Stroke
Historical Background
Stroke has a major impact in the United States, with an estimated yearly incidence of 731,100 new and recurrent strokes1 from 1993-1994. In 1997, 821,760 stroke admissions occurred in this country.2 Stroke constitutes the third leading cause of death and is a major cause of disability.3 Although stroke is a lesser cause of disability than heart disease,4 the population of stroke survivors continues to increase, in part because of a fall in mortality rate.5 Historically, stroke was not emphasized in the critical care setting because of the limited scope of interventions in the past. In the early 1990s, neurologic diseases, including but not limited to stroke, accounted for a mere 6% to 7% of admissions to critical care units.6 Now there is acute treatment for stroke, namely, the use of tissue plasminogen activator (tPA) for ischemic stroke within 3 hours of symptom onset,7 as well as intra-arterial (IA) thrombolysis for as long as 6 hours following stroke onset.8 A clot removal device (mechanical embolus removal in cerebral ischemia, or MERCI)9 has been approved, and newer, likely more effective, devices10 may become available shortly. Thrombolytic agents designed to work within a 9-hour time window, in conjunction with sophisticated imaging, have being studied, although they have not yet been shown to be useful.11 The great danger with the use of these agents is the risk of intracerebral hemorrhage (ICH). Frequent monitoring, as often as every 15 minutes following administration of a thrombolytic, is standard for patients so treated, and observation in a critical care unit is required.
Consequently, critical care physicians need to learn about this condition, which has become a regular part of their professional lives, particularly in centers that devote themselves to the care of stroke patients. In one critical care unit with which the author is familiar, ischemic stroke accounts for 3% of the primary admissions and hemorrhagic stroke for 5.4%.12
Overview
Stroke is traditionally defined as a focal neurologic deficit of presumed vascular onset, lasting 24 hours or longer, as opposed to transient ischemic attack (TIA), which is an episode shorter than 24 hours in duration.13 Many TIAs actually last for less than 60 minutes.14
Carotid symptoms, as listed in Box 63.1, primarily consist of hemisensory loss, hemiparesis, and retinal ischemia (monocular blindness). Left hemispheric ischemia, generally in the perisylvian area, may result in varying degrees of aphasia.15 Involvement of the sensory association areas within the right parietal lobe can produce the phenomenon of neglect.16 In neglect, a stimulus is felt when it is alone but not in the presence of a competing stimulus. For example, a touch on the left hand or an object in the left visual field may be perceived when alone, but not when another stimulus is simultaneously presented, generally on the right side (double simultaneous stimulation). In that instance the right-sided stimulus alone is perceived. In extreme circumstances, affected individuals may not recognize the left side of the body as being theirs (anosognosia), as described memorably by Oliver Sacks in The Man Who Mistook His Wife for a Hat.17
Box 63.1 lists symptoms resulting from ischemia in the vertebrobasilar territory, which includes the cerebellum, brainstem, and the medial aspect of the occipital lobe, as well as the thalamus and the inferomedial portions of the temporal lobe. As a result, vertebrobasilar ischemia can produce cranial nerve dysfunction, nystagmus, cerebellar dysmetria, ataxia, and long tract signs such as sensory loss or motor impairment. These may involve one or both sides of the body. Memory disorders and visual field deficits also occur. In extreme circumstances, when the basilar artery becomes occluded, coma or quadriparesis may develop, although the presentation may vary, as described by Kubik and Adams18 in 1946. As a result of coma or quadriparesis, mechanical ventilation may be required, and the prognosis in such patients is grim. In one study,19 22 of 25 patients died, and the other 3 lingered in the “locked-in syndrome.”20 This frightening manifestation of basilar occlusion, secondary to pontine infarction, leaves patients chronically limited to eye blinking as their sole means of communication.
Likewise, one must not label complaints that are not cerebrovascular in nature as stroke (see Box 63.1). Syncope, wooziness, and the like usually reflect systemic hypotension as opposed to focal ischemia. The still widespread practice of studying carotid vessels—most often through ultrasound—following the development of syncope should be abandoned because syncope does not result from ischemic stroke.
In Box 63.1, item 4 under “Symptoms Not Considered Vascular in Origin” draws attention to the fact that certain symptoms may represent stroke when associated with other symptoms but not in isolation. Vertigo, for instance, can result from disease of the semicircular canals. If other complaints or findings referable to the posterior fossa of the brain (brainstem and cerebellum) coexist, such as those listed in Box 63.1 under “Vertebrobasilar Distribution,” the symptoms may indeed localize there. Similarly, amnesia alone may follow a seizure or result from transient global amnesia as opposed to stroke, and so on.
The items listed in Box 63.2 reflect the presence of seizures. Seizure onset may be unwitnessed, and in the hospital only postictal deficits, such as aphasia or hemiparesis, may be observed. The appearance of any number of positive phenomena will draw attention to the correct diagnosis. These phenomena contrast with the abolition of normal function that happens with stroke and instead represent abnormal activity resulting from uncontrolled electrical discharges. Occasionally, however, limb shaking may represent carotid ischemia, usually as the result of hemodynamic compromise in the territory of the ipsilateral carotid artery.21 Dreifuss22 gives a comprehensive classification of epilepsy types and symptoms.
Migraine can also be associated with focal neurologic complaints. Commonest among these are visual complaints including scotomas, whether scintillating or not. The most dramatic manifestation is hemiplegic migraine, which raises the fear of ICH at first presentation. Aphasia and paresthesia are also described. Silberstein and colleagues23 have reviewed the manifestations of migraine.
Box 63.3 lists those entities that most frequently mimic stroke. Hypoglycemia may produce focal neurologic deficits. Occasionally a mass lesion such as tumor or subdural hematoma may present with fluctuating deficits or be revealed by seizure activity that may be confused with stroke. In the case of subdural hematoma in the elderly, the inciting trauma may have been minor or forgotten. Headache may be prominent, mild, or even absent. Mass lesions and hemorrhage are frequently marked by confusion, decreased level of consciousness, or headache, but if the lesion is small, these symptoms may not appear. Consequently, blood sugar measurements and computed tomography (CT) scans (without contrast) are obligatory in all instances of suspected stroke. Within the first 6 hours of the event, CT may well be negative, even in instances of major infarction such as that involving the entire MCA watershed. Thus CT finds its greatest utility not in confirming the clinical diagnosis but in excluding the presence of small hemorrhages. Neurologists have relied on clinical findings to diagnose stroke, especially in the hyperacute phase (0 to 6 hours), when CT is least helpful.
Advances in Radiology
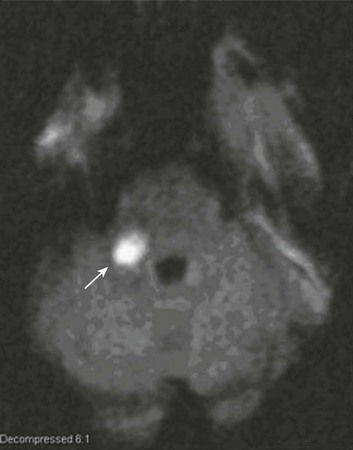
With the advent of diffusion-weighted imaging (DWI), magnetic resonance imaging (MRI) scans (Fig. 63.1) can now be used to detect acute cerebral ischemia within the initial 6-hour period following symptom onset.24 This allows the clinician to confirm or exclude the presence of stroke in doubtful cases. Most commonly, such circumstances involve the possibility of a new lesion in a previously injured area of the brain. For example, in the case of a new seizure originating from the hemisphere affected by a prior stroke, DWI can show whether the new event is seizure alone or caused by a new stroke. By the same token, if a newly delirious or febrile patient should manifest worsening of a preexisting neurologic deficit, DWI will clarify whether the worsening stems from the intercurrent injury or from a coincident new stroke. DWI can also reveal silent areas of cerebral ischemia, which sometimes appear simultaneously with the area of injury that presents symptomatically as stroke. The coincident development of ischemia in different vascular territories may indicate the presence of an unusual mechanism of infarction, such as vasculitis, hypercoagulable state, or cardiac source of emboli. MRI has been proved to be just as effective as CT in detecting ICH,25 thus potentially removing an extra step (the initial head CT) from the stroke evaluation. Unfortunately, MRI is less immediately available than CT, requires more time and cooperation from the patient, and may not be feasible in the face of claustrophobia or of ferromagnetic implants/fragments within the body.
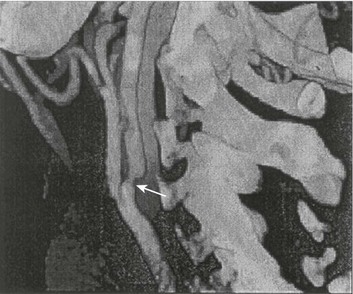
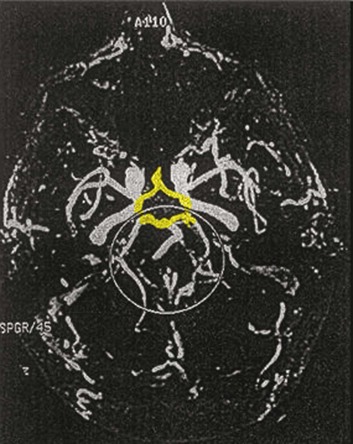
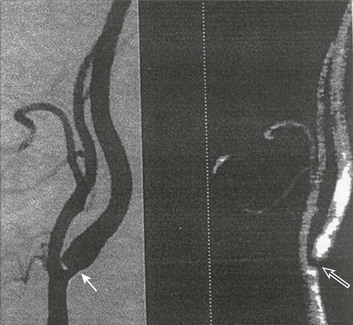
Both CT and MRI technology can delineate the cerebral vasculature in detail, starting from the aortic arch and extending to the vicinity of the circle of Willis. Computed tomographic angiography (CTA) has been shown to be reliable in studying the intracranial vasculature26 and the extracranial segment of the carotid artery (Fig. 63.2).27 Magnetic resonance angiography (MRA) is superior to ultrasound in detecting carotid artery stenosis in the neck28 and is effective as well intracranially29 (Figs. 63.3 and 63.4), although its specificity and sensitivity in both instances are likely to improve. CTA has its own limitations, namely, the difficulty in performing the study in patients with contrast dye allergies. Because of these new techniques, the performance of conventional cerebral angiography is limited to specific indications, as outlined in Box 63.4.
Thrombolysis in Stroke
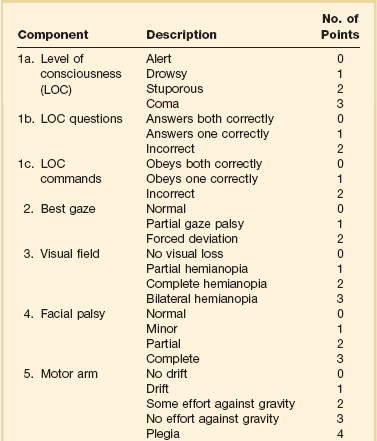
The era of thrombolysis in acute stroke began with the publication of the NINDS (National Institute of Neurological Disorders and Stroke) tPA trial in 1995.7 This groundbreaking study was the first to demonstrate a beneficial effect of tPA when given to patients presenting within 3 hours of the onset of the event. Depending on the criteria used to determine favorable outcome at 3 months, roughly an additional 11% to 13% of subjects receiving tPA recovered with little or no disability. If one uses the National Institutes of Health stroke scale (NIHSS) (Table 63.1) score, a reliable measure30 to measure disability, the improvement was from 20% with minimal or no disability with placebo to 31% with tPA. This was counterbalanced by an increase in the rate of ICH, from 0.6% in the placebo group to 6.4% in the tPA cohort. Half the subjects in the placebo group who suffered ICH died, whereas the mortality rate from ICH in the tPA cohort was 2.9% (less than half). Hemorrhage following tPA use occupies the same area of the brain affected by the initial thrombosis in most but not all cases.31
Strict inclusion (Box 63.5) and exclusion (Box 63.6) criteria are applied to attempt to minimize the risk of ICH. The safety and efficacy of thrombolysis have not been analyzed in children. The time of onset of symptoms is taken to be the last time that the patient was seen to be normal. For example, an individual who went to sleep at 10 PM and awoke at 6 AM, immediately hemiplegic, will not qualify for tPA therapy. One who awoke at 4 AM, went back to sleep, and awoke again at 6 AM with stroke symptoms may be treated with tPA, but only up to 7 AM. A person who awoke at 6 AM, was briefly normal, and developed stroke symptoms at 6:05 AM may be treated until 9:05 AM.
The blood glucose must be determined before initiating tPA therapy to avoid misdiagnosing hypoglycemia as stroke (see Box 63.3). A CT scan of the head is essential to look for a mass, most often an ICH or subdural hematoma. The CT must also be scrutinized for the presence of early ischemic changes, such as sulcal effacement, hypolucency within the brain, or loss of definition between structures within the brain, or for the presence of a hyperdense MCA, suggestive of thrombosis.32 Whether ischemic changes on CT predict a heightened risk of hemorrhagic transformation is controversial.31,33 However, CT findings appear more commonly among subjects with scans performed relatively late in the course of their stroke.33 Hence their appearance should prompt reevaluation of the time of onset of the stroke. Prior ICH, recent stroke, and hypertension at presentation are believed to increase the risk of sustaining ICH. Avoiding systemic bleeding, which may result in hypotension and worsening of the neurologic deficit, is also important. Extremes of blood glucose or the presence of a coincident seizure make the neurologic deficit seem worse than it is, rendering calculation of a risk-benefit ratio more difficult.
Two blood tests must be checked before embarking on thrombolysis: a blood sugar, as mentioned earlier, and a platelet count. If the patient is on anticoagulant therapy, the prothrombin time/international normalized ratio or partial thromboplastin time must be available before deciding whether to proceed with treatment. If a patient is not known to be receiving anticoagulants at baseline, but the coagulation profile proves abnormal, the infusion must be stopped if thrombolysis is still ongoing at the time that the abnormal value returns. If a patient is receiving low-molecular-weight heparin, there is no rapid way of determining the degree of anticoagulation, and intervention must be withheld. Testing for the activity of factor Xa antagonists is not yet standardized, although a normal thrombin time may suggest that thrombolysis is safe.34
If the blood pressure is elevated (>180 mm Hg systolic or 110 mm Hg diastolic), a modest dosage of labetalol, 5 to 10 mg IV (intravenous), may be given and repeated if necessary, up to a maximum dosage of 40 mg. Nitropaste is a less exact alternative but has the advantage that it can be removed. A nicardipine drip may be employed as well, which constitutes a change from prior guidelines. If the blood pressure subsequently rebounds to an undesirable range, tPA should not be given. If the pressure should rise above 180 mm Hg systolic or 105 mm Hg diastolic subsequent to starting the tPA infusion, it is paramount to bring it down using IV infusions of antihypertensive agents (Table 63.2).35 In this context, it is worth emphasizing that blood pressure should be treated acutely only when there exists a specific indication for doing so, as defined in the table.
Table 63.2
Approach to Elevated Blood Pressure in Acute Ischemic Stroke
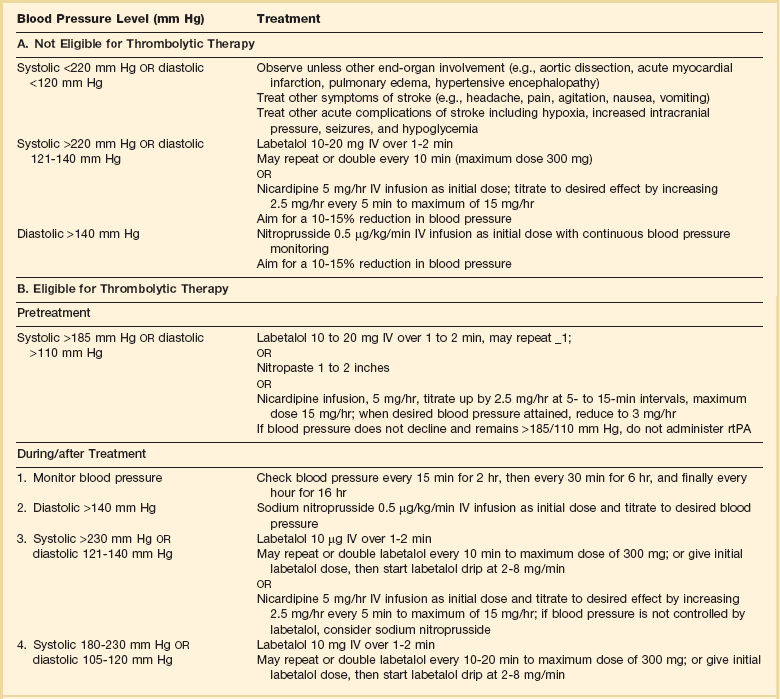
rtPA, recombinant tissue plasminogen activator.
From Adams HP, del Zoppo G, Alberts MJ, et al: Guidelines for the early management of adults with ischemic stroke. Stroke 2007;38:1655-1711.
Hypotension may develop, raising the concern of systemic hemorrhage. Of course, hypotension may have a variety of other causes. The most feared event following tPA use is the development of ICH. The presenting signs and symptoms appear in Box 63.7. Most are self-explanatory. Hypertension is a compensatory response to increased intracranial pressure (ICP), to maintain cerebral perfusion, and bradycardia occurs secondary to it.
The steps to be taken with suspected ICH appear in Box 63.8. It is relatively uncommon for neurosurgeons to intervene on hemorrhages in the setting of tPA because of the risk of further bleeding into the surgical bed. Systemic hemorrhage is handled in a similar fashion, but transfusion may also be necessary. Platelets should be administered if the count is significantly decreased (<50,000 cells/µL).
The benefit of tPA is greatest in the instances of the smallest vascular occlusions (lacunar, as opposed to cortical, infarcts). Older subjects with particularly severe strokes are most likely to have a poor outcome, but even in this group tPA remains beneficial overall.36 The sooner tPA is administered, the higher the likelihood of successful recovery, as shown by a meta-analysis37 of thrombolysis trials. A residual benefit of tPA exists, as far out as 6 hours from the ictus. Two studies38,39 that specifically examined treatment beyond 3 hours failed to show a benefit of administration of tPA within a 5- or 6-hour time frame. More recently, however, the ECASS-III (European Cooperative Acute Stroke Study III) trial40 established the utility of tPA administration in the 3.0- to 4.5-hour period following stroke onset. The inclusion and exclusion criteria are more restrictive than those for treatment within 3.0 hours (Boxes 63.9 and 63.10). Initially, there was some concern about the administration of tPA within the 3.0- to 4.5-hour window to subjects with a history both of prior stroke and of diabetes. A statistical analysis41 based on datasets from large registries suggests that neither factor predisposes to poor outcome in reality. No doubt there exists a subgroup of subjects presenting beyond 4.5 hours that remains amenable to treatment, but this group cannot yet be identified.
Various authors have identified different predictors of ICH following treatment with tPA. Levy and colleagues42 identified the dosage of tPA given, the age of the subjects, and diastolic hypertension as pertinent factors. Larrue and colleagues31 also found age to heighten the risk of development of ICH, but not the degree of hypertension or the time to treatment. It has become clear that tPA can be given safely in the community43 and that complication rates of tPA use can be lowered to a satisfactory level by careful adherence to the exclusion criteria.44
IA thrombolysis can be used to treat acute ischemic stroke beyond the 3-hour time window for IV tPA. Basilar thrombosis has been treated as late as 24 hours after the onset of symptoms using this approach.45 Other subjects with occlusion of the proximal (M1 or M2) segments of the MCA have been successfully treated between 3 and 6 hours following stroke onset. The utility of this approach rests on the PROACT II (Prolyse in Acute Cerebral Thromboembolism II) trial,8 a study using a novel agent, prourokinase (pro-UK). In contrast to the IV tPA trial, aspirin was allowed in the first 24 hours following stroke onset and heparin was used acutely following pro-UK to prevent vascular occlusion. To enter the study, the upper age limit was 85 years and the minimum NIHSS score was 11.
The trial showed a 40% rate of favorable neurologic outcome at 3 months following IA thrombolysis, as opposed to 25% in the control group. The rate of ICH with clinical deterioration at 24 hours following study entry was 10% in the pro-UK group, as opposed to 2% in the placebo group. Although pro-UK itself has not been approved for use in this country, IA tPA is used for subjects who cannot be treated within 3 hours with IV tPA, and, despite the absence of studies, as rescue therapy following IV tPA use. The inclusion and exclusion criteria are similar to those for IV tPA. The availability of mechanical clot extraction, as summarized later, means that subjects who do not qualify for IA tPA may still receive treatment, again in the absence of definitive trials. Apparent predictors of hemorrhagic transformation of cerebral infarct in patients subjected to IA thrombolysis appear in Box 63.11.46 In another study,47 36 subjects underwent IA thrombolysis for stroke within 2 weeks of major surgery (mean time from surgery to treatment of 21.5 hours). Nine of the subjects (25%) died, but only three died of hemorrhagic complications, suggesting that IA thrombolysis may be considered following major surgery when IV thrombolysis is contraindicated. The smaller dosage of thrombolytic (0.2 mg/kg vs. 0.9 mg/kg) used may render the IA approach safe in this setting. IA thrombolysis is also used when cardiac catheterization is complicated by stroke, in large part because of immediate access to the vascular tree. This approach, although reasonable, has not been studied systematically.48
The use of a 0.6-mg/kg systemic bolus of IV tPA acutely, followed by IA thrombolysis if shown necessary by immediate angiography, was examined by the IMS (Interventional Management of Stroke) II trial.49 A microcatheter was employed, when feasible, to allow for ultrasound-assisted clot lysis in addition to local instillation of tPA. There were trends toward higher incidence of ICH, but better clinical outcomes overall, when compared to the original NINDS tPA trial. The IMS III trial,50 a randomized comparison of IV tPA against IV/IA therapy, the latter enhanced by mechanical clot extraction, has begun.
Neurointerventionalists attempt to heighten the effectiveness of local thrombolytics by using catheters to mechanically disrupt clots. Such an approach is difficult to assess in a standardized fashion. There exist data on the MERCI device, designed to extract clot via the endovascular approach without thrombolysis. Initially, this approach was tested in subjects ineligible for tPA,9 but it has also been tested subsequently in subjects previously treated with IV tPA.51 Even though a trend toward increased ICH emerged, vessel recanalization rates were relatively high, approaching 70% in patients also treated with IA tPA. This intervention was used, when needed, to dissolve clot resistant to device intervention or to treat inaccessible distal thrombus. A newer and possibly more effective device, Solitaire,10 may shortly be available.
Such devices have been compared to each other, but not to placebo. Available data consist of series of patients treated with a device compared with historical control subjects. The data focus on the rate of recanalization of the occluded vessel and only secondarily on clinical outcome. The limitations of such an analysis are discussed in detail elsewhere.52 Mechanical clot extraction will find a role in the acute treatment of ischemic stroke, which remains to be defined.
Radiologic imaging has been proposed as a tool to determine when individual subjects may be suitable for IA intervention, regardless of time from stroke onset. Specific patients may, by virtue of good collateral flow, possess a significant area of ischemic but viable brain that may be salvaged by endovascular treatment, even if beyond standard time windows. This algorithm may apply particularly to “wake-up” strokes. Computed perfusion tomography (CT perfusion) can be used to estimate the size of the ischemic area but not infarcted area at presentation,53 and diffusion-weighted/perfusion-weighted MRI may identify those patients who will benefit from intervention.54 However, these tools need validation and standardization in large studies in order to attain clinical efficacy.
Symptomatic Carotid Disease/Stenting
Carotid endarterectomy (CEA) dramatically reduces stroke risk in patients with cerebral or retinal ischemia who prove to harbor a significant carotid stenosis ipsilateral to the affected cerebral hemisphere. Two separate randomized controlled trials55,56 have shown similar results. In NASCET (North American Symptomatic Carotid Endarterectomy Trial),56 the risk of stroke fell from 26% over 2 years in the nonsurgical group to 9% over 2 years in the surgical group, with a surgical morbidity and mortality rate of 5.6%. The patients had stenosis of 70% or greater. A more modest benefit was seen in men with stenoses between 50% and 69%; namely, they showed a reduction in stroke risk from 22.2% over 5 years without surgery to 14.9% over 5 years with surgery. This is an absolute reduction of the stroke rate of 7.3% over 5 years, as opposed to an absolute reduction of 17% over 2 years in the group with stenosis greater than 70%. Superior surgical skill is necessary in order to lower the surgical morbidity and mortality rates to a point that the procedure remains beneficial in this cohort.
Other considerations help determine which patients carry an increased risk of poor outcome acutely from endarterectomy. As far back as 1975, Sundt and colleagues57 recognized that poor or unstable neurologic status, as well as cerebral ischemia within 24 hours of surgery, identified a group at high surgical risk. These authors also related high risk to the following: congestive heart failure, recent cardiac ischemia, marked hypertension, emphysema, age older than 70, and severe obesity. The presence of a contralateral carotid occlusion places subjects at extremely high risk of stroke if left untreated but also carries a high morbidity and mortality risk acutely.58
The likelihood of stroke in patients with carotid stenosis rises steadily with increasing severity of stenosis greater than 70%56 and then decreases again as the stenosis reaches 94% to 99%. However, surgery remains beneficial in this range.59 In addition, hemispheric symptoms predict roughly twice the danger of stroke as do isolated retinal symptoms.60 The presence of ulceration in conjunction with stenosis, as detected angiographically, increases the risk of stroke. This effect is most marked when associated with the highest degrees of stenosis and becomes progressively more modest as the degree of stenosis decreases toward 70%.61
A meta-analysis62 has shown that men benefit more from carotid surgery than women. In contrast to Sundt and colleagues’57 prior findings, there is greater benefit of the procedure in persons older than 75 years. The most important finding to emerge from this report, however, is the dramatic benefit when endarterectomy is performed within 2 weeks of the sentinel event, which diminishes rapidly if surgery is delayed. The previously established surgical practice, to wait for 4 to 6 weeks following stroke or TIA before proceeding with surgery, has become untenable. When severe disability exists, endarterectomy may be reasonably delayed in order to see if sufficient recovery occurs to warrant intervention. However, carotid revascularization, whether by means of endarterectomy or, as discussed later, carotid angioplasty and stenting (CAS), should be undertaken acutely in the absence of major neurologic disability.
Protected CAS has been compared with CEA in a controlled, randomized trial.63 Study participants were 18 years old or older and had either a greater than 50% symptomatic stenosis or a greater than 80% asymptomatic stenosis. They were at high risk, as defined by at least one of the factors listed in Box 63.12.
Subsequently published studies, SPACE (Stent-Protected Angioplasty versus Carotid Endarterectomy)64 and EVA-3S (Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis),65 have been used to cast doubt on the efficacy of CAS in a population of patients with significant symptomatic stenosis, free of such elevated risk. SPACE shows a trend, not reaching statistical significance, toward more stroke and death acutely with CAS than with CEA. Over subsequent 2-year follow-up, the stroke and death risk is the same in either group. However, there appears to be more restenosis in the CAS group, and it is clear that CEA is superior in patients older than 68 years of age. SPACE has flaws. The severity of stenosis was assessed by carotid ultrasound alone, without further validation. Distal protection devices were employed in only a quarter of the CAS patients. Although the authors state that the incidence of periprocedural stroke was not affected by the use of such devices, it is not clear how the decision was taken to use them.
The most recently published trials, CAVATAS (Carotid And Vertebral Artery Transluminal Angioplasty Study)66 and CREST (Carotid Revascularization Endarterectomy versus Stenting Trial),67 are more definitive. They include both symptomatic and asymptomatic subjects. CAVATAS is an earlier study using a mix of angioplasty and stenting. It shows that endovascular treatment carries a higher risk of small perioperative stroke, although it does protect from cranial nerve injury. Overall, there is no substantive difference between the two treatments. There may be a higher risk of stroke, among both older patients and those patients suffering from cardiac disease, and there may be a higher risk of restenosis with angioplasty. CREST, the largest and most recent trial, reveals an increased risk of stroke acutely with stenting, although endarterectomy acutely is associated with an increased risk of heart attack—this latter risk, however, is not associated with increasing mortality risk. Over 2.5 years of follow-up, there is no significant difference in outcome between the two treatments. Endarterectomy appears to be superior, again, in older patients over 70. Recovery from nonfatal MI is less problematic than recovery from stroke, and the increased stroke risk in the CAS group is early in the patients’ course. The results of this study argue against adoption of CAS as an alternative to CEA, particularly as it does not appear to confer a survival benefit, as hoped, to older subjects.
A comment on intracranial angioplasty and stenting is also in order. The realization that warfarin is not superior68 to high-dose aspirin for stroke prevention in the setting of symptomatic intracranial arterial stenosis led to the performance of the SAMMPRIS (Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis) trial.69 This was a randomized comparison of intracranial angioplasty and stenting versus intensive medical therapy, including the use of aspirin in conjunction with clopidogrel, for symptomatic intracranial stenosis. The study was stopped prematurely because of a very high risk of periprocedural stroke following intervention, and a very low risk of stroke in the medically treated group. Consequently, it is difficult to support the routine performance of intracranial angioplasty and stenting on symptomatic vessels, but combined therapy with aspirin and clopidogrel may be considered.
Anticoagulation in Stroke
Anticoagulants occupy an important but limited place in stroke therapy. The acute use of heparin in unselected stroke patients was studied some years ago but not justified.70 More recently, the treatment with warfarin of subjects presenting with symptomatic intracranial stenosis has proved no better than therapy with high-dose (1300 mg daily) aspirin. This use of warfarin produced increased morbidity, in part because of an increased risk of major hemorrhage, primarily gastrointestinal (GI).68 When warfarin is given to unselected stroke patients in a population including a slight majority of subjects with lacunar stroke, it confers no advantage relative to aspirin.71 Warfarin does not reduce the incidence of stroke among subjects with a decreased ejection fraction (<35%) when compared to aspirin.72
Warfarin clearly prevents stroke among subjects suffering from nonrheumatic atrial fibrillation, even if they have never had a stroke or TIA.73 The risk of stroke falls by 64%, a robust and consistent effect that aspirin cannot equal. The CHADS274 score (Box 63.13) is used commonly to assess the risk of stroke; the higher the score, the higher the risk. Individuals younger than 65 years of age with atrial fibrillation who have no significant vascular risk factors are at low risk and do not need anticoagulation. Such individuals are rare in clinical practice. The risk of systemic hemorrhage and of ICH with warfarin use in this setting is low. A combination of clopidogrel and low-dose aspirin75 is inferior to warfarin in stroke prevention among patients with atrial fibrillation. The bleeding risk is actually lower with warfarin than with the antiplatelet combination. However, combination therapy with aspirin and clopidogrel is superior to aspirin alone among patients thought unsuitable for anticoagulant use,76 although the combination does raise the risk of major hemorrhage somewhat.
Individuals with atrial fibrillation who sustain a minor stroke or TIA have a high stroke risk (12% per year), which falls to 4% per year upon treatment with warfarin. A 2.8% yearly risk of all bleeding complications with warfarin exists, as opposed to 0.7% with placebo. Aspirin is nearly as safe as placebo, but it reduces the stroke risk only modestly.77 It remains uncertain when in the course of stroke to initiate anticoagulant therapy. A randomized trial78 has compared a form of low-molecular-weight heparin (dalteparin) to 160 mg of aspirin in patients with stroke and atrial fibrillation. Treatment began within 30 hours of stroke onset, and the risk of recurrent stroke at 14 days was determined. This proved to be 8%. Dalteparin was equivalent to aspirin. This study was not powered to demonstrate a more modest beneficial effect of dalteparin, which may or may not be present. Nevertheless, this study urges caution on those who would treat completed stroke acutely with anticoagulants. The exclusion criteria for the study serve as a guideline as to when not to treat with anticoagulants. Patients with severe strokes and those with marked elevation of blood pressure were not entered. Acute anticoagulation in the face of stroke should be withheld if there is a sizable infarct as measured by the NIHSS (perhaps a score of ≥12) or on CT (>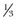 of MCA territory), which could progress to herniation and death as a result of hemorrhage into the infarct bed. The 185/110 mm Hg cutoff measurement used in the NINDS tPA trial may give some idea of the range of hypertension that would cause one to withhold acute treatment with anticoagulants.
of MCA territory), which could progress to herniation and death as a result of hemorrhage into the infarct bed. The 185/110 mm Hg cutoff measurement used in the NINDS tPA trial may give some idea of the range of hypertension that would cause one to withhold acute treatment with anticoagulants.
In contrast, one should move quickly to anticoagulation following a TIA occurring in conjunction with atrial fibrillation or any other recognized indication for anticoagulation. Minor strokes that begin with potentially devastating deficits but go on to resolve substantially should receive anticoagulation if its use is indicated. These recommendations seem appropriate in view of the high risk of stroke recurrence in atrial fibrillation, as cited previously.77
Other indications for anticoagulation appear in Box 63.14. Some, such as the presence of rheumatic heart disease with atrial fibrillation, prosthetic heart valve, or deep venous thrombosis (DVT), are long established. Anticoagulation carries more urgency when a DVT coexists with a communication across the atrial septum, such as patent foramen ovale (PFO), because there is risk of both recurrent stroke and pulmonary embolism.
The utility of warfarin is limited by the need for regular blood work, and by difficulty in regulating its dosage. Factor II and factor Xa inhibitors have recently been studied for stroke prevention in the setting of atrial fibrillation. Dabigatran (Pradaxa) and rivaroxaban (Xarelto) are marketed in the United States. Another, apixaban, reduces the risk of stroke79—when compared to aspirin—in patients deemed unsuitable for warfarin use, without an increase in major or intracranial hemorrhage. When compared directly to warfarin, apixaban reduces the risk not only of stroke but also of both intracranial hemorrhage and of serious hemorrhage.80 Rivaroxaban81 appears equivalent to warfarin in stroke prevention, and it is less likely to produce intracranial hemorrhage but carries a higher risk of major GI hemorrhage. Dabigatran, at the dosage of 150 mg daily,82 is associated with a smaller stroke risk and, like rivaroxaban, a lesser risk of intracranial hemorrhage but a higher risk of major GI hemorrhage, when compared to warfarin.
The main concern with these agents is the lack of means to reverse their effect in the setting of hemorrhage. One patient taking dabigatran has died83 from relatively minor head trauma leading to progressive intracranial hemorrhage. It may be possible to reverse the effects of rivaroxaban, but not dabigatran, through the use of prothrombin complex concentrate,84 although dabigatran may be cleared through dialysis.85 Fortunately, these agents have short half-lives and are cleared rapidly.86 Nevertheless, care is enjoined in their use, both in the setting of renal insufficiency, and in conjunction with aspirin.87 Hemorrhage in patients using factor II and factor Xa antagonists will remain troublesome until means to reverse the anticoagulation produced by these agents are developed, together with the capability to monitor the process in real time. For now, fear of this complication will limit the use of these agents.
Venous sinus thrombosis is an uncommon condition that manifests with headache and papilledema, as well as focal symptoms, and often leads to ICH.88 Diagnosis is by magnetic resonance venography.89 Treatment is with heparin acutely, even in the presence of ICH.90 More recent reports have established the safety of low-molecular-weight heparin followed by 3 months of warfarin therapy for this condition, although only a trend toward favorable outcome emerged.91 Retrograde thrombolysis through the venous system with tPA in conjunction with IV heparin is effective in more serious cases.92
Spontaneous dissection of the carotid and vertebral arteries is the subject of a review by Schievink.93 This is a significant cause of stroke in younger individuals. Carotid dissection often presents with neck pain, radiating to the head, and with components of Horner’s syndrome (ptosis, miosis, and anhidrosis). Vertebral dissection presents with pain in the neck or back of the head initially. Either may proceed to cerebral infarction or to retinal infarction in the case of carotid dissection. MRA diagnoses carotid dissection quite accurately, and vertebral dissection less so.94 Anticoagulation is commonly employed but has not been subjected to a rigorous trial. The presence of dissection does not appear to contraindicate the performance of thrombolysis by whatever route.95
Box 63.15 lists the hypercoagulable states.96 Most of these lead mainly to the development of venous thrombosis, although antiphospholipid antibodies and the lupus anticoagulant may produce both venous and arterial thrombosis. The duration of anticoagulation rests on the judgment of the treating physician. Three to 6 months of therapy is commonly undertaken if the episode of thrombosis is isolated.
Antiplatelet Agents in Stroke
Secondary stroke prevention continues to depend on aspirin, as it has for many years. A meta-analysis carried out in 199497 showed that antiplatelet therapy, primarily with aspirin, reduces the risk of stroke, MI, and vascular death in aggregate by 25%. This analysis did not discriminate among those subjects treated primarily for heart disease and those treated for cerebral ischemia. A similar analysis98 focusing only on the latter found a lesser benefit of aspirin, on the order of 13%. The optimal dosage of aspirin remains unknown. One study99 failed to show a difference in outcome when comparing dosages of 30 mg and 283 mg. Another100 revealed no difference in efficacy between 300 mg and 1200 mg of aspirin a day. In both reports, the lesser dosage led to fewer significant bleeding episodes.
The limited effect of aspirin on stroke recurrence, coupled with the seeming failure of increases in dosage to further reduce stroke risk, led to a search for other antiplatelet agents. Ticlopidine enjoyed brief popularity but was found to produce agranulocytosis (<1500 neutrophils/µL) in 2.4% of subjects, which was severe (<450/µL) in 0.8%.101 Thrombotic thrombocytopenic purpura, fatal in 4 of 13 cases, also occurred.102 Both the need for regular blood monitoring and the risk of a severe and potentially fatal complication led to the virtual abandonment of this agent.
Another thienopyridine, clopidogrel, was studied in the CAPRIE (Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events) trial.103 It exhibited minimal benefit compared with aspirin in preventing vascular events both in patients presenting with stroke and in those manifesting with MI. Nevertheless, it was approved for use primarily on the strength of its ability to reduce the development of vascular events in subjects presenting with peripheral arterial disease. It has won wide support and has subsequently proved quite useful, administered together with aspirin, in the treatment of unstable angina104 and acute MI105,106 and following stent placement in the coronary vessels.107
Physicians have used clopidogrel in conjunction with aspirin for stroke prevention in the expectation that the combination would prove superior to clopidogrel in isolation. However, a comparison between the use of 75 mg of aspirin together with 75 mg of clopidogrel and the use of clopidogrel alone revealed no benefit of combination therapy, just a heightened risk of GI hemorrhage.108 Another study109 failed to show a benefit of combination therapy when compared with the use of aspirin alone in preventing stroke, MI, or vascular death. In addition, the risk of “moderate” hemorrhage was increased in the group receiving combination therapy. Patients, in order to enter this study, had to have either established vascular disease or, in a minority, multiple vascular risk factors. The study as published does not assess the risks or benefits of combination therapy relative to aspirin in subjects randomized for stroke alone. Nevertheless, the results do not support the chronic use of combination therapy in the prevention of acute vascular syndromes and death of vascular origin. Perhaps combination therapy may be useful in the setting of acute cerebral ischemia, as it is for acute coronary syndromes with ST-segment elevation.105,106 Certainly the use of aspirin is recognized as beneficial in acute stroke when instituted within 48 hours,110 and one may imagine, on the basis of the cardiac data, that clopidogrel may also benefit stroke acutely. A study is ongoing111 to address this possibility, but results will not be available soon.
An alternative to the use of aspirin or clopidogrel is the combination of low-dose aspirin in conjunction with extended-release dipyridamole (ASA + ER/DP). The initial aspirin and dipyridamole study, ESPS (European Stroke Prevention Study),112 showed a striking benefit of the combination of 990 mg of aspirin and 75 mg of dipyridamole daily in reducing stroke by 38%. This result raised the question of whether the high aspirin dosage or the addition of dipyridamole was responsible for the dramatic improvement in outcome. A second trial, ESPS-2,113 revealed a similar benefit using a regimen of 50 mg of aspirin and 400 mg of ER/DP per day. The effect of the two agents was additive. However, there was no improvement in mortality rate or in the rate of MI in any of the treatment groups (ASA, ER/DP, or ASA + ER/DP) when compared with placebo in ESPS-2. This contrasts with the results of ESPS, in which combination therapy did reduce both the rate of vascular death and, in the intention-to-treat analysis, that of MI. Thus both the active regimen used in ESPS and the combination therapy used in ESPS-2 significantly reduced the rate of recurrent stroke. However, only in ESPS, which used a much higher aspirin dosage, was there a reduction in the rate of MI and vascular death.
As already noted, previous studies99,100 have not revealed significant differences in outcome on the basis of the aspirin dosage used but the numbers enrolled in these trials are relatively modest and differences cannot be definitively excluded.
A recent large randomized trial with roughly 20,000 patients (PRoFESS [Prevention Regimen for Effectively avoiding Second Strokes]) compared ASA + ER/DP to clopidogrel in the prevention of stroke, MI, and death among patients presenting with stroke.114 The two treatments proved equivalent overall, but the rate of ICH was increased in the ASA + ER/DP group. Because ASA + ER/DP needs to be taken twice daily, has more side effects (headache, primarily), and has no cost advantage, the study has not led to its widespread use.
In conclusion, the area of antiplatelet therapy for stroke has evolved. Clopidogrel remains the drug of choice under certain restricted circumstances (Box 63.16). Clinicians often change therapy from one antiplatelet agent to another following the development of a cerebral ischemic event, but one must expect a significant failure rate with any of these agents. Whether such a strategy is beneficial is unclear. Aspirin will remain the agent of choice for most patients, particularly when one considers the cost of the alternatives. Combination therapy with aspirin and dipyridamole may find a niche in individuals with cerebrovascular disease who are at risk for the development of GI hemorrhage.
Patent Foramen Ovale
PFO is a recently recognized cause of cardiac embolization to the brain. PFO is detected by two-dimensional echocardiography with injection of agitated saline or by transesophageal study. In one report it appeared in 18% of young control subjects and in 40% of subjects with cryptogenic stroke. PFO was more common as well in younger as opposed to older patients with cryptogenic stroke (stroke of unknown origin following standard evaluation).115 The combination of PFO with atrial septal aneurysm in stroke patients younger than the age of 55 is associated with a high (15.2% over 4 years) risk of recurrent stroke.116 Others117 have found a large diameter (>4 mm) of the PFO to predict increased risk of stroke recurrence. PFO can be treated with warfarin, aspirin, surgical closure, and now endovascular closure. No data suggest conclusively that one treatment surpasses the others,118 as confirmed by the recently published CLOSURE I trial.119 In this study, PFO closure did not lead to fewer strokes than did medical therapy. Neither the size of the interatrial shunt nor the presence of an associated atrial septal aneurysm influenced the results. Moreover, PFO closure, aside from procedural complications, was related to a significantly increased risk of atrial fibrillation. At this time, closure of a PFO may be indicated in the absence of another explanation for multifocal infarction.
Massive Hemispheric Cerebral Infarct
Infarction of the entire MCA territory leads to the development of the “malignant MCA” syndrome.120 This major injury leads to death in 78% of cases in the first week after the ictus, as the result of cerebral edema leading to transtentorial herniation, and the survivors are quite disabled. Brain swelling peaks at 3 to 5 days following stroke onset.
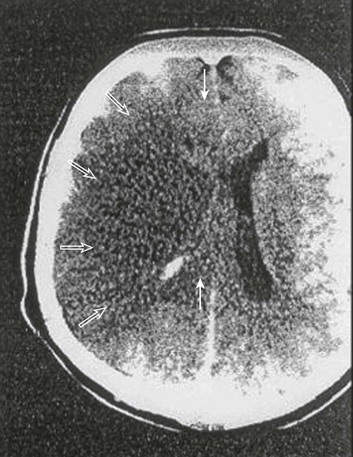
The presence of early CT changes, namely, hypodensity of more than 50% of the MCA territory, indicates a poor prognosis.121 In the absence of appropriate intervention, midline displacement of the pineal gland greater than 4 mm predicts death reliably. Large volume of infarction (Fig. 63.5) and displacement of the septum pellucidum also indicate a poor outcome.122 At initial presentation, aphasia or neglect may be present, depending on the hemisphere affected. Characteristically, the patients display hemiplegia and a dense hemisensory loss, although the leg may be relatively spared. Quite often, there is a forced eye deviation and hemianopia may coexist. Mortality rate is higher in older individuals with this syndrome,123 but transtentorial herniation is a more significant cause of death among younger subjects with ischemic stroke.124 Presumably younger individuals have less cerebral atrophy and less room within the cranial vault to accommodate brain swelling.
Treatment of this syndrome has so far proved disappointing, as reviewed by different authors.125–127 The review by Wijdicks127 is particularly comprehensive. Standard therapies include hyperventilation and the administration of IV mannitol. Mannitol is a dehydrating agent, an osmotic diuretic, which aims at reducing cerebral volume and thus ICP. It is given initially at a dosage of 1 g/kg, followed by recurrent dosing four times daily with 0.25 to 0.5 g/kg. The goal of therapy is a serum osmolality of 315 to 320 mOsm/L. Mechanical ventilation with hyperventilation produces hypocarbia, which leads to cerebral vasoconstriction, reduced cerebral blood volume, and decreased ICP. The target range for PaCO2 is not well defined. Steiner and colleagues126 state that lowering PCO2 from “35 to 29 mm Hg lowers ICP by 25% to 30% in most subjects.” The benefit of these interventions in clinical practice appears to be short-lived. Because hyperventilation is associated with decreased blood flow, it should be viewed as a temporizing measure only.
More promising therapies include hypothermia and decompressive hemicraniectomy. Hypothermia in one pilot trial128 lowered ICP temporarily when instituted within 14 hours of onset of major MCA stroke. Hypothermia has also been employed129 shortly after thrombolysis. The use of a device placed in the inferior vena cava lowers body temperature much more quickly than surface cooling,130 although there is a concern regarding the development of DVT. Hypothermia to 33° C for 24 hours, following tPA administration within 6 hours of stroke onset, is safe, apart from an increased risk of pneumonia.131 A larger randomized trial of hypothermia following thrombolysis, ICTuS2/3 (Intravascular Cooling in the Treatment of Stroke 2/3 Trial),132 is under way.
Decompressive hemicraniectomy with durotomy in early studies appeared beneficial when compared with medical treatment,133 particularly when intervention occurred before the appearance of signs of incipient herniation.134 However, the surgically treated patients in these two reports differed significantly from the control patients. The surgical patients were younger, and subjects with large left hemispheric infarcts producing global aphasia were not considered for surgery. Some, if not all, of the benefit in the surgically treated group may be explained by this imbalance between the control and treated groups. More recently, three randomized trials have been carried out,135–137 and a preplanned pooled analysis138 of their results published. Two of these studies were terminated prematurely,135,136 all the studies revealed reductions in mortality rate, but benefits in functional outcome were lesser and not consistently significant. The pooled analysis, looking at the benefit of hemicraniectomy in patients younger than 60 years of age treated within 48 hours, showed significant benefits both in mortality rate and in functional outcome, such that hemicraniectomy has been adopted into general practice. A further study, DESTINY II,139 will examine the efficacy of this intervention among subjects older than 60.
Surgical intervention also exists for cerebellar infarction, which, when large, may expand and place pressure on the brainstem.140 Both ventriculostomy to relieve hydrocephalus acutely and decompressive craniotomy are employed. The main determinant of survival and recovery is the absence of coma on presentation. Surgery is the accepted treatment, but the exact timing of intervention remains uncertain.141
Critical Care Consultation
Critical care consultation for stroke patients is indicated for a number of reasons (Box 63.17).
References
1. Broderick, J, Brott, T, Kothari, R, et al. The Greater Cincinnati/Northern Kentucky Stroke Study: Preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998; 29:415–421.
2. Fang, J, Alderman, MH. Trend of stroke hospitalization, United States, 1988-1997. Stroke. 2001; 32:2221–2226.
3. Thom, T, Haase, N, Rosamond, W, et al. Heart disease and stroke statistics—Update. Circulation. 2006; 113:e85–e151.
4. Centers for Disease Control and Prevention (CDC). Prevalence of disabilities and associated health conditions among adults: United States, 1999. MMWR. 2001; 50:120–125.
5. Muntner, P, Garrett, E, Klag, MJ, et al. Trends in stroke prevalence between 1973 and 1991 in the US population 25 to 74 years of age. Stroke. 2002; 33:1209–1213.
6. Groeger, JS, Guntupalli, KK, Strosberg, M, et al. Descriptive analysis of critical care units in the United States: Patient characteristics and intensive care unit utilization. Crit Care Med. 1993; 21:279–291.
7. The National Institute of Neurological Disorders and Stroke t-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995; 333:1581–1587.
8. Furlan, A, Higashida, R, Wechsler, L, et al. Intra-arterial prourokinase for acute ischemic stroke. The PROACT II Study: A randomized controlled trial. JAMA. 1999; 282:2003–2011.
9. Smith, WS, Sung, G, Starkman, S, et al. Safety and efficacy of mechanical embolization in acute ischemic stroke: Results of the MERCI Trial. Stroke. 2005; 36:1432–1438.
10. Saver JL, Jahan R, Levy E, et al: Primary Results of the SOLITAIRE with the Intention for Thrombectomy (SWIFT) Multicenter, Randomized Clinical Trial. Abstract presented at the International Stroke Conference, New Orleans, LA, Feb. 3, 2012.
11. Hacke, W, Furlan, AJ, Al-Rawi, Y, et al. Intravenous desmoteplase in patients with acute ischemic stroke (DIAS-2): A prospective, randomized, double-blinded, placebo-conrolled study. Lancet Neurol. 2009; 8:141–150.
12. Dellinger RP: Personal 2008.
13. Albers, GW, Caplan, LR, Easton, JD, et al. Transient ischemic attack-proposal for a new definition. N Engl J Med. 2002; 347:1713–1716.
14. Pessin, MS, Duncan, GW, Mohr, JP, et al. Clinical and angiographic features of carotid transient ischemic attacks. N Engl J Med. 1977; 296:358–362.
15. Caplan, D. Aphasia. In: Heilman KM, Valenstein E, eds. Clinical Neuropsychology. 4th ed. Oxford: Oxford University Press; 2003:14–34.
16. Heilman, KM, Watson, RT, Valenstein, E. Neglect and related disorders. In: Heilman KM, Valenstein E, eds. Clinical Neuropsychology. 4th ed. Oxford: Oxford University Press; 2003:296–346.
17. Sacks, O. The man who fell out of bed. In: The Man Who Mistook His Wife for a Hat. New York: Simon & Schuster; 1998.
18. Kubik, CS, Adams, RD. Occlusion of the basilar artery—A clinical and pathological study. Brain. 1946; 69:73–124.
19. Wijdicks, EFM, Scott, JP. Outcome in patients with acute basilar artery occlusion requiring mechanical ventilation. Stroke. 1996; 27:1301–1303.
20. Plum, F, Posner, JB. The Diagnosis of Stupor and Coma, 3rd ed. Philadelphia: FA Davis; 1980.
21. Baquis, GD, Pessin, MS, Scott, RM. Limb shaking—A carotid TIA. Stroke. 1985; 16:444–448.
22. Dreifuss, FE. Classification of epileptic seizures. In: Engel J, Pedley TA, eds. Epilepsy: A Comprehensive Textbook. Philadelphia: Lippincott-Raven; 1997:517–524.
23. Silberstein, SD, Lipton, RB, Goadsby, PJ. Headache in Clinical Practice, 2nd ed. London: Martin Dunitz; 2002.
24. Tong, DC, Yenari, MA, Albers, GW, et al. Correlation of perfusion- and diffusion-weighted MRI with NIHSS score in acute (<6. 5 hour) ischemic stroke. Neurology. 1999; 50:864–869.
25. Fiebach, JB, Schellinger, PD, Gass, A, et al. Stroke magnetic resonance imaging is accurate in hyperacute intracerebral hemorrhage: A multicenter study on the validity of stroke imaging. for the Kompetenznetzwerk Schlaganfall B5. Stroke. 2004; 35:502–506.
26. Knauth, M, von Kummer, R, Jansen, O, et al. Potential of CT angiography in acute ischemic stroke. Am J Neuroradiol. 1997; 18:1001–1010.
27. Koelemay, MJW, Nederkoorn, PJ, Reitsma, JB, Majoie, CB. Systematic review of CT angiography for assessment of carotid artery disease. Stroke. 2004; 35:2306–2312.
28. Johnston, DCC, Goldstein, LB. Clinical carotid endarterectomy decision making: Noninvasive vascular imaging versus angiography. Neurology. 2001; 56:1009–1015.
29. Tomanek, AI, Coutts, SB, Demchuk, AM, et al. MR angiography compared to conventional selective angiography in acute stroke. Can J Neurol Sci. 2006; 33:58–62.
30. Brott, T, Adams, HP, Jr., Olinger, CP, et al. Measurements of acute cerebral infarction: A clinical examination scale. Stroke. 1989; 20:864–870.
31. Larrue, V, von Kummer, R, del Zoppo, et al. Hemorrhagic transformation in acute ischemic stroke. Potential contributing factors in the European Cooperative Acute Stroke Study. Stroke. 1997; 28:957–960.
32. Von Kummer, R, Bozzao, C, Manelfe, C. Early CT Diagnosis of Hemispheric Brain Infarction. Berlin: Springer Verlag; 1995.
33. Patel, SC, Levine, SR, Tilley, BC, et al. Lack of clinical significance of early ischemic changes on computed topography in acute stroke. JAMA. 2001; 286:2830–2838.
35. Adams, HP, Del Zoppo, G, Alberts, MJ, et al. Guidelines for the early management of patients with ischemic stroke. 2007 Guidelines Update. Stroke. 2007; 38:1655–1711.
36. The NINDS t-PA Stroke Study Group. Generalized efficacy of t-PA for acute stroke. Subgroup analysis of the NINDS t-PA stroke trial. Stroke. 1997; 28:2119–2125.
37. The ATLANTIS, ECASS, and NINDS rt-PA Study Group Investigators. Association of outcome with early stroke treatment: Pooled analysis of ATLANTIS, ECASS, and NINDS rt-PA stroke trials. Lancet. 2004; 363:768–774.
38. Hacke, W, Kaste, M, Fieschi, C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Lancet. 1998; 352:1245–1251.
39. Clark, WM, Wissman, S, Albers, GW, et al. Recombinant tissue-type plasminogen activator (alteplase) for ischemic stroke 3 to 5 hours after symptom onset: The ATLANTIS study—A randomized controlled trial. JAMA. 1999; 282:2019–2026.
40. Hacke, W, Kaste, M, Bluhmki, E, et al. Thrombolysis with alteplase 3 to 4. 5 hours after acute ischemic stroke. N Engl J Med. 2008; 359:1317–1329.
41. Mishra, NK, Ahmed, N, Davalos, A, et al. Thrombolysis outcomes in acute ischemic stroke patients with prior stroke and diabetes mellitus. Neurol. 2011; 77:1866–1872.
42. Levy, DL, Brott, TG, Haley, EC, et al. Factors related to intracranial hematoma formation in patients receiving tissue-type plasminogen activator for acute ischemic stroke. Stroke. 1994; 25:291–297.
43. Graham, GD. Tissue plasminogen activator for acute ischemic stroke in clinical practice. A meta-analysis of safety data. Stroke. 2003; 34:2847–2850.
44. Katzan, IL, Hammer, MD, Furlan, AJ, et al. Quality improvement and tissue-type plasminogen activator for acute ischemic stroke: A Cleveland update. Stroke. 2003; 34:799–800.
45. Levy, EI, Firlik, AD, Wisniewski, S, et al. Factors affecting survival rates for acute vertebrobasilar artery occlusions treated with intra-arterial thrombolytic therapy: A meta-analytical approach. Neurosurgery. 1999; 45:539–548.
46. Kidwell, CS, Saver, JL, Carneado, J, et al. Predictors of hemorrhagic transformation in patients receiving intra-arterial thrombolysis. Stroke. 2002; 33:717–724.
47. Chalela, JA, Katzan, I, Liebeskind, DS, et al. Safety of intra-arterial thrombolysis in the postoperative period. Stroke. 2001; 32:1365–1369.
48. Khatri, P, Kasner, SE. Ischemic strokes after cardiac catheterization: Opportune thrombolysis candidates? Arch Neurol. 2006; 63:817–821.
49. The IMS II Trial Investigators. The Interventional Management of Stroke (IMS) II Study. Stroke. 2007; 38:2127–2135.
50. . http://clinicaltrials.gov/ct2/show/NCT00359424/?term=IMS+III&rank=1
51. Smith, WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the stands Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, Part I. Am J Neuroradiol. 2006; 27:1177–1182.
52. Becker, KJ, Brott, TG. Approval of the MERCI clot retriever: A critical view. Stroke. 2005; 36:400–402.
53. Wintermark, M, Reichhart, M, Thiran, J-P, et al. Prognostic accuracy of cerebral blood flow measurement by perfusion computed tomography, at the time of emergency room admission, in acute stroke patients. Ann Neurol. 2002; 51:417–432.
54. Parsons, MW, Christensen, S, McElduff, P, et al. Pretreatment diffusion- and perfusion-MR lesion volumes have a crucial influence on clinical response to stroke thrombolysis. J Cereb Blood Flow Metab. 2010; 30:1214–1225.
55. European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST). Lancet. 1998; 351:1379–1387.
56. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991; 325:445–453.
57. Sundt, TM, Jr., Sandok, BA, Whisnant, JP. Carotid endarterectomy. Complications and preoperative assessment of risk. Mayo Clin Proc. 1975; 50:301–306.
58. Gasecki, AP, Eliasziw, M, Ferguson, GG, et al. Long-term prognosis and effect of endarterectomy in patients with symptomatic severe carotid stenosis and contralateral carotid stenosis or occlusion: Results from NASCET. North American Symptomatic Carotid Endarterectomy Trial (NASCET) Group. J Neurosurg. 1995; 83:778–782.
59. Morgenstern, LB, Fox, AJ, Sharpe, BL, et al. The risks and benefits of carotid endarterectomy in patients with near occlusion of the carotid artery. Neurology. 1997; 48:911–915.
60. Benavente, OR, Eliasziw, M, Streifler, JY, et al. Prognosis after transient monocular blindness associated with carotid artery stenosis. N Engl J Med. 2001; 345:1084–1090.
61. Eliasziw, M, Streifler, JY, Fox, AJ, et al. Significance of plaque ulceration in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial. Stroke. 1994; 25:304–308.
62. Rothwell, PM, Eliasziw, M, Gutnikov, SA, et al. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004; 363:915–924.
63. Yadav, JS, Wholey, MH, Kuntz, RE, et al. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004; 351:1493–1501.
64. Eckstein, HH, Ringleb, P, Allenberg, JR, et al. Results of the Stent-Protected Angioplasty versus Carotid Endarterectomy (SPACE) study to treat symptomatic stenoses at 2 years: A multinational, prospective, randomised trial. Lancet Neurol. 2008; 7:893–902.
65. Mas, JL, Trinquart, L, Leys, D, et al. Endarterectomy Versus Angioplasty in Patients with Symptomatic Severe Carotid Stenosis (EVA-3S) trial: Results up to 4 years from a randomised, multicentre trial. Lancet Neurol. 2008; 7:885–892.
66. Ederle, J, Bonati, LH, Dobson, L, et al. Endovascular treatment with angioplasty or stenting versus endarterectomy in patients with carotid artery stenosis in the Carotid And Vertebral Artery Transluminal Angioplasty Study (CAVATAS): Long-term follow-up of a randomised trial. Lancet Neurol. 2009; 8:898–907.
67. Brott, TG, Hobson, RW, Howard, G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010; 363:11–23.
68. Chimowitz, MI, Lynn, MJ, Howlett-Smith, H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005; 352:1305–1316.
69. Chimowitz, MI, Lynn, MJ, Derdeyn, CP, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011; 365:993–1003.
70. Duke, RJ, Bloch, RF, Turpie, AG, et al. Intravenous heparin for the prevention of stroke progression in acute partial stable stroke. Ann Intern Med. 1986; 105:825–828.
71. Mohr, JP, Thompson, JLP, Lazar, RM, et al. A comparison of warfarin and aspirin for the prevention of recurrent ischemic stroke. N Engl J Med. 2001; 345:1444–1451.
72. Homma S, Thompson JLP: Results of the warfarin versus aspirin in reduced cardiac ejection fraction (WARCEF) trial. Abstract presented at the 37th International Stroke Conference, New Orleans, LA, Feb. 3, 2012.
73. Hart, RG, Pearce, LA, Aguilar, MI. Meta-analysis: Antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007; 146:857–867.
74. Gage, BF, van Walraven, C, Pearce, L, et al. Selecting patients with atrial fibrillation for anticoagulation. Stroke risk stratification in patients taking aspirin. Circulation. 2004; 110:2287–2292.
75. ACTIVE Writing Group on Behalf of the ACTIVE Investigators. Clopidogrel plus Aspirin versus Oral Anticoagulation for Atrial Fibrillation in the Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE W): A randomized controlled trial. Lancet. 2006; 367:1903–1912.
76. The ACTIVE Investigators. Effect of clopidogrel added to aspirin in patients with atrial fibrillation. N Engl J Med. 2009; 360:2066–2078.
77. EAFT (European Atrial Fibrillation Trial) Study Group. Secondary prevention in nonrheumatic atrial fibrillation after transient ischaemic attack or minor stroke. Lancet. 1993; 342:1255–1262.
78. Berge, E, Abdelnoor, M, Nakstad, PH, Sandset, PM, on behalf of the HAEST Study Group. Low molecular-weight heparin versus aspirin in patients with acute ischemic stroke and atrial fibrillation: A double-blind randomised study. Lancet. 2000; 355:1205–1210.
79. Connolly, SJ, Eikelboom, J, Joyner, C, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011; 364:806–817.
80. Granger, CB, Alexander, JH, McMurray, JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011; 365:981–992.
81. Patel, MR, Mahaffey KW. Garg, J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011; 365:883–891.
82. Connolly, SJ, Ezekowitz, MD, Yusuf, S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009; 361:1139–1151.
83. Garber, S, Sivakumar, W, Schmidt, RH. Neurosurgical complications of direct thrombin inhibitor—Catastrophic hemorrhage after mild traumatic brain injury in a patient receiving dabigatran. J Neurosurg. 2012; 116(5):1093–1096.
84. Eerenberg, ES, Kamphuisen, PW, Sijpkens, MK, et al. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate. Circulation. 2011; 124:1573–1579.
85. Stangier, J, Rathgen, K, Staehle, H, Mazur, D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate. Clin Pharmacokinet. 2010; 49:259–268.
86. Battinelli, EM. Reversal of new oral anticoagulants. Circulation. 2011; 124:1508–1510.
87. Ezekowitz, MD, Reilly, PA, Nehmiz, G, et al. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol. 2007; 100:1419–1426.
88. De Bruijn, SFTM, de Haan, RJ, Stam, J, for the Cerebral Venous Sinus Thrombosis Study Group. Features and prognostic factors of cerebral venous sinus thrombosis in a prospective series of 59 patients. J Neurol Neurosurg Psychiatr. 2001; 70:105–108.
89. Vogl, TJ, Bergman, C, Villringer, A, et al. Dural sinus thrombosis: Value of venous MR angiography for diagnosis and follow-up. AJR Am J Roentgenol. 1994; 162:1191–1198.
90. Einhaupl, KM, Villringer, A, Meister, W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991; 338:596–600.
91. De Bruijn, SFTM, Stam, J, for the Cerebral Venous Sinus Thrombosis Study Group. Randomized, placebo-controlled trial of anticoagulant treatment with low-molecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999; 30:484–488.
92. Frey, JL, Muro, GJ, McDougall, CG, et al. Cerebral venous thrombosis. Combined intrathrombus rtPA and intravenous heparin. Stroke. 1999; 30:489–494.
93. Schievink, WI. Current concepts: Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001; 344:898–906.
94. Levy, C, Laissy, JP, Raveau, V, et al. Carotid and vertebral artery dissections: Three-dimensional time-of-flight MR angiography and MR imaging versus conventional angiography. Radiology. 1994; 190:97–103.
95. Arnold, M, Nedeltchev, K, Sturzenegger, M, et al. Thrombolysis in patients with acute stroke caused by cervical artery dissection: Analysis of nine patients and review of the literature. Arch Neurol. 2002; 59:549–553.
96. Thomas, DP, Roberts, HR. Hypercoagulability in venous and arterial thrombosis. Ann Intern Med. 1997; 26:638–644.
97. Antiplatelet Trialists’ Collaboration. Collaborative overview of randomized trials of antiplatelet treatment: Part 1: Prevention of death, MI and stroke by prolonged antiplatelet therapy in various categories of patients. BMJ. 1994; 308:81–106.
98. Algra, A, Van Gijn, J. Aspirin at any dose above 30 mg offers only modest protection after cerebral ischemia. J Neurol Neurosurg Psychiatr. 1996; 60:197–199.
99. The Dutch TIA Study Group. A comparison of two doses of aspirin (30 mg vs. 283 mg a day) in patients after a transient ischemic attack or minor ischemic stroke. N Engl J Med. 1991; 325:1261–1266.
100. Farrell, B, Goodwin, J, Richard, S, et al. The United Kingdom transient ischemic attack (UK-TIA) aspirin trial: Final results. J Neurol Neurosurg Psychiatr. 1991; 54:1044–1054.
101. Ticlid (Ticlopidine hydrochloride tablets). Complete Prescribing Information. Roche Pharmaceuticals, Nutley, NJ, March 2001.
102. Chen, DK, Kim, JS, Sutton, DMC. Thrombotic thrombocytopenic purpura associated with ticlopidine use: A report of three cases and review of the literature. Arch Intern Med. 1999; 159:311–314.
103. CAPRIE Steering Committee. A randomized, blinded trial of Clopidogrel versus Aspirin in Patients at Risk of Ischemic Events (CAPRIE). Lancet. 1996; 348:1329–1339.
104. The Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndrome without ST-segment elevation. N Engl J Med. 2001; 345:494–502.
105. Chen, ZM, Jiang, LX, Chen, YP, et al. Addition of clopidogrel to aspirin in patients with acute myocardial infarction: Randomized placebo-controlled trial. Lancet. 2005; 366:1607–1621.
106. Sabatine, MS, Cannon, CP, Gibson, CM, et al. Addition of clopidogrel to aspirin and fibrinolytic therapy for myocardial infarction with ST-segment elevations. N Engl J Med. 2005; 352:1179–1189.
107. Mehta, SM, Yusuf, S, Peters, RJG, et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: The PCI-CURE Study. Lancet. 2001; 358:527–533.
108. Diener, HC, Bogousslavsky, J, Brass, LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): Randomized, double-blinded, placebo-controlled trial. Lancet. 2004; 364:331–337.
109. Bhatt, DL, Fox, KA, Hacke, W, et al. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006; 354:1706–1717.
110. Chen, ZM, Sandercock, P, Pan, HC, et al. Indications for early aspirin use in acute ischemic stroke: A combined analysis of 40,000 randomized patients from the Chinese Acute Stroke Trial and the International Stroke Trial. Stroke. 2000; 31:1240–1249.
111. Yoo, DH, Anticytokine therapy in systemic lupus erythematosus. Lupus. 2010; 19(12):1460–1467. http://clinicaltrials.gov/ct2/show/NCT00991029
112. ESPS Group. European Stroke Prevention Study. Stroke. 1990; 21:1122–1130.
113. Diener, HC, Cunha, L, Forbes, C, et al. European Stroke Prevention Study 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996; 143:1–13.
114. Sacco, RL, Diener, H-C, Yusuf, S, et al. Aspirin and Extended-Release Dipyridamole versus Clopidogrel for Recurrent Stroke. N Engl J Med. 2008; 359:1238–1251.
115. Di Tullio, M, Sacco, RL, Gopal, A, et al. Patent foramen ovale as a risk factor for cryptogenic stroke. Ann Intern Med. 1992; 117:461–465.
116. Mas, J-L, Arquizan, C, Lamy, C, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med. 2001; 345:1740–1746.
117. Schuchlenz, HW, Weihs, W, Horner, S, Quehenbeger, F. The association between the diameter of a patent foramen ovale and the risk of embolic cerebrovascular events. Am J Med. 2000; 109:456–462.
118. Messe, SR, Silverman, IE, Kizer, JR, et al. Practice parameter: Recurrent stroke with patent foramen ovale and atrial septal aneurysm. Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2004; 62:1042–1050.
119. Furlan, AJ, Reisman, M, Massaro, J, et al. Closure or medical therapy for cryptogenic stroke with patent foramen ovale. N Engl J Med. 2012; 366:991–999.
120. Hacke, W, Schwab, S, Horn, M, et al. “Malignant” middle cerebral artery territory infarction: Clinical course and prognostic signs. Arch Neurol. 1996; 53:309–315.
121. Wijdicks, EF, Diringer, MN. Middle cerebral artery territory infarction and early brain swelling: Progression and effect of age on outcome. Mayo Clin Proc. 1998; 73:829–836.
122. Biller, J, Adams, HP, Jr., Bruno, A, et al. Mortality in acute cerebral infarction in young adults: A ten-year experience. Angiology. 1991; 42:224–230.
123. Krieger, DW, Demchuk, AM, Kasner, SE, et al. Early clinical and radiological predictors of fatal brain swelling in ischemic stroke. Stroke. 1999; 30:287–292.
124. Pullicino, PM, Alexandrov, AV, Shelton, JA, et al. Mass effect and death from severe acute stroke. Neurology. 1997; 49:1090–1095.
125. Hofmeijer, J, van der Worp, B, Kapelle, LJ. Treatment of space-occupying cerebral infarction. Crit Care Med. 2003; 31:617–625.
126. Steiner, T, Ringleb, P, Hacke, W. Treatment options for large hemispheric stroke. Neurology. 2001; 57(Suppl 2):S61–S68.
127. Wijdicks, EFM. Management of massive hemispheric cerebral infarct: Is there a ray of hope? Mayo Clin Proc. 2000; 75:945–952.
128. Schwab, S, Schwarz, S, Spranger, M, et al. Moderate hypothermia in the treatment of patients with severe middle cerebral artery infarction. Stroke. 1998; 29:2461–2466.
129. Krieger, DW, De Georgia, MA, Abou-Chebl, A, et al. Cooling for acute ischemic brain damage (COOL AID): An open pilot study of induced hypothermia in acute ischemic stroke. Stroke. 2001; 32:1847–1854.
130. De Georgia, MA, Krieger, DW, Abou-Chebl, A, et al. Cooling for acute ischemic brain damage (COOL AID): A feasibility trial of endovascular cooling. Neurology. 2004; 63:312–317.
131. Hemmen, TA, Raman, R, Guluma, KZ, et al. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L). Stroke. 2010; 41:2265–2270.
132. . http://clinicaltrial.gov/ct2/show/NCT01123161?term=ictus&rank=2
133. Rieke, K, Schwab, S, Krieger, D, et al. Decompressive surgery in space-occupying hemispheric infarction: Results of an open, prospective trial. Crit Care Med. 1995; 23:1576–1587.
134. Schwab, S, Steiner, T, Aschoff, A, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke. 1998; 29:1888–1893.
135. Juettler, E, Schwab, S, Schmiedek, P, et al. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY). Stroke. 2007; 38:2518–2525.
136. Vahedi, K, Vicaut, E, Mateo, J, et al. Sequential-design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL trial). Stroke. 2007; 38:2506–2517.
137. Hofmeijer, J, Kappelle, LJ, Algra, A, et al. Surgical decompression for space-occupying cerebral infarction (the hemicraniectomy after middle cerebral artery infarction with life-threatening edema trial [HAMLET]): A multicentre, open, randomised trial. Lancet Neurol. 2009; 8:326–333.
138. Vahedi, K, Hofmeijer, J, Juettler, E, et al. Early decompressive surgery in malignant infarction of the middle cerebral artery: A pooled analysis of three randomized controlled trials. Lancet Neurol. 2007; 6:215–222.
139. Juettler, E, Boesel, J, Amiri, H, et al. DESTINY II: DEcompressive Surgery for the Treatment of malignant INfarction of the middle cerebral artery II. Int J Stroke. 2011; 6:79–86.
140. Hornig, CR, Rust, DS, Busse, O, et al. Space-occupying cerebellar infarction. Clinical course and prognosis. Stroke. 1994; 25:372–374.
141. Jauss, M, Krieger, D, Hornig, C, et al. Surgical and medical management of patients with massive cerebellar infarctions: Results of the German-Austrian Cerebellar Infarction Study. for the GASCIS Study Centers. J Neurol. 1999; 246:257–264.