Chapter 175 Streptococcus pneumoniae (Pneumococcus)
Epidemiology
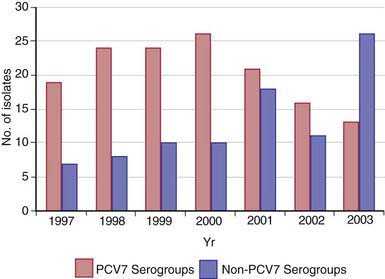
Most healthy individuals carry various S. pneumoniae serotypes in their upper respiratory tract; >90% of children between 6 mo and 5 yr of age harbor S. pneumoniae in the nasopharynx at some time. A single serotype usually is carried by a given individual for an extended period (45 days to 6 mo). Carriage does not consistently induce local or systemic immunity sufficient to prevent later reacquisition of the same serotype. Rates of pneumococcal carriage peak during the 1st and 2nd yr of life and decline gradually thereafter. Carriage rates are highest in institutional settings and during the winter, and rates are lowest in summer. Nasopharyngeal carriage of pneumococci is common among young children attending out-of-home care, with rates of 21-59% in point prevalence studies and 65% in longitudinal studies. During the past 4 decades, serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F have constituted the majority of invasive isolates in children in the U.S. and other developed countries; strains belonging to serotypes 6B, 9V, 14, and 19F frequently have reduced susceptibility to penicillin. Since licensure of the PCVs, the prevalence of carriage and infection with vaccine serotypes has substantially declined and a shift to increased carriage or infections with nonvaccine serotypes has occurred (Fig. 175-1). Indirect protection of unvaccinated persons has occurred since PCV introduction, and this herd protection is likely due to decreases in nasopharyngeal carriage of virulent pneumococcal vaccine serotypes.
Pneumococcal disease usually occurs sporadically but can be spread from person to person by respiratory droplet transmission. The frequency and severity of pneumococcal disease are increased in patients with sickle cell disease, asplenia, deficiencies in humoral (B cell) and complement-mediated immunity, HIV infection, certain malignancies (e.g., leukemia, lymphoma), chronic heart, lung, or renal disease (particularly the nephrotic syndrome), cerebrospinal fluid (CSF) leak, and cochlear implants. Other high-risk groups are noted in Table 175-1. S. pneumoniae is an important cause of secondary bacterial pneumonia in patients with influenza. During influenza epidemics and pandemics, most deaths result from bacterial pneumonia, and pneumococcus is the predominant bacterial pathogen isolated in this setting. Pneumococcal co-pathogenicity may be important in disease caused by other respiratory viruses as well.
Table 175-1 CHILDREN AT HIGH OR MODERATE RISK OF INVASIVE PNEUMOCOCCAL INFECTION
HIGH RISK (INCIDENCE OF INVASIVE PNEUMOCOCCAL DISEASE = 150 CASES/100,000 PEOPLE PER YEAR)
Children with:
PRESUMED HIGH RISK (INSUFFICIENT DATA TO CALCULATE RATES)
Children with:
MODERATE RISK (INCIDENCE OF INVASIVE PNEUMOCOCCAL DISEASE = 20 CASES/100,000 PEOPLE PER YEAR)
From American Academy of Pediatrics: Red book: 2006 report of the Committee on Infectious Diseases, ed 27, Elk Grove Village, IL, 2006, American Academy of Pediatrics, p 527.
Clinical Manifestations
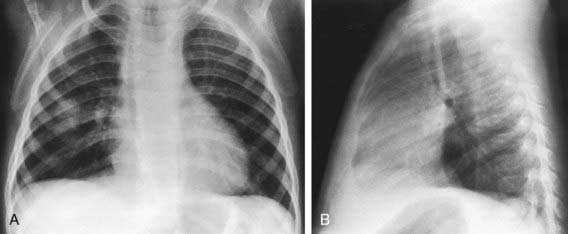
The signs and symptoms of pneumococcal infection are related to the anatomic site of disease. Common clinical syndromes include otitis media (Chapter 632), sinusitis (Chapter 372), pneumonia (Fig. 175-2) (Chapter 392), and sepsis (Chapter 64). Before routine use of PCVs, pneumococci caused >80% of bacteremia episodes in infants 3-36 mo of age with fever without an identifiable source (i.e., occult bacteremia). Bacteremia may be followed by meningitis (Chapter 595), osteomyelitis (Chapter 676), suppurative arthritis (Chapter 677), endocarditis (Chapter 431), and rarely, brain abscess (Chapter 596). Primary peritonitis (Chapter 363) may occur in children with peritoneal effusions due to nephrotic syndrome and other conditions. Local complications of infection may occur, causing empyema, pericarditis, mastoiditis, epidural abscess, or meningitis. Hemolytic-uremic syndrome (Chapter 478.4) and disseminated intravascular coagulation also occur as rare complications of pneumococcal infections. Epidemic conjunctivitis caused by nonencapsulated or encapsulated pneumococci occurs as well.
Prevention
Immunologic responsiveness and efficacy following administration of pneumococcal polysaccharide vaccines is unpredictable in children <2 yr of age. PPSV23 contains purified polysaccharide of 23 pneumococcal serotypes responsible for >95% of cases of invasive disease. The clinical efficacy of these vaccines is controversial and studies have yielded conflicting results. In contrast, PCVs (see Table 175-2) provoke “protective” antibody responses in 90% of infants given these vaccines at 2, 4, and 6 mo of age, and greatly enhanced responses (e.g., immunologic memory) are apparent after “booster” doses given at 12-15 mo of age. In addition, PCVs reduce nasopharyngeal carriage of vaccine serotypes by up to 60-70%. In efficacy trials in the USA, infant immunization with PCV7 decreased invasive infections from pneumococcal vaccine serotypes by >93% and lobar pneumonias by >73%. Its administration was associated with a 6-7% decrease in otitis media, but greater reduction in complications of otitis media such as tympanostomy tube placement. From 2000 (PCV7 introduction) to 2005, invasive pneumococcal disease in U.S. children <5 yr of age decreased by 94%. PCV7 has significantly decreased rates of invasive pneumococcal disease in children with sickle cell disease, and preliminary studies suggest substantial protection for HIV-infected children and splenectomized adults. Adverse events after the administration of PCV7 have included local swelling and redness and slightly increased rates of fever, when used in conjunction with other childhood vaccines. There have been multiple reports of increases of empyema due to serotypes 1, 3, and 19A; necrotizing pneumonia due to serotype 3 and serogroup 19; bacteremia due to serotypes 3 and 8; and mastoiditis and recalcitrant acute otitis media due to MDR serotype 19A. These observations inform the serotype composition of new PCVs (Table 175-2).
Table 175-2 COMPARISON OF PNEUMOCOCCAL VACCINES LICENSED IN USA OR IN ADVANCED DEVELOPMENT (PCV7 SEROTYPES IN BOLD)
| CARRIER PROTEIN | PNEUMOCOCCAL CAPSULAR POLYSACCHARIDES | MANUFACTURER |
|---|---|---|
| Diphtheria CRM197 protein | 4,6B, 9V, 14, 18C, 19F, 23F | Wyeth Lederle (PCV7, Prevnar) |
| Diphtheria CRM197 protein | 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, 23F | Wyeth Lederle (PCV13, Prevnar 13) |
| Haemophilus influenzae protein D Tetanus and diphtheria toxoids |
1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F | GlaxoSmithKline (PCV10, Synflorix) |
| None | 1, 2, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 10A, 11A, 12F, 14, 15B, 17F, 18C, 19A, 19F, 20, 22F, 23F, 33F | Sanofi Pasteur MSD (PPSV23, Pneumovax II) |
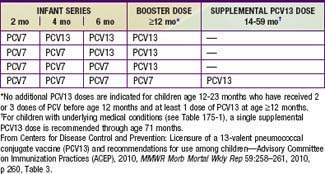
Immunization with PVC13 is recommended for all infants on a schedule for primary immunization, in previously unvaccinated infants, and for transition for those partially vaccinated with PCV7 (Tables 175-3 and 175-4). High-risk children ≥2 yr of age, such as those with asplenia, sickle cell disease, some types of immune deficiency (e.g., antibody deficiencies), HIV infection, cochlear implant, CSF leak, diabetes mellitus, and chronic lung, heart, or kidney disease (including nephrotic syndrome), may benefit also from PPSV23 administered after 2 yr of age following priming with the scheduled doses of PCV13. Thus, it is recommended that children ≥2 yr of age with these underlying conditions receive supplemental vaccination with PPSV23. A second dose of PPSV23 is recommended 5 yr after the first dose of PPSV23 for persons aged ≥2 yr who are immunocompromised, have sickle cell disease, or functional or anatomic asplenia.
Table 175-3 RECOMMENDED ROUTINE VACCINATION SCHEDULE FOR 13-VALENT PNEUMOCOCCAL CONJUGATE VACCINE (PCV13) AMONG INFANTS AND CHILDREN WHO HAVE NOT RECEIVED PREVIOUS DOSES OF 7-VALENT VACCINE (PCV7) OR PCV13, BY AGE AT FIRST DOSE—ADVISORY COMMITTEE ON IMMUNIZATION PRACTICES (ACIP), USA, 2010
| AGE AT FIRST DOSE (MO) | PRIMARY PCV13 SERIES* | PCV13 BOOSTER DOSE† |
|---|---|---|
| 2-6 | 3 doses | 1 dose at age 12-15 mo |
| 7-11 | 2 doses | 1 dose at age 12-15 mo |
| 12-23 | 2 doses | — |
| 24-59 (healthy children) | 1 dose | — |
| 24-71 (children with certain chronic diseases or immunocompromising conditions) | 2 doses | — |
* Minimum interval between doses is 8 weeks except for children vaccinated at age <12 months for whom minimum interval between doses is 4 weeks. Minimum age for administration of first dose is 6 weeks.
† Given at least 8 weeks after the previous dose.
From Centers for Disease Control and Prevention: Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children—Advisory Committee on Immunization Practices (ACEP), 2010, MMWR Morb Mortal Wkly Rep 59:258–261, 2010, p 260, Table 3.
American Academy of Pediatrics. Recommendations for the prevention of Streptococcus pneumoniae infections in infants and children: use of 13-valent pneumococcal conjugate vaccine (PCV13) and pneumococcal polysaccharide vaccine (PPSV23). Pediatrics. 2010;126:186-190.
American Academy of Pediatrics. Red book: 2009 report of the Committee on Infectious Diseases, ed 28. Elk Grove Village, IL: American Academy of Pediatrics; 2009. 524–535
Centers for Disease Control and Prevention. Licensure of 1 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children—ACIP, 2010. MMWR Morb Mortal Wkly Rep. 2010;59:258-261.
Centers for Disease Control and Prevention. Effects of new penicillin susceptibility breakpoints for Streptococcus pneumoniae—United States, 2006–2007. MMWR Morb Mortal Wkly Rep. 2008;57:1353-1357.
Centers for Disease Control and Prevention. Direct and indirect effects of routine vaccination of children with 7-valent pneumococcal conjugate vaccine on incidence of invasive pneumococcal disease—United States, 1998–2003. MMWR Morb Mortal Wkly Rep. 2005;54:893-897.
Centers for Disease Control and Prevention. Emergence of antimicrobial-resistant serotype 19A Streptococcus pneumonia—Massachusetts, 2001–2006. MMWR Morb Mortal Wkly Rep. 2007;56:1077-1080.
Halasa NB, Shankar SM, Talbot TR, et al. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44:1428-1433.
Hendrickson DJ, Blumberg DA, Joad JP, et al. Five-fold increase in pediatric parapneumonic empyema since introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2008;27:1030-1032.
Hsu HE, Shutt KA, Moore MR, et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med. 2009;360:244-256.
Huss A, Scott P, Stuck AE, et al. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ. 2009;180:48-58.
Kaplan SL, Barson WJ, Lin PL, et al. Serotype 19A is the most common serotype causing invasive pneumococcal infections in children. Pediatrics. 2010;125:429-436.
Kaplan SL, Mason EO, Barson WJ, et al. Outcome of invasive infections outside the central nervous system caused by Streptococcus pneumoniae isolates nonsusceptible to ceftriaxone in children treated with beta-lactam antibiotics. Pediatr Infect Dis J. 2001;20:392-396.
Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumonia. N Engl J Med. 2006;354:1455-1463.
Lucero MG, Nohynek H, Williams G, et al. Efficacy of an 11-valent pneumococcal conjugate vaccine against radiologically confirmed pneumonia among children less than 2 years of age in the Philippines. Pediatr Infect Dis J. 2009;28:455-462.
Lynch JP, Zhanel GG. Streptococcus pneumonia: does antimicrobial resistance matter? Semin Respir Crit Care Med. 2009;30:210-238.
Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis. 2008;198:962-970.
Musher DM, Rueda-Jaimes AM, Graviss EA, et al. Effect of pneumococcal vaccination: a comparison of vaccination rates in patients with bacteremic and nonbacteremic pneumococcal pneumonia. Clin Infect Dis. 2006;43:1004-1008.
Neuman MI, Harper MB. Time to positivity of blood cultures for children with Streptococcus pneumoniae bacteremia. Clin Infect Dis. 2001;33:1324-1328.
O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893-902.
Ongkasuwan J, Valdez TA, Hulten KG, et al. Pneumococcal mastoiditis in children and the emergence of multidrug-resistant serotype 19A isolates. Pediatrics. 2008;122:34-39.
Park SY, Van Beneden CA, Pilishvili T, et al. invasive pneumococcal infections among vaccinated children in the United States. J Pediatr. 2010;156:478-483.
Pelton SI, Huot H, Finkelstein JA, et al. Emergence of 19A as virulent and multidrug resistant pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26:468-472.
Pichichero ME, Casey JR. Emergence of a multiresistant serotype 19A pneumococcal strain not included in the 7-valent conjugate vaccine as an otopathogen in children. JAMA. 2007;298:1772-1778.
Poehling KA, Lafleur BJ, Szilagyi PS, et al. Population-based impact of pneumococcal conjugate vaccine in young children. Pediatrics. 2004;114:755-761.
Reefhuis J, Honein MA, Whitney CG, et al. Risk of bacterial meningitis in children with cochlear implants. N Eng J Med. 2003;349:335-345.
Reinert P, Benkerrou M, Montalembert M, et al. Immunogenicity and safety of a pneumococcal conjugate 7-valent vaccine in infants with sickle cell disease. Pediatr Infect Dis J. 2007;26:1105-1109.
Rello J, Lisbon T, Lujan M, et al. Severity of pneumococcal pneumonia associated with genomic bacterial load. Chest. 2009;136:832-840.
Talbot TR, Poehling KA, Hartert T, et al. Reduction in high rates of antibiotic-nonsusceptible invasive pneumococcal disease in Tennessee after introduction of the pneumococcal conjugate vaccine. Clin Infect Dis. 2004;39:641-651.
Von Gottberg A, Klugman KP, Cohen C, et al. Emergence of levofloxacin-non-susceptible Streptococcus pneumoniae and treatment for multidrug-resistant tuberculosis in children in South Africa: a cohort observational surveillance study. Lancet. 2008;371:1108-1113.
Waters AM, Kerecuk L, Luk D, et al. Hemolytic uremic syndrome associated with invasive pneumococcal disease: the United Kingdom experience. J Pediatr. 2007;151:140-144.