CHAPTER 81 Subthalamotomy in Parkinson’s Disease
Indications and Outcome
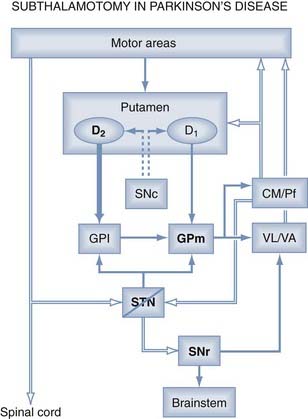
Subthalamic Nucleus as Target
In a classic paper, Whittier and Mettler1 described the consequences of an electrolytic lesion in the STN in monkeys. They induced hemiballism (which they termed hyperkinesia choreica) contralateral to the STN lesion and showed that two elements must be present for hyperkinetic movement to occur: minimal (>20%) destruction of the structure, and integrity of the globus pallidus (GP) and pallidothalamic pathway. One year later, Carpenter and coworkers2 showed that lesions of the GP or pallidal efferent eliminated the dyskinesias induced by STN lesions. These studies demonstrated the importance of this small structure in the origin of hyperkinesias. This correlation was already known in clinical practice, because patients with vascular insults in the STN developed a hemichorea-ballism (HCB) contralateral to the side of the injury.3 Accordingly, for a long time the STN was thought to exert an inhibitory effect on target nuclei because their lesioning induced a movement disorder. Subsequently in 1988, Smith and Parent4 first reported that the STN efferent projection shows glutamate inmunoreactivity, and Albin and associates5 confirmed the glutamate subthalamic pathway 1 year later. This finding was relevant because the STN is the only excitatory structure of the basal ganglia (BG) and consequently is capable of exciting BG output nuclei, GPi, and substantia nigra pars reticularis to thalamus.
Mitchell and coworkers6 from Manchester University described metabolic studies in the STN of monkeys using 2-deoxyglucose (2-DG) autoradiography with carbon 14 to map the metabolic changes associated with dyskinesia. Uptake of 2-DG is believed to represent an index of regional afferent synaptic activity. Their study demonstrated that the external globus pallidus (GPe)-STN pathway is overactive on the side contralateral to dyskinesia, causing inhibition of the STN and leading to reduced pallidal output to the thalamus.6 The same group found that monkeys previously treated with 1-methyl, 4-phenyl, 1,2,3,6-tetrahydropyridine (MPTP) showed a major decrease in 2-DG uptake, indicating reduced inhibition from the GPe to the STN in parkinsonian primates’ brains.7 The authors concluded that the STN plays a key role in both experimental parkinsonism and dyskinesias, operating in opposite functional directions.6,7 At the same time, Miller and DeLong8 used neuronal recording to demonstrate that the STN exhibits increased neuronal activity in MPTP-treated monkeys. Subthalamic hyperactivity was associated with increased GPi neuronal activity, which led to excessive inhibition of the motor thalamus and thalamocortical projections. Overall, these studies provided the basis for the pathophysiologic model of the BG, where STN activity plays a pivotal and opposite role in the origin of the major BG motor disorders.
The next strategy was to create an STN lesion in MPTP-treated monkeys to alleviate the parkinsonian condition. This was first reported by Bergman and colleagues,9 who found considerable alleviation of akinesia, rigidity, and tremor on the contralateral side of a chemically (ibotenic acid) induced lesion in two parkinsonian monkeys. One year later, Aziz and associates10 reported similar findings in six parkinsonian monkeys with STN thermolesions. Finally, Guridi and coworkers11 showed that subthalamic chemical lesions in parkinsonian monkeys improved their motor features and reduced the increased BG output activity, as shown by in situ hybridization for glutamic acid decarboxylase messenger RNA, in the GPi and substantia nigra pars reticularis. All these studies determined that ablation of the STN to reduce or eliminate hyperactivity in the parkinsonian state led to behavioral motor improvement (Fig. 81-1). Thanks to experimental research, a new target had been discovered, and it was paradoxical, given that the STN had been the most feared anatomic structure during early surgery for PD, before the introduction of levodopa.
Benazzouz and associates12 implanted electrodes in the STN for high-frequency stimulation in two parkinsonian monkeys and found a marked clinical improvement. Soon after, the Benabid group13 reported the first parkinsonian patient treated with STN-DBS, and in 1995 they described three cases showing a great improvement in motor symptoms.14 This report was the beginning of the STN-DBS era, which is where functional neurosurgery for PD still stands today. Subthalamic lesions and STN-DBS generally produce the same clinical response in terms of motor disability improvement. However, stimulation procedures do not result in lesions in the brain, and the reversibility and adjustability of such procedures are potential advantages. In contrast, ablative surgery does not require a second operation for battery implantation, and device-related complications, such as infection or adverse stimulation effects, are not a factor. In some patients, however, the ablative lesion may cause contralateral dyskinesia, which means that subthalamic nucleotomy is performed in relatively few patients compared with DBS procedures, owing to the fear of inducing HCB.
All these research findings led to a revitalization of surgery for PD and paved the way for a reevaluation of the STN as a potential surgical target.15 However, pallidotomy was the preferred approach when surgery for PD was revitalized in the early 1990s. This was based mainly on the surgical dogma that STN lesions are always associated with severe dyskinesias. Thus, early surgery during the 1960s relied on subthalamic area lesions, termed campotomies or subthalamotomy, as an alternative to thalamotomy for alleviating tremor and rigidity in parkinsonian surgical candidates.16–18 The surgical targets were Forel’s field, the zona incerta, and the prerubral field, but never the STN itself, for fear of inducing HCB. Indeed, early stereotactic surgery for PD (before the introduction of levodopa as medical treatment) established that surgeons should not perform any lesion below the intercommissural line because of the high risk of inducing dyskinetic movement. Postoperative HCB was a severe complication in some cases, and surgeons were reluctant to touch the STN.19,20 In patients with parkinsonism after encephalitis or after bilateral thalamotomies, the risk of developing dyskinesia was increased.20
Laitinen and coworkers’21,22 experience with pallidal lesions using Leksell’s target reestablished the GPi as a surgical target for advanced PD. All the cardinal features, such as tremor, rigidity, and bradykinesia, as well as levodopa-induced dyskinesias (LIDs), were improved on the side contralateral to the lesion. Pallidotomy was performed in the posteroventral and lateral portions of the GP, close to the putamen.22 However, it soon became apparent that the improvement conveyed by unilateral pallidotomy fell short of the needs of patients with advanced PD. This coincided with the early development of DBS, summarized earlier.
Surgical Procedure
Imaging and Target Selection
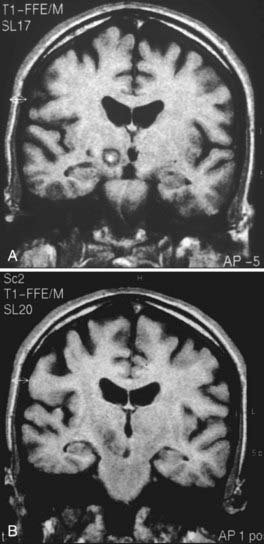
Magnetic resonance imaging (MRI) is performed 24 to 48 hours before surgery. T1- and T2-weighted sequences are used, with 2-mm-thick slices and no interspaces. On the day of surgery, the stereotactic frame is positioned with the use of local anesthesia, and a computed tomography (CT) scan is obtained (slices 2 mm thick with no interspaces); this information is sent to the neuronavigation workstation.23 The CT scan is fused with the T2-weighted image, and the target is selected by indirect and direct procedures. With indirect targeting, the anterior commissure–posterior commissure (AC-PC) line is measured, and the mid-intercommissural point (ICP) is located. The target located is 12 mm lateral (X coordinate) and 2 to 3 mm posterior to the ICP (Y coordinate) and 4 mm below the AC-PC line (Z coordinate). The STN can be identified as a hypointense almond-shaped nucleus located lateral to the red nucleus (RN) in the mesencephalon on axial T2-weighted images. This image target may be moved to a line crossing the anterior border of the RN approximately 3 mm from its lateral wall. The RN can be used as a landmark for the anteroposterior coordinate of the STN on axial T2-weighted images (direct targeting).24 After target selection, the anteroposterior and coronal arc is chosen, avoiding the ventricle wall.
Access and Electrophysiology
After the STN target has been chosen, the patient is placed in a semisitting position and administered local anesthesia. A bur hole (14 mm) is created 15 to 20 mm from the midline. Dural opening and cortical coagulation are performed while the surgical field is continuously sealed to prevent air embolism. The first electrophysiologic recording track is advanced until it reaches the nucleus. The beginning of the STN is defined by an abrupt and significant increase in electrical activity in the recording. During this part of the procedure, we look for the sensorimotor portion of the STN. Kinesthetic neurons with a response to contralateral limb movements and tremor cells (in patients with tremor) are located in the dorsolateral portion of the anatomic structure. Somatotopically, lower limb units are more medial than upper limb units, which are in the most lateral part of the STN, near the internal capsule.25 After recording with microelectrodes or semi-microelectrodes, a mapping of the nucleus is obtained.23 Nuclear length is an important parameter for lesion placement. Currently, there is no consensus on the best target, and different groups use different criteria to determine lesion location. The lesion must be placed in the sensorimotor portion of the STN (dorsolateral region), which is where the kinesthetic units are recorded; this is the same place used for electrode introduction in stimulation surgery.
Clinical Results
Antiparkinsonian Effect
Unilateral Subthalamotomy
Subthalamotomy produces a significant improvement in the cardinal features of PD and reduces by about 50% (P < .001) the Unified Parkinson’s Disease Rating Scale (UPDRS) score in parts III (motor) and II (activities of daily living) in those not taking medication.26–30 The clinical benefit is mainly on the side contralateral to surgery, but axial features also improve.26,27 Tremor, rigidity, and bradykinesia were alleviated, and the benefit was maintained at 6 to 24 months (P < .001), as described by Patel and coworkers27 in 21 patients. However, disease progression was evidenced by an increase in the UPDRS ipsilateral evaluation in patients taking and not taking medication at 2 years.27 A Cuban group assessed 57 patients at 12 months, 36 patients at 24 months, and 25 patients at 36 months and found a significant reduction in the off-medication UPDRS at 12 (50% improvement), 24 (30% improvement), and 36 (18% improvement) months postoperatively.31 They observed that a significant alleviation in the cardinal features contralateral to the lesion persisted, but the ipsilateral side and axial signs worsened during the assessment period. The UPDRS at 36 months postoperatively increased by 36.6%, compared with the evaluation at 12 months.31 This worsening in UPDRS part III at 3 years was mainly due to a significant increase in scores on the ipsilateral side and for axial items on the scale. The major antiparkinsonian impact of the lesion is achieved with a reduction in daily levodopa intake in a large percentage of patients.27,28,31
The on-medication UPDRS score also improved significantly. A 39% reduction (P < .01) was reported by different authors at 12 months, indicating that tremor on the contralateral side was alleviated by the STN lesion.26–28 Patel and coworkers27 also reported that UPDRS part II scores were reduced both in those taking and those not taking medication at 12 and 24 months owing to cardinal factors on the contralateral side. However, disease progression and worsening subscores for ipsilateral and axial features were also detected.
The conclusion is that the main clinical benefits of a unilateral STN lesion persist up to 3 years, but disease progression increases patients’ disability whether they are taking or are not taking medication. This evolution is observed in the ipsilateral and axial worsening in patients during follow-up, which may be similar to that seen during the long follow-up in PD patients after unilateral pallidotomy.32,33 Although some patients obtained very good results and great clinical improvement in the UPDRS assessment, another group found suboptimal alleviation of symptoms despite a similar surgical procedure. We are currently analyzing lesion placement in the STN as it correlates with clinical assessment.
Bilateral Subthalamotomy
Experience with bilateral surgery is limited, owing to the fear of inducing adverse cognitive effects. Alvarez and colleagues34 reported an open-label pilot study of the effect of bilateral STN lesions in 18 patients. In 7 patients, they performed a unilateral lesion first, followed by contralateral surgery in 12 to 24 months (staged surgery); in 11 patients, they carried out bilateral subthalamic nucleotomy during one procedure (simultaneous surgery). They described a significant improvement in the off-medication (49.5%) and on-medication (35.5%) UPDRS motor score at 12 months. The main parkinsonian features of PD (UPDRS part III) and activities of daily living (UPDRS part II) also improved (P < .01). The authors reported that the benefits of bilateral surgery were maintained over a long follow-up period (3 to 6 years). Su and associates28 also reported on four patients with bilateral subthalamotomy, describing greater benefits than in those with unilateral lesions. They performed the second surgery 4 to 6 months after the first surgery and concluded that the benefits in terms of gait and postural stability were significant in the off-medication patients but not in the on-medication patients.
Levodopa-Induced Dyskinesias
LIDs are significantly reduced after STN lesions. However, a number of factors affect this aspect of the disease, such as the main clinical features of LID before the procedure and the surgical variability among different groups. Initially, Alvarez and coworkers26 reported that unilateral lesions did not change LIDs in 11 patients evaluated at 12 months by UPDRS part IV (scores of 1.5 preoperatively versus 1.6 postoperatively), despite no change in levodopa therapy. In contrast, Su and associates28 reported that STN lesions reduced LIDs by 75% (P < .01) in three of four patients with severe bilateral preoperative LIDs, although the levodopa dosage was maintained at the preoperative level for 3 months. Finally, Patel and coworkers27 reported that contralateral LIDs were significantly reduced at 12 and 24 months after surgery (P < .01) in 21 patients; in this group, levodopa therapy was approximately halved. More recently, Alvarez and coworkers34 described a significant reduction in LIDs associated with an important levodopa reduction (P < .01) after bilateral surgical procedures.
Complications: Hemichorea
Some complications of subthalamotomy occur in percentages similar to those associated with STN-DBS electrode placement, such as bleeding along the recording track (2% to 3.3%), infection, epileptic seizures during the procedure, and dysarthria (5% to 10%). However, hemichorea after lesion placement is correlated with this type of surgery and the anatomic structure. The percentage of patients who experience HCB after STN lesions is approximately 10%.26,29,30,35 Su and associates28 reported three cases of HCB in seven patients after STN lesions, one of whom died of aspiration pneumonia. Alvarez and coworkers31 assessed HCB in a series of patients who underwent subthalamotomy and found that 58% developed dyskinesia during the first 24 hours after the procedure. In most of these cases the HCB had a focal distribution and eventually resolved. In 16% of patients, however, the HCB persisted for months; in 9% of patients the HCB was severe, with no response to levodopa withdrawal or tetrabenazine treatment. In these cases, a pallidotomy was performed to eliminate the dyskinesia.31,36,37
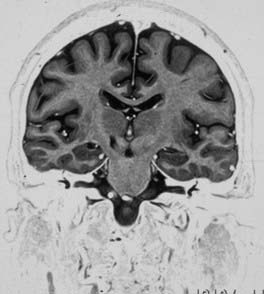
The factors affecting the development of HCB after subthalamotomy are not completely understood (Fig. 81-3). The location of the lesion in the STN, lesion volume, previous history of LIDs and their severity, and bilateral procedures have all been suggested as factors. Su and associates28 reported that patients with HCB had a lesion volume greater than those who did not develop the complication. Volume may be important, because some lesions may reach the fasciculus lenticularis, which is dorsal to the STN; their interruption should eliminate the dyskinesia, as Carpenter and colleageus2 described in 1950. In this case, subthalamotomy could induce a pallidotomy-like effect, reaching Forel field H2 (pallido-fugal pathway) and the zona incerta, and would be associated with a lower incidence of dyskinesia, although this has not been demonstrated in the literature.20,38,39 In Vilela and da Silva’s29 series, no patients with lesions confined to the STN presented with dyskinesia.
However, in another analysis of lesion placement, it was found that patients who developed HCB were more likely to have lesions clustered in a dorsomedial location of the nucleus than were those who did not develop the complication (Alvarez, personal observation); lesion volume was not a significant factor. We believe that there may be critical subregions within the STN that are prone to induce HCB. In addition, the threshold for HCB associated with STN lesions seems to be greater in the parkinsonian condition than in normal individuals, as described by Guridi and associates15,20 in a monkey model, owing to functional modifications in striatopallidal circuits as a consequence of dopamine depletion.15,20 Another factor relevant to the development of HCB may be the severity of preoperative LIDs. In patients with severe LIDs, the STN lesion may induce the complication, and targeting the GPi may be a better option.31
Cognitive Function
There are very few studies describing the cognitive impact of STN lesions in patients with PD. One group reported no deficits in 12 patients after unilateral procedures, except for a reduction in verbal fluency.40 In a later report, the same group analyzed 17 patients reporting an 18% deterioration in the Rivermead Story Memory Test. They found that deterioration was less likely after right subthalamotomy (5% decline in cognitive test scores) than left subthalamotomy (25% decline).27 Alvarez and coworkers34 reported that after bilateral procedures, neuropsychological assessment in 10 patients revealed no cognitive deterioration. These patients also showed a significant improvement in apathy and depression test scores (Hamilton Depression Rating Scale) after surgery. Hyperactive behaviors, such as lack of inhibition and hypomania, occurred in five patients. This new behavior or mood change was severe 1 month after the procedure but gradually diminished by 1 year after surgery.
In conclusion, neither unilateral nor bilateral ablative surgery of the STN is associated with any major disorders of cognition or speech. However, bilateral lesions should be carefully considered in patients who exhibit speech problems preoperatively.34
Neuroprotective Effect of the Lesion
Current reports suggest that glutamatergic excitotoxicity from the STN may contribute to dopaminergic cell death in the substantia nigra pars compacta in parkinsonian patients. There is experimental evidence that several glutamatergic nuclei and their pathways, such as the centromedian parafascicular area, STN, and pedunculopontine nucleus, are functionally hyperactive in the depleted BG in parkinsonian conditions. Studies in rats have demonstrated that a prior STN lesion reduces cell loss in the substantia nigra pars compacta after hydroxydopamine (6-OHDA) treatment.41–44 Recently, Wallace and colleagues45 reported that after either STN lesions or DBS in MPTP-treated monkeys, there was 20% to 24% preservation of dopaminergic cells on analysis with Nissl staining and tyrosine hydroxylase immunocytochemistry. These authors suggested that this neuroprotective effect is correlated with a reduction in glutamate excitotoxicity from STN input to the substantia nigra pars compacta, but valid evidence in patients is difficult if not impossible to obtain.46 In addition, the 6-OHDA and MPTP animal models may be misleading, inasmuch as they do not mimic the process and evolution of a neurodegenerative disease.
Alvarez L, Macias R, Guridi J, et al. Dorsal subthalamotomy for Parkinson’s disease. Mov Disord. 2001;16:72-78.
Alvarez L, Macias R, Pavón N, et al. Therapeutic efficacy of unilateral subthalamotomy in Parkinson’s disease: results in 89 patients followed for up to 36 months. J Neurol Neurosurg Psychiatry. 2009;80:979-985.
Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436-1438.
Guridi J, Herrero MT, Luquin MR, et al. Subthalamotomy in parkinsonian monkeys. Behavioural and biochemical analysis. Brain. 1996;119:1717-1727.
Guridi J, Obeso JA. The subthalamic nucleus, hemiballismus and Parkinson’s disease: reappraisal of a neurosurgical dogma. Brain. 2001;124:5-19.
Lozano AM. The subthalamic nucleus. Myth and opportunities. Mov Disord. 2001;16:183-184.
Patel NK, Heywood P, O’Sullivan K, et al. Unilateral subthalamotomy in the treatment of Parkinson’s disease. Brain. 2003;126:1136-1145.
Rodriguez-Oroz MC, Rodriguez M, Guridi J, et al. The subthalamic nucleus in Parkinson’s disease: somatotopic organization and physiological characteristics. Brain. 2001;124:1777-1790.
Wallace BA, Ashkan K, Heise CE, et al. Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain. 2007;130:2129-2145.
1 Whittier JR, Mettler FA. Studies on subthalamus of rhesus monkey: hyperkinesia and other physiologic effects of subthalamic lesions, with special reference to the subthalamic nucleus of Luys. J Comp Neurol. 1949;90:319-372.
2 Carpenter MB, Whittier JR, Mettler FA. Analysis of choreoid hyperkinesia in the rhesus monkey. J Comp Neurol. 1950;92:293-322.
3 Purdon Martin J, Alcock NS. Hemichorea associated with a lesion of the corpus Luysii. Brain. 1934;57:504-516.
4 Smith Y, Parent A. Neurons of the subthalamic nucleus in primates display glutamate but not GABA immunoreactivity. Brain Res. 1988;453:353-356.
5 Albin RL, Aldridge JW, Young AB, Gilman S. Feline subthalamic nucleus neurons contain glutamate-like but not GABA-like or glycine-like immunoreactivity. Brain Res. 1989;491:185-188.
6 Mitchell IJ, Jackson SA, Sambrook MA, Crossman AR. Common neuronal mechanism in experimental chorea and hemiballismus in the monkey: evidence from 2-deoxyglucose autoradiography. Brain Res. 1985;339:346-350.
7 Mitchell IJ, Clarke CE, Boyce S, et al. Neural mechanism underlying parkinsonian symptoms based upon regional uptake of 2-deoxyglucose in monkeys exposed to 1-methyl, 4-phenyl, 1,2,3,6-tretrahydropyridine. Neuroscience. 1989;32:213-226.
8 Miller WC, DeLong MR. Altered tonic activity of neurons in the globus pallidus and subthalamic nucleus in the primate model of parkinsonism. In: Carpenter MB, Jayaraman A, editors. The Basal Ganglia II. Structure and Function. New York: Plenum; 1987:415-427.
9 Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436-1438.
10 Aziz TZ, Peggs D, Sambrook MA, Crossman AR. Lesion of the subthalamic nucleus for the alleviation of 1-methyl, 4-phenyl, 1,2,3,6-tetrahydropyridine (MPTP)-induced parkinsonism in the primate. Mov Disorder. 1991;6:288-292.
11 Guridi J, Herrero MT, Luquin MR, et al. Subthalamotomy in parkinsonian monkeys. Behavioural and biochemical analysis. Brain. 1996;119:1717-1727.
12 Benazzouz A, Gross C, Féger J, et al. Reversal of rigidity and improvement in motor performance by subthalamic high-frequency stimulation in MPTP-treated monkeys. Eur J Neurosci. 1993;5:382-389.
13 Benabid AL, Pollak P, Gross C, et al. Acute and long-term effects of subthalamic nucleus stimulation in Parkinson’s disease. Stereotact Funct Neurosurg. 1994;62:76-84.
14 Limousin P, Krack P, Pollak P, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson’s disease. Lancet. 1995;345:91-95.
15 Guridi J, Herrero MT, Luquin MR, Obeso JA. The subthalamic nucleus: a new possible stereotaxic target for Parkinson’s disease. Mov Disord. 1993;8:421-429.
16 Andy OJ, Jurko MF, Sias FR. Subthalamotomy in treatment of parkinsonian tremor. J Neurosurg. 1963;20:860-870.
17 Fager CA. Evaluation of thalamic and subthalamic surgical lesions in the alleviation of Parkinson’s disease. J Neurosurg. 1963;28:145-149.
18 Mundinger F. Stereotaxic interventions on the zona incerta area for treatment of extrapyramidal motor disturbances and their results. Confin Neurol. 1965;26:222-230.
19 Woringer E, Hopf A, Hamou Y, et al. L’hemiballisme post-stereotaxique. Neurochirurgie. 1968;14:567-576.
20 Guridi J, Obeso JA. The subthalamic nucleus, hemiballismus and Parkinson’s disease: reappraisal of a neurosurgical dogma. Brain. 2001;124:5-19.
21 Laitinen LV. Brain targets in surgery for Parkinson’s disease. Results of a survey of neurosurgeons. J Neurosurg. 1985;62:349-351.
22 Laitinen LV, Bergenheim AT, Hariz MI. Leksell’s posteroventral pallidotomy in the treatment of Parkinson’s disease. J Neurosurg. 1992;76:53-61.
23 Lopez-Flores G, Morales J, Tejeiro J, et al. Anatomic and neurophysiological methods for the targeting and lesioning of the subthalamic nucleus. Cuban experience and review. Neurosurgery. 2003;52:817-831.
24 Bejjani BP, Dormont D, Pidoux B, et al. Bilateral subthalamic stimulation for Parkinson’s disease by using three-dimensional stereotactic magnetic resonance imaging and electrophysiological guidance. J Neurosurg. 2000;92:615-625.
25 Rodriguez-Oroz MC, Rodriguez M, Guridi J, et al. The subthalamic nucleus in Parkinson’s disease: somatotopic organization and physiological characteristics. Brain. 2001;124:1777-1790.
26 Alvarez L, Macias R, Guridi J, et al. Dorsal subthalamotomy for Parkinson’s disease. Mov Disord. 2001;16:72-78.
27 Patel NK, Heywood P, O’Sullivan K, et al. Unilateral subthalamotomy in the treatment of Parkinson’s disease. Brain. 2003;126:1136-1145.
28 Su PC, Tseng HM, Liu HM, et al. Subthalamotomy for advanced Parkinson disease. J Neurosurg. 2002;97:598-606.
29 Vilela F, da Silva DJ. Unilateral subthalamic nucleus lesioning: a safe and effective treatment for Parkinson’s disease. Arq Neuropsiquiatr. 2002;60:935-948.
30 Gill SS, Heywood P. Bilateral dorsolateral subthalamotomy for advanced Parkinson’s disease. Lancet. 1997;350:1224.
31 Alvarez L, Macias R, Pavón N, et al. Therapeutic efficacy of unilateral subthalamotomy in Parkinson’s disease: results in 89 patients followed for up to 36 months. J Neurol Neurosurg Psychiatry. 2009;80:979-985.
32 Lang AE, Lozano AM, Montgomery E, et al. Posteroventral pallidotomy in advanced Parkinson’s disease. N Engl J Med. 1997;337:1036-1042.
33 Baron MS, Vitek JL, Bakay RAE, et al. Treatment of advanced Parkinson’s disease by unilateral posterior GPi pallidotomy: 4 years results of a pilot study. Mov Disord. 2000;15:230-237.
34 Alvarez L, Macias R, Lopez G, et al. Bilateral subthalamotomy in Parkinson’s disease: initial and long-term response. Brain. 2005;128:570-583.
35 Barlas O, Hanagasi HA, Imer M, et al. Do unilateral ablative lesions of the subthalamic nucleus in parkinsonian patients lead to hemiballism? Brief report. Mov Disord. 2001;16:306-310.
36 Suarez JI, Metman LV, Reich SG, et al. Pallidotomy for hemiballismus: efficacy and characteristics of neuronal activity. Ann Neurol. 1997;42:807-811.
37 Vitek JL, Chockkan V, Zhang JY, et al. Neuronal activity in the basal ganglia in patients with generalized dystonia and hemiballismus. Ann Neurol. 1999;46:22-35.
38 Lozano AM. The subthalamic nucleus. Myth and opportunities. Mov Disord. 2001;16:183-184.
39 Dierssen G, Bergman L, Gioino G, Cooper IS. Hemiballism following surgery for Parkinson’s disease. Arch Neurol. 1961;5:627-637.
40 McCarter RJ, Walton NH, Rowan AF, et al. Cognitive functioning after subthalamic nucleotomy for refractory Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2000;69:60-66.
41 Piallat B, Benazzouz A, Benabid AL. Subthalamic nucleus lesion in rats prevents dopaminergic nigral neuron degeneration after striatal 6-OHDA injection: behavioural and immunohistochemical studies. Eur J Neurosci. 1996;8:1408-1414.
42 Nakao N, Nakai E, Nakai K, Itakura T. Ablation of the subthalamic nucleus supports the survival of nigral dopaminergic neurons after nigrostriatal lesions induced mitochondrial toxin 3-nitropropionic acid. Ann Neurol. 1999;45:640-651.
43 Chen L, Liu Z, Tian Z, et al. Prevention of neurotoxic damage of 6-OHDA to dopaminergic nigral neuron by subthalamic nucleus lesions. Stereotact Funct Neurosurg. 2000;75:66-75.
44 Carvalho GA, Nikkhah G. Subthalamic nucleus lesion are neuroprotective against terminal 6-OHDA-induced striatal lesions and restores postural balancing reactions. Exp Neurol. 2001;171:405-417.
45 Wallace BA, Ashkan K, Heise CE, et al. Survival of midbrain dopaminergic cells after lesion or deep brain stimulation of the subthalamic nucleus in MPTP-treated monkeys. Brain. 2007;130:2129-2145.
46 Hilker R, Portman AT, Voges J, et al. Disease progression continues in patients with advanced Parkinson’s disease and effective subthalamic nucleus stimulation. J Neurol Neurosurg Psychiatry. 2005;76:1217-1221.