Chapter 54
Superficial Thrombophlebitis
Suman Rathbun
Based on a chapter in the seventh edition by Anil P. Hingorani and Enrico Ascher
Superficial venous thrombophlebitis (SVT) has been the focus of increased attention because of recognition of the potential morbidity and mortality associated with it. A global disorder, SVT develops in approximately 125,000 people per year in the United States but is nonetheless underestimated because many cases go unreported.1
Traditional teaching suggested that SVT is a self-limited process of little consequence and of small risk; however, new evidence on the natural history of SVT has led to improvements in evaluation and treatment. A meta-analysis reported a 6% to 44% incidence of deep venous thrombosis (DVT), a 20% to 33% incidence of asymptomatic pulmonary embolism (PE), and a 2% to 13% incidence of symptomatic PE in patients in whom SVT is diagnosed.2 Improved diagnostic evaluation of SVT with duplex scanning, lung scanning, and blood tests has helped identify predisposing risk factors and potential complications.
This chapter examines current data regarding SVT and its management with the goal of improving recognition and treatment of the underlying disorders to prevent recurrence and its life-threatening complications.
Epidemiology and Pathogenesis
Although SVT is a frequently observed condition, its incidence and prevalence have never been adequately assessed. The classic Tecumseh Community Health Study from 1973 reported that the incidence of SVT increases with age in men from 0.05 per 1000 per year in their third decade to 1.8 per 1000 per year in their eighth decade. In women, the incidence similarly rises from 0.31 per 1000 per year to 2.2 per 1000 per year from their third decade to their eighth decade.3 Moreover, other studies have also demonstrated an increased prevalence of SVT in women.4,5 Overall, the incidence of lower extremity SVT has been reported to be 3% to 11% in the general population.
A useful classification is the recognition that SVT may occur in two forms, with and without varicose veins; alternatively, SVT may be primary, involving the vein wall only, or secondary, involving a more systemic inflammatory process. Primary SVT is most common in the saphenous veins and their tributaries, followed by the upper extremity cephalic and basilic veins. The great saphenous vein (GSV) is affected in 60% to 80% of cases, followed by the small saphenous vein (SSV) in 10% to 20% and bilateral lower extremity SVT in 5% to 10%.6 Patients with varicose veins are affected far more frequently than in the general population, with a prevalence of SVT ranging from 4% to 62%.3,5,6
Risk Factors
Risk factors can be classified in accordance with Virchow’s triad: endothelial injury from trauma or insertion of venous catheters; venous stasis as seen in varicosities; and hypercoagulable states such as factor V Leiden, prothrombin G mutation, protein C and protein S deficiency, antithrombin III abnormalities, and malignant neoplasms including both solid tumors and hematologic conditions of Hodgkin’s lymphoma, leukemia, thrombocytosis, polycythemia vera, cryoglobulinemia, and nocturnal paroxysmal hemoglobinuria.6,7 Patients in whom SVT develops without an inciting physical event or varicosities may need to be fully evaluated for the presence of such disorders.
Other secondary causes for the development of SVT include the use of oral contraceptives, hormonal replacement therapy, pregnancy, obesity, prolonged immobilization, recent surgery, trauma, sclerotherapy, history of venous thromboembolism (VTE), some drugs (e.g., diazepam, amiodarone, vancomycin, heroin, some chemotherapy), and intravenous catheter use with or without bacterial infection.3,5–8 In addition, patients with autoimmune disorders including systemic lupus erythematosus and vasculitis, such as Behçet’s disease and Buerger’s disease, have also been identified as being susceptible to the development of SVT. A 2006 review of 2319 patients with Behçet’s disease found that 14.3% of these patients had vascular involvement. Of these 332 patients, 53.3% had SVT and 29.8% had DVT.9 Patients with Buerger’s disease appear to have an increased incidence of SVT because the inflammatory process involves small arteries and veins of the extremities. It can be diagnosed from biopsy findings of acute superficial thrombophlebitis showing the characteristic acute-phase lesion—inflammation of all three layers of the vessel wall with occlusive cellular thrombosis.10
Involvement of Deep Veins
The main concern related to SVT is the likelihood that the thrombus will extend into the deep veins, causing DVT and potential PE. Several older studies have evaluated this risk especially in relation to the proximity of GSV SVT to the deep veins. Chengelis and colleagues observed 263 patients with isolated SVT and performed follow-up ultrasound at 2 to 10 days (mean, 6.3 days).11 Thirty (11%) had progression to DVT while not receiving anticoagulation. The most common site was propagation of the SVT in the GSV into the common femoral vein. In a small retrospective review, 185 patients with SVT and 370 age- and sex-matched controls were evaluated.12 A minority (13%) received any nonsteroidal anti-inflammatory drug (NSAID) or rarely low-molecular-weight heparin (LMWH) therapy. At 6 months, overall 2.7% had developed DVT and 0.5% PE. SVT conferred a 10 times increased risk for development of DVT compared with controls without SVT. This study represents an estimate of the natural history of SVT because few patients received pharmacologic management. Another focused study evaluated the incidence of PE in 21 patients with isolated SVT in the thigh.13 Nuclear perfusion lung scans were performed within 3 hours of SVT diagnosis and demonstrated PE in seven patients (33%), including one symptomatic patient. Of note, there were no significant differences in the distance of the SVT from the common femoral vein or the presence of common risk factors for thrombosis compared with those with SVT who did not have PE. Although the study was small, these findings highlight the potential serious consequences of SVT and suggest the need for anticoagulant therapy to prevent these complications.
Two large studies have evaluated the natural history of SVT in patients, most of whom received medical therapy. In the POST (Prospective Observational Superficial Thrombophlebitis) trial, Decousus and colleagues prospectively observed a cohort of 844 consecutive patients with symptomatic SVT of the lower limbs confirmed by ultrasound testing.14 Patients with surgery within 10 days or SVT due to sclerotherapy in the previous 30 days were excluded. Patients were initially assessed for concomitant DVT, and then those with isolated SVT (634 patients) were observed with ultrasound again at 10 days and then at 3 months. A secondary outcome was overall mortality at 3 months. DVT or PE was confirmed in 24.9% of patients with SVT (82 patients with proximal DVT, 33 patients with PE). In 41.9% of patients with DVT, both proximal and distal, the DVT was not contiguous with the SVT. Of 634 patients with SVT, 90.5% received one or more anticoagulant medications, mostly LMWH administered at therapeutic doses (62.9%). Sixty patients (10.2%) had venous surgery. Fourteen patients were lost to follow-up. Of the remaining 586 patients, 58 (10.2%) had thromboembolic complications, including 7 with symptomatic proximal DVT, 3 with PE, and 5 with extension of SVT to DVT. Multivariate analysis showed that male sex, history of DVT or PE, previous cancer, and no varicose veins were independent risk factors for symptomatic VTE at 3 months, including recurrence or extension of SVT.
The OPTIMEV study evaluated 788 patients with SVT of 8256 patients who were referred for VTE.15 The median age of these patients was 65 years; 36% were men, and 16% were inpatients. Similar to the POST study, 29% were found to have concomitant DVT. Patients with both SVT and DVT had risk factors of presence of non-varicose veins involved, age older than 75 years, inpatient status, and active cancer compared with isolated SVT, which was independently associated with anticoagulant treatment at inclusion and pregnancy or postpartum state. Compared with SVT of varicose veins, SVT in non-varicose veins was a strong risk factor for concurrent DVT but did not confer a higher risk of 3-month adverse outcomes of death, VTE recurrence, and bleeding. In this study, 76% of those with isolated SVT were treated with anticoagulants, 92.5% with full-dose LMWH.
Thrombophilia
More recently, there has been greater awareness of the presence of hypercoagulable states in patients with SVT. These patients have a higher probability for development of DVT and recurrent SVT and may require long-term anticoagulation to prevent complications. Milio and colleagues evaluated 107 patients with unprovoked SVT for common thrombophilic conditions.16 The patients underwent duplex evaluation at baseline and every 48 hours for 8 days with notation of whether the SVT occurred in varicose or non-varicose veins. In the overall cohort, factor V Leiden was detected in 22.4%, MTHFR in 17.7%, and factor II G20210A mutation in 8.4%. Patients with thrombophilia and SVT in non-varicose veins had a higher rate of extension of thrombus to deep veins. However, because all patients were treated with either LMWH or NSAIDs and the results were not analyzed by treatment group, it is difficult to make definitive conclusions about the role of thrombophilia and DVT extension. Similar studies have supported the presence of acquired and inherited thrombophilic disorders (such as factor V Leiden and prothrombin G20210A gene mutations; deficiencies of antithrombin, heparin cofactor 2, protein C, and protein S; lupus anticoagulant; anticardiolipin antibodies; and abnormal fibrinolytic activity) as being a risk factor for the development of SVT.3,5,9,10,17–19
Even though a great deal of literature has described the pathophysiologic mechanism and various changes that take place in leukocyte–vessel wall interactions, cytokines/chemokines, and various other factors involved in the development and resolution of DVT, data on changes in patients with SVT were not identified. Although some authors have alluded to the observation that the underlying pathologic processes of SVT and DVT may be analogous, this viewpoint remains mostly unsupported to date.
Clinical Presentation
Evaluation for SVT by physical examination is based on the presence of erythema and tenderness in the distribution of the superficial veins, with the thrombosis being suspected by a palpable cord. Pain, erythema, and swelling are the most common symptoms.20 A number of conditions have unique risk factors for clinical presentations of SVT.
Superficial Thrombophlebitis with Varicose Veins
The most common predisposing risk factor for the development of SVT is varicose veins. It has been reported that DVT will develop in only 3% to 20% of SVT patients with varicose veins compared with 44% to 60% of SVT patients without varicose veins.21,22 Therefore, it appears that the pathophysiologic mechanism in patients with varicose veins may be different from that in patients without varicose veins.
It is essential to address patients with SVT involving varicose veins. This type of SVT may remain localized to the cluster of tributary varicosities or may extend into the GSV.5,6,10,23,24 SVT of varicose veins themselves may occur without antecedent trauma. SVT is frequently found in varicose veins in conjunction with venous stasis ulcers. This diagnosis should be confirmed by duplex ultrasound because the degree of SVT may be much greater than that based solely on clinical examination. SVT in varicosities may be manifested as tender nodules with localized induration and erythema.
Traumatic Thrombophlebitis
Traumatic SVT is often seen in individuals using drugs or undergoing drug therapy in a hospital or outpatient setting. It is associated with direct endothelial injury from the intravenous catheter used for the infusion of medications and irritating solutions, particularly when the indwelling catheter has been in place for long periods. Its onset is usually heralded by the development of pain, tenderness, and erythema at the site of catheter insertion or infusion. Treatment usually consists of cessation of the infusion, removal of the offending access device, and sometimes anticoagulation, depending on the severity of symptoms and underlying hypercoagulable condition. The induration may take up to weeks to months to resolve.
Septic and Suppurative Thrombophlebitis
Suppurative SVT is also associated with the use of an intravenous cannula; however, it may cause additional morbidity because of its association with septicemia. Signs and symptoms of suppurative SVT include pus at an intravenous site, fever, leukocytosis, and local intense pain.25 Aerobic, anaerobic, and mixed infections have been reported. Organisms associated with suppurative SVT include Staphylococcus aureus, Pseudomonas spp, Klebsiella spp, Enterococcus spp, Fusobacterium spp, and recently fungi such as Candida spp.24,25 Treatment begins with removal of the foreign body and intravenous administration of antibiotics. Excision of the vein is rarely needed to clear the infection.
Migratory Thrombophlebitis
Migratory thrombophlebitis was first described by Jadioux in 1845 as an entity characterized by repeated thrombosis developing in superficial veins at varying sites but most commonly in the lower extremity.24 This entity may be associated with carcinoma (Trousseau’s syndrome) and may precede diagnosis of the carcinoma by several years. Consequently, evaluation for occult malignant disease is warranted when the diagnosis of migratory thrombophlebitis is made. Migratory thrombophlebitis also occurs in the presence of some forms of vasculitis, such as Behçet’s disease, Buerger’s disease, and polyarteritis nodosa.7
Mondor’s Disease
Mondor’s disease is defined as thrombophlebitis of the thoracoepigastric vein of the breast and chest wall. It is thought to be associated with breast carcinoma or hypercoagulable states, although cases have been reported with no identifiable cause.18 Tender, cordlike structures can be seen extending from the lower portion of the breast toward the costal margin or in the anterolateral aspect of the breast.26 The condition is considered benign and self-limited and treatment is conservative, rarely involving systemic anticoagulation.27
The term Mondor’s disease has also been applied to SVT of the dorsal vein of the penis. This phenomenon occurs in patients with DVT, after hernia operations, and in association with excessive sexual intercourse. Treatment consists of NSAIDs and dorsal penile vein resection if the symptoms are refractory.28
Small Saphenous Vein Superficial Thrombophlebitis
Although the bulk of attention has been focused on SVT of the GSV, SVT of the SSV is also of clinical import. SSV SVT may progress to popliteal DVT. In a group of 56 patients with SSV SVT, 16% had PE or DVT.23 In one study, it was found that 65.6% of 32 patients with SSV SVT also had DVT during a 1-year period.29 Therefore, it is crucial that patients with SSV SVT be treated similarly to those in whom GSV SVT is diagnosed—the same careful duplex examination, follow-up, and anticoagulation or ligation if the SVT approaches the popliteal vein.
Upper Extremity Superficial Thrombophlebitis
Although very little appears in the literature, upper extremity SVT is believed to be the result of intravenous cannulation and infusion of caustic substances that damage the endothelium. Typically, the basilic or cephalic veins are involved, particularly if the catheter is inserted at or near the antecubital fossa. Interestingly, progression of upper extremity SVT to upper extremity DVT or PE is less common compared with lower extremity SVT.30 Initial treatment of upper extremity SVT is catheter removal followed by conservative measures, with anticoagulation occasionally required.
Superficial Thrombophlebitis after Endovascular Venous Obliteration
Minimally invasive endovascular techniques have emerged and gained popularity as alternatives to GSV ligation and stripping for GSV insufficiency and treatment of varicose veins. Endovenous laser treatment (EVLT) and radiofrequency ablation (RFA) have become widespread because of superior pain scores and feasibility as office-based procedures. In a prospective study of 67 patients undergoing EVLT and 66 patients undergoing RFA of the GSV or SSV, the rate of SVT was 1.5% in the EVLT group compared with 0% in the RFA group.31 In a prospective study of 155 patients who underwent RFA of the GSV or SSV, only 1 patient (1.4% treated veins) developed SVT, which occurred at a puncture site.32 The incidence of SVT was higher in 150 patients who underwent SSV RFA (4%).33 Zuniga and colleagues also reported a higher incidence of SVT of 10% to 15% in 667 RFA procedures of the GSV.34 A meta-analysis of 104 studies evaluating the use of endovenous foam sclerotherapy for the treatment of venous disorders found an incidence of SVT of 11%.35 This is somewhat higher compared with what has been reported for other endovenous procedures. Similarly, in a large randomized prospective study of 500 consecutive patients comparing EVLT, RFA, foam sclerotherapy, and stripping for GSV varicose veins, SVT occurred in 4 patients (3.2%) undergoing EVLT, 12 (9.6%) patients undergoing RFA, 17 patients (13.7%) with foam sclerotherapy, and 5 patients (4%) with surgical stripping.36
Diagnosis
A positive diagnosis of SVT is primarily established by identifying the clinical and physical signs described earlier. Typically, pain, erythema, tenderness, or induration corresponding to one or more superficial veins or dilated varicose veins is evidence with possible edema over the inflamed vein. Painful “cords” are palpable, and fever or leukocytosis may be present in association with a systemic infection. The inflammatory reaction may last 2 to 3 weeks, and either vein fibrosis or vein recanalization occurs in the ensuing 6 to 8 weeks. Postinflammatory hyperpigmentation may occur and last several months.7 However, the potential presence of occult or symptomatic DVT or PE concomitantly with SVT warrants further imaging studies. A small study found that recurrent SVT correlated with thrombus in tributary veins, but other risk factors of varicose veins, previous DVT, cancer, and hypercoagulable state were not predictive.37
Duplex ultrasound scanning has become the initial test of choice for the diagnosis of DVT and evaluation of SVT since it was first introduced by Talbot in 1982 (Fig. 54-1). The availability of reliable duplex ultrasonography of the deep and superficial venous systems has made routine determination of the location and incidence of DVT in association with SVT precise and practical (see Chapter 18). Furthermore, the extent of involvement of the deep and superficial systems can be more accurately assessed with this protocol. Duplex ultrasound imaging also offers the advantage of being inexpensive and noninvasive and can be repeated for follow-up examination.
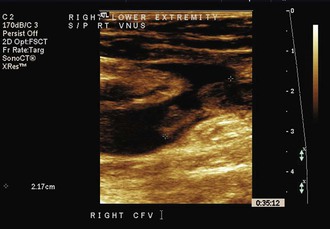
Figure 54-1 Ultrasound image of thrombophlebitis with free-floating thrombus extending from the great saphenous vein into the femoral vein after radiofrequency ablation of the saphenous vein.
Because venography may contribute to the onset of phlebitis and duplex imaging affords an accurate diagnosis, venography is not a first choice for investigation.
Although initial studies generated enthusiasm for the use of scintigraphy and computed tomographic venography, their poor specificity and lack of anatomic detail have led to duplex ultrasonography’s remaining the primary diagnostic modality for SVT.
D-dimer levels may or may not be elevated in patients with SVT, thus offering little assistance with the negative or positive predictive value of the disease. Blood tests in patients with SVT should be directed toward testing for acquired and congenital hypercoagulable states or malignant diseases if it is clinically indicated, particularly in patients with non-varicose vein SVT.
Treatment
Treatment depends on the cause and location of the SVT. The goals of therapy are to decrease the acute symptoms of SVT, including pain and erythema, and to prevent potential serious complications, such as DVT and PE as well as extension or recurrence of SVT. Historically, treatment was dictated by the proximity of the thrombus to the deep veins. Treatment for SVT has evolved from primarily surgical options to emphasis on medical treatment with the introduction of LMWH. Although warm compresses and compression hose have been recommended, there are few data supporting their use. Following is a discussion of the effectiveness of the most commonly used treatments for SVT.
Low-Molecular-Weight Heparin
One of the first trials evaluating modern medical management of SVT was the STENOX (Superficial Thrombophlebitis Treated by Enoxaparin) trial, a randomized double-blind trial of 427 patients with acute SVT of the legs at least 5 cm long.38 Patients were randomized to receive enoxaparin 40 mg daily, enoxaparin 1.5 mg/kg daily, oral tenoxicam 20 mg daily, or placebo for 8 to 12 days. Follow-up ultrasound was performed between days 8 and 12.
The study excluded patients who were at high risk. Reasons for exclusion were thrombophilia; a history of DVT, PE, or SVT; two or more episodes of SVT; a history of venous sclerotherapy; and pregnancy. The overall rate of DVT by day 12 was 3.6% in the placebo group, 0.9% in the 40-mg enoxaparin group, 1% in the 1.5-mg/kg enoxaparin group, and 2.1% in the tenoxicam group. The study found that 30% of patients in the placebo group eventually had the combined outcome of extension of the SVT toward the saphenofemoral junction, recurrent SVT, and progression to DVT or PE by day 12. Fifteen percent of the NSAID group, 8% of those receiving enoxaparin 40 mg, and 7% of those receiving enoxaparin 1.5 mg/kg had the combined outcome for thrombotic events, showing that all active treatment groups significantly reduced DVT and SVT progression. At 3-month follow-up after cessation of therapy, there was still a significant decrease in the combined outcome of DVT and SVT in the active treatment groups, but not in DVT alone. There were no episodes of major bleeding or death during the study. Overall, this study showed a significant risk for DVT and SVT extension in the absence of medical treatment and suggested that LMWH (either prophylactic or treatment dose) is the most effective therapy for prevention of thrombotic complications. The results suggested that the duration of therapy requires further evaluation because most of the recurrent VTE events in the treatment groups occurred after active treatment halted.
Since publication of the STENOX trial in 2003, there have been four additional randomized trials evaluating the use of medical therapy for SVT. The Vesalio Investigators randomized 164 consecutive patients with acute SVT of the GSV not within 3 cm of the saphenofemoral junction to nadroparin fixed prophylactic dose (2850 units) subcutaneously versus body weight–adjusted therapeutic doses of nadroparin for 1 month in a double-blind double-dummy fashion.39 The main outcome of VTE complications at 3 months occurred in seven patients receiving prophylactic doses compared with six patients receiving treatment doses (not significant). No patients in either group developed major bleeding. The CALISTO study group randomized 3002 patients with acute SVT not within 3 cm of the saphenofemoral junction to fondaparinux 2.5 mg subcutaneously daily or placebo for 45 days.40 On follow-up at day 47, the primary combined outcome of symptomatic VTE, death, extension to the saphenofemoral junction, or symptomatic SVT recurrence occurred in 13 or 1502 patients (0.9%) of the fondaparinux group and 88 of 1500 patients (5.9%) receiving placebo. Major bleeding occurred in one patient in each group. Except for the outcome of death, all components of the primary outcomes were significantly reduced in the fondaparinux group.
Rathbun and coworkers randomized 72 patients with acute SVT of the upper or lower limb regardless of proximity to the deep veins to dalteparin 200 units/kg body weight (maximum dose, 18,000 units) on day 1, then 10,000 units for up to 13 additional days, versus ibuprofen 800 mg three times daily for up to 14 days, with the primary outcome of SVT extension or VTE at 14 days and 3 months.41 Four patients receiving ibuprofen compared with no patients receiving dalteparin had thrombus extension at 14 days (P = .05); however, there was no difference in thrombus extension at 3 months. Both treatments significantly reduced pain. There were no episodes of major or minor bleeding during the trial. Of note, extension of thrombus in the time after ibuprofen and dalteparin were discontinued suggested that the treatment duration of 14 days may have been too brief or that there is a rebound phenomenon after the medication is halted. Finally, most recently, Cosmi and associates randomized 664 patients with SVT of the GSV or SSV or tributaries of at least 4 cm in length to parnaparin, 8500 units daily for 10 days followed by placebo for 20 days (group A), or 8500 units daily for 10 days followed by 6400 units daily for 20 days (group B), or 4250 units daily for 30 days (group C) in a double-blind fashion. The primary outcome of the composite of symptomatic and asymptomatic DVT, symptomatic PE, and relapse or symptomatic or asymptomatic SVT recurrence in the first 33 days with 60 days of follow-up occurred in 15.6% of group A, 1.8% of group B, and 7.3% of group C. No major hemorrhages occurred. The authors concluded that an intermediate dose of parnaparin for 30 days is superior to either a 30-day prophylactic dose or a 10-day intermediate dose for treatment of SVT.42
Nonsteroidal Anti-inflammatory Drugs
In addition to the trials discussed before (STENOX and Rathbun), the effectiveness of NSAIDs for SVT treatment has been the focus of a Cochrane systematic review.2 Five trials overall (including STENOX) were evaluated. This analysis found that NSAIDs significantly reduced the risk of SVT extension or recurrence by 67% (odds ratio, 0.33; 95% CI, 0.16-0.68), compared with placebo. However, there were no differences in the rate of VTE or resolution of symptoms. No episodes of major bleeding were recorded in these trials.
Surgery
For SVT within 1 cm of the saphenofemoral junction, management by high saphenous ligation with or without saphenous vein stripping has been suggested to be the treatment of choice because of the recognized potential for extension into the deep system and embolization.11,43–46 There have been only two randomized trials during the past 15 years that have evaluated surgery for the treatment of SVT. The first was a prospective study consisting of 444 evaluable patients randomized to six different treatment plans for the management of superficial thrombophlebitis: compression only; early surgery, with and without stripping; low-dose subcutaneous heparin; LMWH; and oral anticoagulant treatment.47 Patients with SVT and large varicose veins but without any suspected or documented systemic disorder were included in this study. Criteria for inclusion were venous incompetence (by duplex investigation), a tender indurated cord along a superficial vein, and redness and heat in the affected area. Exclusion criteria were obesity, cardiovascular or neoplastic diseases, nonambulatory status, bone or joint disease, problems requiring immobilization, age older than 70 years, and presence of superficial thrombophlebitis without varicose veins. Color duplex ultrasound scans were used to detect concomitant DVT and to determine extension or reduction of SVT at 3 and 6 months.
The incidence of SVT extension at 3 and 6 months was higher in the elastic compression alone group and in the saphenous ligation alone group (P < .05) compared with the groups with medical therapy or surgical therapy with ligation and stripping. There was no significant difference in the incidence of DVT at 3 months in any of the treatment groups. The results of this study are difficult to evaluate because the details of the treatment protocols were lacking. Furthermore, the exclusion criteria would eliminate many of the patients in whom SVT is routinely diagnosed in clinical practice, limiting the generalizability of the findings.
Lozano and Almazan randomized 60 patients with above-knee GSV SVT to saphenofemoral disconnection under local anesthesia with short-term compression bandage or enoxaparin 1 mg/kg twice weekly for 1 week, then once daily for 3 weeks.48 All patients in the trial were instructed to use compression stockings. Although the rate of VTE was less in the enoxaparin group (relative risk, 2; 95% CI, 0.01-4), it did not reach statistical significance. The rate of wound infections was 6.7% (2/30 patients) in the surgical group.
A systematic review of 6 studies (Belcaro et al47 and five small case series) including 246 patients altogether who received GSV ligation with or without stripping compared with 88 patients who received intravenous heparin followed by vitamin K antagonist for 6 weeks to 6 months showed no difference in SVT progression, DVT, or PE. The overall surgical complication rate including hematoma, seroma, and infection was 7.7%.
Topical Therapy
As part of the Cochrane systematic review mentioned earlier, seven trials that included topical treatment for SVT were evaluated.2 One of these small trials compared the use of a novel liposomal heparin spray versus LMWH (enoxaparin 40 mg daily subcutaneously) in 46 outpatients. The main outcomes measures of erythema size and duplex assessment of thrombus regression were comparable in the two groups; however, there were three cases of DVT in the heparin spray gel group compared with one in the LMWH group. To date, no further follow-up studies have been published, and the spray is not available for commercial use. Other topical treatments that have been evaluated include methylthioadenosine, diclofenac gel, and etofenak gel. Although these treatments showed reduction in local signs and symptoms, none of these studies evaluated the effect on VTE or SVT extension.
Other therapies that have been evaluated in small trials include oral vasotonin, venoruton, oral heparan sulfate, oral sulodexide, oxyphenbutazone, oral vitamin K antagonists, enzyme therapy, and desmin.2 Most provided local symptomatic relief.
To summarize, LMWH seems to provide the best outcomes with the fewest complications. However, questions remain about the duration of therapy, with 1 week likely being too short. The most recent American College of Chest Physicians guidelines give the following recommendations for treatment of patients with SVT of the lower limb49:
Selected Key References
Decousus H, Prandoni P, Mismetti P, Bauersachs RM, Boda Z, Brenner B, Laporte S, Matyas L, Middeldorp S, Sokurenko G, Leizorovicz A. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. [CALISTO Study Group] N Engl J Med. 2010;363:1222–1232.
This study reports the results of a large double-blind randomized trial evaluating low-dose fondaparinux for the treatment of superficial thrombophlebitis.
Decousus H, Quéré I, Presles E, Becker F, Barrellier MT, Chanut M, Gillet JL, Guennequez H, Leandri C, Mismetti P, Pichot O, Leizorovicz A. Superficial venous thrombosis and venous thromboembolism. [POST (Prospective Observational Superficial Thrombophlebitis) Study Group] Ann Intern Med. 2010;152:218–224.
This manuscript reports the prevalence of venous thromboembolism in a large prospective cohort of patients with superficial thrombophlebitis and describes risk factors for the disease.
Galanaud JP, Genty C, Sevestre MA, Brisot D, Lausecker M, Gillet JL, Rolland C, Righini M, Leftheriotis G, Bosson JL, Quere I. Predictive factors for concurrent deep-vein thrombosis and symptomatic venous thromboembolic recurrence in case of superficial venous thrombosis. The OPTIMEV study. [OPTIMEV SFMV investigators] Thromb Haemost. 2011;105:31–39.
This study evaluated the predictive risk factors for concurrent deep venous thrombosis for patients with superficial thrombophlebitis.
Kearon C, Akl EA, Comerota AJ, Prandoni P, Bounameaux H, Goldhaber SZ, Nelson ME, Wells PS, Gould MK, Dentali F, Crowther M, Kahn SR. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. [American College of Chest Physicians] Chest. 2012;141:e419S.
This guideline gives the most recent recommendations from the American College of Chest Physicians evidence-based practice guidelines regarding the treatment of superficial thrombophlebitis.
Prandoni P, Tormene D, Pesavento R. High vs. low doses of low-molecular-weight heparin for the treatment of superficial vein thrombosis of the legs: a double-blind randomized trial. [Vesalio Investigators Group] J Thromb Haemost. 2005;3:1152–1157.
Superficial Thrombophlebitis Treated by Enoxaparin Study Group. A pilot randomized double-blind comparison of a low-molecular-weight heparin, a nonsteroidal anti-inflammatory agent, and placebo in the treatment of superficial vein thrombosis. Arch Intern Med. 2003;163:1657–1663.
This study reports the results of a double-blind randomized controlled trial of LMWH and NSAIDs versus placebo for the treatment of superficial thrombophlebitis.
The reference list can be found on the companion Expert Consult website at www.expertconsult.com.
References
1. DeWeese M. Nonoperative treatment of acute superficial thrombophlebitis and deep femoral venous thrombosis. Ernst CB, Stanley JS. Current therapy in vascular surgery. ed 2. Mosby: Philadelphia; 1996.
2. Di Nisio M, et al. Treatment for superficial thrombophlebitis of the leg. Cochrane Database Syst Rev. 2007;(2).
3. Coon WW, et al. Venous thromboembolism and other venous disease in the Tecumseh community health study. Circulation. 1973;48:839–846.
4. Marchiori A, et al. Superficial vein thrombosis: risk factors, diagnosis, and treatment. Semin Thromb Hemost. 2006;32:737–743.
5. Leon L, et al. Clinical significance of superficial vein thrombosis. Eur J Vasc Endovasc Surg. 2005;29:10–17.
6. Meissner MH, et al. Acute venous disease: venous thrombosis and venous trauma. J Vasc Surg. 2007;46(Suppl S):25S–53S.
7. Kalodiki E, et al. Superficial vein thrombosis: a consensus statement. Int Angiol. 2012;31:203–216.
8. Quenet S, et al. Factors predictive of venous thrombotic complications in patients with isolated superficial vein thrombosis. J Vasc Surg.. 2003;38:944–949.
9. Sarica-Kucukoglu R, et al. Vascular involvement in Behçet’s disease: a retrospective analysis of 2319 cases. Int J Dermatol. 2006;45:919–921.
10. Olin JW, et al. Thromboangiitis obliterans (Buerger’s disease). Curr Opin Rheumatol. 2006;18:18–24.
11. Chengelis D, et al. Progression of superficial venous thrombosis to deep vein thrombosis. J Vasc Surg. 1996;24:745–749.
12. van Weert H, et al. Spontaneous superficial venous thrombophlebitis: does it increase risk for thromboembolism. J Fam Pract. 2006;55:52–57.
13. Verlato F, et al. An unexpectedly high rate of pulmonary embolism in patients with superficial thrombophlebitis of the thigh. J Vasc Surg. 1999;30:1113–1115.
14. Decousus H, et al. Superficial venous thrombosis and venous thromboembolism. [POST (Prospective Observational Superficial Thrombophlebitis) Study Group] Ann Intern Med. 2010;152:218–224.
15. Galanaud JP, et al. Predictive factors for concurrent deep-vein thrombosis and symptomatic venous thromboembolic recurrence in case of superficial venous thrombosis. The OPTIMEV study. Thromb Haemost. 2011;105:31–39.
16. Milio G, et al. Superficial venous thrombosis: prevalence of common genetic risk factors and their role on spreading to deep veins. Thromb Res. 2008;123:194–199.
17. Hanson JN, et al. Saphenous vein thrombophlebitis (SVT): a deceptively benign disease. J Vasc Surg. 1998;27:677–680.
18. Marchiori A, et al. Low doses of unfractionated heparin for the treatment of superficial thrombophlebitis of the leg. A prospective, controlled, randomized study. Haematologica. 2002;87:523–527.
19. de Moerloose P, et al. Superficial vein thrombosis of lower limbs: influence of factor V Leiden, factor II G20210A and overweight. Thromb Haemost. 1998;80:239–241.
20. Gorski G, et al. Progress of local symptoms of superficial vein thrombosis vs. duplex findings. VASA. 2004;33:219–225.
21. Prountjos P, et al. Superficial venous thrombosis of the lower extremities co-existing with deep venous thrombosis. A phlebographic study on 57 cases. Int Angiol. 1991;10:63–65.
22. Bergqvist D, et al. Deep vein thrombosis in patients with superficial thrombophlebitis of the leg. Br Med J (Clin Res Ed). 1986;292:658–659.
23. Lutter KS, et al. Superficial thrombophlebitis diagnosed by duplex scanning. Surgery. 1991;110:42–46.
24. De Palma RG. Superficial thrombophlebitis. Rutherford R. Vascular surgery. ed 6. Elsevier Saunders: Philadelphia; 2005:2216–2220.
25. Hammond JS, et al. Suppurative thrombophlebitis: a new look at a continuing problem. South Med J. 1988;81:969–971.
26. Mayor M, et al. Mondor’s disease. Int J Dermatol. 2000;39:922–925.
27. Cesarone M, et al. Management of superficial vein thrombosis and thrombophlebitis: status and expert opinion document. Angiology. 2007;58:7s–15s.
28. Sasso F, et al. Penile Mondor’s disease: an underestimated pathology. Br J Urol. 1996;77:729–732.
29. Ascher E, et al. Lesser saphenous vein thrombophlebitis: its natural history and implications for management. Vasc Endovascular Surg. 2003;37:421–427.
30. Sassu GP, et al. A rare etiology for pulmonary embolism: basilic vein thrombosis. J Emerg Med. 1990;8:45–49.
31. Tesmann J, et al. Radiofrequency induced thermotherapy (RFITT) of varicose veins compared to endovenous laser treatment (EVLT): a non-randomized prospective study concentrating on occlusion rates, side-effects and clinical outcome. Eur J Dermatol. 2011;21:945–951.
32. Bisang U, et al. Results of endovenous ClosureFast treatment for varicose veins in an outpatient setting. Phlebology. 2012;27:118–123.
33. Huisman L, et al. Endovenous laser ablation of the small saphenous vein: prospective analysis of 150 patients, a cohort study. Eur J Vasc Endovasc Surg. 2009;38:199–202.
34. Zuniga J, et al. Short-term outcome analysis of radiofrequency ablation using ClosurePlus vs ClosureFast catheters in the treatment of incompetent great saphenous vein. J Vasc Surg. 2012;55:1048–1051.
35. Rathbun S, et al. Efficacy and safety of endovenous foam sclerotherapy: meta-analysis for treatment of venous disorders. Phlebology. 2012;27:105–117.
36. Rasmussen L, et al. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg. 2011;98:1079–1087.
37. Gorty S, et al. Superficial venous thrombosis of the lower extremities; analysis of risk factors, and recurrence and role of anticoagulation. Vasc Med. 2004;9:1–6.
38. Superficial Thrombophlebitis Treated by Enoxaparin Study Group. A pilot randomized double-blind comparison of a low-molecular-weight heparin, a nonsteroidal anti-inflammatory agent, and placebo in the treatment of superficial vein thrombosis. Arch Intern Med. 2003;163:1657–1663.
39. Prandoni P, et al. High vs. low doses of low-molecular-weight heparin for the treatment of superficial vein thrombosis of the legs: a double-blind randomized trial. [Vesalio Investigators Group] J Thromb Haemost. 2005;3:1152–1157.
40. Decousus H, et al. Fondaparinux for the treatment of superficial-vein thrombosis in the legs. N Engl J Med. 2010;363:1222–1232.
41. Rathbun S, et al. A randomized trial of dalteparin compared with ibuprofen for the treatment of superficial thrombophlebitis. J Thromb Haemost. 2012;10:833–839.
42. Cosmi B, et al. A randomized double-blind study of low-molecular-weight heparin (parnaparin) for superficial vein thrombosis: STEFLUX (Superficial ThromboEmbolism and Fluxum). [STEFLUX Investigators] J Thromb Haemost. 2012;10:1026–1035.
43. Husni EA, et al. Superficial thrombophlebitis of lower limbs. Surgery. 1982;91:70–73.
44. Lofgren EP, et al. The surgical treatment of superficial thrombophlebitis. Surgery. 1981;90:49–54.
45. Gjores JE. Surgical therapy of ascending thrombophlebitis in the saphenous system. Angiology. 1962;13:241–243.
46. Plate G, et al. Deep venous thrombosis, pulmonary embolism and acute surgery in thrombophlebitis of the long saphenous vein. Acta Chir Scand. 1985;151:241–244.
47. Belcaro G, et al. Superficial thrombophlebitis of the legs: a randomized, controlled, follow-up study. Angiology. 1999;50:523–529.
48. Lozano F, et al. Low-molecular weight heparin versus saphenofemoral disconnection for the treatment of above-knee greater saphenous thrombophlebitis: a prospective study. Vasc Endovascular Surg. 2003;37:415–420.
49. Kearon C, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141:e419S.