!DOCTYPE html>
Stratum Corneum and Sensitive Skin
Stratum Corneum and Sensitive Skin
Subjects with sensitive skin report exaggerated reactions when their skin is in contact with cosmetics, soaps, and sunscreens, and they often report worsening after exposure to dry and cold climates. Epidemiologic studies have been carried out to assess whether there is a correlation with sex, age, skin type, or race and are described elsewhere in this book.
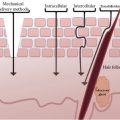
Subjects with sensitive skin may have a thinner SC with a reduced corneocyte area causing a higher transcutaneous penetration of water-soluble chemicals (1). Frosch and Kligman (2), by testing different irritants, showed a 14% incidence of sensitive skin in the normal population, likely correlated to a thin permeable SC, which makes these subjects more susceptible to chemical irritation.
Moreover, the declined barrier function in sensitive skin has already been reported as the result of an imbalance of intercellular lipid of SC (3). Although impaired barrier function is easily understood as a mechanism of sensitive skin, other factors are also possible implications such as changes in the nerve system and/or the structure of the epidermis. In a study (4), detailed characteristics of sensitive skin have been investigated using noninvasive methods. Sensitive skin has been classified into three different types based on their physiological parameters. Type 1 has been defined as the low barrier function group. Type 2 has been defined as the inflammation group with normal barrier function and inflammatory changes. Type 3 has been specified as the pseudohealthy group in terms of normal barrier function and no inflammatory changes. In all types, a high content of nerve growth factor has been observed in the SC, relative to that of nonsensitive skin. In both types 2 and 3, the sensitivity to electrical stimuli was high (4). Since these data suggest that the hypersensitive reaction of sensitive skin is closely related to nerve fibers innervating the epidermis, Yamasaki and Gallo (5) proposed that the innate immune system triggers an abnormal inflammatory reaction that mediates the symptoms of rosacea and sensitive skin. If so, flushing and blushing erythema may be due to chronic inflammation. In particular, cathelicidin may play a role in inducing the cytokine cascade. Indeed, some forms of cathelicidin peptides were known to have a unique capacity to be both vasoactive and proinflammatory (5).
Direct connections were observed between unmyelinated nerve fibers and mast cells; stress in animal models induces substance P (SP) in unmyelinated nerve fibers, which triggers mast cell degranulation with subsequent histamine release (6). Stress is commonly reported as a trigger for sensitive skin, and mast cell degranulation is supported by the finding that sensitive skin sufferers had higher density of mast cells and size of lymphatic microvasculature (7). Neurogenic inflammation probably results from the release of neurotransmitters such as SP, calcitonin gene-related peptide (CGRP), and vasoactive intestinal peptide, which induce vasodilatation and mast cell degranulation. Nonspecific inflammation may also be associated with the release of interleukins. Indeed, sensitive skin could be the result of an inflammatory process resulting from the abnormal penetration in the skin of potentially irritating substances because of skin barrier dysfunction (8). In addition, the presence of a nonspecific reaction has been related to cutaneous sensory innervation in the establishment of skin sensitivity (9,10). Neuropeptides released from cutaneous nerves and skin resident cells such as SP, CGRP, and POMC peptides (such as β-endorphin and encephalin) are mandatory for a fine-tuned regulation of cutaneous immune responses and tissue maintenance and repair (11,12). In response to noxious stimuli, SP and CGRP lead to vasodilatation and mast cell degranulation, originating in a process called neurogenic inflammation. Classical pathways are then activated causing a nonspecific inflammation in consequence of released cytokines and eicosanoids such as interleukin-1 a (IL-1a), tumor necrosis factor alpha, prostaglandin E2, and prostaglandin F2 (13). On the other hand, POMC activities include antagonism and downregulation of adhesion molecules and reduced inflammation by the modulation of IL-10 production, which contributes to the amelioration of the subjective neurosensory forms of discomfort.
Several research studies, however, are investigating the molecular basis for sensory hyperreactivity. Transient receptor potential, vanilloid family 1 (TRPV1) is a nonreceptive, thermosensitive ion channel which reacts to noxious stimuli, most notably noxious heat and low pH. TRPV1 is expressed on fibroblasts, mast cells, and endothelial cells; activation results in pain or pruritus with a burning component. TRPV1 is also dramatically upregulated by inflammatory mediators (14) as well as heat and capsaicin. It has been hypothesized that the development of sensitive skin may be related to the dysregulation of muscle contraction and relaxation process (15); actin-bound myosin cross bridges in sensitive skin had more compacted shape than those in nonsensitive skin, indicating more contracted cross-bridge state in sensitive skin tissues. This could also be linked to altered adenosine triphosphate metabolism and response of skin pH. These data demonstrated that subjects with sensitive skin showed impaired pH homeostasis after lactic acid stimulation and increase of detection ability for pH upon internal or external stimuli such as lactic acid (15). Enhanced acidity might induce pain via the stimulation of TRPV1, acid-sensing ion channel subunit 3, and CGRP in the human sensitive skin. SC microbiome has also been investigated in subjects affected by sensitive skin, and no differences versus normal controls have been reported (16).
The existing overlap between atopic population and subjects affected by sensitive skin is well documented. Using TEWL modeling, statistically significant differences have been detected in the parameters obtained in the sensitive skin group, which supports the thesis that individuals with increased skin susceptibility have impaired barrier function (17). However, few studies have investigated the SC lipid composition in subjects affected by sensitive skin. Cho et al. (18) compared the average amounts of ceramides in the SC on various parts of the body (right cheek, forearm, thigh, leg, back, palm) between the sensitive group and the nonsensitive group. The results indicated that the mean values of the amounts of ceramides in other parts of the body surface except the face were lower in the sensitive group than in the nonsensitive group, but the difference was not statistically significant. However, on the face, the sensitive group showed a statistically significant decrease in the mean value of the amounts of ceramides compared to the nonsensitive, indicating that the amount of ceramides in the SC on the facial skin has a correlation with skin sensitivity.
Changes in SC thickness and therefore of transcutaneous penetration may explain regional differences or specialized areas of sensitive skin. The face has demonstrated to be the most common site of skin sensitivity, physiologically predictable due to the larger and multiple number of products used on the face (particularly in women), a thinner barrier in facial skin, and a greater density of nerve endings (19). The nasolabial fold was reported to be the most sensitive region of the facial area, followed by the malar eminence, chin, forehead, and upper lip (20,21). Saint-Martory et al. (22) found that hand, scalp, feet, neck, torso, and back sensitivity followed facial sensitivity in descending order of prevalence. Significant numbers of individuals experience sensitivity of the scalp (23,24). One-third of the population interviewed reported sensitive scalp with higher levels in women than in men. Interestingly, the prevalence declared that sensitivity of the scalp increases with age. The authors explain that this could be due to alterations of nerve endings due to the aging process or increased proclivity to irritation as a consequence of chronic exposure to surfactants contained in shampoos. The genital area is another site frequently affected by sensitive skin.
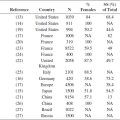
In a study of 1039 men and women, 56.2% reported sensitivity of genital skin (25), an area of particular interest since it is formed partially from embryonic endoderm and therefore differs from the skin at other body sites (26). A surprising 56.2% of responders claimed sensitive genital skin, with significantly more African Americans than Caucasians (66.4%; P < .0001) claiming sensitivity of this area. Rough fabrics were found to be the most common offender for sensitive skin in the genital area (27).
In conclusion, SC plays a central role in the determination of sensitive skin. This could be due to differences not only in structure and intercellular lipids, but also in proclivity to release neuromodulators and proinflammatory agents causing hyperreactions and sensations. Further studies are needed to fully elucidate these mechanisms.
REFERENCES
1. Berardesca E, Cespa M, Farinelli N et al. In vivo transcutaneous penetration of nicotinates and sensitive skin. Contact Dermatitis (1991) 25, 35–38.
2. Frosch PJ, Kligman AM. A method for appraising the stinging capacity of topically applied substances. J Soc Cosmet Chem (1977) 28, 197–209.
3. Ohta M, Hikima R, Ogawa T. Physiological characteristics of sensitive skin classified by stinging test. J Cosmet Sci Soc Jpn (2000) 23, 163–167.
4. Yokota T, Matsumoto M, Sakamaki T et al. Classification of sensitive skin and development of a treatment system appropriate for each group. IFSCC Magazine (2003) 6, 303–307.
5. Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatol Sci (2009) 55, 77–81.
6. Kumagai M, Nagano M, Suzuki H et al. Effects of stress memory by fear conditioning on nerve-mast cell circuit in skin. J Dermatol (2011) 38, 553–561.
7. Quatresooz P, Piérard-Franchimont C, Piérard GE. Vulnerability of reactive skin to electric current perception—A pilot study implicating mast cells and the lymphatic microvasculature. J Cosmet Dermatol (2009) 8, 186–189.
8. Yosipovitch G, Yarnitzky D. Quantitative sensory testing. In: Maibach HI, Marzulli FN, eds. Dermatotoxicology Methods: The Laboratory Worker’s Vade Mecum. New York: Taylor & Francis (1997) 120–135.
9. Primavera G, Berardesca E. Sensitive skin: Mechanisms and diagnosis. Int J Cosmet Sci (2005) 27, 1–10.
10. Misery L, Myon E, Martin N et al. Sensitive skin: Psychological effects and seasonal changes. J Eur Acad Dermatol Venereol (2007) 21, 620–628.
11. Peters EMJ, Ericson ME, Hosoi J et al. Neuropeptide control mechanisms in cutaneous biology: Physiological and clinical significance. J Invest Dermatol (2006) 126, 1937–1947.
12. Luger TA, Lotti T. Neuropeptides: Role in inflammatory skin diseases. J Eur Acad Dermatol Venereol (1998) 10, 207–211.
13. Luger TA. Neuromediators—A crucial component of the skin immune system. J Dermatol Sci (2002) 30, 87–93.
14. Kueper T, Krohn M, Haustedt LO et al. Inhibition of TRPV1 for the treatment of sensitive skin. Exp Dermatol (2010) 19, 980–986.
15. Kim EJ, Lee DH, Kim YK et al. Decreased ATP synthesis and lower pH may lead to abnormal muscle contraction and skin sensitivity in human skin. J Dermatol Sci (2014) 76, 214–221.
16. Hillion M, Mijouin L, Jaouen T et al. Comparative study of normal and sensitive skin aerobic bacterial populations. Microbiologyopen (2013) 2, 953–961.
17. Pinto P, Rosado C, Parreirão C et al. Is there any barrier impairment in sensitive skin?: A quantitative analysis of sensitive skin by mathematical modeling of transepidermal water loss desorption curves. Skin Res Technol (2011) 17, 181–185.
18. Cho HJ, Chung BY, Lee HB et al. Quantitative study of stratum corneum ceramides contents in patients with sensitive skin. J Dermatol (2012) 39, 295–300.
19. Chew A, Maibach H. Sensitive skin. In: Loden M, Maibach H, eds. Dry Skin and Moisturizers: Chemistry and Function. Boca Raton, FL: CRC Press (2000) 429–440.
20. Marriott M, Holmes J, Peters L et al. The complex problem of sensitive skin. Contact Dermatitis (2005) 53, 93–99.
21. Distante F, Bonfigli A, Rigano L et al. Intra- and inter-individual differences in facial skin biophysical properties. Cosmet Toiletries (2002) 7, 149–158.
22. Saint-Martory C, Roguedas-Contios AM, Sibaud V et al. Sensitive skin is not limited to the face. Brit J Dermatol (2008) 158, 130–133.
23. Misery L, Sibaud V, Ambronati M et al. Sensitive scalp: Does this condition exist? An epidemiological study. Contact Dermatitis (2008) 58, 234–238.
24. Misery L, Rahhali N, Ambonati M et al. Evaluation of sensitive scalp severity and symptomatology by using a new score. J Eur Acad Dermatol Venereol (2011) 25, 1295–1298.
25. Frosch PJ, Kligman AM. A method for appraising the stinging capacity of topically applied substances. J Soc Cosmet Chem (1977) 28, 197–209.
26. Farage M, Maibach HI. The vulvar epithelium differs from the skin: Implications for cutaneous testing to address topical vulvar exposures. Contact Dermatitis (2004) 51, 201–209.
27. Farage MA. Perceptions of sensitive skin of the genital area. Curr Probl Dermatol (2011) 40, 142–154.