Stroke
Stroke is an important cause of mortality and long-term neurologic morbidity in children. In the pediatric age group, it is defined as a cerebrovascular event occurring between 14 weeks of gestation and 18 years of life. It ranks among the top 10 causes of death in children,1 with the highest incidence observed in the perinatal period. It occurs in approximately 25 per 100,000 live births in the neonatal population and in 2 to 3 per 100,000 in children between 30 days and 18 years. Recurrence is estimated to be around 3% to 5% in neonates and ranges from 20% to 40% in older children. Among those who survive, more than 50% progress to the development of permanent neurologic or cognitive sequelae.2 The required treatment and rehabilitation programs usually result in a large economic burden to the family and society (Box 37-1).
Fetal Stroke
Maternal, placental, and fetal risk factors have been reported, but in more than 50% of cases, no obvious cause is found. The common maternal conditions associated with fetal stroke are alloimmune thrombocytopenia, diabetes, anticoagulant or antiepileptic therapy, and trauma. Placental factors include placental hemorrhage, abruption, and thromboemboli.3 It is unclear whether coagulopathy is a risk factor, but a case of fetal protein C deficiency has been reported.
Once an abnormality is found on a prenatal ultrasound examination, fetal magnetic resonance imaging (MRI) usually is performed; it is the imaging modality of choice for assessing fetal brain injury (Fig. 37-1). Hemorrhagic lesions have been reported in more than 90% of cases, compared with porencephalic cysts, which are reported in 10% of cases. Arterial ischemic stroke (AIS) typically involves the major arterial territories, most commonly the middle cerebral artery (MCA). Arterial ischemic insults occurring in the second trimester can cause cortical disorganization, resulting in polymicrogyria. If fetal hemorrhagic strokes are similar in origin to the vast majority of preterm and term hemorrhages, it is likely that many fetal hemorrhagic strokes are venous strokes. When tissue destruction occurs as a result of a fetal stroke, the type of tissue response identified on postnatal imaging can help determine the time of the intrapartum event. Porencephalic cysts lack a surrounding astroglial response and develop with injuries between 22 and 27 weeks of gestation. Thereafter, cystic encephalomalacia with gliosis is seen on pathology and MRI. Unlike in neonates and adults, diffusion-weighted imaging (DWI) may not be reliable in predicting the approximate date of an event.4
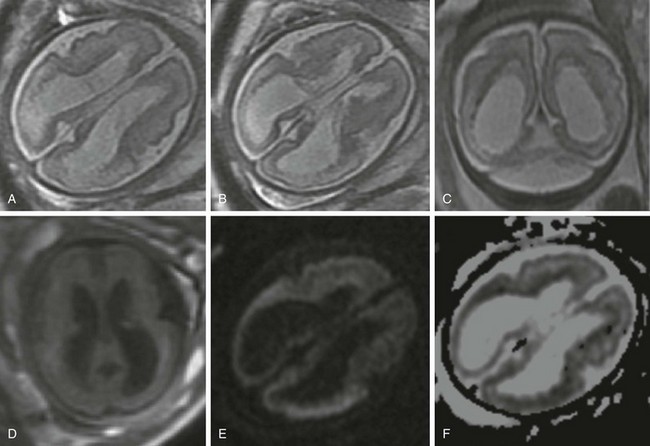
Figure 37-1 Fetal stroke.
A 30-year-old woman underwent a magnetic resonance imaging scan after fetal ventriculomegaly was found on a routine prenatal ultrasound at 29 weeks’ gestational age. A, B, and C, T2-weighted images show dilatation of the lateral ventricles associated with T2 prolongation, thinning, and irregularity of the periatrial white matter. D, T1-weighted image. E, Diffusion-weighted image. F, Apparent diffusion coefficient map. Reduced diffusion also is observed within the periventricular white matter, suggesting evolving ischemic necrosis.
Perinatal or Neonatal Stroke
Perinatal or neonatal stroke is an event that occurs between the late third trimester and the first month of life. The pathophysiology is complex and typically multifactorial. Recently, prothrombotic abnormalities of the coagulation pathway have been of particular interest because of the evolving role and potential use of antithrombotic agents for both treatment and prevention.5
Arterial Ischemic Stroke
Perinatal AIS leads to focal ischemic necrosis in an arterial distribution, most commonly in the MCA territory. The cause is undetermined in more than half of all cases. In the remainder of cases, the source of the thromboemboli may be an intracranial or extracranial vessel, the heart, or the placenta. An increased incidence of dehydration and sepsis also is found, along with cardiac and coagulation disorders.2 AIS may be clinically subtle, and newborns often present with seizures without encephalopathy 2 to 3 days after birth. At the time of clinical presentation, ultrasound of the head can be have false-negative results. Computed tomography (CT) can detect hemorrhage and areas of advanced infarction but also may have false-negative results. Furthermore, ionizing radiation exposure is discouraged in neonates. Acute AIS is easily identified on MRI as regions of bright signal on DWI and decreased signal on apparent diffusion coefficient (ADC) maps within a vascular territory. The reduction in ADC values results from the presence of acute ischemic necrosis and the associated physiologic changes, such as cellular swelling, increased tortuosity of the extracellular space, decreased intracellular cytosolic streaming, and increased intracellular viscosity. The reduction in ADC can persist for up to 2 weeks, being more conspicuous during the first 4 days.6
On T2-weighted images, subtle loss of gray-white matter differentiation often is identified, although it may be negative within the first hours after clinical presentation. MR angiography (MRA) may be helpful in excluding complete occlusion of a major intracranial artery, but turbulent or fast flow often can result in signal dropout, which generates a concern for partially occlusive thrombus in this clinical context. Cerebral perfusion can be obtained using arterial spin labeling. This technique uses arterial blood water magnetically labeled by a radiofrequency pulse to obtain cerebral blood flow measurements; it does not require intravenous injection of contrast media (see Chapter 28). This technique can be particularly useful in determining the presence of reperfusion in areas of abnormal ADC (Fig. 37-2).7
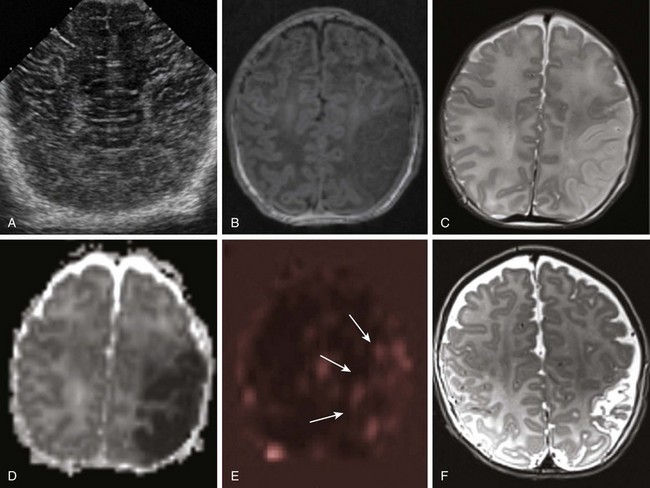
Figure 37-2 Neonatal stroke.
A 1-day-old full-term infant with seizures. A, Ultrasound of the head shows normal cerebral architecture. A magnetic resonance imaging scan performed 10 hours later shows edema (B, T1-weighted image; C, T2-weighted image) and decreased diffusion (D, apparent diffusion coefficient map) involving gray and white matter in the left middle cerebral artery (MCA) territory. Perfusion-weighted images demonstrate relative increased blood flow in the same region (arrows in E, arterial spin labeling). A follow-up T2-weighted image at 3 months (F) shows encephalomalacic changes and volume loss within the area of the prior MCA territory infarct.
AIS also can develop as a result of bacterial meningitis as inflammatory cells infiltrate the vessel wall, leading to foci of necrosis that incite thrombosis of the arteries or veins coursing through the infected space (Fig. 37-3).
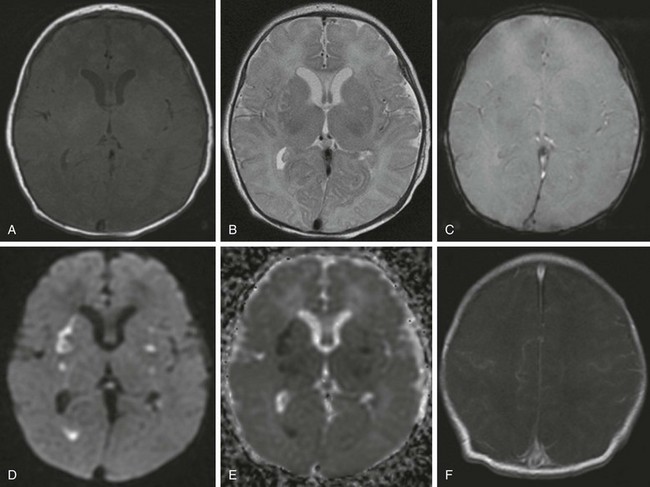
Figure 37-3 Postinfectious arteritis.
A 5-day-old girl with group B streptococcus meningitis (A, T1-weighted image; B, T2- weighted image; C, T2 planar gradient recalled; D, diffusion-weighted image; E, apparent diffusion coefficient map; F, postcontrast T1-weighted image). Small regions of T2 signal abnormality and bilateral foci of decreased diffusion are seen within the basal ganglia and thalami, consistent with ischemic necrosis. In addition, material isointense to gray matter is layered dependently within the atria of the right lateral ventricle, which demonstrates decreased diffusion, but no evidence is seen of a susceptibility artifact on multiple planar gradient recalled images, suggesting pyogenic material. Subtle, scattered prominence of leptomeningeal enhancement also is seen along the cortical surfaces.
Venous Stroke
Venous strokes are associated with vasogenic edema, hemorrhage, and ischemic necrosis in a venous distribution. A venous stroke can occur as a result of transient mechanical or thrombotic occlusion of a vein or venous sinus. Newborns present with nonspecific symptoms related to increased intracranial pressure, lethargy, or seizures. A significant proportion of neonatal sinovenous thrombosis (SVT) is classified as idiopathic, but risk factors include dehydration, sepsis, asphyxia, maternal diabetes, and thrombophilia.8 Isolated SVT has a good prognosis, except in rare cases when the deep venous system becomes involved.
CT may show a hyperdense clot in the involved vein or venous sinus and can identify intraventricular hemorrhage seen with involvement of the deep venous system. MRI is the preferred modality for confirming the diagnosis and determining the presence and extent of the associated brain injury. T2* gradient-echo or susceptibility-weighted images are particularly useful in demonstrating the thrombus as a region of “blooming” in the venous system and detecting intraparenchymal hemorrhage (Fig. 37-4 and Boxes 37-2 and 37-3). On follow-up examinations, brain parenchyma affected by a venous stroke can show atrophy or can almost completely resolve, depending on the severity and duration of the injury.
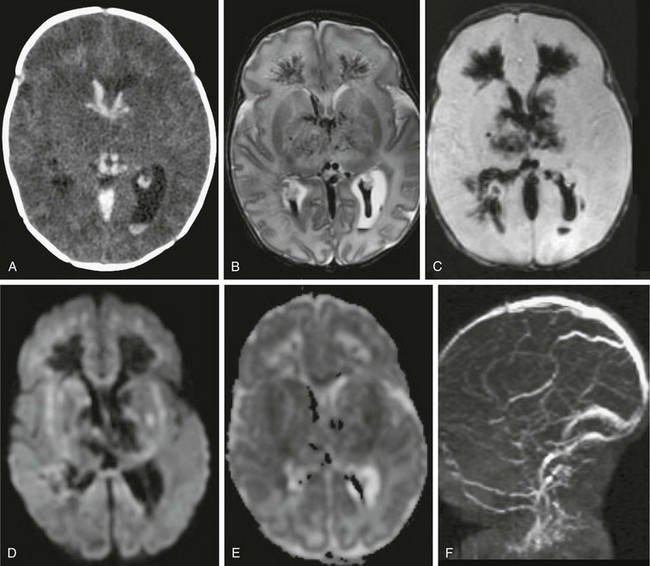
Figure 37-4 An 11-day-old newborn with seizures for 24 hours.
An axial computed tomography (CT) scan (A), T2-weighted magnetic resonance (MR) image (B), susceptibility sequence (C), diffusion-weighted image (DWI) (D) with apparent diffusion coefficient (E), and two-dimensional time-of-flight MR venography (MRV) (F) from CT shows hyperdense clots involving the deep venous system with extensive cerebral edema. MR imaging with MRV confirms lack of enhancement of the entire deep venous system, consistent with thrombosis with extension into the perimedullary veins. Extensive edema is present involving the deep gray nuclei and white matter with associated bithalamic and intraventricular hemorrhage. On DWI, the cerebral edema shows both decreased and increased diffusion, consistent with a combination of cytotoxic and vasogenic edema. Factor V Leiden mutation was the cause of the hypercoagulable state.
Childhood Stroke
The incidence of childhood stroke based on imaging findings is estimated to be 2.4 cases per 100,000 patient population.9 Cerebrovascular insults in children can be categorized as AIS or SVT.
Arterial Ischemic Stroke
In more than half of childhood cases of AIS, the precise etiology is never determined. In the remaining cases, a variety of pathologies are found, including thromboembolism (from intracranial or extracranial vessels, cardiac disorders such as congenital or acquired heart disease, intracardiac shunts, and procedures), arteriopathy (arterial dissection, moyamoya disease, vasculitis, sickle cell disease [SCD] arteriopathy, postvaricella angiopathy, and idiopathic focal cerebral arteriopathy), and hypercoagulable states (protein C or S deficiency, antithrombin III, and factor V Leiden mutation).10,11
On imaging, acute infarction is usually superimposed on a diseased brain with atrophy and chronic changes. MRA commonly shows irregular stenotic arteries involving the anterior circulation with leptomeningeal collaterals, often longstanding. CT angiography (CTA) use is limited because iodinated contrast predisposes to sickling crises. Low osmolar agents should be used when iodinated contrast is deemed necessary, along with transfusion and hydration to reduce the risk of complications. Biannual screening with transcranial Doppler imaging is routinely performed, and abnormally elevated time-averaged mean velocities (>200 cm/sec) from stenotic arteries necessitates blood transfusion to prevent stroke.12 Brain MRI is usually performed to evaluate children with sickle cell anemia presenting with seizures or motor or sensory deficit.
Moyamoya disease accounts for approximately 6% of AIS incidence in Western countries. Moyamoya disease is a progressive vasculopathy causing stenosis of intracranial arteries with a predilection for terminal portions of the internal carotid arteries (Fig. 37-5). It can be associated with neurofibromatosis type 1, radiation vasculitis, Down syndrome, and SCD; if the cause remains undetermined, it is referred to as moyamoya disease. Collateral formation from the lenticulostriate vessels and thalamoperforators lead to a classic “puff-of-smoke” appearance on angiography. MRI can demonstrate loss of the normal flow void in the distal carotid branches on T2-weighted images, as well as development of abnormally large and irregular collateral vessels. Fluid attenuated inversion recovery images often show increased signal in distal vessels with decreased flow. Carotid stenosis also can be observed on MRA, but its severity can be overestimated because of slow and turbulent flow.13 Postcontrast MRA and, in particular, CTA can improve accuracy. DWI demonstrates acute areas of ischemic necrosis, whereas perfusion imaging and arterial spin labeling can demonstrate peripheral areas of delayed cerebral flow.
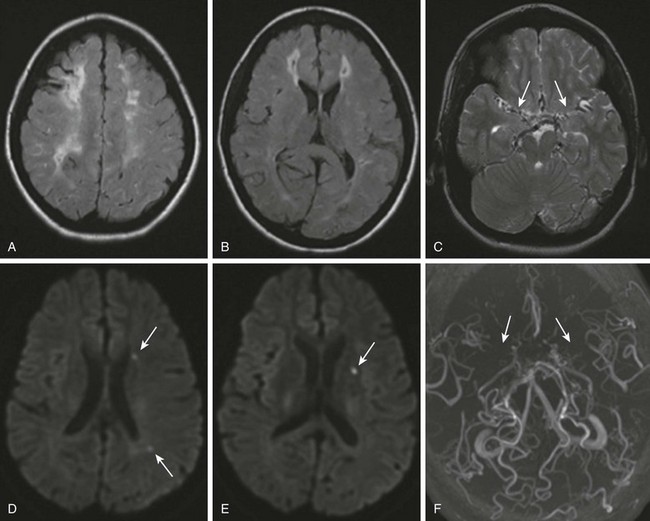
Figure 37-5 Moyamoya disease.
A 16-year-old girl with a history of sickle cell disease and multiple cerebrovascular accidents has new onset right-sided numbness. Axial fluid attenuated inversion recovery images (A and B) demonstrate multiple small areas of old infarcts surrounded by gliosis in a watershed distribution. A T2-weighted image (C) shows prominence of extraaxial vessels (arrows), suggesting collateral circulation. Diffusion-weighted images (D and E) show tiny foci of decreased diffusion (arrows), compatible with small acute ischemic strokes. Magnetic resonance angiography (F) shows lack of flow related enhancement of the supraclinoid internal carotid arteries and middle cerebral arteries (arrows).
Arterial dissection may occur spontaneously or after trauma. Arterial dissection leads to formation of an intramural thrombus, which can propagate and embolize distally, or it can lead to vascular occlusion. The common locations are at the junction between relatively fixed and mobile segments of arteries and among the intracranial arteries; the supraclinoid internal carotid artery often is affected. T1-weighted fat-saturated sequences performed from the aortic arch to the cavernous sinus can demonstrate concentric hyperintense signal because of methemoglobin in the vessel wall in the subacute phase. If the dissection is recent, close inspection of the images is necessary to rule out isointense concentric wall thickening. If MRA is degraded by flow artifacts, CTA can be performed (Fig. 37-6). The angiographic findings are abrupt segmental narrowing with an intimal flap, a beaded appearance, or pseudoaneurysm formation.14
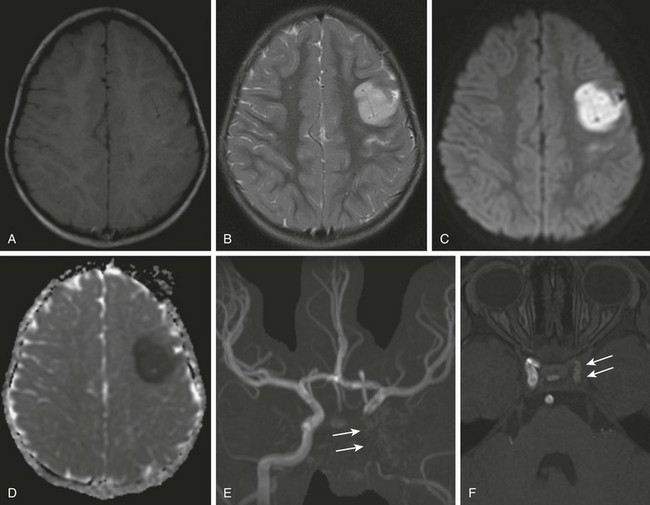
Figure 37-6 Carotid dissection.
A 3-year-old girl with right facial drop and right upper extremity weakness after blunt trauma to the neck. A magnetic resonance imaging scan shows evidence of cortical and subcortical edema within the left frontal lobe (A, T1-weighted image; B, T2-weighted image) with reduced diffusion associated (C, diffusion-weighted image; D, apparent diffusion coefficient map) compatible with ischemic infarction. Maximum intensity projection magnetic resonance angiography (MRA) shows absence of flow within the left internal carotid artery (arrows in E). An MRA source image (F) shows irregular and diminutive flow signal within the cavernous segment of the left carotid artery (arrows).
Mitochondrial disorders usually involve multiple systems, but the strokelike events that appear as focal neurologic deficits of abrupt onset can mimic and be clinically indistinguishable from AIS. Mitochondrial injuries do not follow the vascular boundaries, and on MRI, the ADC values are typically normal to elevated, as opposed to the classically low values seen with AIS6 (see Chapter 33).
The presence of transient neurologic symptoms in patients with migraine also can simulate stroke. Migraine with aura has been associated with increased risk for stroke in the adult population, and the literature includes some case reports of migrainous infarct in adolescents. The association between these two entities is thought to be related to a dysfunction of cerebral arteries during migraine bouts (Fig. 37-7).15
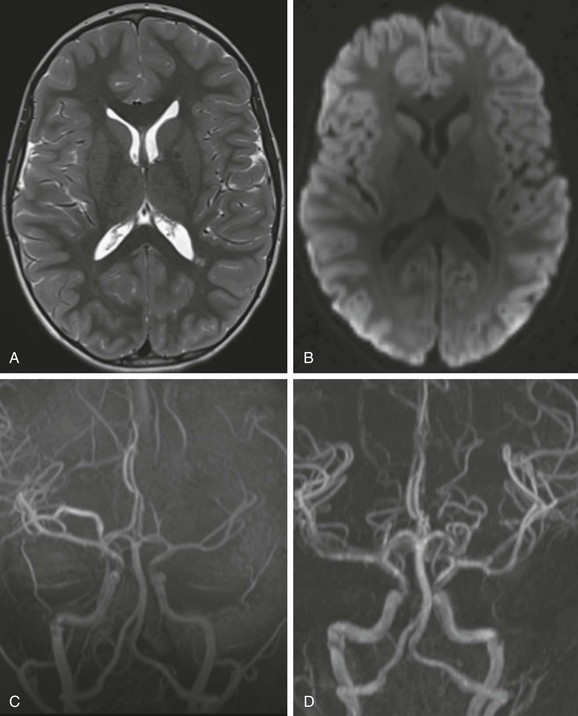
Figure 37-7 Vasospasm.
A 7-year-old boy has headache, ataxia, and confusion. A T2-weighted image (A) and diffusion-weighted image (B) are unremarkable. Magnetic resonance angiography (MRA) (C) demonstrates asymmetry of the intracranial vascularity with left middle cerebral artery branches less prominent than the contralateral side. Follow-up MRA (D) 21 hours later shows significantly improved visualization of the distal M3 and M4 segments of the left middle cerebral artery.
Venous Stroke
In children, SVT is the primary cause of venous stroke. Dehydration, complicated otitis media, and sinusitis are the major risk factors in older children. Less commonly, a prothrombotic disorder, trauma, or medication is the identified cause. If the superficial cortical veins are involved, SVT leads to regional cerebral edema, which has a good prognosis. SVT is demonstrated as hyperdensity on CT and variable signal intensity on conventional MRI sequences. MRI with susceptibility-weighted images, DWI, MR venography, and postcontrast volumetric T1 imaging are the sequences of choice. DWI is helpful for determining whether vasogenic edema or ischemic necrosis is present. Postcontrast volumetric T1 images provide direct visualization of filling defects in the venous system and allow secondary evaluation of regions of signal dropout on MR venography.16 Identification of the clot on susceptibility sequences is the best confirmation of SVT. If MRI is equivocal, CT venography can give a better delineation of the venous system, at the expense of radiation exposure (Fig. 37-8).
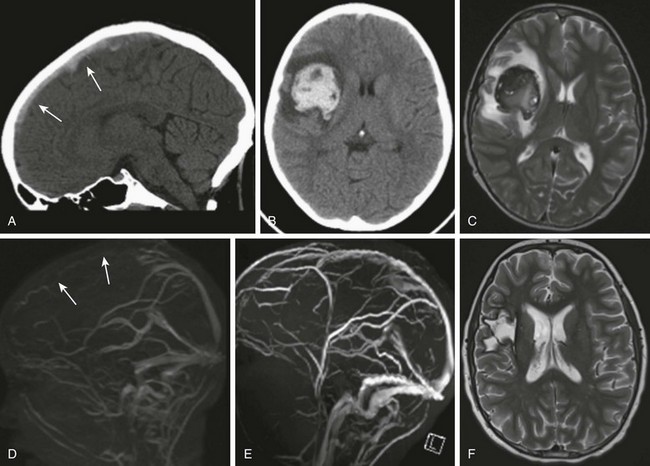
Figure 37-8 Hemorrhagic venous infarct.
An 11-year-old boy with acute lymphocytic leukemia, status postinduction, presented with nausea, vomiting, left upper limb weakness, and left facial weakness. A computed tomography (CT) scan in the sagittal view (A) shows hyperdense material compatible with a clot within the superior sagittal sinus (arrows). An axial view CT image (B) and T2-weighted magnetic resonance (MR) image (C) demonstrate a large intraparenchymal hemorrhage in the right frontoparietal region consistent with hemorrhagic venous infarct, associated with effect of a mass causing effacement of the right lateral ventricle and mild leftward midline. Surrounding edema also is noticed. MR venography (MRV) (D) shows lack of flow-related enhancement of the superior sagittal sinus (arrows). A follow-up scan 1 year later (E) shows a patent superior sagittal sinus on MRV (E), as well as encephalomalacic changes on a T2-weighted image in the area of the previous hemorrhagic venous infarct (F).
Jackson, BF, Porcher, FK, Zapton, DT, et al. Cerebral sinovenous thrombosis in children: diagnosis and treatment. Pediatr Emerg Care. 2011;27(9):874–880.
Mackay, MT, Wiznitzer, M, Benedict, SL, et al. Arterial ischemic stroke risk factors: the International Pediatric Stroke Study, International Pediatric Stroke Study Group. Ann Neurol. 2011;69(1):130–140.
Rodrigues, K, Grant, PE. Diffusion-weighted imaging in neonates. Neuroimaging Clin N Am. 2011;21(1):127–151.
References
1. Office of Statistics and Programming, National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. 10 Leading causes of death by age group, United States—2009. http://www.cdc.gov/Injury/wisqars/pdf/10LCD-Age-Grp-US-2009-a.pdf, 2012. [Accessed October 22].
2. Lynch, JK, Hirtz, DG, DeVeber, G, et al. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109(1):116–123.
3. Ozduman, K, Pober, BR, Barnes, P, et al. Fetal stroke. Pediatr Neurol. 2004;30(3):151–162.
4. Tarui, T, Khwaja, OS, Estroff, JA, et al. Fetal MR imaging evidence of prolonged apparent diffusion coefficient decrease in fetal death. AJNR Am J Neuroradiol. 2011;32(7):E126–E128.
5. Deveber, G. Paediatric stroke. Who should be treated? Hamostaseologie. 2009;29(1):88–90.
6. Rodrigues, K, Grant, PE. Diffusion-weighted imaging in neonates. Neuroimaging Clin N Am. 2011;21(1):127–151.
7. Chen, J, Licht, DJ, Smith, SE, et al. Arterial spin labeling perfusion MRI in pediatric arterial ischemic stroke: initial experiences. J Magn Reson Imaging. 2009;29(2):282–290.
8. deVeber, G, Andrew, M, Adams, C, et al. Canadian Pediatric Ischemic Stroke Study Group: Cerebral sinovenous thrombosis in children. N Engl J Med. 2001;345(6):417–423.
9. Agrawal, N, Johnston, SC, Wu, YW, et al. Imaging data reveal a higher pediatric stroke incidence than prior US estimates. Stroke. 2009;40(11):3415–3421.
10. Beslow, LA, Jordan, LC. Pediatric stroke: the importance of cerebral arteriopathy and vascular malformations. Childs Nerv Syst. 2010;26(10):1263–1273.
11. Mackay, MT, Wiznitzer, M, Benedict, SL, et al. International Pediatric Stroke Study Group: Arterial ischemic stroke risk factors: the International Pediatric Stroke Study. Ann Neurol. 2011;69(1):130–140.
12. Adams, RJ, McKie, VC, Hsu, L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339(1):5–11.
13. Smith, ER, Scott, RM. Spontaneous occlusion of the circle of Willis in children: pediatric moyamoya summary with proposed evidence-based practice guidelines. J Neurosurg Pediatr. 2012;9(4):353–360.
14. Stence, NV, Fenton, LZ, Goldenberg, NA, et al. Craniocervical arterial dissection in children: diagnosis and treatment. Curr Treat Options Neurol. 2011;13(6):636–648.
15. Ming, X, Yacoub, H, Khanna, A, et al. Two young patients with stroke in conjunction with migraineus headache. Open Neurol J. 2010;15(4):111–116.
16. Jackson, BF, Porcher, FK, Zapton, DT, et al. Cerebral sinovenous thrombosis in children: diagnosis and treatment. Pediatr Emerg Care. 2011;27(9):874–880.






