Chapter 20 Sunscreens
CHEMICAL SUNSCREENS
The FDA-approved chemical sunscreens and the maximum allowed concentration for each are listed in Table 20.1. These ‘sunscreen active ingredients’ are defined as absorbing, reflecting, or scattering radiation in the ultraviolet range at wavelengths of 290–400 nm. The chemical (also called organic or soluble) sunscreen active ingredients prevent sunburn by absorbing UV radiation as photons of light energy that are transformed into harmless long wave radiation and then re-emitted as heat. The FDA defined maximum, rather than minimum, concentrations of each to avoid subjecting consumers to unnecessarily high levels of any active ingredient in sunscreen combination products. This provision also recognizes that final product testing, not the concentration of each active ingredient, determines efficacy.
| Active ingredient | Maximum concentration (%) |
| Aminobenzoic acid (PABA) | 15 |
| Avobenzone | 3 |
| Cinoxate | 3 |
| Dioxybenzone | 3 |
| Ecamsule | 10 |
| Homosalate | 15 |
| Methyl anthranilate | 5 |
| Octocrylene | 10 |
| Octyl methoxycinnamate | 10 |
| Octyl salicylate | 5 |
| Oxybenzone | 6 |
| Padimate O | 8 |
| Phenylbenzimidazole sulfonic acid | 4 |
| Sulisobenzone | 10 |
| Trolamine salicylate | 12 |
PHYSICAL SUNSCREENS
Traditionally, physical agents used to prevent sunburn were called ‘sunblocks’ while chemical agents were ‘sunscreens’. The terminology is misleading because it suggests that the former merely scatter or reflect UV radiation. In fact, the physical (also called inorganic or insoluble) agents, titanium dioxide and zinc oxide, also act as semiconductors that absorb UV radiation and release it as heat. The use of the term ‘chemical-free’ for sunscreens containing only physical, not chemical, sunscreen agents is also confusing for consumers, since all active and inactive ingredients have been obtained and/or combined through some chemical process. The FDA-approved maximum concentration of these agents in sunscreen is listed in Table 20.2.
| Active ingredient | Maximum concentration (%) |
| Titanium dioxide | 25 |
| Zinc oxide | 25 |
RATING EFFICACY
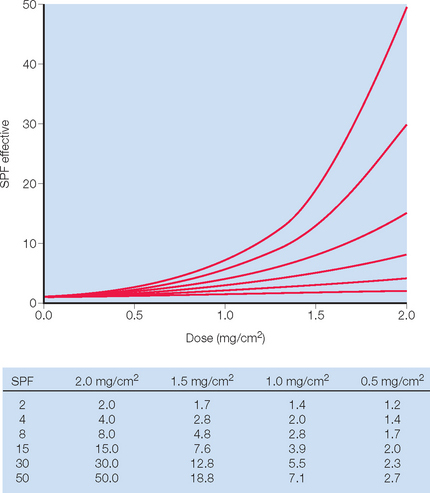
Sunscreen ingredients differ in their absorption spectrum, as shown in Table 20.3. Ideally a sunscreen should provide protection against the full spectrum of ultraviolet radiation. Until now most of the focus of the FDA’s attention has been on reducing exposure to UVB light. The sun protection factor (SPF), which measures UVB protection, was the only internationally standardized measure of a sunscreen’s ability to filter UV radiation. It is the ratio of the UV energy required to produce a minimal erythema dose (MED) on sunscreen-protected skin to the UV energy required to produce a MED on unprotected skin (Box 20.1). The MED is the quantity of energy required to produce the first perceptible redness reaction of the skin with clearly defined borders. Energy is delivered utilizing a filtered light source simulating the solar emission spectrum, with 94% of its output between 290 and 400 nm. (This mimics sunlight at sea level at a zenith angle of 10°.) For any given product, measurement must be done on between 20 and 25 test subjects of Fitzpatrick skin types I, II, and III. Test material is applied to an area of at least 50 cm2 at a thickness of 2 mg/cm2.
Table 20.3 Absorbance range of selected sunscreen active ingredients
| Sunscreen | Absorbance range (nm) |
| Homosalate | 300–310 |
| Octyl salicylate | 300–310 |
| Aminobenzoic acid (PABA) | 260–313 |
| Padimate O | 290–315 |
| Methyl anthralinate | 290–320 |
| Phenylbenzimidazole sulfonic acid | 290–320 |
| Trolamine salicylate | 260–320 |
| Cinoxate | 270–328 |
| Octyl methoxycinnamate | 270–328 |
| Oxybenzone | 270–350 |
| Sulisobenzone | 270–360 |
| Dioxybenzone | 260–380 |
| Zinc oxide | 250–380 |
| Avobenzone | 310–400 |
| Ecamsule | 290–400 |
| Titanium dioxide | 250–400 |
The SPF of a given OTC topical sunscreen is determined by testing of that product as above. In accordance with FDA regulations, multiple sun protective active ingredients can be combined as long as each contributes a minimum SPF of at least 2 to the finished product. This requirement is meant to avoid the addition of unnecessary ingredients. FDA will revise some of the existing SPF testing procedures to decrease the health risk to persons enrolled in the SPF test and further enhance accuracy of SPF values.
Regulations concerning UVA were delayed until recently since reliable testing methodologies were not available. However, the new proposed regulations address manufacturing, testing, and labeling of UVA sunscreens. Sunscreen manufacturers will now have to post the degree of protection afforded by a particular product against UVA rays. The scale of one to four stars corresponding to low, medium, high or very high UVA protection is to be prominently displayed on OTC sunscreen products near the SPF rating (Figs 20.1 and 20.2). Because consumers are familiar with SPF numbers, the FDA believes there may be confusion if UVB and UVA protection levels were both identified by numbers. Since star rating has been used in a variety of industries (e.g. hotels and restaurants), the FDA expects consumers will learn how to use this information to select the appropriate sunscreen, as they have done with SPF values. Furthermore, the product will bear a ‘no UVA protection’ marking on the front label if the sunscreen does not provide even a low level of protection.
Fig. 20.1 The principal display panel.
Adapted from http://www.fda.gov/cder/drug/infopage/sunscreen/qa.htm

Fig. 20.2 Proposed labeling configuration for UVA protection.
Adapted from http://www.fda.gov/cder/drug/infopage/sunscreen/qa.htm
Table 20.4 outlines the in vivo and in vitro options available. Immediate pigment darkening (IPD) measures the transient brown color that appears and fades within minutes of UVA exposure in vivo in darker skin types. The persistent pigment darkening (PPD) technique has an easier to assess endpoint: pigment due to melanin oxidation that can be measured 24 hours after exposure in vivo. The protection factor in the UVA (PFA) method is also read in vivo at 24 hours and assesses either erythema or tanning. In vitro, the critical wavelength (λc) below which 90% of a sunscreen’s UV absorbance occurs is between 290 and 400 nm. That means that a product with a λc of 340 nm would filter UVB and UVA II, but not UVA I.
Table 20.4 Methods of testing UVA protection
| Test | Skin types used | Time of reading |
| In vivo | ||
| Immediate pigment darkening (IPD) | III–V | Immediate |
| Persistent pigment darkening (PPD) | II–IV | 24 hours |
| Protection factor in the UVA (PFA or APF) | I–IV | 24 hours |
| In vitro | ||
| Critical wavelength (λc) | ||
DOSAGE AND USAGE
The current FDA standard for sunscreen application is 2 mg/cm2 and yet studies suggest that actual usage is only 25–50% of the amount used to rate sunscreen SPF. The main problem with sunscreen effectiveness is the nonlinear relationship between the SPF rating and the amount applied to the skin (Fig. 20.3). Sunscreen application has been shown to be as little as 0.5 mg/cm2 which would translate to an SPF of 8 or 15 when applying an SPF product of 30. To cover the average 1.73 m2 adult, a total of 35 mL of sunscreen is required.
The question then centers on why there is such a large discrepancy between how much sunscreen is needed and the amount actually used. There are numerous possibilities, starting with the consumer. Some sunscreen users feel uncomfortable at the recommended doses. Products may feel thick and occlusive, and at the appropriate dose can look opaque. There is a lack of education and specific instructions. Directions on sunscreen labels can be vague, with language such as ‘apply liberally to all exposed areas prior to sun exposure’. The public may not understand how much is actually required, or may not have a good grasp on what 35 mL translates to clinically. This should be countered by relating the amount of product required with a common measurement that individuals can understand. Schneider (Fig. 20.4) suggests the ‘teaspoon rule’ based on the rule of nines used for calculating burn areas. With the teaspoon rule, an adult should apply approximately one-half teaspoon to each arm and to the face and neck. Six milliliters (just a little more than a teaspoon) should be applied to each leg, the chest, and to the back. This would amount to 33 mL being used. Another measurement most adults understand is the shot glass used to mix drinks. A shot glass is 30 mL and would approximate the requirement. Another method employed by some physicians is to recommend that patients apply twice or to put on two layers. This should increase the amount applied and may help to reduce the number of ‘missed’ or ‘skipped’ areas. One of the biggest obstacles to proper dosing, however, is the desire by many Americans and Europeans to have tanned skin.
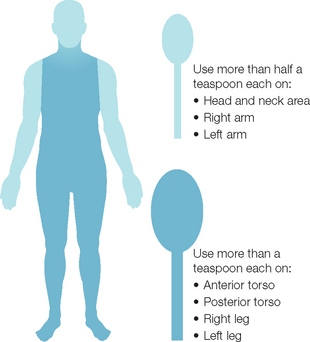
Fig. 20.4 The ‘teaspoon rule’ can be used to guide patients on the appropriate amount of sunscreen required
Besides the amount of sunscreen applied, there are a number of factors that also influence the protection equation and the best dosing regimen. These include resistance to water immersion and sand abrasion, reapplication, and the way products are applied. A mathematical study examined the relative importance of three sunscreen related factors: the amount of sunscreen applied, how the sunscreen was spread, and the UVA absorbing property of sunscreen. Diffey calculated that approximately 75% of the variance in UVA photoprotection achieved clinically depends upon how much product is applied. How well the product is applied and how well it absorbs UVA contributed almost equally to the remaining 25% of the variance.
ADVERSE EVENTS
Adverse events can be divided into direct reactions (Box 20.2) such as contact allergic reactions, and indirect sequelae such as increased UV exposure. The latter are more difficult to quantify and, to date, are less well defined.
• Direct adverse events
Contact irritant reactions are by far the most common adverse event. Over 90% of cosmetic side effects are irritant reactions and half of these are subjective only. Also, some products may aggravate pre-existing conditions such as acne rosacea, atopic dermatitis, and seborrheic dermatitis. A true contact allergic reaction is typically seen 48 hours after exposure and, like other delayed hypersensitivity reactions, presents with a spongiotic dermatitis. The offending agent acts as a hapten to bind with endogenous proteins and activates T lymphocytes. An immediate contact urticaria can be caused by sunscreens, with a wheal and flare reaction developing within 30–60 minutes after exposure. In one study using sunscreen or its vehicle, 19% (114 of 603 subjects) of the study population had adverse reactions. True allergic sensitization was seen in 6, while 45 patients developed aggravation of their atopic dermatitis, 39 had a contact irritation, and 22 had nonspecific cosmetic intolerance.
• Indirect adverse events
There has been additional concern that the use of sunscreen may actually increase the risk for developing melanoma, but an analysis of 14 studies published to date could not confirm an increased risk for melanoma developing after sunscreen use. There is concern that fair skinned individuals will increase their exposure to the sun with a false sense of security when using sunscreens. This is especially a problem if the sunscreen used has a high SPF rating but lacks adequate UVA coverage. Statistically US adolescents still have one or more summer sunburns and many adolescents reported having used sunscreen with a SPF of 15 or higher before receiving their most serious summer sunburn. These types of studies fail to demonstrate whether the teenagers would have sunburned without the use of a sunscreen, and would they indeed decrease risky sun behaviors if they did not have the aid of a sunscreen agent.
Thus the debate about indirect side effects is sure to continue for some time.
NEW RESEARCH
The most promising research, however, lies in the use of additives that have a synergistic property with the sunscreen. Most notably, the addition of antioxidants could potentially limit the damage from UV photons and help to repair some of the genetic damage induced by UV photons that have penetrated the skin. Vitamin C has been demonstrated to modestly protect against UVB photodamage and UVA-induced phototoxic responses. When combined with vitamin E there may be an even greater protection against cellular insult. Beta-carotene has been reported to be of value in the treatment of erythropoietic protoporphyria and may be able to inhibit UV-promoted carcinogenesis. Selenium compounds, when topically applied in concentrations of less than 0.05%, reduce UV skin damage measured as less inflammation, less pigmentation, and retardation of skin cancer. Chelates such as ortho-phenanthroline, edetic acid, and dipyridylamine bind metals such as iron, thus limiting their interactions with other materials and protecting against cellular damage from free radical oxygen. Topical chelate application prior to UV exposure is reported to reduce or delay visible skin wrinkling caused by UV exposure along with tumor formation.
Albert MR, Ostheimer KG. The evolution of current medical and popular attitudes toward ultraviolet light exposure: Part 3. Journal of the American Academy of Dermatology. 2003;49:1096–1106.
Al Mahroos MA, Yaar M, Phillips TJ, et al. Effect of sunscreen application on UV-induced thymine dimers. Archives of Dermatology. 2002;138:1480–1485.
Bastuji-Garin S, Diepgen T. Cutaneous malignant melanoma, sun exposure, and sunscreen use: epidemiological evidence. British Journal of Dermatology. 2002;146(Suppl 61):24–30.
Benech-Kieffer F, Meuling WJ, Leclerc C, et al. Percutaneous absorption of Mexoryl SX in human volunteers: comparison with in vitro data. Skin Pharmacology and Applied Skin Physiology. 2003;16:343–355.
Bischoff-Ferrari HA, Willett WC, Wong JB, et al. Fracture prevention with vitamin D supplementation: a meta-analysis of randomized controlled trials. Journal of the American Medical Association. 2005;293:2257–2264.
Bissonnette R, Allas S, Moyal D, et al. Comparison of UVA protection afforded by high sun protection factor sunscreens. Journal of the American Academy of Dermatology. 2000;43:1036–1038.
Chatelain E, Gabard B. Photostabilization of butyl methoxydibenzoylmethane (Avobenzone) and ethylhexyl methoxycinnamate by bis-ethylhexyloxyphenol methoxyphenyl triazine (Tinosorb S), a new UV broadband filter. Photochemistry and Photobiology. 2001;74:401–406.
Damiani E, Greci L, Parsons R, et al. Nitroxide radicals protect DNA from damage when illuminated in-vitro in the presence of dibenzoylmethane and a common ingredient. Free Radical Biology and Medicine. 1999;26:809–816.
Darlington S, Williams G, Neale R, et al. A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Archives of Dermatology. 2003;139:451–455.
Davies MJ. Reactive species formed on proteins exposed to singlet oxygen. Photochemical and Photobiological Sciences. 2004;3:17–25.
Deflandre A, Lang G. Photostability assessment of sunscreens. Benzylidene camphor and dibenzoylmethane derivatives. International Journal of Cosmetic Science. 1988;10:53–62.
DeLeo VA, Suarez SM, Maso MJ. Photoallergic contact dermatitis. Archives of Dermatology. 1992;128:1513–1518.
Diffey BL. Sunscreens, suntans, and skin cancer. People do not apply enough sunscreen for protection. British Journal of Dermatology. 1996;313:942.
Diffey BL. Sunscreen isn’t enough. Journal of Photochemistry and Photobiology B. 2001;64:105–108.
Diffey BL. Sunscreens and UVA protection: a major issue of minor importance. Photochemistry and Photobiology. 2001;74:61–63.
Diffey BL. When should sunscreen be reapplied? Journal of the American Academy of Dermatology. 2001;45:882–885.
Foley P, Nixon R, Marks R, Frowen K, Thompson S. The frequency of reactions to sunscreens: results of a longitudinal population-based study on the regular use of sunscreens in Australia. British Journal of Dermatology. 1993;128:512–518.
Fourtanier A, Labat-Robert J, Kern P, et al. In vivo evaluation of photoprotection against chronic ultraviolet-A irradiation by a new sunscreen Mexoryl SX. Photochemistry and Photobiology. 1992;55:549–560.
Farrerons J, Barnadas M, Rodríguez J, et al. Clinically prescribed sunscreen (sun protection factor 15) does not decrease serum vitamin D concentration sufficiently either to induce changes in parathyroid function or in metabolic markers. British Journal of Dermatology. 1998;139:422–427.
Green A, Williams G, Neale R, et al. Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet. 1999;354:723–729.
Hawk JL. Cutaneous photoprotection. Archives of Dermatology. 2003;139:527–530.
Kielbassa C, Epe B. DNA damage by UV and visible light and its wavelength dependence. Methods in Enzymology. 2000;319:436–445.
LeBoff MS, Kohlmeier L, Hurwitz S, et al. Occult Vitamin D deficiency in postmenopausal US women with acute hip fracture. Journal of the American Medical Association. 1999;281:1505–1511.
Lim HW, Naylor M, Honigsmann H, et al. American Academy of Dermatology Consensus Conference on UVA protection of sunscreens: summary and recommendations. Journal of the American Academy of Dermatology. 2001;44:505–508.
Marks R, Foley PA, Jolley D, et al. The effect of regular sunscreen use on vitamin D levels in an Australian population. Results of a randomized controlled trial. Archives of Dermatology. 1995;131:415–421.
Marrot L, Belaidi JP, Chaubo C, et al. An in vitro strategy to evaluate the phototoxicity of solar UV at the molecular and cellular level: application to photoprotection assessment. European Journal of Dermatology. 1998;8:403–412.
Meves A, Repacholi MH, Rehfuess EA. Promoting safe and effective sun protection strategies. Journal of the American Academy of Dermatology. 2003;49:1203–1204.
Mitchnick MA, Fairhurst D, Pinnell SR. Microfine zinc oxide (Z-cote) as a photostable UVA/UVB sunblock agent. Journal of the American Academy of Dermatology. 1999;40:85–90.
Moloney F, Collins S, Murphy G. Sunscreens safety, efficacy and appropriate use. American Journal of Clinical Dermatology. 2002;3:185–191.
Naylor M, Boyd A, Smith D, Cameron GS, Hubbard D, Neldner K. High sun protection factor sunscreens in the suppression of actinic neoplasia. Archives of Dermatology. 1995;131:170–175.
Osborne J, Hutchinson P. Vitamin D and systemic cancer: is this relevant to malignant melanoma? British Journal of Dermatology. 2002;147:197–213.
Ravanat JL, Martinez GR, Medeiros MH, et al. Mechanistic aspects of the oxidation of DNA constituents mediated by singlet molecular oxygen. Archives of Biochemistry and Biophysics. 2004;423:23–30.
Schneider J. The teaspoon rule of applying sunscreen. Archives of Dermatology. 2002;138:838–839.
Seite S, Moyal D, Richard S, et al. Mexoryl SX: a broad absorption UVA filter protects human skin from the effects of repeated suberythemal doses of UVA. Journal of Photochemistry and Photobiology B. 1998;44:69-76.
Sollitto RB, Kraemer KH, DiGiovanna JJ. Normal vitamin D levels can be maintained despite rigorous photoprotection: six years’ experience with xeroderma pigmentosum. Journal of the American Academy of Dermatology. 1997;37:942–947.
Szczurko C, Dompmartin A, Michel M, Moreau A, Leroy D. Photocontact allergy to oxybenzone: ten years of experience. Photodermatology, Photoimmunology and Photomedicine. 1994;10:144–147.
Tarras-Wahlberg N, Stenhagen G, Larko O, et al. Changes in ultraviolet absorption of sunscreens after ultraviolet irradiation. Journal of Investigative Dermatology. 1999;113:547–553.
US Food and Drug Administration. Center for Food Safety and Applied Nutrition, Office of Cosmetics and Colors Fact Sheet: Sunscreens, tanning products, and sun safety. Online. Available: http://www.cfsan.fda.gov/∼dms/cos-sun.html, 2003.
US Food and Drug Administration, HHS. Sunscreen drug products for over-the-counter human use; final monograph. Federal Register. 64(98), 1999.
Wolpowitz D, Gilchrest BA. The vitamin D questions: how much do you need and how should you get it? Journal of the American Academy of Dermatology. 2006;54:301–317.
Internet resources for FDA updates on sunscreens: http://www.fda.gov/consumer/updates/sunscreen082307.html