CHAPTER 57 Strabismus surgery
Terminology, history, and epidemiologic considerations
The term strabismus is derived from a Greek word, strabismos, which means to squint. Strabismus has been recognized since antiquity. However, the first strabismus surgery was not reported until 1839 – a medial rectus myotomy by Johann Dieffenbach on a 7-year-old child with esotropia1. Within a few years, strabismus surgery was widely practiced throughout Europe. Strabismus is one of the most common ocular diseases in children. It is also present in many adults either because it was not corrected during childhood or secondary to a condition having an adult onset. The most common types of strabismus vary depending on the ethnicity of the population. In North America and Europe, esotropia is the most common type of strabismus. This probably reflects the high incidence of hyperopia in this population that predisposes patients to accommodative esotropia. However, in Asia, exotropia is the most common type of strabismus, perhaps because of the lower incidence of hyperopia in this population2.
Anatomical considerations
Gross anatomy of the extraocular muscles
There are six extraocular muscles – the medial, lateral, superior and inferior rectus muscles, and the superior and inferior oblique muscles. The rectus muscles all have insertions that are 10–11 mm in width. The four rectus muscles all arise from the apex of the orbit at the annulus of Zinn and then insert at varying distances from the limbus. The medial rectus muscle inserts closest to the limbus and the superior rectus muscle furthest from the limbus. The outward spiraling of the insertions of the rectus muscles, beginning with the medial rectus muscle and ending with the superior rectus muscle, is referred to as the spiral of Tillaux (Fig. 57.1). The inferior and superior rectus muscle insertions are slanted nasally whereas the medial and lateral rectus muscles are not usually slanted. The rectus muscles have varying arcs of contact with the globe – the longest being 10 mm for the lateral rectus muscle. Certain disorders can be associated with ectopia of the rectus muscles. For example, patients with craniosynostosis often have medial rectus muscles which are abnormally high and lateral rectus muscles which are abnormally low, which can result in a V-pattern mimicking overaction of the inferior oblique muscles.
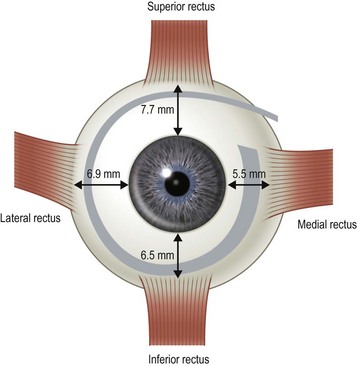
Fig. 57.1 The spiral of Tillaux.
From Coats DK, Olitsky SE, editors. Strabismus Surgery and its Complications. Berlin: Springer, 2007.
There are two distinct layers to the rectus muscles – the global and orbital layers. The orbital layer inserts directly into the muscle pulleys while the global layer inserts into the globe3 (Fig. 57.2).
Physiology of the extraocular muscles
The extraocular muscles are some of the fastest and most fatigue resistant muscles. Unlike skeletal muscles, which are exclusively singly innervated, the extraocular muscles are both singly and multiply innervated4. Singly innervated muscle fibers are thicker and produce rapid all-or-none contractions, while multiply innervated fibers are thinner and produce slow, graded contractions. Thick fibers are primarily located in the global layer of rectus muscles while thin, tonic fibers are primarily located in the orbital layer of rectus muscles. In the oblique muscles, a core of thick fibers is surrounded by an outer layer of thin fibers. Thick fibers are believed to be primarily responsible for saccades while thin, tonic fibers are responsible for small, refixation movements of the eye.
The extraocular muscles have complicated actions based on the angle and location of their insertions. While the medial and lateral rectus muscles only have the primary actions of adducting and abducting the eyes respectively, the other four extraocular muscles also have secondary and tertiary actions in primary position (Table 57.1). All of these actions must be taken into account when planning strabismus surgery. The eyes are yoked together and the movement of one eye is closely linked to the movement of the fellow eye. Sherrington’s law of reciprocal innervations states that, when the agonist muscle receives a stimulatory impulse, the antagonist muscle in the same eye receives an inhibitory impulse or no impulse. Herring’s law of equal innervations states that the muscles responsible for rotating the eyes in a certain direction (yoke muscles) receive equal innervation thereby assuring that the eyes move in the same direction.
Tenon’s fascia and muscle pulleys
The paths of the rectus muscles have been shown to be highly uniform in normal patients in primary position using high resolution magnetic resonance imaging5. In secondary positions of gaze, the paths of the rectus muscles exhibit small but consistent shifts opposite to the direction of gaze. Patients with strabismus generally have little if any change in their rectus muscle paths either in primary position or in other positions of gaze. Rectus muscles pass through connective tissue sleeves in Tenon’s fascia near the equator of the globe. Demer et al6. have labeled these fascial connections which suspend the rectus muscles in the orbit as ‘muscle pulleys’. Ectopic pulleys are a rare cause of strabismus and may be identified by high resolution magnetic resonance imaging7.
Indications for strabismus surgery
The primary indications for strabismus surgery are: (i) diplopia, (ii) reduced or threatened binocularity, (iii) an abnormal head position secondary to ocular misalignment or nystagmus, (iv) a constricted visual field secondary to strabismus, and (v) poor cosmesis secondary to ocular misalignment. The indications for strabismus surgery vary depending on the age and the particular needs of the patient. In children, the primary indication for strabismus surgery is often to align the eyes to permit the development of binocular vision. Other common indications in children include anomalous head postures that can be associated with nystagmus or ocular misalignment. In adults, perhaps the most common indication for extraocular muscle surgery is the elimination or reduction of diplopia or asthenopia. Finally, an important indication for surgery for patients of all ages is the restoration of normal ocular alignment since discrimination in the workplace, and psychological and physical dysfunction are all well-documented consequences of uncorrected ocular misalignment8–15.
Preoperative assessment
Examination
The majority of the preoperative examination should focus on the sensory–motor evaluation. This evaluation should be tailored to the patient and his or her individual problem. It is important to identify whether an abnormal angle kappa or eccentric fixation is present, which can be done by having the patient fix on a light with each eye separately and noting the position of the corneal light reflex relative to the center of the pupil. A normal angle kappa is slightly positive (displaced slightly toward the nose). Ductions and versions are tested, and recorded. Overacting or underacting muscles should be identified and documented. In very young children, or in patients with very poor vision, the Hirschberg light reflex test or Krimsky test is used to estimate the ocular deviation, but when possible alternate prism and cover testing are preferred to document the magnitude of the deviation in the nine diagnostic positions of gaze and at near. In patients with vertical deviations, the alignment should also be tested with right and left head tilt. The AC/A ratio should be assessed in patients with a distance–near disparity with either the lens gradient or the heterophoria method. For patients with intermittent exotropia, the alignment should also be assessed at near with +3.00 D lenses and at distance with −2.00 D lenses to determine if a high AC/A ratio is present. Testing in the far distance and after monocular occlusion is also helpful since it may bring out a larger deviation16. Common patterns that can be elucidated with motility testing include ‘A’ patterns, which are often associated with superior oblique muscle overaction, and ‘V’ patterns, which are often associated with inferior oblique muscle overaction. Other common patterns include ‘T’ or ‘Y’ patterns, ‘X’ patterns, and lambda patterns. Orbital pulley and innervational anomalies can give rise to atypical strabismus patterns17.
Sensory testing can be helpful in predicting postoperative outcomes and in directing surgical management. For example, adult patients with anomalous retinal correspondence should be warned of an increased possibility of postoperative diplopia. Decreasing distance stereoacuity can be an indication that control of an intermittent exotropia is worsening and that surgical intervention is warranted18. Surgery on the superior oblique muscles should be approached cautiously in adult patients with high grade stereopsis because of the risk of inducing vertical or torsional diplopia. The sensory evaluation should be tailored to the individual patient.
Orbital imaging is not routinely performed in all strabismus cases, but can be helpful in identifying anomalous anatomy, identifying slipped or lost muscles, or assisting with surgical planning in complicated cases7.
Surgical decision making
Table 57.2 summarizes some of the most common forms of strabismus and outlines accepted treatment practices. Table 57.3 gives guidelines for surgical dosages based on Parks’ recommendations. Adjustable sutures can be used in cooperative patients when surgical outcomes are likely to be unpredictable. The use of adjustable sutures is discussed in the section on p. 28. Each surgeon should modify these tables based on their own outcomes.
Informed consent of the strabismus patient
Informed consent is a process of communication between a patient and physician whereby the patient is informed of their diagnosis, the nature and purpose of the proposed procedure, the risks, benefits, and alternatives to the procedure, and the risks and benefits of not undergoing the treatment. This communication process is both ethically and legally required before a surgical procedure can take place. Although the physician is not required to disclose every conceivable risk, he or she should disclose any and all risks that a reasonable person would consider important in deciding whether or not to undergo a procedure. Once this communication has taken place, then the patient or guardian must sign a written consent as evidence that proper informed consent has taken place. This written consent becomes a permanent part of the medical record. More detailed information about the legal aspects of informed consent can be found in legal textbooks19.
Anesthesia
Postoperative pain and nausea are common following strabismus surgery particularly when general anesthesia is used. Box 57.1 lists precautions that can be taken to minimize postoperative pain and nausea in children.
Operative techniques
Forced ductions and traction testing
Traction testing is helpful for diagnosing restricted rectus muscles. It can also be helpful in diagnosing Brown syndrome or a lax superior oblique muscle. When performed under general anesthesia, depolarizing muscle relaxants should be avoided since they can make it more difficult to interpret forced ductions. When testing the horizontal rectus muscles, the conjunctiva is grasped with fine forceps at 6 and 12 o’clock 1–2 mm posterior to the limbus. The globe is then abducted and adducted without retropulsion. It is often helpful to compare the forced ductions in both eyes since a comparison between the two eyes will often allow a subtle difference to be detected. When testing the vertical rectus muscles, the conjunctiva is grasped at 3 and 9 o’clock and the globe is elevated and depressed. The restriction can be confirmed when the muscle is secured on a muscle hook. When testing the superior oblique muscle, the globe is grasped at 4 and 10 o’clock for the right eye and 2 and 8 o’clock for the left eye (Fig. 57.3). The globe is then retropulsed and elevated in a superonasal direction. Once in this position, it is moved back and forth temporally and nasally to determine how taut the tendon is. In an eye with Brown syndrome, it is generally difficult to fully elevate the eye. In an eye with a lax superior oblique tendon the eye can not only be fully elevated, but there is little resistance to rocking the globe while it is retropulsed and elevated20,21. The degree of laxity can be graded and used to determine whether to tuck the superior oblique tendon. Traction testing can result in conjunctival hemorrhages and tears and corneal abrasions, and thus should be performed carefully.
Conjunctival incisions and methods of muscle reattachment
Choice of type and location of incision
Fornix conjunctival incisions are made through the bulbar conjunctiva between adjacent rectus muscles at a point 8–9 mm posterior to the limbus to avoid the extraconal fat pad that begins 10 mm posterior to the limbus22. Fornix incisions are almost always used for surgery on the oblique muscles, and can also be used for surgery on the rectus muscles. Advantages of the fornix incision include access to a greater number of extraocular muscles through a single incision, ease of construction and closure of the incision, less scarring, and greater patient comfort. Disadvantages of the fornix incision include poorer exposure, especially when exposure to the posterior orbit is needed, an increased risk of conjunctival tearing, inability to resect conjunctiva in cases of large rectus muscle resections, and greater reliance on a skilled assistant.
Fornix incision technique
A fornix incision can be made in any of the quadrants between adjacent rectus muscles (Video: Medial rectus recession using fornix incision.). The following describes the procedure used to create an inferonasal conjunctival incision, which is the most common site used for accessing the medial rectus or inferior rectus muscles. The description is for a right-handed surgeon operating on a right eye. The fornix incision is initiated by having the assistant grasp the perilimbal conjunctiva at the 4:30 o’clock position; the assistant elevates and abducts the eye. The surgeon angles the blunt tipped Westcott scissors perpendicular to the globe and initiates the incision at a point 8 mm posterior to the limbus, extending the incision approximately 8 mm (Fig. 57.4A). Next the surgeon makes a second 8 mm incision through Tenon’s capsule and intermuscular septum, which is oriented perpendicular to the conjunctival incision (Fig. 57.4B).
Limbal incision technique
Limbal incisions are commonly used when performing surgery on the rectus muscles (Video: Medial rectus muscle using a limbal incision.). The conjunctiva is grasped with forceps along one border of the muscle undergoing surgery, and blunt Westcott scissors are used to make a radial incision that begins at the limbus and extends about 3–4 mm posteriorly (Fig. 57.5A). It is important to not extend the conjunctival incision to the plica when operating on the medial rectus muscle and to the lateral canthus when operating on the lateral rectus muscle to avoid the formation of symblepharon. The scissors are then used to extend the incision along the limbus through an angle of approximately 3 clock-hours. Tenon’s capsule should be bluntly dissected from the conjunctiva prior to performing the conjunctival peritomy. A second radial incision is then created at the opposite end of the limbal incision (Fig. 57.5B).
Conjunctival closure
Limbal conjunctival incisions require suture closure. It is important not to suture Tenon’s capsule to the conjunctiva since this can result in unsightly hyperemia. Tenon’s capsule can be distinguished from conjunctiva by irrigating saline solution on its surface. Tenon’s capsule will absorb the solution and become thickened whereas the appearance of conjunctiva remains unchanged. The corners of the conjunctival flap should be identified with forceps. A suture is then passed through each corner of the conjunctival flap and sutured back to the conjunctiva adjacent to the limbus with interrupted absorbable sutures (Fig. 57.6). If the conjunctival flaps are large, more than one interrupted suture may be needed to close each flap.
Surgery on the rectus muscles
Hooking and dissecting rectus muscles using a limbal approach
Prior to hooking the rectus muscles, the blunt Westcott scissors should be used to separate the intermuscular membrane and Tenon’s capsule from the rectus muscles. A muscle hook with a blunt end (Graefe or Stevens hook) should then be advanced parallel to the margin of the rectus muscle and then rotated behind the muscle. It is important that the toe of the muscle hook be firmly pressed against the globe during this maneuver since the rectus muscle lies flat against the globe and the hook will otherwise only capture Tenon’s capsule. It is also important to consider the varying distances of the rectus muscles from the limbus. In addition, since the nasal edges of the superior and inferior rectus muscle insertions are generally closer to the limbus than the temporal edges, it is generally preferable to hook these muscles nasally rather than temporally. Once it has been determined that the muscle is securely hooked, the intermuscular membrane should be dissected off the insertion of the muscle. This can be facilitated by lifting the conjunctiva overlying the muscle with a Manson double ended strabismus hook (Fig. 57.7). The poles of the muscle should then be inspected to ensure that the entire muscle has been captured by the muscle hook. The Graefe or Stevens muscle hook should then be replaced with a Green or Jameson muscle hook, which have a toe at one end of the hook that will prevent the muscle from slipping off of the hook. When performing a recession, just enough of the intermuscular membrane should be removed to place the polyglactin suture in the muscle insertion. However, a more extensive dissection of the intermuscular membrane is required when performing a resection. In addition when performing a resection, a second Green or Jameson hook is placed to spread out the muscle.
Hooking and dissection of rectus muscles using a fornix approach
Hooking and dissecting the rectus muscles using a fornix incision is performed similarly to that described for a limbal incision. Blunt Westcott scissors are used to separate the intermuscular membrane and Tenon’s capsule in the quadrants between adjacent rectus muscles. A Stevens hook is used to identify the insertion of the rectus muscle, the muscle is placed on a Green or Jameson hook (Fig. 57.8A), and the conjunctiva is reflected over the hook (Fig. 57.8B). Blunt Westcott scissors are used to dissect Tenon’s fascia from the tip of the hook, and a ‘pole test’ is performed by sweeping a small hook around the pole of the muscle to assure that all rectus muscle fibers are contained on the Green hook (Fig. 57.8C). The small hook can then be used to pull Tenon’s fascia away from the rectus muscle so that is can be dissected off of the muscle with blunt Westcott scissors (Fig. 57.8D). For recessions of the horizontal muscles, dissection of the Tenon’s fascia is limited to the anterior portion of the muscles. For resections or for vertical muscles, the muscle is dissected posteriorly to allow for the desired amount of resection, and to separate the vertical muscles from the eyelid retractors.
Methods of muscle reattachment
There are two commonly used methods to reattach the extraocular muscle to the globe. Either the muscle can be sutured directly to the sclera at its desired attachment point, or it can be sutured back to its original insertion and allowed to ‘hang back.’ Figure 57.9A and B shows the ‘crossed swords’ technique popularized by Parks to reattach the muscle directly to the sclera23. Figure 57.10A and B shows the ‘hang back’ technique24. The ‘hang back’ technique is used only when a rectus muscle is recessed. While initially the muscle is attached to the globe only by the sutures sewn to the original muscle insertion, over 1–2 weeks the muscle forms a new attachment to the sclera at a point dictated by the length of the hang back sutures. There are a number of advantages of the hang back technique for recessing a muscle including ease of suture placement, the safety of placing sclera passes through the thicker sclera lying anterior to the muscle insertion, and the ease of postoperative muscle adjustments. It can be particularly difficult to suture a rectus muscle to the sclera at the desired point of reattachment when performing large recessions in young children with small orbits. In addition, the sclera is much thinner directly behind the rectus muscle insertions so there is a greater risk of a scleral perforation with suture placement. Since the insertion of the rectus muscle roughly delineates the ora serrata of the retina, an inadvertent full-thickness scleral pass posterior to the muscle insertion can also result in a retinal tear.
Special considerations of individual rectus muscles
Rectus muscle recession
Rectus muscles are recessed by weaving a double armed 6-0 polyglactin suture in the muscle insertion and then disinserting the muscle. The suture can be woven in the muscle insertion a variety of ways. One popular technique is to pass the suture full thickness through the central third of the muscle tendon to create a central ‘security knot’ and then tying a square knot in the suture. By placing a ‘security knot’ the suture will not pull out of the muscle if one arm of the suture is inadvertently cut when the tendon is disinserted. The suture should be placed as close as possible to the insertion of the tendon in the sclera, while still allowing enough space to disinsert the tendon without cutting the suture. Each arm of the suture is then woven from the center of the tendon near the ‘security knot’ to both sides of the muscle (Fig. 57.11). A locking bite extending 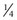 to
to 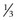 of the distance into the tendon is then placed on both sides of the tendon. The tendon is then disinserted with Manson–Aebli scissors with the long blade positioned behind the tendon (Fig. 57.12). The tendon can then be disinserted being careful to keep the scissor’s blades lying flat against the sclera. Usually there is bleeding from the cut ends of the anterior ciliary arteries lying at the muscle insertion or anterior to it. When performing a ‘hang back’ recession, it is helpful to cauterize the individual bleeding sites anterior to the muscle stump with bipolar cautery, since it is difficult to visualize the needle passing through the sclera unless adequate hemostasis has been achieved. Caution should be exercised when performing cautery to avoid excessively cauterizing the sclera, which may cause it to contract and to become charred. The muscle may then be reattached to the globe using one of the methods of muscle reattachment illustrated by Figures 57.9 and 57.10. The conjunctiva is then closed with 7-0 or 8-0 absorbable sutures.
of the distance into the tendon is then placed on both sides of the tendon. The tendon is then disinserted with Manson–Aebli scissors with the long blade positioned behind the tendon (Fig. 57.12). The tendon can then be disinserted being careful to keep the scissor’s blades lying flat against the sclera. Usually there is bleeding from the cut ends of the anterior ciliary arteries lying at the muscle insertion or anterior to it. When performing a ‘hang back’ recession, it is helpful to cauterize the individual bleeding sites anterior to the muscle stump with bipolar cautery, since it is difficult to visualize the needle passing through the sclera unless adequate hemostasis has been achieved. Caution should be exercised when performing cautery to avoid excessively cauterizing the sclera, which may cause it to contract and to become charred. The muscle may then be reattached to the globe using one of the methods of muscle reattachment illustrated by Figures 57.9 and 57.10. The conjunctiva is then closed with 7-0 or 8-0 absorbable sutures.
Rectus muscle resection
Rectus muscles are resected by weaving a double armed 6-0 polyglactin suture through the muscle using the same technique described above for a rectus muscle recession (Video: Medial rectus muscle resection.). A caliper should be set for the desired distance of the resection, and then ink from a marking pen is placed on the tips of the caliper. One end of the caliper is then placed at the base of the muscle insertion and the other on the muscle belly’s at the desired point of resection. This is repeated at several points so as to create a line in the muscle delineating the amount of muscle to be resected (Fig. 57.13). A suture is then sewn into the muscle along this line using the same technique as described for a rectus muscle recession. Once the suture has been secured in the muscle, the muscle is clamped anterior to the suture using a straight hemostat. After crushing the muscle for 15–30 seconds, the hemostat is then removed and the muscle is cut where it was crushed being careful to not cut the polyglactin suture. The muscle stump is then excised with blunt Westcott scissors and hemostasis is achieved with bipolar cautery. The muscle is then reattached to the sclera and advanced to the original insertion (Fig. 57.14). Care should be taken when advancing the muscle to not erode the scleral tunnels. It is helpful to rotate the globe in the direction the muscle is being advanced prior to advancing the muscle to reduce the tension on the scleral tunnels. Once the muscle is positioned at the muscle insertion site, two additional single throws are placed on top of the double throw and the ends of the sutures are trimmed 1 mm above the knot. The conjunctiva is then closed with 7-0 or 8-0 interrupted absorbable sutures.
Rectus muscle tuck
A rectus muscle can be tucked if there is a need to preserve the anterior ciliary vessels in the muscle. A double-armed suture is woven into the muscle the desired distance from its insertion (Fig. 57.15)25. Great care should be taken to avoid disrupting the anterior ciliary vessels. For this reason, locking bites should be taken only on both sides of the muscle and not in the center. Scleral passes are then made 1 mm anterior to the muscle insertion at both ends of the muscle insertion. The scleral passes should be deep enough that they will support the muscle when it is advanced. The muscle is then advanced and tied down, resulting in a plication in the muscle. The overlying conjunctiva is then closed.
Z-myotomy
A marginal myotomy is rarely indicated, but it can be helpful to weaken a muscle that has already been maximally recessed. Exposure can be difficult when operating on a previously recessed muscles. Hemostats are placed across the muscle in the area where the muscle is to be cut for hemostasis26. Two overlapping myotomies are then created on opposite sides of the muscle with blunt Westcott scissors (Fig. 57.16). Care should be taken when manipulating the globe after the procedure because of the risk of rupturing the remaining muscle fibers.
Reoperations
Reoperations on the rectus muscles are generally more difficult because the conjunctiva and Tenon’s capsule overlying the muscle are often scarred down to the muscle or sclera so it can be difficult to locate the muscle. Great care must be taken to carefully dissect the conjunctiva free from the sclera and the muscle to prevent a button hole or tears from forming in the conjunctiva. If attention is not paid to preserving the conjunctiva, the area overlying the muscle may remain hyperemic and chemotic for an extended period of time after surgery. When possible, old operative reports should be reviewed to ascertain the probable location of the muscle. It can be difficult to isolate muscles after large recessions or transpositions. It can be helpful to dissect free the tissue behind the recessed muscle with blunt Westcott scissors prior to passing a muscle hook27. Muscles which are shortened and inelastic should not be advanced to the orignal insertion if full advancement results in restriction on forced duction testing. In some instances the muscle will have slipped and only the muscle sheath will be attached to the sclera28. The muscle should be carefully inspected prior to placing sutures in the muscle to determine the position of the muscle in the sheath. Ludwig and Chow29 have also described a lengthened or stretched remodeled scar in between an operated muscle tendon and the sclera, which she has postulated contributes to the variability of outcomes following strabismus surgery.
Surgery on the inferior oblique muscle
Hooking and dissecting the inferior oblique muscle
When operating on the inferior oblique muscle, a traction suture should be placed at 6 o’clock and the eye should be rotated in a superonasal direction and then clamped into position with a hemostat (Video: Inferior Oblique Muscle Recession.). A 2 clock hour inferotemporal fornix incision is then created 8–9 mm posterior to the limbus. Muscle hooks are then used to hook the inferior and lateral rectus muscles. The hooks should be passed at a tangent to the limbus so as not to prematurely hook the inferior oblique muscle. A Desmarres lid retractor is then placed underneath Tenon’s capsule to expose the inferior oblique muscle. The vortex vein should be identified first and then a Stevens muscle hook should be placed immediately posterior to the inferior oblique muscle. The muscle hook should then be rotated away from the globe (Fig. 57.17). The muscle hooks underneath the lateral and inferior rectus muscles and the Desmarres lid retractor are then removed and the inferior oblique muscle is drawn anteriorly. The overlying orbital fat is carefully dissected away from the muscle and a button hole is created over the Stevens tenotomy hook at the edge of the muscle. A second Stevens tenotomy hook should then be placed adjacent to the first hook and both hooks should be lifted up to ensure that the entire muscle has been hooked. If the whole muscle has been hooked, Tenon’s capsule will be visible behind the muscle. If the muscle has been split the split muscle, which is pink rather than white, will be visible behind the hooked portion of the inferior oblique muscle. Once it has been determined that the entire muscle has been hooked, the intermuscular membrane is dissected off the muscle back to its insertion.
Inferior oblique recession and anterior transposition
Recession of the inferior oblique muscle is a procedure used to weaken the inferior oblique muscle. Once the inferior oblique muscle has been exposed, the muscle is disinserted using blunt Westcott scissors. Some surgeons clamp the muscle with a hemostat prior to disinserting the muscle with high temperature hand held cautery (Fig. 57.18). A cotton Q-tip should be placed behind the muscle while it is being transected with cautery to protect the globe since the inferior oblique generally inserts over the macula. Once the muscle has been transected, a single armed 6-0 absorbable suture is passed through the anterior corner of the muscle. Some surgeons place locking bites at both ends of the muscle. For a standard 10 mm recession, the muscle is sutured back to the sclera at a point 3 mm posterior and 2 mm lateral to the lateral border of the inferior rectus muscle (Fig. 57.19). A standard recession is usually ‘self-titrating’ and can be used to treat mild, moderate, and severe overaction of the inferior oblique muscle. An anterior transposition of the inferior oblique muscle is sometimes used to treat severe inferior oblique overaction, often in the setting of dissociated vertical deviation. Anterior transposition of the inferior oblique muscle is achieved by suturing the anterior corner of the muscle to the sclera adjacent to the lateral insertion of the inferior rectus muscle. Patients may develop restricted elevation, the ‘anti-elevation syndrome’, if the inferior oblique muscle is attached too anteriorly30.
Inferior oblique myectomy
Inferior oblique myectomy is an alternative inferior oblique weakening procedure. A myectomy has the advantage over an inferior oblique recession of being faster to perform, and does not require that the muscle be sutured to the sclera, so there is less risk of scleral perforation during the procedure. The primary disadvantage of myectomy is that the site of reattachment of the inferior oblique muscle to the sclera is unpredictable31.
To perform an inferior oblique myectomy, two hemostats are placed on the inferior oblique muscle, one at the insertion of the muscle, and the other 5–10 mm more medially (Fig. 57.20). Westcott scissors are then used to remove the segment of muscle between the two hemostats, and the cut ends of the muscle are cauterized. The muscle is then allowed to retract into Tenon’s capsule and the conjunctiva is closed with absorbable sutures.
Denervation and extirpation of the inferior oblique muscle
To perform a denervation and extirpation of the inferior oblique muscle, the muscle is identified and transected from the globe. A hook is then placed under the inferior rectus muscle to elevate the eye. Next, the neurovascular bundle for the inferior oblique muscle is identified as a fusiform expansion on the lateral border of the muscle (Fig. 57.21A). The bundle is hooked and cauterized (Fig. 57.21B), which results in a release of the restriction of the muscle allowing the dissection to continue to the point where the muscle penetrates Tenon’s capsule. A 3.0 absorbable suture is placed around the muscle, and cautery is used to extirpate the muscle (Fig. 57.21C). The muscle stump is then allowed to retract through Tenon’s capsule, and the conjunctiva is then closed with absorbable sutures.
Inferior oblique strengthening procedures
Inferior oblique muscle strengthening procedures include an inferior oblique muscle tuck and advancement of the inferior oblique muscle. These procedures are performed infrequently, but can be useful to treat severe incyclotorsion occurring after macular translocation surgery32 or with an isolated inferior oblique muscle palsy.
Inferior oblique tuck procedure
After isolating the inferior oblique muscle, the muscle is plicated, and a 6.0 absorbable suture is used to secure each end of the plicated muscle (Fig. 57.22A).
Inferior oblique muscle advancement
After isolating the inferior oblique muscle, the muscle is advanced along its usual course, passed under the lateral rectus muscle, and then reattached to the sclera at the superior border of the lateral rectus muscle (Fig. 57.22B).To obtain added excyclotorsion, the inferior oblique muscle can be resected before reattaching the muscle.
Surgery on the superior oblique muscle
Hooking and dissecting the superior oblique muscle
The superior oblique muscle is best accessed through a fornix incision temporal to the superior rectus muscle (Fig. 57.23A). After creating the fornix incision, the superior rectus muscle should be hooked with a Jameson muscle hook (Fig. 57.23B). If surgery is planned on the tendon insertion, the lid speculum should be removed and a Desmarres lid retractor is inserted in the fornix incision underneath Tenon’s capsule (Fig. 57.23C). The sclera adjacent to the temporal margin of the superior rectus muscle is then carefully inspected. The insertion site of the superior oblique tendon is quite variable making it difficult to localize. In addition, the tendon fans out at its insertion and can be quite diaphanous. It is sometimes helpful to drag a Stevens tenotomy hook across the sclera over the area where the tendon is suspected to facilitate its visualization. If the tendon still can’t be located, a Stevens tenotomy hook may be used to retract the superior rectus muscle nasally, since the insertion of the superior oblique tendon is sometimes covered by the superior rectus muscle. Once the superior oblique tendon has been visualized, it is hooked posteriorly (Fig. 57.23D). Since the anterior fibers of the superior oblique tendon insert more temporally, it is important to pass the hook sufficiently nasally and posteriorly so the entire tendon is hooked. It is carefully inspected once it is on the Stevens tenotomy hook to be certain that the tendon has not been split.
Superior oblique tenotomy, tenectomy
The superior oblique tenotomy or tenectomy can be performed either nasally or temporally to the superior rectus muscle. The superior oblique tendon is attached to the superior rectus muscle by a frenulum which limits the effect of a weakening procedure on the superior oblique tendon temporal to the superior rectus muscle33. The tendon can either be transected (tenotomy) or a portion of the tendon is excised (tenectomy). A posterior 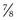 tenotomy is performed to weaken abduction in downgaze while not altering torsion. The overlying conjunctiva is then closed.
tenotomy is performed to weaken abduction in downgaze while not altering torsion. The overlying conjunctiva is then closed.
Superior oblique expander
Wright34 first described the use of a silicone expander to weaken the superior oblique muscle. It has been used primarily to treat patients with Brown syndrome and superior oblique overaction35. The superior oblique tendon is isolated using a fornix incision temporal to the superior rectus muscle. After hooking the superior rectus muscle with a Jameson hook, a Stevens tenotomy hook is used to pull the conjunctiva over the superior rectus muscle exposing its nasal border. The intermuscular membrane should not be disturbed. The lid speculum is then removed and a Desmarres retractor is placed nasal to the superior rectus muscle to expose the superior oblique tendon. A small opening is then made in the fascia with blunt Westcott scissors to expose the superior oblique tendon (Fig. 57.24A). The tendon is then hooked with a Stevens tenotomy hook. The superior oblique fascia is then gently lifted off of the tip of Stevens hook and a second Stevens hook is placed adjacent to it. Two non-absorbable double arm sutures are then sewn securely into the superior oblique tendon leaving enough space between the sutures so that the tendon can be transected without cutting the sutures. The first suture should be placed 3 mm nasal to the superior rectus muscle. The tendon is then transected between the sutures and a segment of 240 or 40 retinal band which has been presoaked in antibiotic solution and cut to the desired length is attached to the tendon using horizontal mattress sutures (Fig. 57.24B). After trimming the sutures, the silicone expander is positioned between the cut ends of the tendon. The fascial capsule and conjunctiva are then separately closed over the silicone band.
Harada–Ito procedure
The Harada–Ito procedure is used to correct large degrees of excyclotropia with minimal effect on the vertical or horizontal alignment of the eyes. It is used most commonly in patients with bilateral superior oblique palsies when there is no or only a minimal vertical deviation in primary position. The classical Harada–Ito procedure is performed by hooking the superior oblique tendon temporally. The anterior quarter of the tendon is then split off from the rest of the tendon for 6–8 mm. A 6-0 Mersilene double arm suture is then passed underneath the anterior tendon fibers and placed in the sclera 8 mm posterior to the superior margin of the lateral rectus muscle (Fig. 57.25A). The anterior tendon fibers are then transposed toward the lateral rectus muscle (Fig. 57.25B).
Fells, modification of the Harada–Ito procedure is performed by hooking the superior oblique tendon and the splitting the tendon in half. A 6-0 polyglactin double arm suture is then woven into the anterior half of the tendon. Next, both ends of the suture are passed through the sclera 8 mm posterior to the superior margin of the lateral rectus muscle. The anterior half of the superior oblique tendon is then transposed to this new location (Fig. 57.26). An intraoperative assessment of objective torsion of the fundus can be used to titrate the procedure.
Tucking procedure
The superior oblique tendon is generally tucked temporal to the superior rectus muscle using a tendon tucker. After hooking the superior oblique tendon, the hook is replaced with a tendon tucker (Fig. 57.27A). The tendon tucker is then adjusted the desired amount. The number on the tucker should be doubled to calculate the total amount of tucking. The size of the tuck will be dictated by the amount of laxity in the tendon. A 5-0 or 6-0 Mersilene double arm suture is then passed through the mid portion of both halves of the tendon inferior to the tendon tucker (Fig. 57.27B). After securing the two halves of the tendon, forced ductions should then be performed to assess the tautness of the superior oblique tendon. This should be performed by grasping the globe in the inferotemporal quadrant and then elevating it in a superonasal direction without retropulsion. Ideally, resistance should be felt when the limbus is just above an imaginary line drawn between the medial and lateral canthus. If resistance is felt before the eye reaches this point, the tuck is too tight and the size of the tuck should be reduced. Alternatively, resistance of the operated eye to elevation can be compared with that in the non-operated eye, aiming to achieve similar resistance in both eyes, which will minimize the likelihood of inducing iatrogenic Brown syndrome.
Transposition procedures
Transposition of the rectus muscles to treat A or V pattern strabismus
A and V pattern strabismus that occurs in the absence of oblique muscle dysfunction can be corrected by transposing or offsetting the rectus muscles undergoing surgery. Offseting the muscle weakens the effect of that muscle when the eye is moved in the direction in which it is offset. (Fig. 57.28). For example, in a patient with a V pattern esotropia, both medial rectus muscles are moved 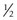 –1 tendon width inferiorly when the muscles are recessed, thereby diminishing the medial rectus muscles’ strength in downgaze, which reduces the V pattern.
–1 tendon width inferiorly when the muscles are recessed, thereby diminishing the medial rectus muscles’ strength in downgaze, which reduces the V pattern.
Full tendon transposition
This procedure can be performed to treat severe paralysis of any rectus muscle, although it is used most commonly to treat lateral rectus muscle palsy by transposing the superior and inferior rectus muscles laterally. The original procedure described by Knapp was used to treat ‘double elevator palsy’ by transposing the medial and lateral rectus muscles superiorly36. The full tendon transposition procedure helps to move the eye into a more central position, and will sometimes provide a small amount of improvement in the duction of the paralyzed muscle.
To perform the full tendon transposition procedure, the surgeon must first make an incision in the conjunctiva, and then access the muscles to be transposed (Fig. 57.29), which would be the inferior and superior rectus muscles in the case of a lateral rectus muscle palsy. The intermuscular septum is then dissected free for 12–15 mm. Vessel sparing methods can be used if the patient is at high risk for anterior segment ischemia37. Once the muscles have been detached from their insertions, they are moved laterally. The temporal border of the superior rectus muscle is sutured to the superior border of the lateral rectus muscle, and the temporal border of the inferior rectus muscle is sutured to the inferior border of the lateral rectus muscle. The muscles are generally positioned along the spiral of Tillaux (Fig. 57.29), although alternatively they may be positioned perpendicular to the lateral rectus muscle (Fig. 57.30). The effect of the transposition procedure can be augmented by attaching a portion of the transposed muscle to the sclera (Fig. 57.31) with a posterior fixation suture38 or to the belly of the paretic muscle (Fig. 57.32)39. Since recession of the medial rectus muscle increases the risk of anterior segment ischemia if performed simultaneously as a full tendon transposition procedure, it is usually performed at a later date. Botulinum toxin is sometimes injected into the antagonist of the paretic muscle preoperatively, at the time of full tendon transposition, or postoperatively40.
Jensen procedure
The Jenson procedure is an alternative to the full tendon transposition to treat severe muscle paresis41 that reduces the likelihood of anterior segment ischemia42.
To perform the Jensen procedure (in the case of a lateral rectus muscle palsy) the superior, inferior, and lateral rectus muscles are split longitudinally into two halves extending 12 to 15 mm posteriorly. The nasal anterior ciliary arteries of the vertical rectus muscles are left intact, and the temporal portions of the superior and inferior rectus muscles are joined with a non-absorbable 6-0 suture to the superior and inferior portions of the lateral rectus muscle (Fig. 57.33). Since this procedure spares a portion of the ciliary vasculature, simultaneous recession of the medial rectus muscle can be performed with less risk of anterior segment ischemia.
Hummelsheim procedure
The Hummelsheim procedure, like the Jensen procedure, is a partial tendon transposition procedure used to treat patients with severe muscle paresis. The original procedure, used to treat lateral rectus muscle paresis, involved suturing the transposed portions of the superior and inferior rectus muscles directly to the lateral rectus muscle at its insertion. Currently, the procedure has been modified such that the transposed portions of the muscle are sutured to the sclera adjacent to the lateral rectus muscle insertion (Fig. 57.34). In a modification by Brooks, 4–5 mm of the transposed muscle half is resected prior to attaching the muscle to the sclera, to augment the effect of the transposition43. The effect can be further augmented by suturing the transposed superior and interior rectus muscles to the lateral edge of the lateral rectus muscle ≥10 mm posterior to its insertion (lateral fixation).
Superior oblique tendon transposition procedure
The superior rectus muscle is approached nasally, through either a limbal or a superonasal fornix incision. The superior oblique muscle tendon is transected near the nasal border of the superior oblique muscle (Fig. 57.35A and B). The eye is adducted, and the tendon is sutured to the sclera at the superior border of the medial rectus muscle. Some surgeons fracture the superior oblique trochlea prior to suturing the tendon to the sclera.
Adjustable sutures
Adjustable sutures were popularized by Arthur Jampolsky as a means of increasing the success rate of strabismus surgery with a single surgery44. They may be performed as either a one-stage or a two-stage procedure. The one-stage procedure is performed using only topical anesthesia, while the two-stage procedure uses a combination of regional or general anesthesia and topical anesthesia.
Rationale and indications for the use of adjustable sutures
Adjustable sutures have been touted as a means of more consistently obtaining satisfactory ocular alignment with a single strabismus operation27. They are most commonly used in patients who are undergoing a reoperation or in patients who have paretic or restrictive disease45. Some strabismus surgeons use them with nearly all of their adult patients and some even with children46. In non-randomized series, the use of adjustable sutures has been reported to reduce the incidence of reoperation from nearly 30% to less than 10%47. However, adjustable sutures have never been studied in a randomized clinical trial to prove their efficacy and many strabismologists believe that they can achieve results as good as those obtained with adjustable sutures using fixed sutures.
Sliding knot adjustable suture
A sliding knot (also commonly referred to as noose or cinch) adjustable suture is created by tying a remnant of the 6-0 polyglactin suture to the two ends of the polyglactin suture attached to the extraocular muscle. The suture should be tied with a series of single knots as tightly as possible so that the noose will slide only when tugged on. The ends of the suture are then tied over an instrument to create a small noose (Fig. 57.36). The knot should then be moved up and down after applying balanced salt solution to the knot to smooth out the suture surface and to assure that the knot slides easily. If the knot is too loose, it should be replaced with a more secure knot. A traction suture should then be placed at the limbus, adjacent to the muscle undergoing recession, to provide countertraction during the postoperative adjustment procedure. The traction suture can be created with a piece of the 6-0 polyglactin suture by passing the needle in the sclera near the limbus. A deep scleral pass is necessary to prevent the traction suture from tearing through the sclera during the adjustment procedure. The sutures attached to the extraocular muscle can be left long and secured to the face of the patient with Steri-stripsTM, buried underneath the conjunctiva, or fashioned into a ‘short tag noose’48.
If the recession is found to be inadequate postoperatively, the knot should be loosened. The muscle can then be recessed further by grasping the traction suture with a surgical instrument while the patient is asked to rotate the eye in the opposite direction. This will allow the extraocular muscle to retract the distance the knot has been loosened. Once the surgeon is pleased with the patient’s ocular alignment, a square knot should be tied over the noose and the long ends of the polyglactin suture and the noose should be excised and covered with conjunctiva. When using a ‘short tag noose,’ the ends of the suture do not need to be trimmed (Fig. 57.37). The ‘short tag noose’ technique has the advantage that no further manipulations of the eye are necessary if it is determined postoperatively that the eyes are aligned satisfactorily.
Bowknot adjustable suture
A bowknot adjustable suture differs from the sliding knot technique in that a bowtie is created over a double throw in the polyglactin suture after positioning the extraocular muscle (Fig. 57.38A). When the alignment is ready to be adjusted, the bow is untied and the double throw knot is loosened if the muscle is to be recessed further or tightened if the muscle is to be advanced (Fig. 57.38B). Once satisfactory ocular alignment has been achieved, a square knot is tied and the ends of the suture are excised (Fig. 57.38C). This technique has the advantage that less suture is left buried underneath the conjunctiva.
Other types of adjustable suture (semi-adjustable)
Other techniques may be used for limited adjustments of the extraocular muscles postoperatively. The ‘ripcord’ adjustable suture technique is used for patients for whom it is deemed likely that they will not cooperate fully with the traditional adjustable suture procedure49. The muscle to be adjusted is recessed 1.5–2 mm more than the desired position using fixed sutures. The muscle is then advanced 1.5–2 mm by passing a piece of suture underneath the fixed sutures attached to the muscle, which is then attached to the sclera anterior to the muscle insertion (Fig. 57.39A). If the patient is found to be under-corrected postoperatively, the ripcord suture is cut allowing the muscle to fall back 1.5–2 mm (Fig. 57.39B). Kushner50 has described a semi-adjustable suture designed to reduce the risk of muscle slippage with an adjustable suture. After recessions of the inferior rectus muscle. Double-armed 6-0 polyglactin sutures are placed through the nasal and temporal corners of the muscles. Next, a third double-armed 6-0 polyglactin suture is passed through the center of the muscle. The muscle is then disinserted and the nasal and temporal corners of the muscle are sewn to the sclera at the desired point of recession. The double-armed suture in the center of the muscle is then passed through the muscle stump and a sliding knot is place on the suture (Fig. 57.40A). The center of the muscle is then advanced the distance desired (see Fig. 57.40B). Postoperatively the center of the muscle is adjusted. The sutures in the corner of the muscles ensure that the muscle does not slip postoperatively.
Posterior fixation suture
Posterior fixation sutures, also referred to as a ‘fadenoperation’ or as a retroequatorial myopexy, can be used to treat incomitant strabismus and accommodative esotropia with a high AC/A ratio. In addition, it can be combined with transpositional procedures to augment the effect of the transposition (Figures 57.31 and 57.32). The procedure is performed by suturing the muscle belly to the sclera 12 mm posterior to the muscle insertion so that its functional insertion is posterior to the equator of the eye (Fig. 57.41). This can be combined with a recession of the muscle (Fig. 57.42A,B). The end result is to disproportionately weaken the muscle in its field of action, while having little (if combined with recession) or no (if performed alone) effect in primary position. Adequate exposure can be difficult to achieve.
The mechanism of action of the posterior fixation suture is disputed. Originally, the posterior fixation suture was thought to have its effect by shortening the effective lever arm of the rectus muscle, thus reducing the torque on the globe51,52. More recently, there is evidence that posterior fixation sutures do not, in fact, reduce muscle torque, but have their effect by displacing the muscle’s pulley sleeve posteriorly when the muscle is contracting, thereby producing a mechanical restriction of the muscle in its field of gaze53. This has led to the creation of a new procedure, described in the next section.
Surgery of the muscle pulleys and connective tissue
Pulley fixation
An alternative technique for performing posterior fixation on a medial rectus muscle in a patient with a high AC/A ratio is to pass fixation sutures through the medial rectus pulleys54. This technique has the advantage that no scleral passes are required. Its effectiveness has been reported to be equivalent to posterior fixation sutures. After hooking the medial rectus muscle, the muscle may be recessed if needed. If no recession is needed, Westcott scissors are used to bluntly dissect the intermuscular membrane from the anterior surface of the medial rectus muscle (Fig. 57.43A). A Stevens tenotomy hook is then slid 10 mm down one side of the medical rectus muscle. The hook is rotated anteriorly and pulled forward to capture the anterior medial rectus pulley (Fig. 57.43B). An interrupted 6-0 Mersilene suture is then passed backhanded underneath the tenotomy hook to capture the superior border of the pulley. The suture is then passed through the superior third of the medial rectus muscle adjacent to the pulley and tied (Fig. 57.43C). The same procedure is then performed on the inferior half of the medial rectus muscle. At the end of the procedure, forced ductions should reveal moderate restriction to adduction.
Botulinum toxin injection
Botulinum was developed as a pharmacological treatment for strabismus by Alan Scott et al55. It has the advantage of being less invasive than strabismus surgery, but the effects are less predictable and the treatment often has to be repeated. In addition, the success of the procedure may not be known for several months until the paralytic effect of the botulinum toxin has worn off.
Indications
Botulinum toxin it is now used primarily for other indications56, but it continues to have a limited role in the treatment of strabismus57. It is particularly helpful in treating patients with high grade stereopsis who are over-corrected following strabismus surgery. An ideal candidate for botulinum treatment would have small angle esotropia or exotropia causing diplopia following horizontal strabismus surgery. It may also be used to treat patients with acute sixth nerve palsies. However, most studies have not shown that treatment with botulinum toxin alters the natural history of ocular misalignment in these patients58. Nevertheless, these patients can often be helped symptomatically in the short-term by aligning their eyes with botulinum toxin59. Botulinum toxin is also used by some ophthalmologists to treat children with infantile esotropia. It works best in children with small to moderate angle esotropia, and multiple treatments are often necessary60–62.
Technique
Botulinum toxin was developed as an outpatient treatment that could be administered in an office setting without incisional surgery. Electromyographic (EMG) guidance is very helpful in delivering the botulinum toxin to the neuromuscular junction where it will have the greatest effect. EMG guidance is particularly helpful in patients who have had previous strabismus surgery since the location of the extraocular muscles may not be known. When using EMG guidance to administer botulinum toxin to a horizontal rectus muscle, an electrode pad is placed on the forehead. After instilling local anesthestic drops, a 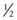 inch 27-gauge Teflon coated needle with a bare tip is placed in the bulbar conjunctiva near the muscle insertion. The patient is then instructed to abduct the eye if the medial rectus is being injected or to adduct the eye if the lateral rectus is being injected. The needle is then advanced along the surface of the sclera until the needle encounters the orbital wall (Fig. 57.44). The patient is then instructed to adduct the eye if the medial rectus is being injected or to abduct the eye if the lateral rectus is being injected. The needle is then advanced perpendicularly (medial rectus muscle) or diagonally (lateral rectus muscle) to run parallel to the orbital wall until the EMG signal is maximized (either the auditory signal or the signal amplitude on a screen). The toxin is then slowly injected into the muscle over 5–10 seconds to minimize leakage out of the muscle being injected. The patient is then asked to remain in an upright position for several hours so the excess toxin will not diffuse posteriorly onto the levator palpebrae superioris muscle, which can result in blepharoptosis. Generally, the paralysis induced by botulinum toxin will begin to be noted 2–3 days after a muscle is treated and the maximal effect will occur in about 1 week. All of the paralytic effect of the toxin usually wears off in 3–6 months, but ocular alignment can be improved on a long-term basis secondary to strengthening of the muscle antagonistic to the muscle injected with toxin.
inch 27-gauge Teflon coated needle with a bare tip is placed in the bulbar conjunctiva near the muscle insertion. The patient is then instructed to abduct the eye if the medial rectus is being injected or to adduct the eye if the lateral rectus is being injected. The needle is then advanced along the surface of the sclera until the needle encounters the orbital wall (Fig. 57.44). The patient is then instructed to adduct the eye if the medial rectus is being injected or to abduct the eye if the lateral rectus is being injected. The needle is then advanced perpendicularly (medial rectus muscle) or diagonally (lateral rectus muscle) to run parallel to the orbital wall until the EMG signal is maximized (either the auditory signal or the signal amplitude on a screen). The toxin is then slowly injected into the muscle over 5–10 seconds to minimize leakage out of the muscle being injected. The patient is then asked to remain in an upright position for several hours so the excess toxin will not diffuse posteriorly onto the levator palpebrae superioris muscle, which can result in blepharoptosis. Generally, the paralysis induced by botulinum toxin will begin to be noted 2–3 days after a muscle is treated and the maximal effect will occur in about 1 week. All of the paralytic effect of the toxin usually wears off in 3–6 months, but ocular alignment can be improved on a long-term basis secondary to strengthening of the muscle antagonistic to the muscle injected with toxin.
Intraoperative complications
Anesthesia related complications
Postoperative nausea and vomiting are especially common following strabismus surgery in children, ranging from 37% to 80%, and many different anesthesia techniques and anti-emetics have been studied63. Although no one protocol has been proven superior to another, we have found the protocol in Table 57.2 to be helpful64. Postoperative anti-emetics are prescribed only when needed.
The oculocardiac reflex is a trigeminal–vagal reflex that can be elicited by traction on the extraocular muscles and can result in profound bradycardia including asystole. Rapid acting narcotics can enhance the degree of bradycardia65. The oculocardiac reflex is less likely to be elicited when sevoflurane (as opposed to other inhalation agents) is used66. Avoiding sudden and excessive traction on the extraocular muscles, and pretreatment with atropine can minimize the occurrence of the oculocardiac reflex.
Malignant hyperthermia is a rare but potentially fatal complication of anesthesia occurring in genetically predisposed individuals, who, when exposed to certain triggers including some types of anesthetic agents, will develop a hypermetabolic crisis, leading to heat production, tachycardia, muscle rigidity, and extremely high fever which can cause cardiac arrest, brain damage, organ failure and death67.
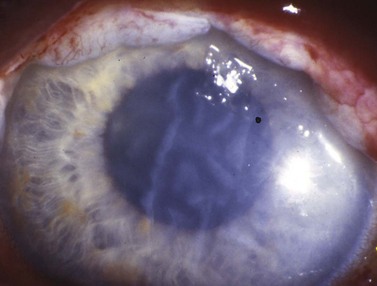
Scleral perforation
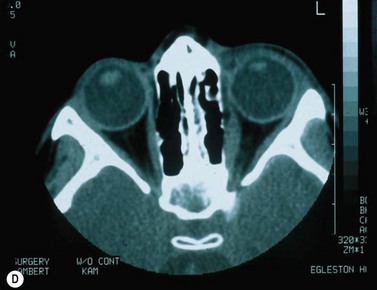
Scleral perforation is a potentially serious complication of strabismus surgery, which most commonly occurs when a needle is unintentionally passed through full-thickness sclera into the suprachoroidal space or through the retina, while attempting to reattach the extraocular muscle to the wall of the eye (Fig. 57.45A,B). Perforation has also been reported to occur during other steps of strabismus surgery including muscle disinsertion, muscle dissection, placement of traction sutures, and even with application of locking forceps onto the sclera68.
The reported incidence of scleral perforation varies from <1% to 10%, but is probably decreasing with the use of smaller, spatulated needles69–74. Risk factors for scleral perforation include thinner sclera, younger patient age74, use of the S-14 needle74, when not using a ‘hang back’ technique, rectus muscle recession (as opposed to resection)74, and a novice surgeon)68.
Complications of scleral perforation are rare, but can include retinal detachment73, hemorrhage73,75, endophthalmitis76, and phthisis bulbi77. Treatment of scleral perforation is controversial. Cryotherapy has been shown to increase the risk of retinal detachment in an animal model78. Less heavy cryotherapy may be better tolerated, but is considered by some to be unwarranted unless the patient has substantial risk factors for retinal detachment69. Laser retinopexy has been shown to be less likely than cryotherapy to release pigment cells into the vitreous, and is advocated by some when a large retinal break surrounded by subretinal fluid is present. Smaller breaks are often observed79.
Postoperative care
The efficacy of preoperative and postoperative ophthalmic medications in preventing postoperative infections is unproven80,81. One drop of povidine iodine 5% solution can be administered during the preoperative surgical preparation and at the conclusion of the procedure, since this has been shown to reduce both conjunctival bacterial flora and the incidence of postoperative endophthalmitis in cataract patients82. We recommend combination antibiotic–steroid drops or ointment, for cooperative patients.
Postoperative complications
Anterior segment ischemia
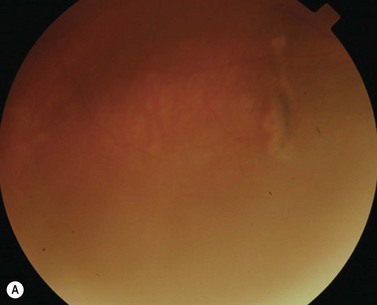
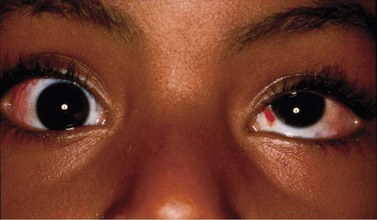
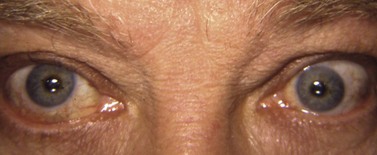
During strabismus surgery on the rectus muscles, the anterior ciliary arteries are disrupted. If the vascular supply to the eye is compromised, in some cases this can result in anterior segment ischemia. Signs and symptoms of anterior segment ischemia include reduced iris perfusion on angiography, changes in reactivity and shape of the pupil, uveitis, and corneal edema (Fig. 57.46).
The treatment of anterior segment ischemia is largely anecdotal. Topical corticosteroids are used commonly. Oral corticosteroids and hyperbaric oxygen therapy have also been used in some cases83. Prostaglandin synthetase inhibitors have been studied in animals, and may have a role in treatment84. Prevention is aimed at limiting the number of rectus muscles operated on at a single setting and using vessel sparing surgical techniques37.
Corneal and conjunctival complications
Advancement of the plica semilunaris is sometimes seen when a limbal incision is used, and the surgeon inadvertently sutures the plica semilunaris, rather than the anterior edges of the conjunctival flap, to close the incision (Fig. 57.47). To prevent this complication, the surgeon should take great care to identify the corners of the conjunctival flap prior to suturing them back to the eye.
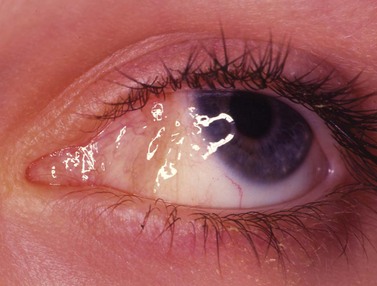
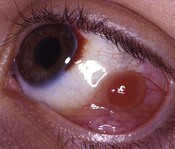
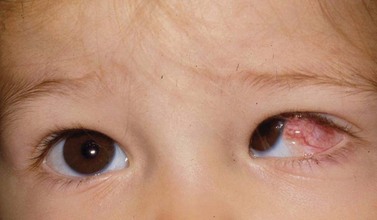
Pyogenic granulomas are benign pink, fleshy tumors that generally arise adjacent to the conjunctival incision, often overlying an area of exposed Tenon’s fascia, about 3–4 weeks after surgery (Fig. 57.48). They consist of a fibrovascular response to surgical trauma, and often resolve spontaneously, but can be easily removed with simple excision.
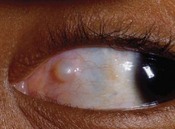
Epithelial inclusion cysts usually occur at the incision site or adjacent to the new insertion of the extraocular muscle (Fig. 57.49), but intramuscular and intrascleral cysts have also been reported. They are thought to arise from the implantation of conjunctival epithelial cells at the time of strabismus surgery. Epithelia inclusion cysts will enlarge over time, so they should be removed surgically when they are identified. Caution must be exercised when dissecting the cysts since they are sometimes incorporated into the muscle. Treatment of intrascleral cysts with sclerosing agents has been reported to be successful in selected cases85.
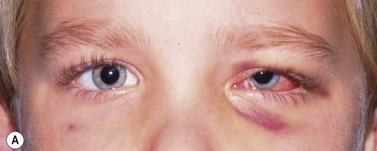
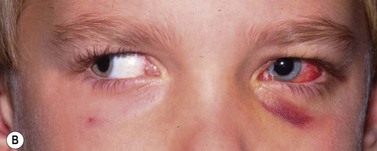
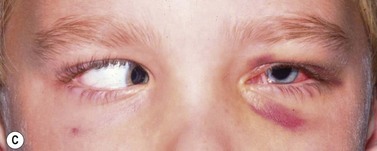
Infection
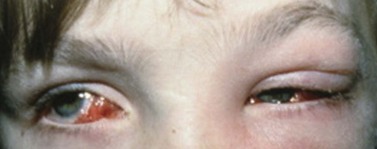
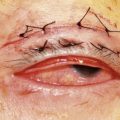
Periocular infections consisting of orbital and preseptal cellulitis are thought to occur in about 1 in 1100 cases86. Presenting signs and symptoms include marked swelling, severe pain, light sensitivity, marked redness, discharge, tearing, fever, irritability, lethargy, nausea, and insomnia from pain (Fig. 57.50). Onset of symptoms is usually 1–5 days after surgery. Predisposing factors are thought to be excessive eye rubbing, unsuspected sinusitis, and poor hygiene. Patients usually respond to antibiotics. Prompt hospitalization for intravenous antibiotic therapy is recommended if the patient does not respond quickly to oral antibiotics.
Endophthalmitis is a rare complication after strabismus surgery68. It has been reported to occur in 1 in 18 500 cases. Although scleral perforation is thought to increase the risk for endophthalmitis, it is possible for a patient to develop endopthalmitis without a recognized perforation. Delay in diagnosis is common. Patients present with pain, eyelid swelling, and erythema. Endophthalmitis can have its onset from 1 day to 2 weeks postoperatively. Visual outcomes are generally poor. The Endophthalmitis Vitrectomy Study Guidelines are recommended for treatment: if visual acuity is light perception or worse, then pars plana vitrectomy is usually recommended. If vision is hand motions or better, an intravitral injection of antibiotics is recommended87. The role of routine postoperative antibiotics to prevent endophthalmitis is unproven80,81.
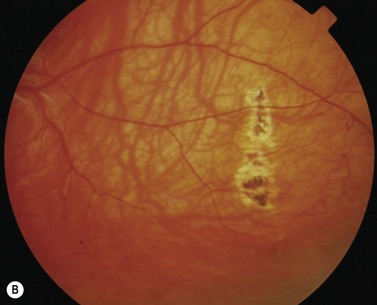
Slipped and lost muscles
Lost muscles can occur as a result of trauma or a variety of surgical procedures including both ophthalmic and ENT procedures. If possible, an attempt should be made to retrieve the muscle immediately, although in the case of severe damage to the muscle during an ENT surgery, or in the case of a ‘pulled in two’ syndrome, this may be impossible. The superior and inferior rectus muscles can usually be found because of their attachment to an oblique muscle (Fig. 57.51). However, it is very difficult to find the medial rectus muscle or the lateral rectus muscle if it is cut posteriorly during an inferior oblique recession (Fig. 57.52). It is usually better to refer a patient with a lost muscle to an experienced strabismus surgeon rather than performing an extensive exploration, which could lead to additional complications such as fat adherence syndrome (Fig. 57.53A–D). A lost muscle can often be retrieved using either a transnasal endoscopic approach with assistance from an ENT surgeon88, or an orbital wall approach with assistance from an orbital surgeon89.
Fat adherence syndrome
Fat adherence syndrome is a restrictive strabismus that occurs when extraconal orbital fat enters the subTenon’s space as a result of accidental or surgical trauma. Surgical treatment is difficult since scar tissue often re-forms after dissection. The goal is to return the eye to the primary position with restoration of as much normal motility as possible, but the results are usually sub-optimal. Attaching amniotic membrane to the underlying sclera may facilitate the treatment of this condition90.
Changes in eyelid position
Strabismus surgery on the vertical rectus muscles often leads to changes in the position of the eyelids. This occurs because there are fascial connections between the vertical rectus muscles and the adjacent eyelid retractor muscles. Patients undergoing recession of the vertical rectus muscles should be warned of the possibility of postoperative eyelid retraction. These changes are most common after large recessions of the inferior rectus muscle (Fig. 57.54). Careful dissection of the fascial connections between the rectus muscles and eyelid retractors can minimize eyelid changes. Reattachment of the head of the capsulopalpebral fascia to the anterior margin of a recessed inferior rectus muscle may reduce the amount of lid retraction91.
1 Medow N. Strabismus: the first 3500 years. J Pediatr Ophthalmol Strabismus. 1999;36(1):30-34.
2 Lambert SR. Are there more exotropes than esotropes in Hong Kong? Br J Ophthalmol. 2002;86(8):835-836.
3 Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41(6):1280-1290.
4 Porter JD, Baker RS, Ragusa RJ, et al. Extraocular muscles: basic and clinical aspects of structure and function. Surv Ophthalmol. 1995;39(6):451-484.
5 Clark RA, Miller JM, Demer JL. Location and stability of rectus muscle pulleys. Muscle paths as a function of gaze. Invest Ophthalmol Vis Sci. 1997;38(1):227-240.
6 Demer JL, Miller JM, Poukens V, et al. Evidence for fibromuscular pulleys of the recti extraocular muscles. Invest Ophthalmol Vis Sci. 1995;36(6):1125-1136.
7 Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2004;45(3):729-738. 8
8 Jackson S, Harrad RA, Morris M, et al. The psychosocial benefits of corrective surgery for adults with strabismus. Br J Ophthalmol. 2006;90(7):883-888.
9 Paysse EA. Adult strabismus: goals of realignment surgery. Binocul Vis Strabismus Q. 2001;16(1):9-10.
10 Coats DK, Paysse EA, Towler AJ, et al. Impact of large angle horizontal strabismus on ability to obtain employment. Ophthalmology. 2000;107(2):402-405.
11 Rosenbaum AL. The goal of adult strabismus surgery is not cosmetic. Arch Ophthalmol. 1999;117(2):250.
12 Olitsky SE, Sudesh S, Graziano A, et al. The negative psychosocial impact of strabismus in adults. J AAPOS. 1999;3(4):209-211.
13 Burke JP, Leach CM, Davis H. Psychosocial implications of strabismus surgery in adults. J Pediatr Ophthalmol Strabismus. 1997;34(3):159-164.
14 Keltner JL. Strabismus surgery in adults. Functional and psychosocial implications. Arch Ophthalmol. 1994;112(5):599-600.
15 Burden AL.Jr. The stigma of strabismus: an ophthalmologist’s perspective. Arch Ophthalmol. 1994;112(3):302.
16 Hutchinson AK. Intermittent exotropia. Ophthalmol Clin North Am. 2001;14(3):399-406.
17 Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus: the Friedenwald lecture.[see comment]. Invest Ophthalmol Vis Sci. 2004;45(3):729-738. 8
18 O’Neal TD, Rosenbaum AL, Stathacopoulos RA. Distance stereo acuity improvement in intermittent exotropic patients following strabismus surgery. J Pediatr Ophthalmol Strabismus. 1995;32(6):353-357. discussion 8
19 Pozgar GD. Legal Aspects of Health Care Administration, 10th edn. Sudbury, MA: Jones and Bartlett; 2007.
20 Guyton DL. Exaggerated traction test for the oblique muscles. Ophthalmology. 1981;88(10):1035-1040.
21 Plager DA. Traction testing in superior oblique palsy. J Pediatr Ophthalmol Strabismus. 1990;27(3):136-140.
22 Parks MM. Fornix incision for horizontal rectus muscle surgery. Am J Ophthalmol. 1968;65:907-915.
23 Parks MM, Parker JE. Atlas of Strabismus Surgery. Philadelphia: Harper and Row; 1983.
24 Repka MX, Guyton DL. Comparison of hang back medial rectus muscle recession with conventional recession. Ophthalmology. 1988;95:782-787.
25 Wright KW. Color Atlas of Strabismus Surgery. Strategies and Techniques. New York: Springer; 2007.
26 Coats DK, Olitsky SE. Strabismus Surgery and its Complications. Berlin: Springer-Verlag; 2007.
27 Jampolsky A. Strabismus reoperation techniques. Trans Sect Ophthalmol Am Acad Ophthalmol Otolaryngol. 1975;79(5):704-717.
28 Lenart TD, Lambert SR. Slipped and lost extraocular muscles. Ophthalmol Clin North Am. 2001;14(3):433-442.
29 Ludwig IH, Chow AY. Scar remodeling after strabismus surgery. J AAPOS. 2000;4(6):326-333.
30 Kushner BJ. Torsion as a contributing cause of the anti-elevation syndrome. J AAPOS. 2001;5(3):172-177.
31 Wetz RD, Romano PE, Wright P. Inferior oblique myectomy, disinsertion and recession in Rhesus monkeys. Arch Ophthalmol. 1977;95:857-860.
32 Freedman SF, Rojas M, Toth CA. Strabismus surgery for large angle cyclotorsion after macular translocation surgery. J Aapos. 2002;6:154-162.
33 Iizuka M, Kushner B. Surgical implications of the superior oblique frenulum. J AAPOS. 2008 Feb;12(1):27-32.
34 Wright KW. Superior oblique silicone expander for Brown syndrome and superior oblique overaction. J Pediatr Ophthalmol Strabismus. 1991;28(2):101-107.
35 Wright KW, Min BM, Park C. Comparison of superior oblique tendon expander to superior oblique tenotomy for the management of superior oblique overaction and Brown syndrome. J Pediatr Ophthalmol Strabismus. 1992;29(2):92-97. discussion 8–9
36 Knapp P. The surgical treatment of double elevator paralysis. Trans Am Ophthalmol Soc. 1969;67:304.
37 McKeown CA, Lambert HM, Shore JW. Preservation of the anterior ciliary vessels during extraocular muscle surgery. Ophthalmology. 1989;96:498-506.
38 Foster RS. Vertical muscle transposition augmented with lateral fixation. J AAPOS. 1997;1:20-30.
39 Buckley EG. Paralytic strabismus. In: Plager DA, editor. Strabismus Surgery: Basic and Advanced Strategies. New York: Oxford University Press; 2004:88-89.
40 Fitzsimons R, Lee JP, Elston J. Treatment of sixth nerve palsy in adults with combined botulinum toxin chemodenervation and surgery. Ophthalmology. 1988;95(11):1535-1542.
41 Jensen CD. Rectus muscle union: a new operation for paralysis of the rectus muscles. Trans Pac Coast Otoophthalmol Soc Annu Meet. 1964;45:359-387.
42 Bleik JH, Cherfan GM. Anterior segment ischemia after the Jensen procedure in a 10-year old patient. Am J Ophthalmol. 1995;119:524-525.
43 Brooks SE, Olitsky SE, deB Ribeiro G. Augmented Hummelsheim procedure for paralytic strabismus. J Pediatr Ophthalmol Strabismus. 2000;37(4):189-195.
44 Jampolsky A, editor. Adjustable Strabismus Surgical Procedures. Saint Louis: C.V. Mosby, 1978.
45 Lueder GT, Scott WE, Kutschke PJ, et al. Long-term results of adjustable suture surgery for strabismus secondary to thyroid ophthalmopathy. Ophthalmology. 1992;99(6):993-997.
46 Awadein A, Sharma M, Bazemore MG, et al. Adjustable suture strabismus surgery in infants and children. J AAPOS. 2008;12(6):585-590.
47 Tripathi A, Haslett R, Marsh IB. Strabismus surgery: adjustable sutures – good for all? Eye. 2003;17(6):739-742.
48 Nihalani BR, Whitman MC, Salgado CM, et al. Short tag noose technique for optional and late suture adjustment in strabismus surgery. Arch Ophthalmol. 2009;127(12):1584-1590.
49 Coats DK. Ripcord adjustable suture technique for use in strabismus surgery. Arch Ophthalmol. 2001;119(9):1364-1367.
50 Kushner BJ. An evaluation of the semiadjustable suture strabismus surgical procedure. J AAPOS. 2004;8(5):481-487.
51 Cuppers C, editor. The so-called ‘fadenoperation’ (surgical considerations by well defined changes of the arc of contact). Marseille: Diffusion Generale de Librairie, 1976.
52 Scott AB. The faden operation: mechanical effects. Am Orthopt J. 1977;27:44-47.
53 Clark RA, Isenberg SJ, Rosenbaum AL, et al. Posterior fixation sutures: a revised mechanical explanation for the fadenoperation based on rectus extraocular muscle pulleys. Am J Ophthalmol. 1999;128:702-714.
54 Clark RA, Ariyasu R, Demer JL. Medial rectus pulley posterior fixation: a novel technique to augment recession. J AAPOS. 2004;8(5):451-456.
55 Scott AB, Rosenbaum A, Collins CC. Pharmacologic weakening of extraocular muscles. Invest Ophthalmol. 1973;12(12):924-927.
56 Dutton JJ, Fowler AM. Botulinum toxin in ophthalmology. Surv Ophthalmol. 2007;52(1):13-31.
57 Rowe FJ, Noonan CP. Botulinum toxin for the treatment of strabismus. Cochrane Database Syst Rev 2009(2):CD006499.
58 Holmes JM, Beck RW, Kip KE, et al. Botulinum toxin treatment versus conservative management in acute traumatic sixth nerve palsy or paresis. J AAPOS. 2000;4(3):145-149.
59 Hung HL, Kao LY, Sun MH. Botulinum toxin treatment for acute traumatic complete sixth nerve palsy. Eye (Lond). 2005;19(3):337-341.
60 McNeer KW, Tucker MG, Spencer RF. Botulinum toxin management of essential infantile esotropia in children. Arch Ophthalmol. 1997;115(11):1411-1418.
61 Ruiz MF, Alvarez MT, Sanchez-Garrido CM, et al. Surgery and botulinum toxin in congenital esotropia. Can J Ophthalmol. 2004;39(6):639-649.
62 Thouvenin D, Lesage-Beaudon C, Arne JL. Botulinum injection in infantile strabismus. Results and incidence on secondary surgery in a long-term survey of 74 cases treated before 36 months of age]. J Fr Ophtalmol. 2008;31(1):42-50.
63 Kovac AL. Management of postoperative nausea and vomiting in children. Pediatric Drugs. 2007;9(1):47-69.
64 Williams AR, Controy JM. The anesthetic management of the pediatric strabismus patient. J AAPOS. 1998;2(2):113-115.
65 Arnold RW, Jensen PA, Kovtoun TA, et al. The profound augmentation of the oculocardiac reflex by fast acting opiods. Binocul Vis Strabismus Q. 2004;19:215-222.
66 Allison CE, De Lange JJ, Koole FD, et al. A comparison of the incidence of the oculocardiac and oculorespiratory reflexes during sevoflurane or halothane anesthesia for strabismus surgery in children. Anesth Analg. 2000;90:306-310.
67 Marmor M. Malignant hyperthermia. Surv Ophthalmol. 1983;28(2):117-127.
68 Simon JW, Lininger LL, Scheraga JL. Recognized scleral perforation during eye muscle surgery: incidence and sequelae. J Pediatr Ophthalmol Strabismus. 1992;29:273-275.
69 Taherian K, Sharma P, Prakash P, et al. Scleral perforations in strabismus surgery: incidence and role of prophylactic cryotherapy – a clinical and experimental study. Strabismus. 2004;12:17-25.
70 Gottleib F, Castro JL. Perforation of the globe during strabismus surgery. Arch Ophthalmol. 1970;84:151-157.
71 Morris RJ, Rosen PH, Fells P. Incidence of inadvertent globe perforation during strabismus surgery. Br J Ophthalmol. 1990;74:490-493.
72 Noel LP, Bloom JN, Clarke WN, et al. Retinal perforation in strabismus surgery. J Pediatr Ophthalmol Strabismus. 1997;34:115-117.
73 Awad AH, Mullaney PB, Al-Hazmi S, et al. Recognized globe perforation during strabismus surgery: incidence, risk factors, and sequelae. J AAPOS. 2000;43(3):150-153.
74 Dang Y, Racu C, Isenberg SJ. Scleral penetrations and perforations in strabismus surgery and associated risk factors. J AAPOS. 2004;4:325-331.
75 Greenberg DR, Ellenhorn NL, Chapman LI, et al. Posterior chamber hemorrhage during strabismus surgery. Am J Ophthalmol. 1988;106:634-635.
76 Salamon SM, Friberg TR, Luxenburg MN. Endophthalmitis after strabismus surgery. Am J Ophthalmol. 1982;93:39-41.
77 Apple DJ, Jones GR, Reidy JJ, et al. Ocular perforation and phthisis bulbi secondary to strabismus surgery. J Pediatr Ophthalmol Strabismus. 1985;22:184-187.
78 Mittleman D, Bakos IM. The role of retinal cryopexy in the management of experimental perforation of the eye during strabismus surgery. J Pediatr Ophthalmol Strabismus. 2004;21:186-189.
79 Coats DK, Olitsky SE. Scleral perforation and penetration. In: Coats DK, Olitsky SE, editors. Strabismus surgery and its complications. Berlin-Heidelberg: Springer; 2007:211-221.
80 Olitsky SE. Perioperative care of the strabismus patient. J Pediatr Ophthalmol Strabismus. 1997;34:126-128.
81 Wortham ET, Anandakrishnan I. Are antibiotic-steroid drops necessary following strabismus surgery? A prospective, randomized masked trial. J Pediatr Ophthalmol Strabismus. 1990;27:205-207.
82 Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone-iodine. Ophthalmology. 1991;98(12):1769-1775.
83 de Smet MD, Carruthers J, Lepawsky M. Anterior segment ischemia treated with hyperbaric oxygen. Can J Ophthalmol. 1987;22:381-383.
84 Inoue M, Shirabe H, Yamamoto M. Blood aqueous barrier disruption in experimental anterior segment ischemia in rabbit eyes. Ophthalmic Res. 1999;31:213-219.
85 Graubart EB, Hubbard GB. Giant intrascleral cyst treated with trichloacetic acid. Arch Ophthalmol. 2008;126(3):438-439.
86 Kivlin JD, Wilson ME, Group TPIS. Periocular infection after strabismus surgery. J Pediatr Ophthalmol Strabismus. 1995;32:42-49.
87 Group TEVS. A randomized trial of immediate vitrectomy and intravenous antibiotics for the treatment of post-operative bacterial endophthalmitis. Arch Ophthalmol. 1995;113:1479-1496.
88 Lenart TD, Reichman OS, McMahon SJ, et al. Retrieval of lost medial rectus muscles with a combined ophthalmologic and otolaryngologic surgical approach. Am J Ophthalmol. 2000;130(5):645-652.
89 Underdahl JP, Demer JL, Goldberg RL, et al. Orbital wall approach with preoperative orbital imaging for identification and retrieval of lost or transected extraocular muscles. J AAPOS. 2001;5(4):230-237.
90 Sheha H, Casas V, Hayashida Y. The use of amniotic membrane in reducing adhesions after strabismus surgery. J AAPOS. 2009;13(1):99-101.
91 Pacheco EM, Guyton DL, Repka MX. Changes in eyelid position accompanying vertical rectus muscle surgery and prevention of lower lid retraction with adjustable surgery. J Pediatr Ophthalmol Strabismus. 1992;29:265-272.

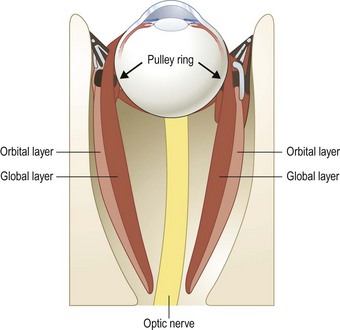
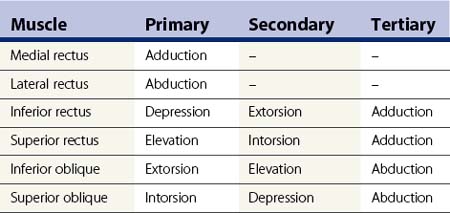
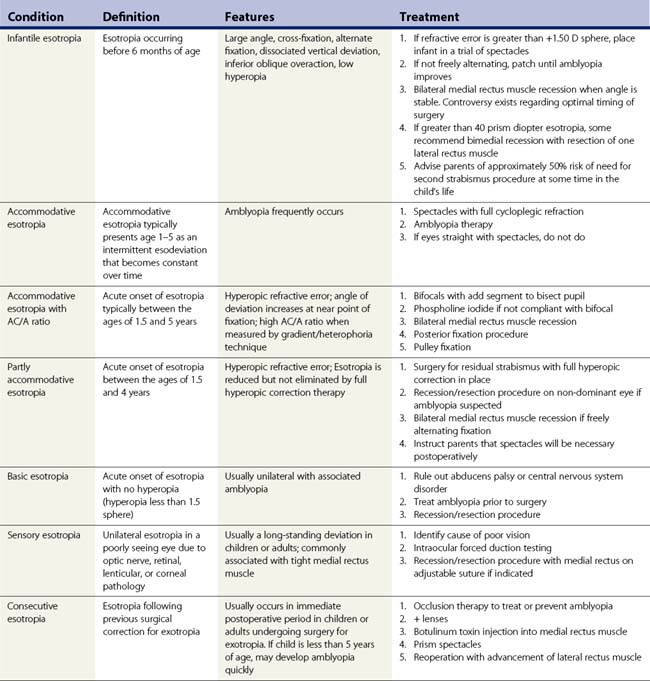
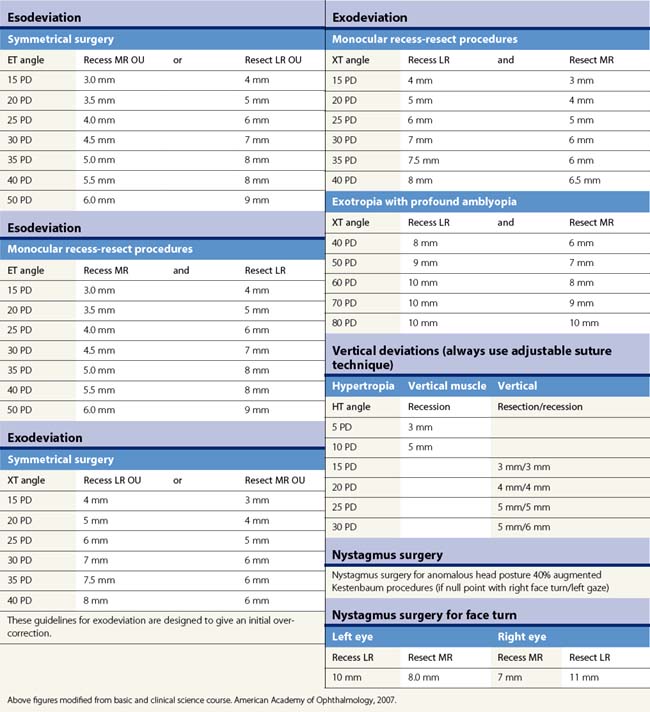
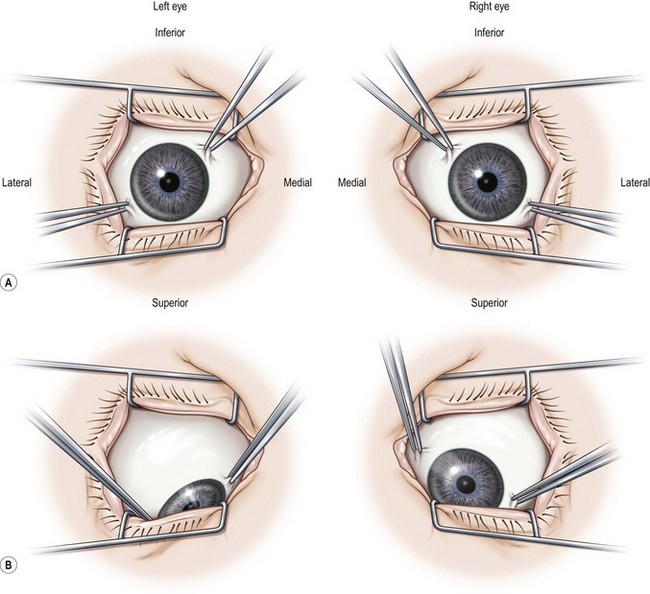
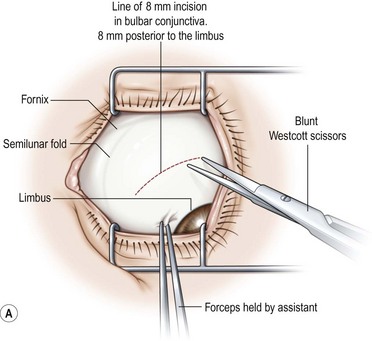
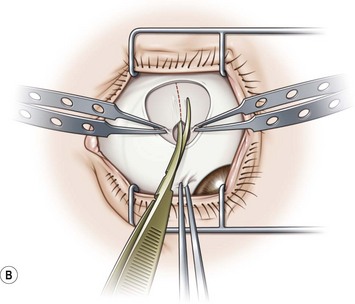
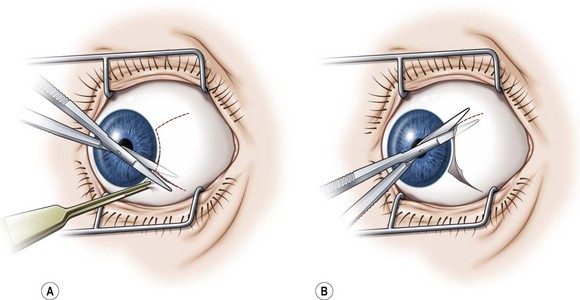
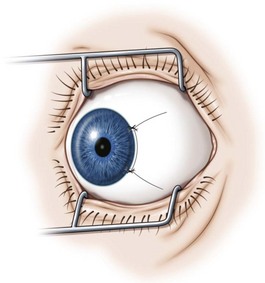
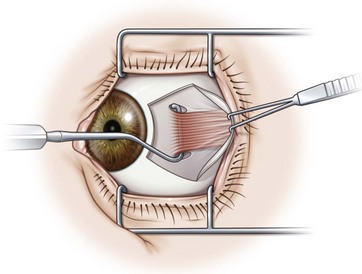
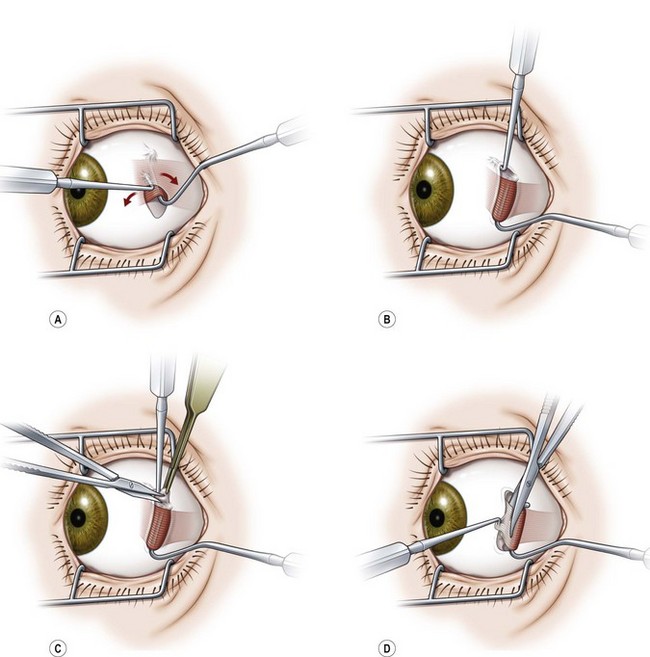
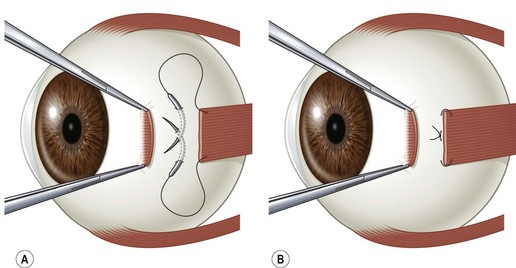
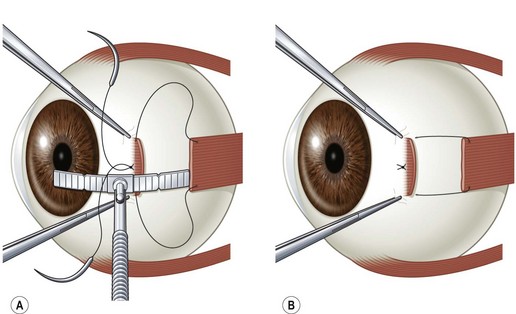
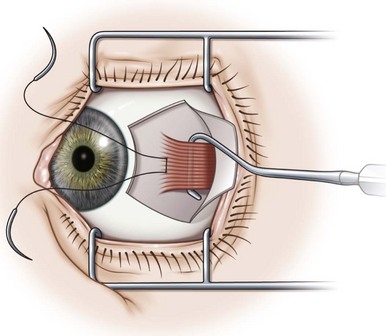
 to
to 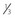 of the distance into the tendon is then placed on both sides of the tendon.
of the distance into the tendon is then placed on both sides of the tendon.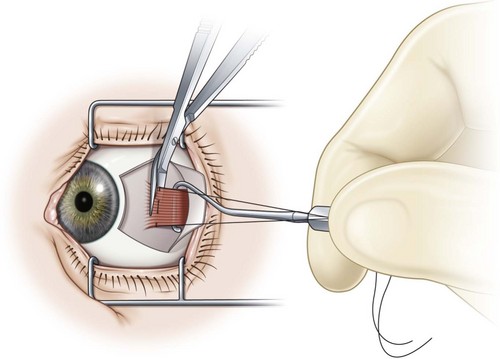
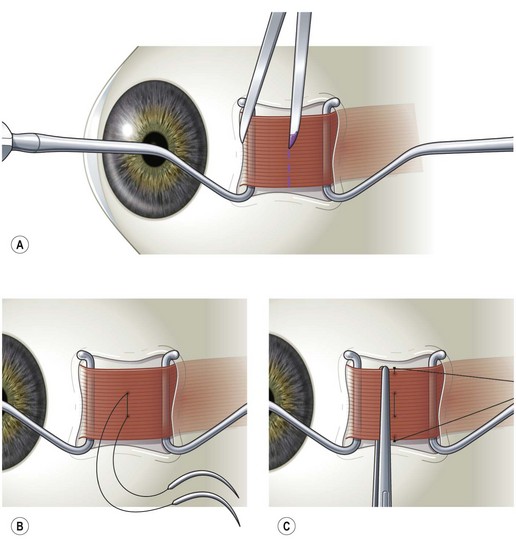
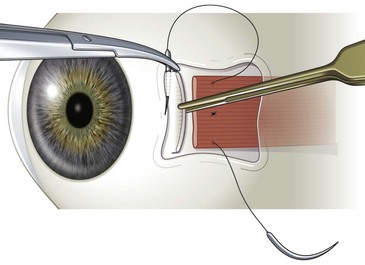
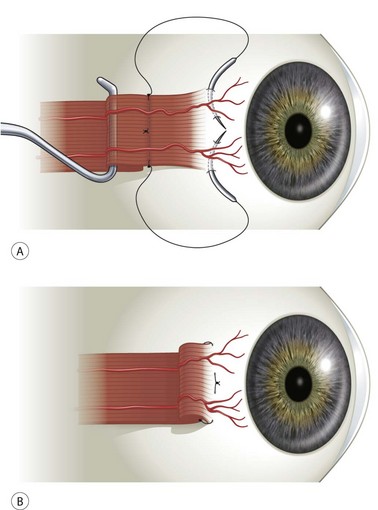
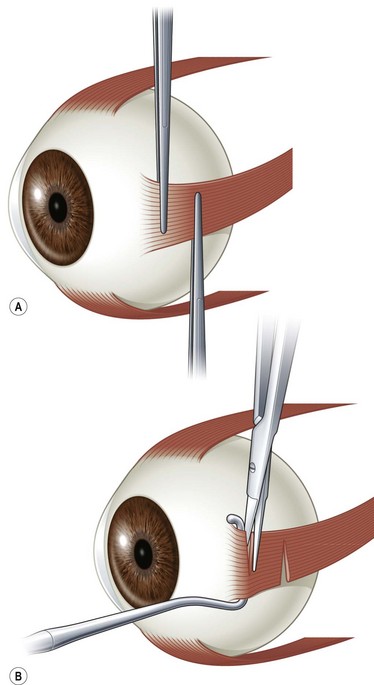
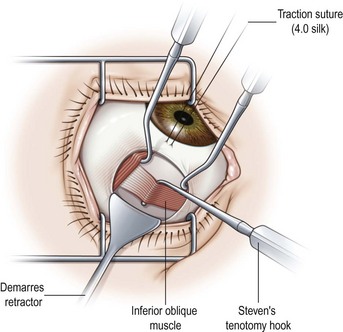
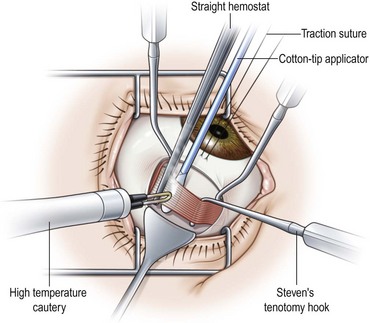
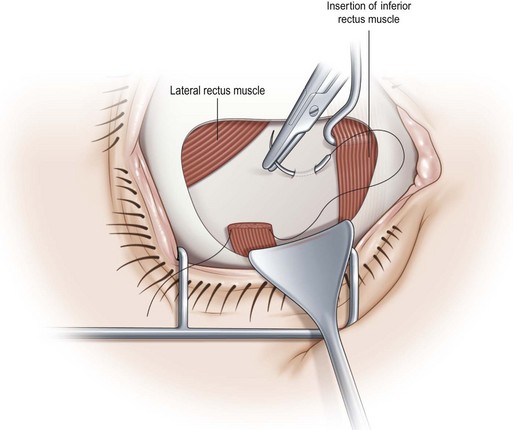
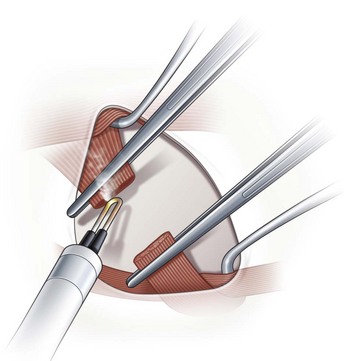
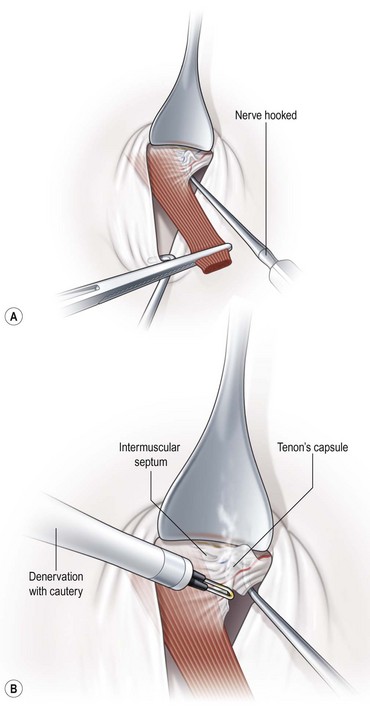
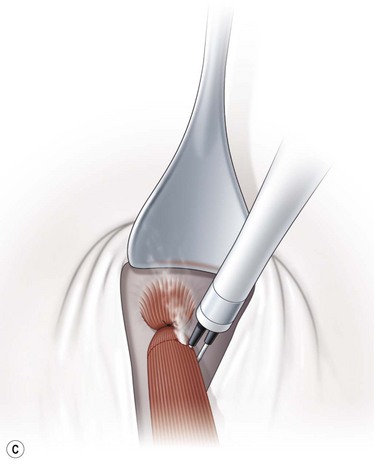
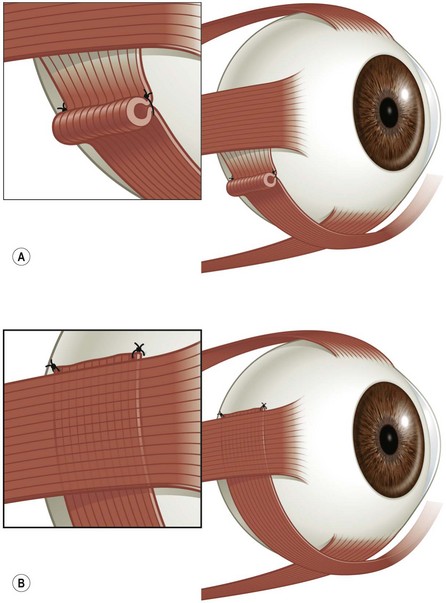
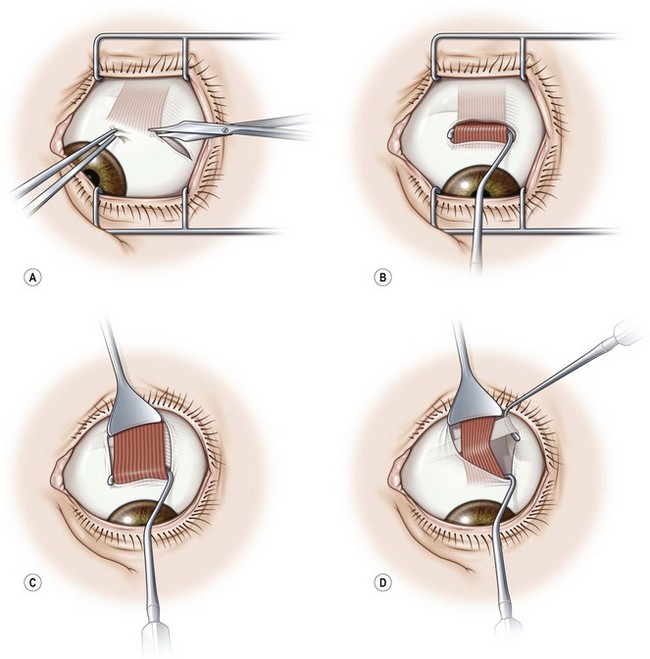
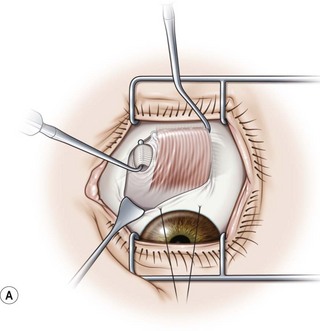
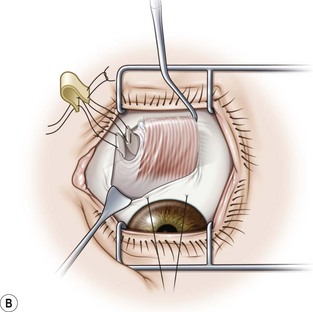
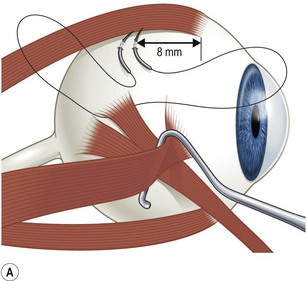
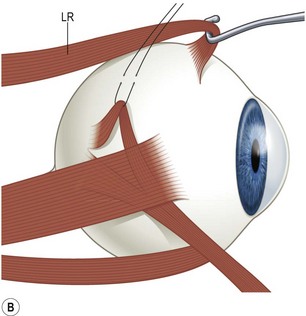
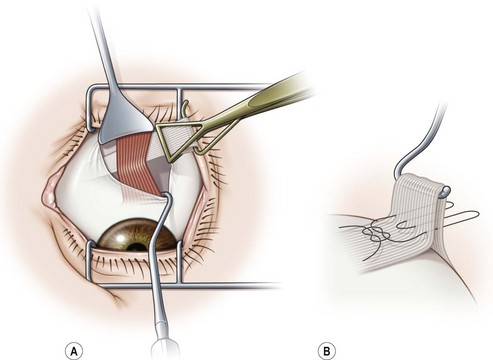
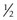 or full tendon width transposition of a rectus muscle to treat A or V pattern strabismus, either a standard limbal or fornix incision may be used, depending on the surgeon’s preference. When using a fornix incision, the surgeon may find it easier to make the incision in the direction that the muscle is to be transposed. For example, in the case of a lateral rectus muscle recession to treat a V pattern strabismus, the surgeon should make a superotemporal incision instead of an inferotemporal incision. When performing a transposition procedure, the surgeon may use either a large limbal incision or two separate fornix incisions (one on either side of the paretic muscle).
or full tendon width transposition of a rectus muscle to treat A or V pattern strabismus, either a standard limbal or fornix incision may be used, depending on the surgeon’s preference. When using a fornix incision, the surgeon may find it easier to make the incision in the direction that the muscle is to be transposed. For example, in the case of a lateral rectus muscle recession to treat a V pattern strabismus, the surgeon should make a superotemporal incision instead of an inferotemporal incision. When performing a transposition procedure, the surgeon may use either a large limbal incision or two separate fornix incisions (one on either side of the paretic muscle).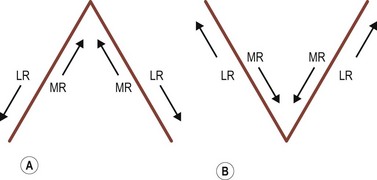
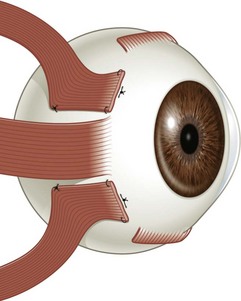
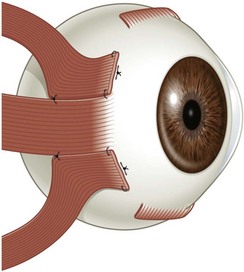
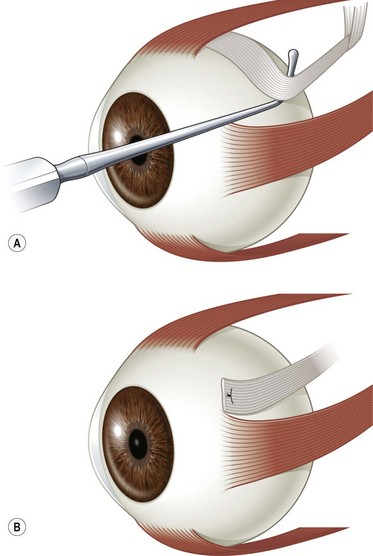
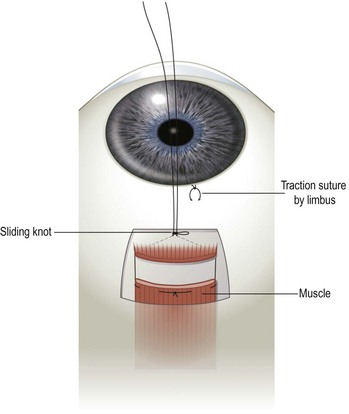
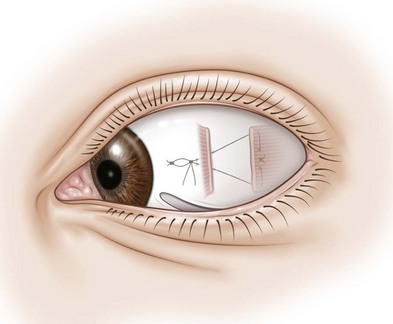
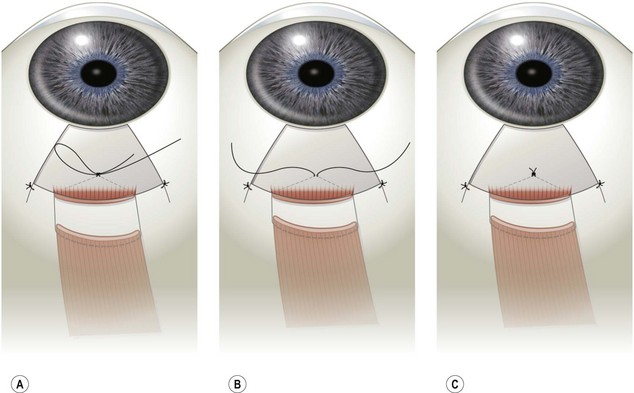
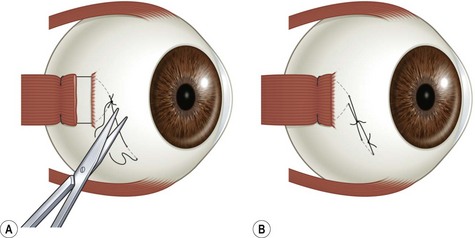
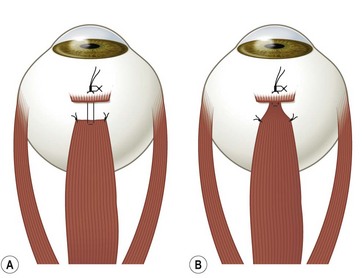
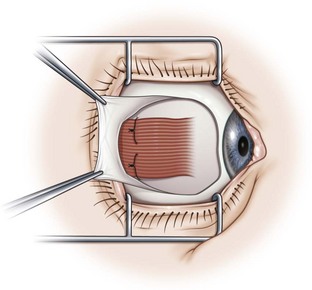
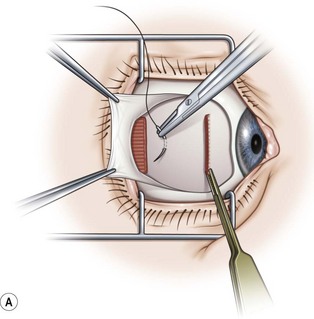
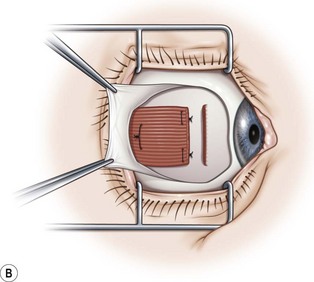
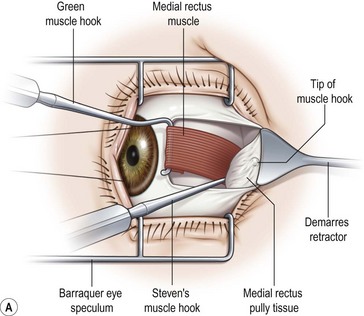
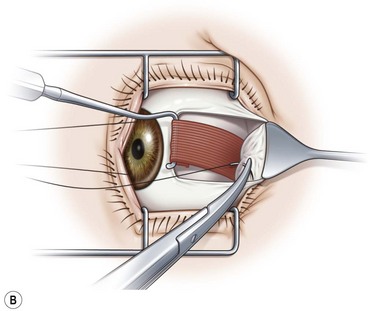
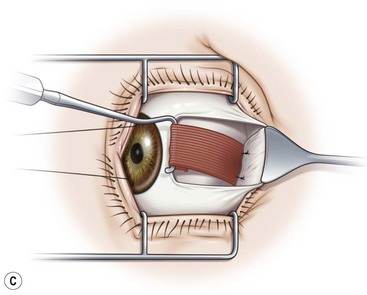
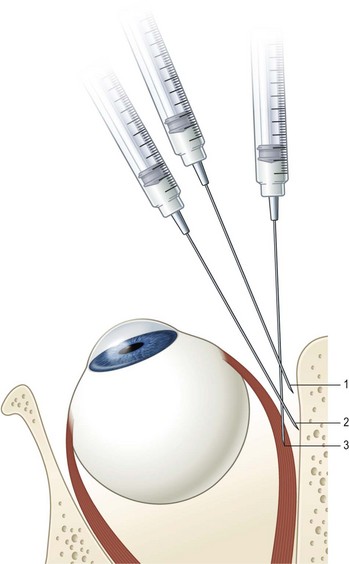
 inch 27-gauge Teflon coated needle with a bare tip along the surface of the sclera until the needle encounters the orbital wall. The needle is then rotated posteriorly to enter the muscle belly.
inch 27-gauge Teflon coated needle with a bare tip along the surface of the sclera until the needle encounters the orbital wall. The needle is then rotated posteriorly to enter the muscle belly.