rates were 3% and 10%, respectively. Rosch et al19 reported one recurrence in a group of 20 patients during a follow-up period of 7 months (range 1 to 11 months). In one of the largest series, in which 76 patients with malignant SVC syndrome were treated with Wallstents with or without radiotherapy, stent therapy provided a faster symptom relief, and 90% of the patients remained symptom free until death.58 Only 12% in this study who underwent radiotherapy alone remained symptom free until death, so the investigators recommended that stent insertion be the first line of therapy. Moreover, complete SVC obstruction should not be considered a contraindication to endovascular therapy. Multimodality therapy including thrombolysis, angioplasty, and stent insertion was successful in 85% of the cases.58 Dinkel et al26 hypothesized that bilateral stent placement is superior to unilateral stent placement in keeping tributaries to the SVC open and preventing recurrent occlusion. To their surprise, however, bilateral Wallstent placement had shorter patency with more complications.
Treatment Complications
Although complications related to SVC stenting remain uncommon, they can have serious consequences. The complications include mediastinal hematoma, stent migration, infection, pulmonary embolus, stent thrombosis and SVC perforation, and pericardial puncture resulting in cardiac tamponade.25,59–61 The newer generation of stents with improved radiopacity, lower device profile, and accurate delivery system have reduced the likelihood of stent malposition or migration during deployment. Like other researchers, we advocate routine SVC balloon angioplasty prior to stent deployment to facilitate accurate stent placement and minimize stent migration.22,24,25 In the unusual event of stent infection or migration, multiple strategies for stent removal or salvage techniques for migrated stents have been described.61,62 These techniques include snaring the stent directly, crushing the stent followed by snaring and removing it and then deploying a migrated stent in a different venous system.61,62
Fatal hemorrhagic complications were typically attributed to administration of thrombolytic agents, including streptokinase, urokinase, and tissue plasminogen activator, to dissolve intracaval clot prior to insertion of a vascular prosthesis. Cerebral hemorrhage has also been reported in patients with intracranial metastases arising from SVC malignancy during thrombolytic therapy.63 Massive hemoptysis can also occur during thrombolytic therapy or during maintenance anticoagulation therapy.54,64
Malignancy-related hypercoagulable state combined with the presence of an intra-vascular stent can increase the risk of thrombosis after stent implantation. Researchers have postulated that this occurrence is due to the exposed stent metal in a low-pressure venous circulation, which can exacerbate thrombus formation. Because the ideal anticoagulation regimen following SVC stent remains controversial, many patients are not given appropriate antithrombotic therapy following stent placement, undoubtedly contributing to the incidence of stent thrombosis. Nonetheless, ideal anticoagulation therapy following stent placement in patients with SVC malignancy remains to be determined. Some researchers recommended no antithrombotic therapy after stenting,65 but most advocate prolonged anticoagulation, including the use of heparin, warfarin, and antiplatelet agents.54,66,67 In the event of a stent thrombosis, immediate intervention with stent catheterization following by thrombolysis or thrombectomy can be effective in restoring lumen patency.
Selected Key References
Barshes NR, Annambhotla S, El Sayed HF, Huynh TT, Kougias P, Dardik A, Lin PH. Percutaneous stenting of superior vena cava syndrome: treatment outcome in patients with benign and malignant etiology. Vascular. 2007;15:314.
Ganeshan A, Hon LQ, Warakaulle DR, Morgan R, Uberoi R. Superior vena caval stenting for SVC obstruction: current status. Eur J Radiol. 2009;71:343.
An excellent article on the utility of stenting in patients with malignant SVC obstruction..
Gwon DI, Ko GY, Kim JH, Shin JH, Yoon HK, Sung KB. Malignant superior vena cava syndrome: a comparative cohort study of treatment with covered stents versus uncovered stents. Radiology. 2013;266:979.
Kim YI, Kim KS, Ko YC, Park CM, Lim SC, Kim YC, Park KO, Yoon W, Kim YH, Kim JK, Ahn SJ. Endovascular stenting as a first choice for the palliation of superior vena cava syndrome. J Korean Med Sci. 2004;19:519.
Nguyen NP, Borok TL, Welsh J, Vinh-Hung V. Safety and effectiveness of vascular endoprosthesis for malignant superior vena cava syndrome. Thorax. 2009;64:174.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. Kamiya K, et al. Superior vena caval syndrome: review of the literature and a case report. Vasc Dis. 1967;4:59.
2. Schechter MM. The superior vena cava syndrome. Am J Med Sci. 1954;227:46.
3. Wilson LD, et al. Clinical practice: superior vena cava syndrome with malignant causes. N Engl J Med. 2007;356:1862.
4. Nieto AF, et al. Superior vena cava obstruction: clinical syndrome, etiology, and treatment. Curr Probl Cancer. 1986;10:441.
5. Rowell NP, et al. Steroids, radiotherapy, chemotherapy and stents for superior vena caval obstruction in carcinoma of the bronchus: a systematic review. Clin Oncol (R Coll Radiol). 2002;14:338.
6. Van Putten JW, et al. Superior vena cava obstruction caused by radiation induced venous fibrosis. Thorax. 2000;55:245.
7. Rice TW, et al. The superior vena cava syndrome: clinical characteristics and evolving etiology. Medicine (Baltimore). 2006;85:37.
8. Slonim SM, et al. Placement of SVC stents over pacemaker wires for the treatment of SVC syndrome. J Vasc Interv Radiol. 2000;1:215.
9. Woodyard TC, et al. Acute superior vena cava syndrome after central venous catheter placement. Cancer. 1993;15:2621.
10. Laguna Del Estal P, et al. Superior vena cava syndrome: a study based on 81 cases. An Med Interna1. 1998;5:470.
11. Fa Qin LV, et al. Doppler superior vena cava flow evolution and respiratory variation in superior vena cava syndrome. Echocardiography. 2008;25:360.
12. Eren S, et al. The superior vena cava syndrome caused by malignant disease: imaging with multi-detector row CT. Eur J Radiol. 2006;59:93.
13. Cihangiroglu M, et al. Collateral pathways in superior vena caval obstruction as seen on CT. J Comput Assist Tomogr. 2001;25:1.
14. Thornton MJ, et al. A three-dimensional gadolinium-enhanced MR venography technique for imaging central veins. AJR Am J Roentgenol. 1999;173:999.
15. Marckmann P, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359.
16. Mahmud AM, et al. Follow-up of patients with superior vena cava syndrome by functional analysis of radionuclide venography. Nucl Med Commun. 1998;19:417.
17. Stanford W, et al. The role of venography and surgery in the management of patients with superior vena cava obstruction. Ann Thorac Surg. 1986;41:158.
18. Bierdrager E, et al. Endovascular stenting in neoplastic superior vena cava syndrome prior to chemotherapy or radiotherapy. Neth J Med. 2005;63:20.
19. Rosch J, et al. Gianturco-Rosch expandable Z-stents in the treatment of superior vena cava syndrome. Cardiovasc Interv Radiol. 1992;15:319.
20. Kim YI, et al. Endovascular stenting as a first choice for the palliation of superior vena cava syndrome. J Korean Med Sci. 2004;19:519.
21. Dyet JF, et al. Use of the Wallstent endovascular prosthesis in the treatment of malignant superior vena caval obstruction. J Vasc Interv Radiol. 1994;5:2.
22. Barshes NR, et al. Percutaneous stenting of superior vena cava syndrome: treatment outcome in patients with benign and malignant etiology. Vascular. 2007;15:314.
23. Tzifa A, et al. Endovascular treatment for superior vena cava occlusion or obstruction in a pediatric and young adult population. J Am Coll Cardiol. 2007;49:1003.
24. Ariza GA, et al. Percutaneous treatment of superior vena cava syndrome using metallic stents. Eur Radiol. 2003;13:853.
25. Sheikh MA, et al. Endovascular stenting of nonmalignant superior vena cava syndrome. Catheter Cardiovasc Interv. 2005;65:405.
26. Dinkel HP, et al. Endovascular treatment of malignant superior vena cava syndrome: is bilateral Wallstent placement superior to unilateral placement? J Endovasc Ther. 2003;10:788.
27. Iafrati M, et al. Radiofrequency thermal wire is a useful adjunct to treat chronic central venous occlusions. J Vasc Surg. 2012;55:603.
28. Williams DR, et al. Thrombosis of superior vena cava caused by pacemaker wire and managed with streptokinase. J Thorac Cardiovasc Surg. 1974;68:134.
29. Kee ST, et al. Superior vena cava syndrome: treatment with catheter-directed thrombolysis and endovascular stent placement. Radiology. 1998;206:187.
30. Kalman PG, et al. Management of upper extremity central venous obstruction using interventional radiology. Ann Vasc Surg. 1998;12:202.
31. Blackburn T, et al. Pacemaker-induced superior vena cava syndrome: consideration of management. Am Heart J. 1988;116:893.
32. de Gregorio MA, et al. Interventional radiologic techniques in thoracic emergencies. Arch Bronconeumol. 2000;36:51.
33. Gray BH, et al. Safety and efficacy of thrombolytic therapy for superior vena cava syndrome. Chest. 1991;99:54.
34. Rosenblum J, et al. Intravascular stents in the management of acute superior vena cava obstruction of benign etiology. J Parenter Enteral Nutr. 1994;18:362.
35. Lin PH, et al. Catheter-direct thrombolysis versus pharmacomechanical thrombectomy for treatment of symptomatic lower extremity deep venous thrombosis. Am J Surg. 2006;192:782.
36. Courtheoux P, et al. Stent placement in superior vena cava syndrome. Ann Thorac Surg. 2003;75:158.
37. Singh KP, et al. Peripheral arterial disease: an overview of endovascular therapies and contemporary treatment strategies. Rev Cardiovasc Med. 2006;7:55.
38. Shah R, et al. Stenting in malignant obstruction of superior vena cava. J Thorac Cardiovasc Surg. 1996;112:335.
39. Petersen BD, et al. Long-term results of treatment of benign central venous obstructions unrelated to dialysis with expandable Z stents. J Vasc Interv Radiol. 1999;10:757.
40. Miller JH, et al. Malignant superior vena cava obstruction: stent placement via the subclavian route. Cardiovasc Interv Radiol. 2000;23:155.
41. Watkinson AF, et al. Expandable Wallstent for the treatment of obstruction of the superior vena cava. Thorax. 1993;48:915.
42. Nguyen NP, et al. Safety and effectiveness of vascular endoprosthesis for malignant superior vena cava syndrome. Thorax. 2009;64:174.
43. Ganeshan A, et al. Superior vena caval stenting for SVC obstruction: current status. Eur J Radiol. 2009;71:343.
44. Edwards RD, et al. Case report: superior vena cava obstruction complicated by central venous thrombosis—treatment with thrombolysis and Gianturco-Z stents. Clin Radiol. 1992;45:278.
45. Gaines PA, et al. Superior vena caval obstruction managed by the Gianturco Z stent. Clin Radiol. 1994;49:202.
46. Glanz S, et al. Axillary and subclavian vein stenosis: percutaneous angioplasty. Radiology. 1998;168:371.
47. Qanadli SD, et al. Subacute and chronic benign superior vena cava obstructions: endovascular treatment with self-expanding metallic stents. Am J Roentgenol. 1999;173:159.
48. Stock KW, et al. Treatment of malignant obstruction of the superior vena cava with the self-expanding Wallstent. Thorax. 1995;50:1151.
49. Gil lK, et al. Recurrent superior vena caval obstruction due to invasion by malignant thymoma: treatment using a stent-graft. Br J Radiol. 2001;73:414.
50. Chin DH, et al. Stent-graft in the management of superior vena cava syndrome. Cardiovasc Interv Radiol. 1996;19:302.
51. Hennequin LM, et al. Superior vena cava stent placement: results with the Wallstent endoprosthesis. Radiology. 1995;196:353.
52. Schindler N, et al. Superior vena cava syndrome: experience with endovascular stents and surgical therapy. Surg Clin North Am. 1999;79:683.
53. Oudker KM, et al. Self expanding metal stents for palliative treatment of superior vena caval syndrome. Cardiovasc Interv Radiol. 1996;19:146.
54. Rizvi AZ, et al. Benign superior vena cava syndrome: stenting is now the first line of treatment. J Vasc Surg. 2008;47:372.
55. Charnsangavej C, et al. Stenosis of the vena cava: preliminary assessment of treatment with expandable metallic stents. Radiology. 1986;161:295.
56. Kovalik EC, et al. Correction of central venous stenosis: use of angioplasty and vascular Wallstents. Kidney Int. 1994;45:1177.
58. Nicholson AA, et al. Treatment of malignant superior vena cava obstruction: metal stents or radiation therapy. J Vasc Interv Radiol. 1997;8:781.
59. Brant J, et al. Hemopericardium after superior vena cava stenting for malignant SVC obstruction: the importance of contrast-enhanced CT in the assessment of postprocedural collapse. Cardiovasc Interv Radiol. 2001;24:353.
60. Martin M, et al. Fatal pericardial tamponade after Wallstent implantation for malignant superior vena cava syndrome. J Endovasc Ther. 2002;9:680.
61. Taylor JD, et al. Strategies for the management of SVC stent migration into the right atrium. Cardiovasc Interv Radiol. 2007;30:1003.
62. Srinathan S, et al. Radiological management of superior vena caval stent migration and infection. Cardiovasc Interv Radiol. 2005;28:127.
63. Nicholson AA, et al. Treatment of malignant superior vena cava obstruction: metal stents or radiation therapy. J Vasc Int Radiol. 1997;8:781.
64. Urruticoechea A, et al. Treatment of malignant superior vena cava syndrome by endovascular stent insertion: experience on 52 patients with lung cancer. Lung Cancer. 2004;43:209.
65. Bierdrager E, et al. Endovascular stenting in neoplastic superior vena cava prior to chemotherapy or radiotherapy. Neth J Med. 2005;63:20.
66. Lanciego C, et al. Stenting as first option for endovascular treatment of malignant superior vena cava syndrome. AJR Am J Roentgenol. 2001;177:585.
67. Lopez-Muniz JIC, et al. Treatment of superior and inferior vena cava syndrome of malignant cause with Wallstent catheter placed percutaneously. Am J Clin Oncol. 1997;20:293.