).
Gastric Disease
Congenital Anomalies
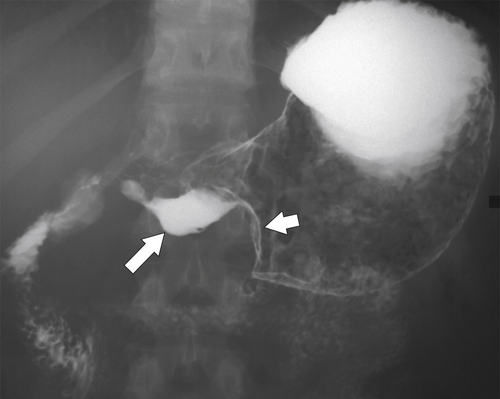
Antral Diaphragm
Pyloric Stenosis
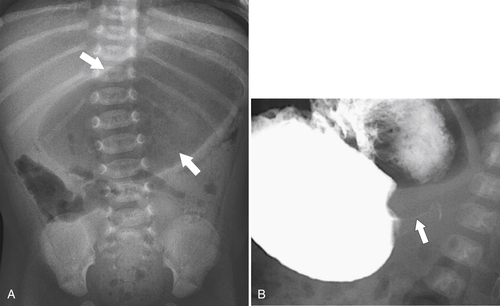
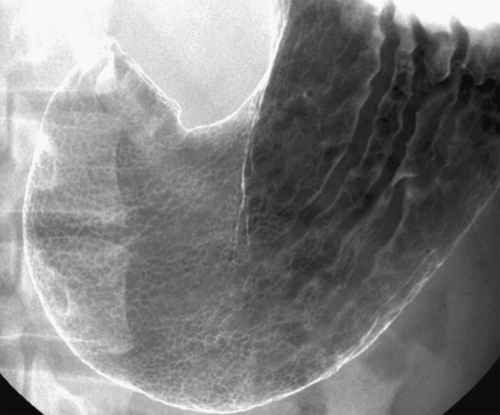
Gastric Diverticulum
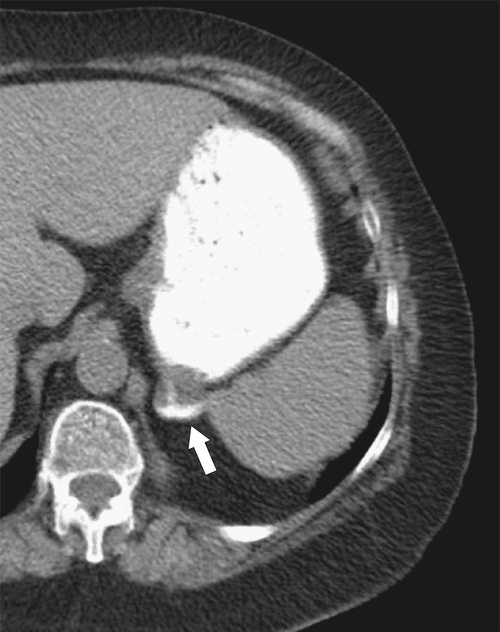
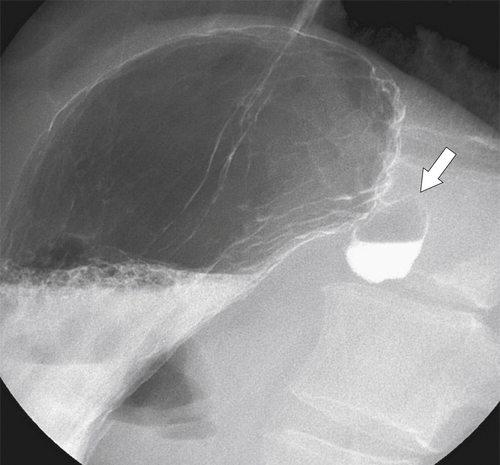
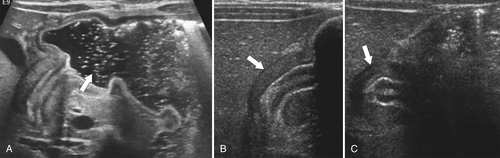
Hiatal Hernias
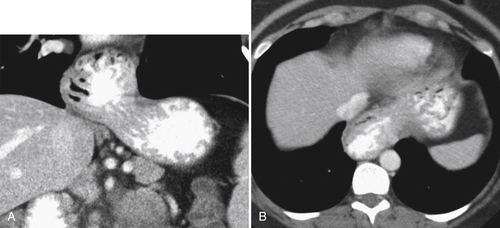
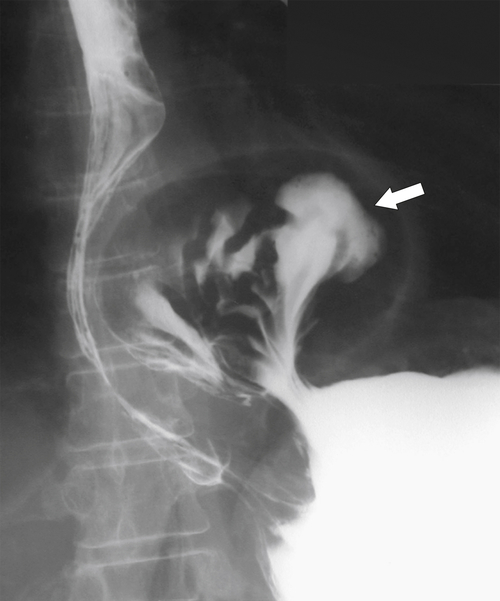
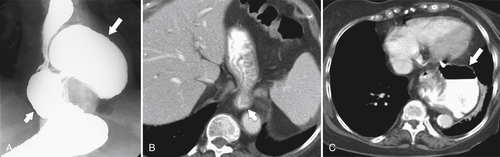
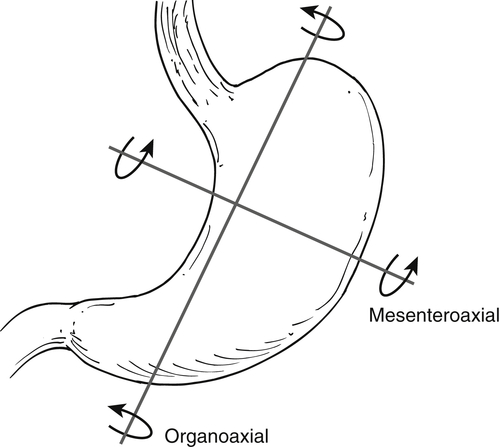
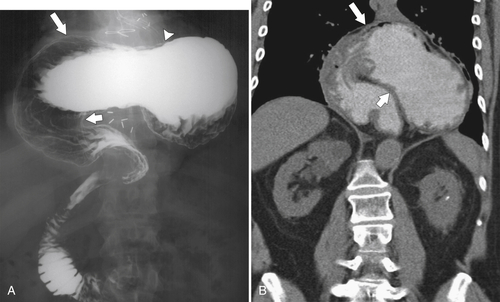
Gastric Volvulus
Diffuse Gastric Mucosal Thickening
Table 2-1
Diffuse Gastric Mucosal Thickening
| Benign | Malignant |
| Gastritis (see Table 2-2) Pseudolymphoma Varices |
Carcinoma Lymphoma Metastases |
Gastritis
Table 2-2
Causes of Gastritis
| Type | Features | Location |
| H. pylori/peptic | Thick lobulated folds, increased areae gastricae | Antrum and body |
| Drugs | Often causes erosions when acute | Antrum, body, fundus |
| Caustic | Thickened folds, ulcers with narrowing when healed | Antrum and body |
| Radiation | Thickened folds and ulcers and antral narrowing when healed | Antrum, body, fundus |
| Eosinophilic | Thickened and nodular folds and antral narrowing | Body and antrum |
| Inflammatory | Aphthous ulcers, thickened folds with larger ulcers in Crohn disease, sarcoid, Behçet syndrome, amyloid | Antrum and body |
| Infectious | Tuberculosis; syphilis can cause linitis plastica | Antrum and body |
| Emphysematous | Thickened folds and gas in wall | Antrum and body |
| Pancreatitis | Thickened folds along greater curvature; gastric narrowing from fluid collections | Body and antrum |
| Hypertrophic | Large lobulated folds | Body and fundus |
| Atrophic | Featureless mucosa with decreased folds and narrowed stomach | Antrum, body, fundus |
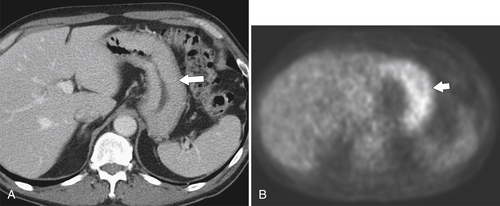
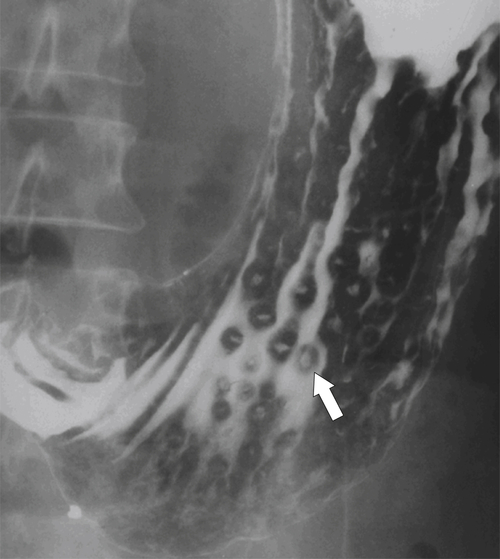
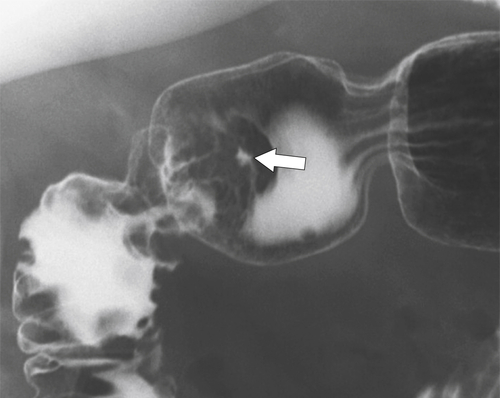
Erosive Gastritis
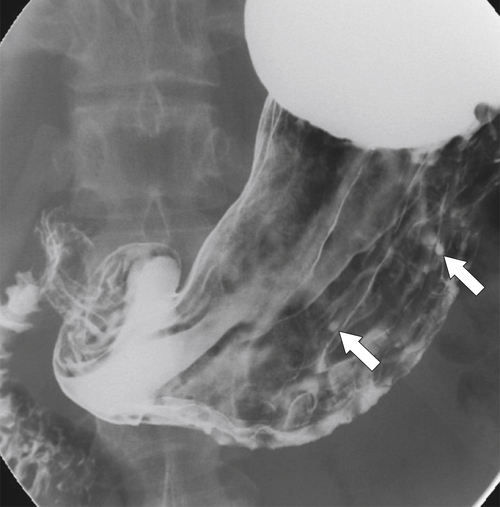
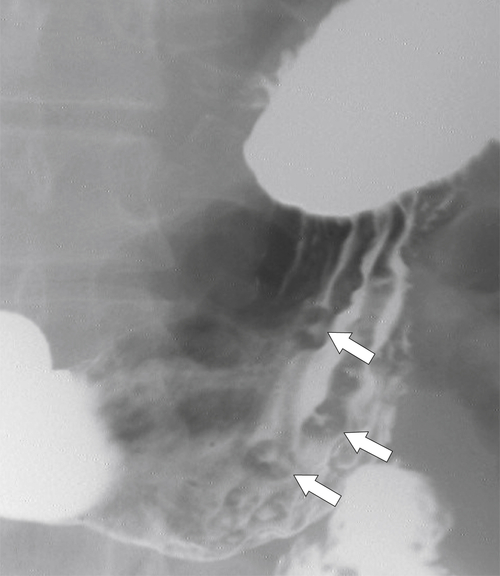
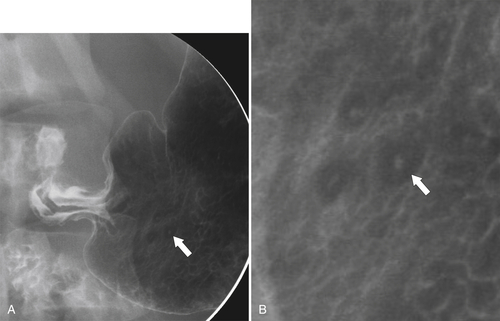
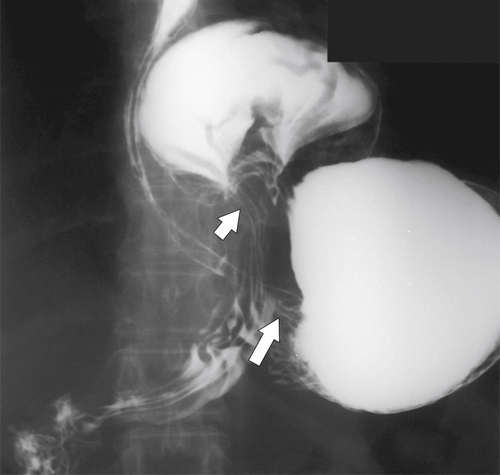
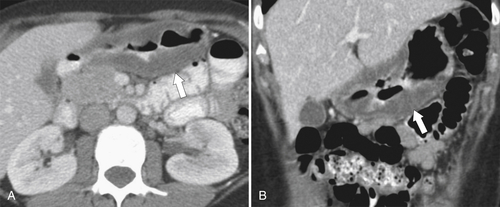
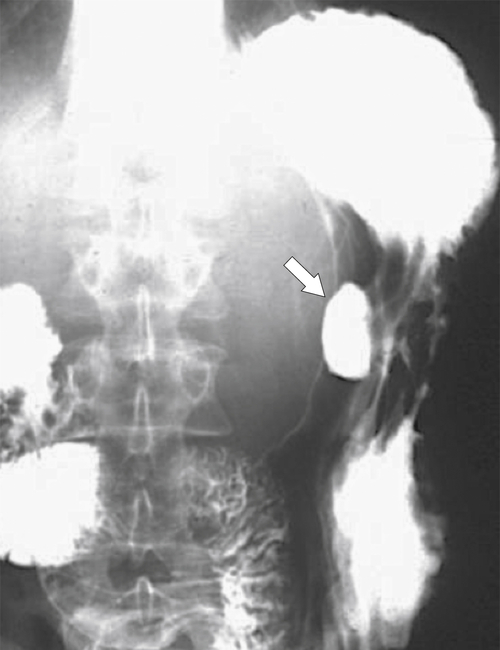
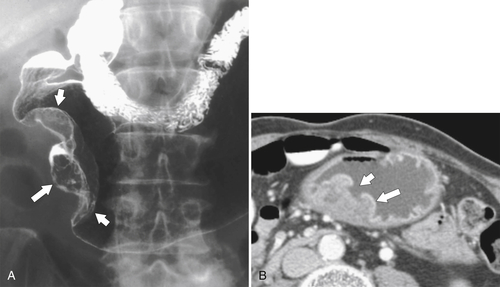
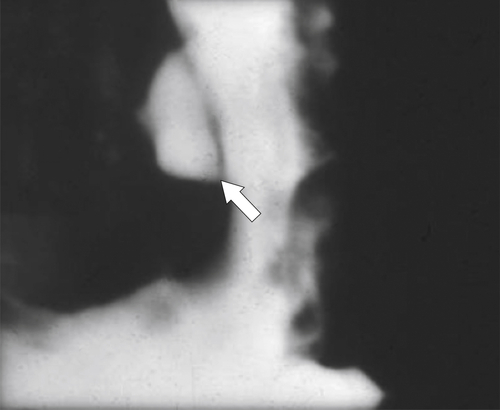
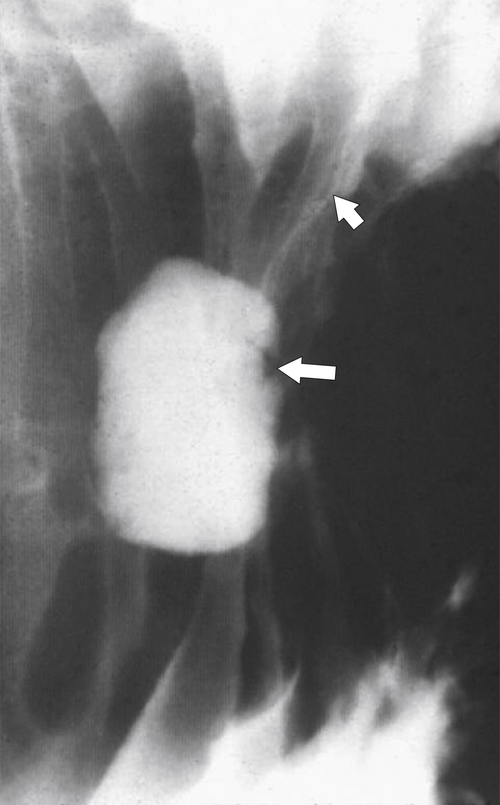
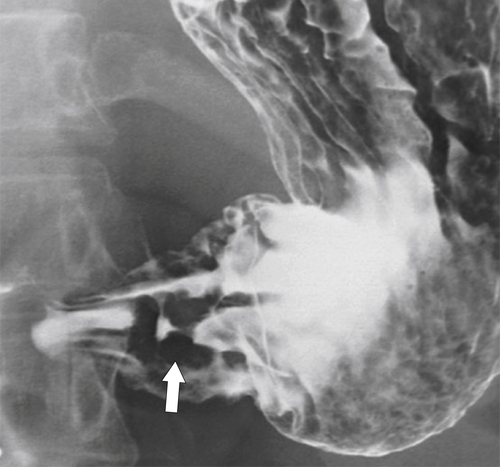
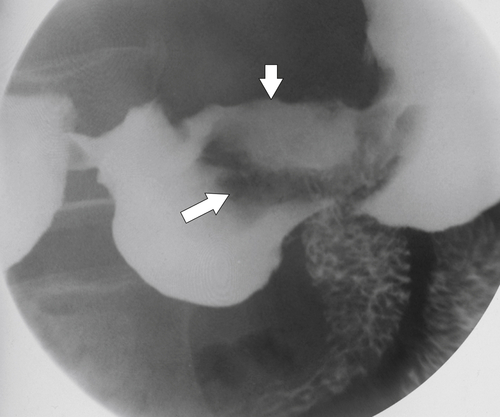
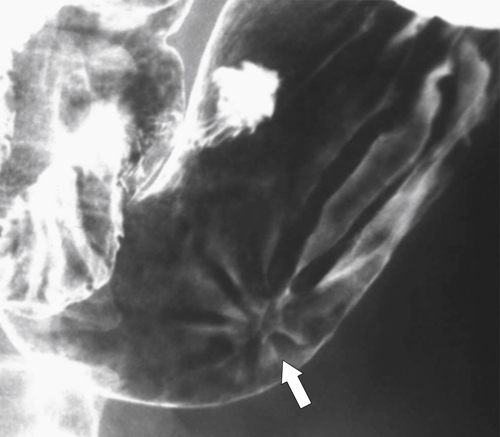
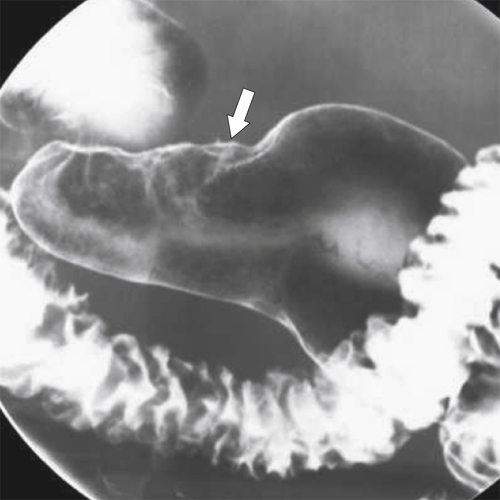
Peptic Gastritis and Ulcer Disease
Table 2-3
Imaging Differentiation of Benign and Malignant Gastric Ulcer Disease
| Features | Benign | Malignant |
| Age | All adult ages | Elderly |
| Sex | Equal between males and females | Males more than females |
| Location | 90% antrum (75% lesser curve) | Antrum, but can occur elsewhere |
| Ulcer position | Central | Eccentric |
| Ulcer shape | Round | Irregular |
| Ulcer collar | Uniform (Hampton line) | Irregular |
| Fold shape | Uniform | Irregular and distorted |
| Fold convergence | To edge of crater | Does not reach ulcer margins |
| Projections beyond gastric wall | Yes | No |
| Multiple | Up to 30% | Uncommon |
| Associated duodenal ulcer | Frequent | Uncommon |
| Carman sign | No | Yes |
| Crescent sign | Yes | No |
| Response to peptic ulcer treatment | Yes | No |
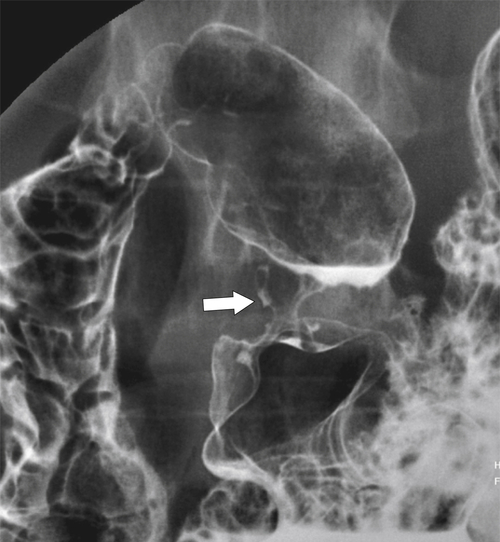
Drug-Induced Gastritis
Corrosive Gastritis
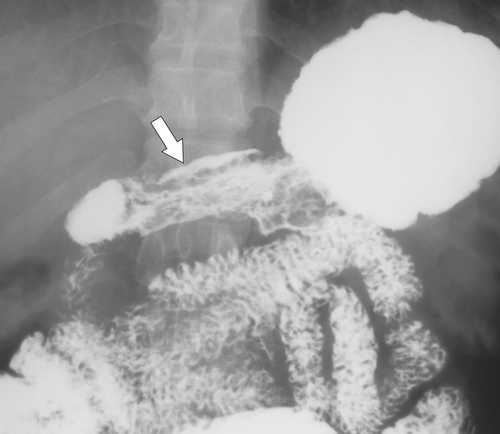
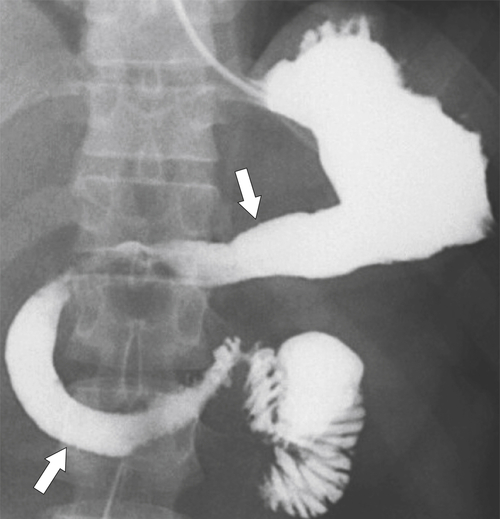
Radiation Gastritis
Eosinophilic Gastroenteritis
Crohn Disease (see Chapters 4 and 5)
Sarcoidosis
Infectious Gastritis
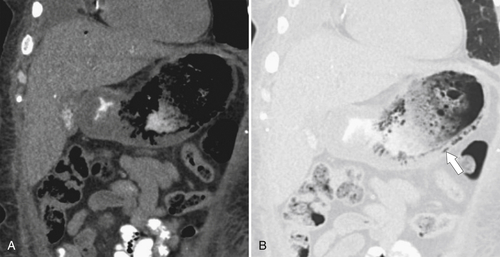
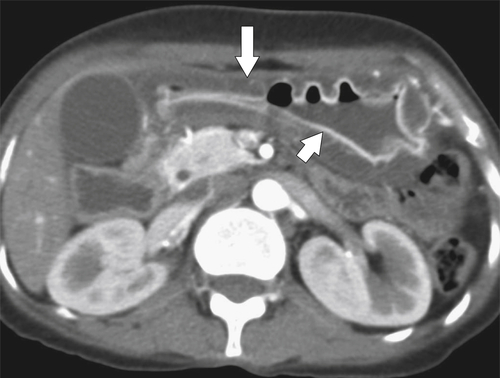
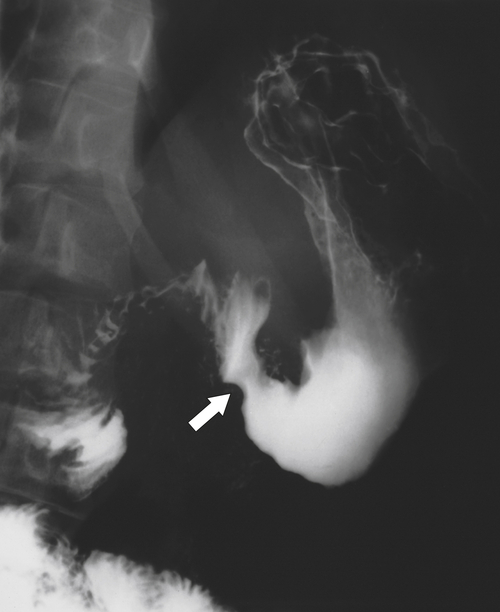
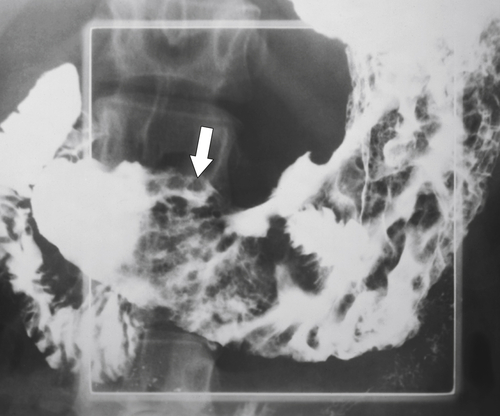
Amyloidosis
Pancreatitis
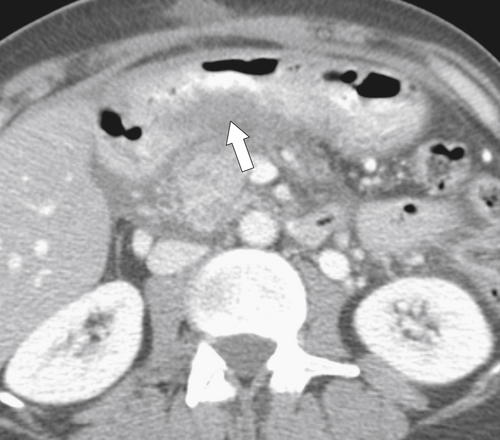
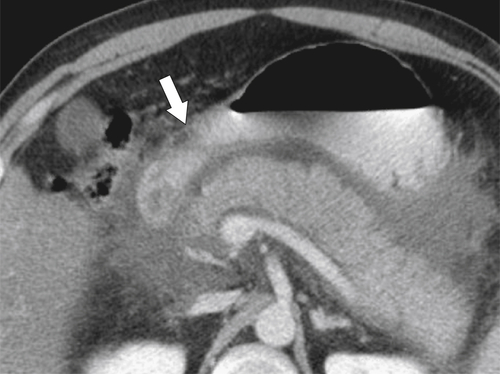
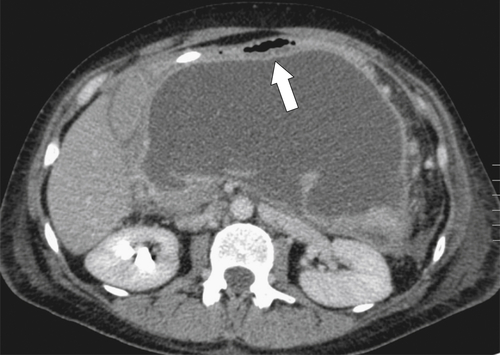
Ménétrier Disease (Hypertrophic Gastritis)
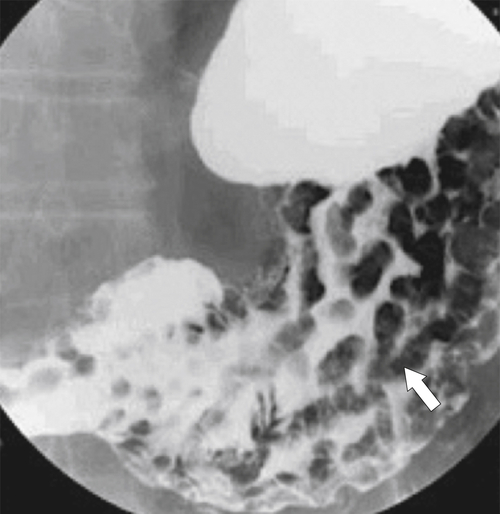
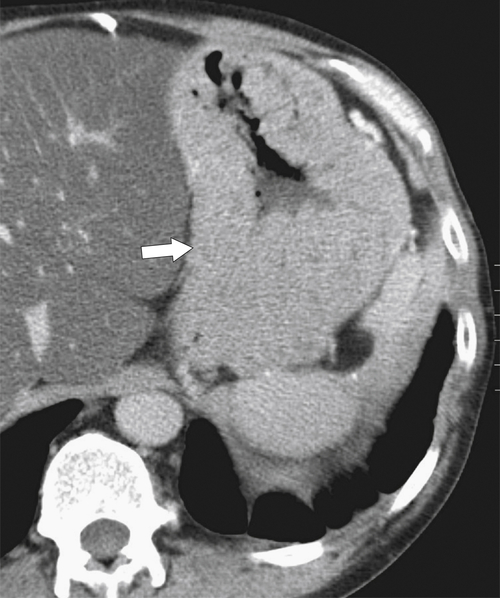
Pseudolymphoma
Zollinger-Ellison Syndrome
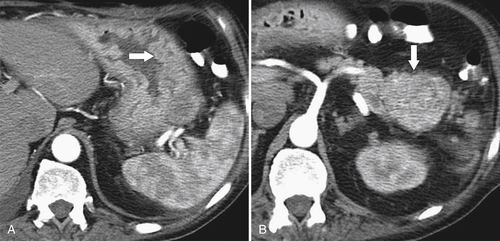
Atrophic Gastritis
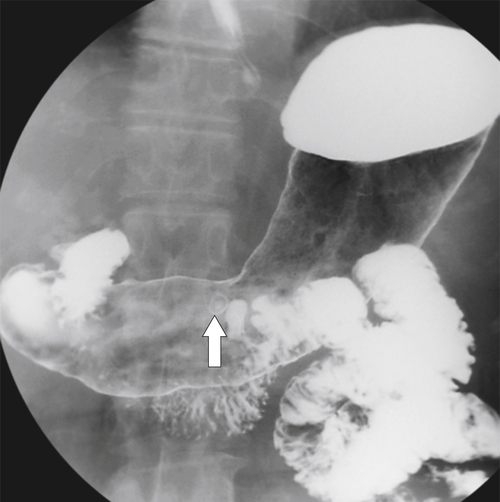
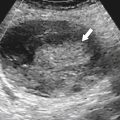
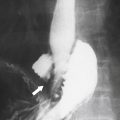
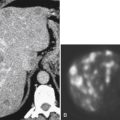
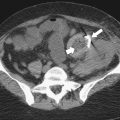
Solitary Gastric Masses
Gastric Polyps
Hyperplastic Polyps
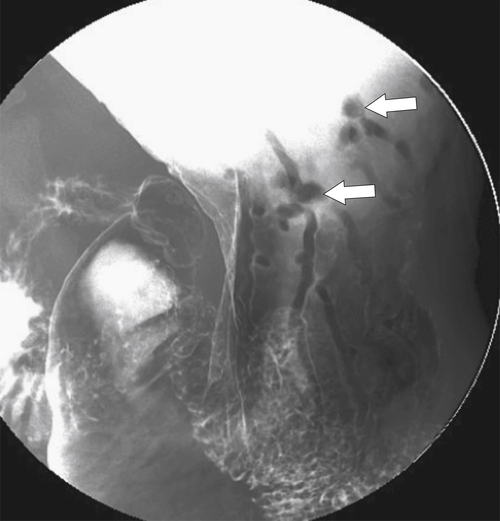
Adenomatous Polyps
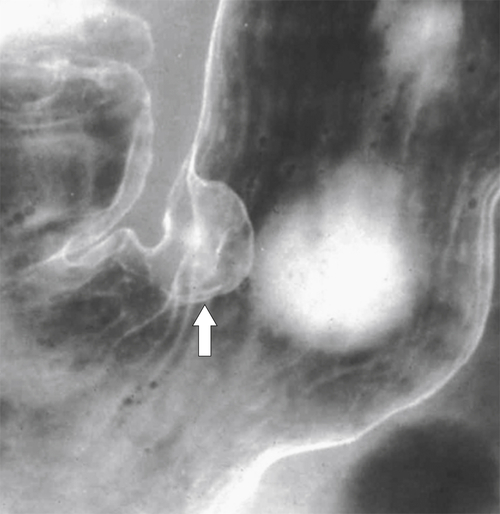
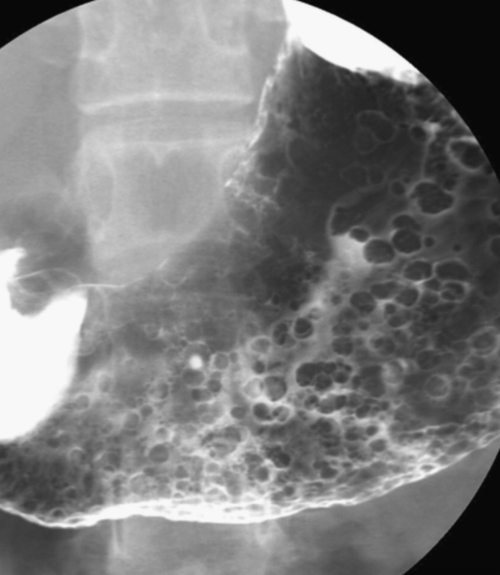
Hamartomatous Polyps
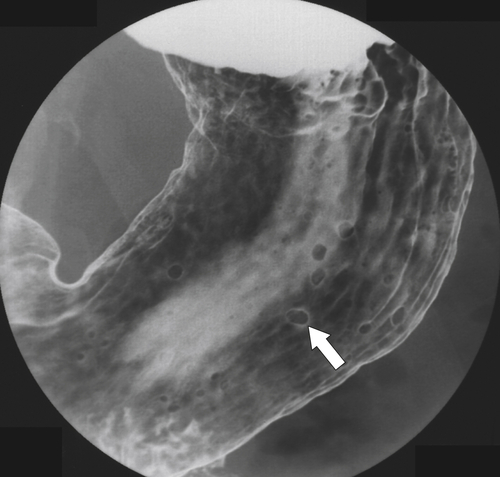
Inflammatory Fibroid Polyps
Benign Intramural Gastric Tumors
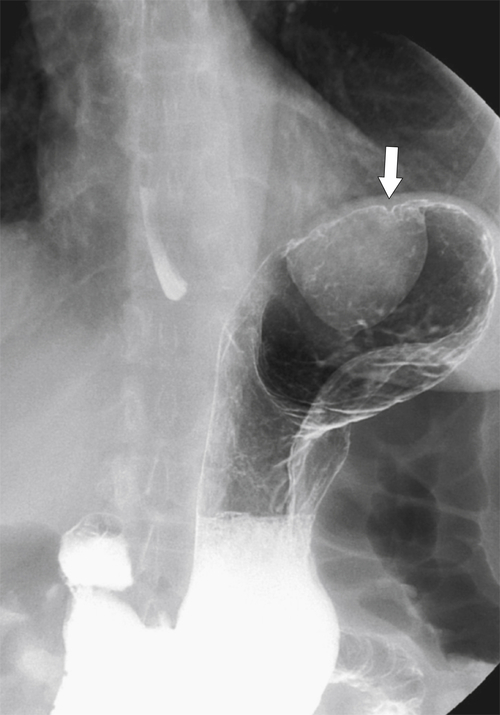
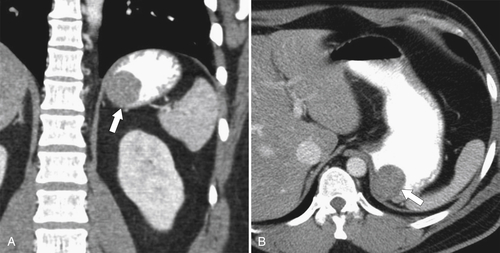
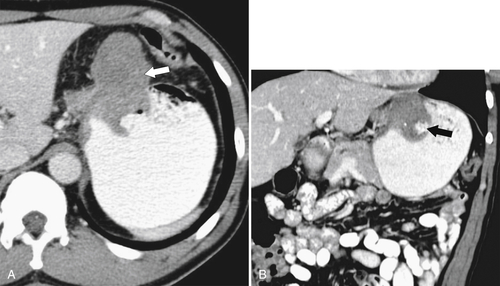
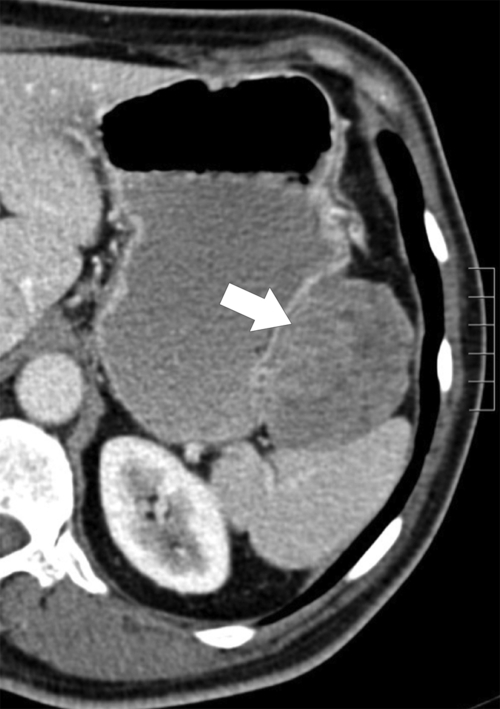
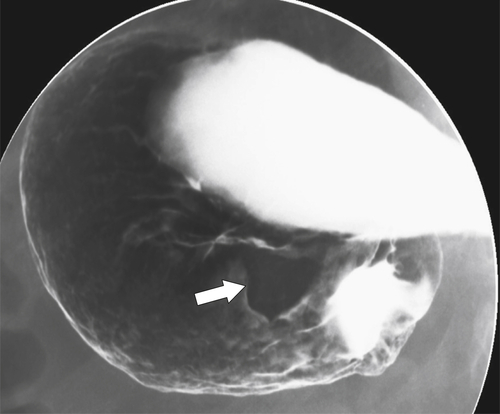
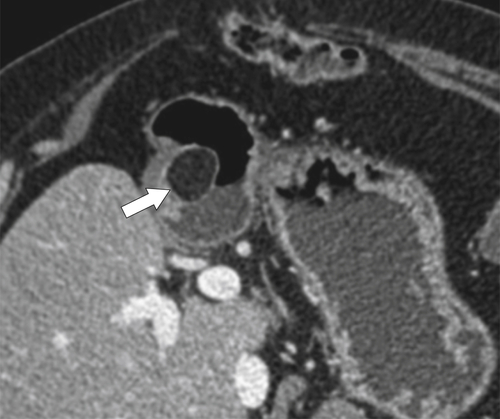
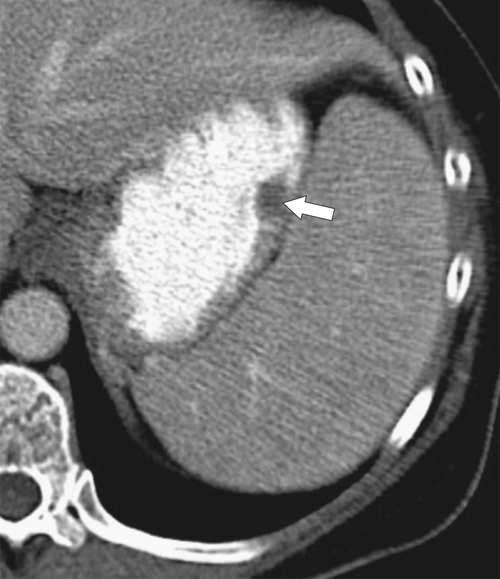
Duplication Cysts
Ectopic Pancreas
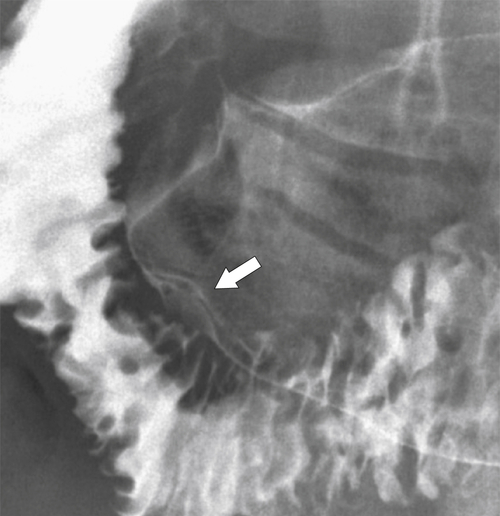
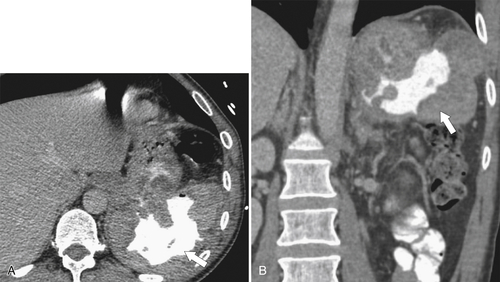
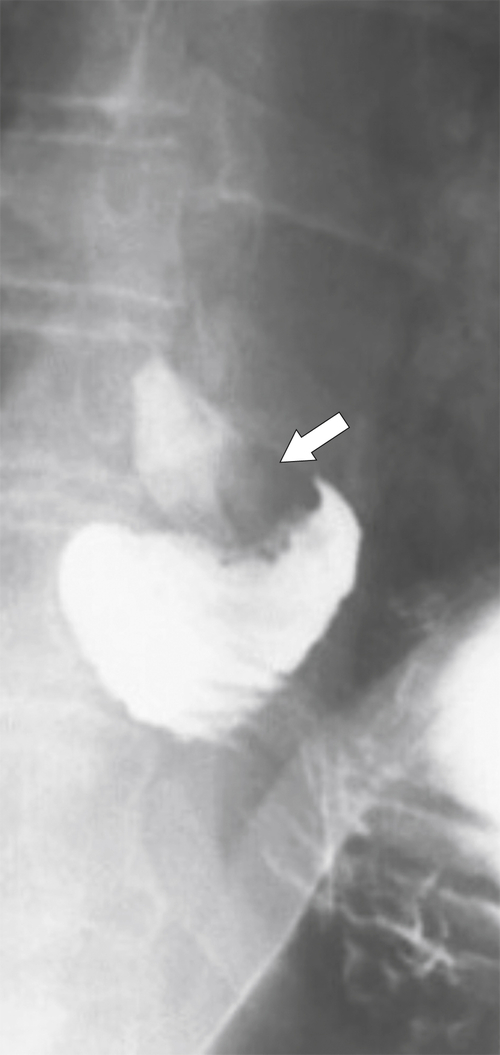
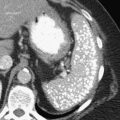
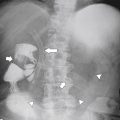
Carcinoid
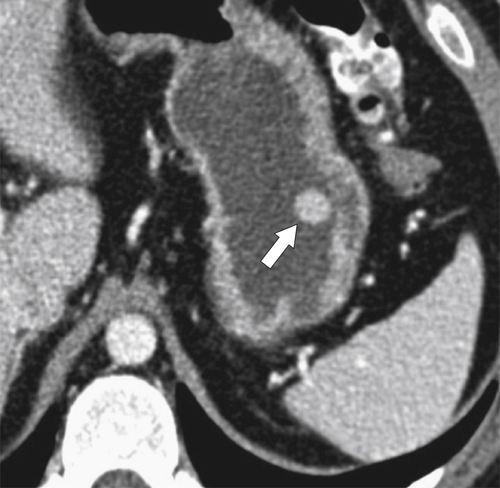
Gastric Varices
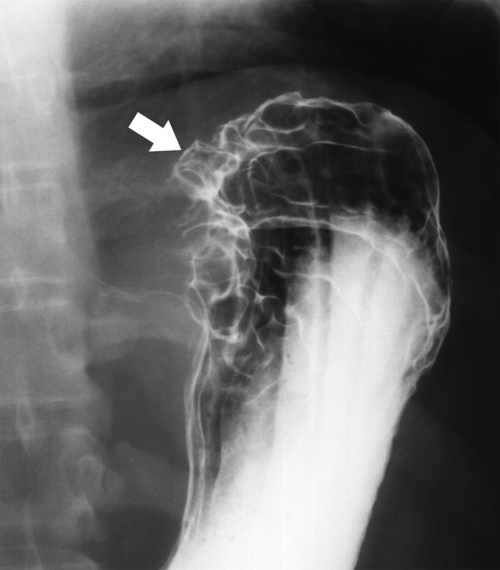
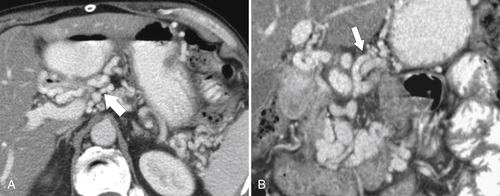
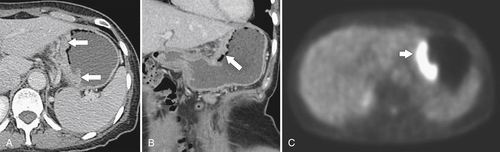
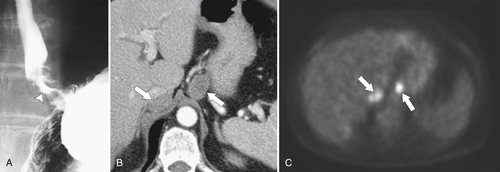
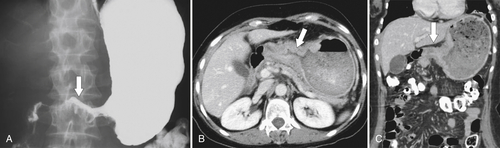
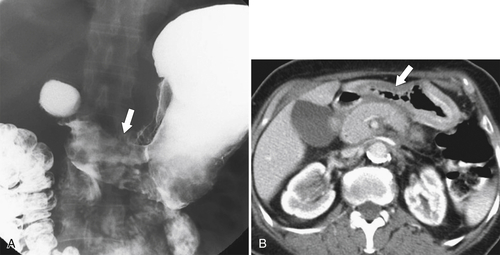
Gastric Carcinoma
Table 2-4
Staging of Gastric Carcinoma
| Stage | Findings |
| 0 | Carcinoma in situ; limited to mucosa |
| 1A | Transmucosal (5-year survival 85%) |
| 1B | Transmucosal and up to 6 regional lymph nodes involved or muscularis invaded |
| II | Transmucosal with 7-15 regional lymph nodes involved or muscularis involvement with 6 regional lymph nodes or serosal involvement without regional lymph nodes |
| IIIA | Muscularis involvement with 7-15 adjacent nodes; serosal invasion with up to 6 local nodes (5-year survival 50%); local organ invasion but no nodes |
| IIIB | Serosal involvement with 7-15 regional nodes |
| IV | Adjacent organs and at least 1 regional lymph node; more than 15 regional nodes; distant metastases |
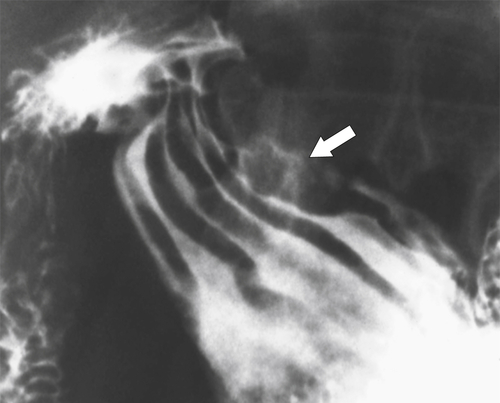
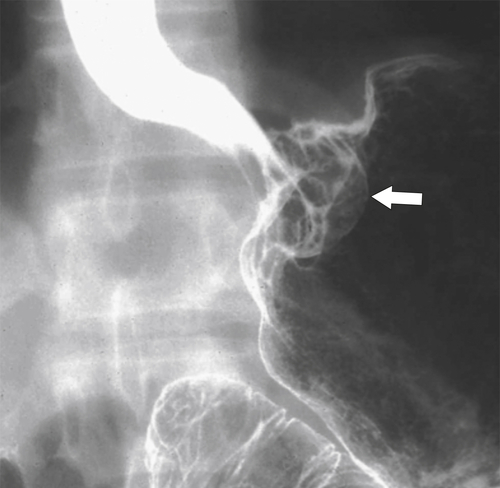
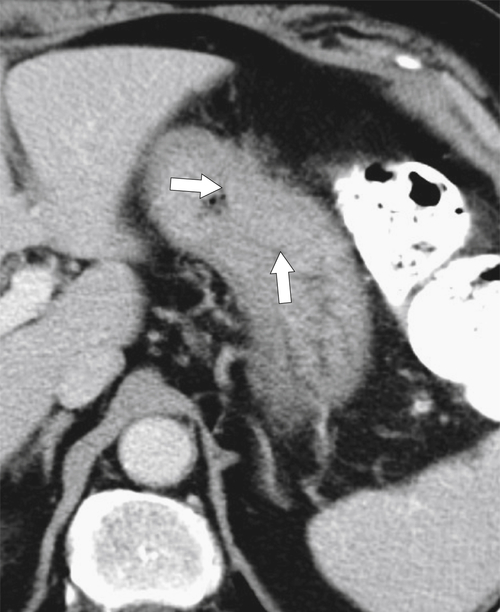
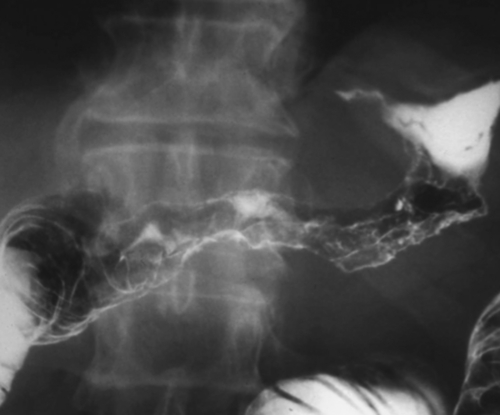
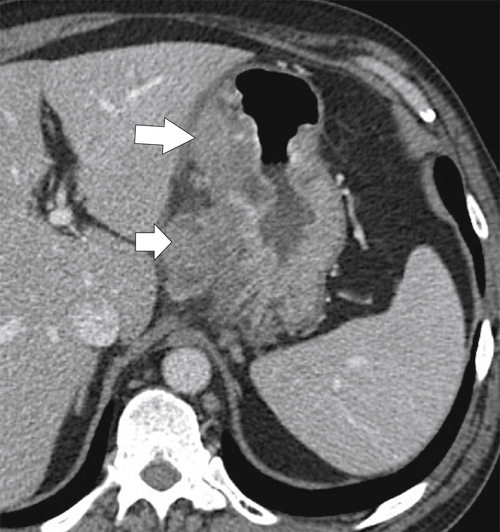
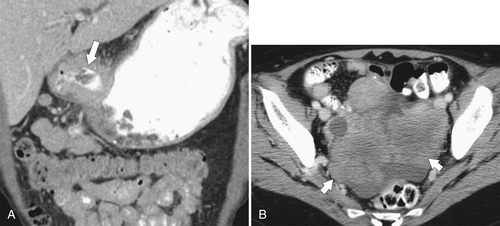
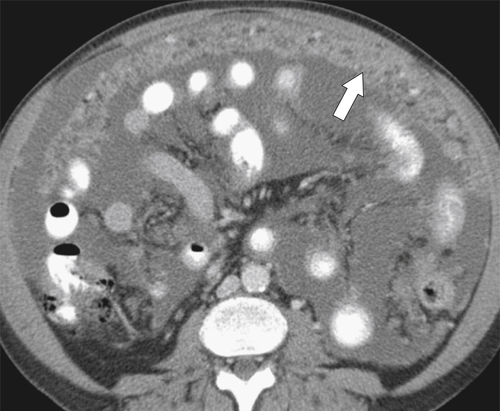
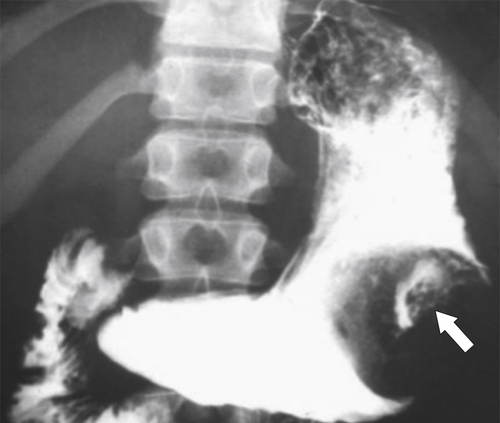
Malignant Gastrointestinal Stromal Tumors
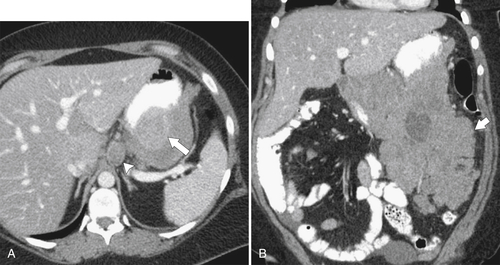
Lymphoma
Table 2-5
Lymphomas Usually Associated with Gastrointestinal Malignancy
| B-cell Lymphoma | Grade | Features |
| Diffuse large B-cell | High grade | Low-grade MALT that has transformed diffuse large B-cell; most common type—anywhere along GI tract |
| MALT-type | Low grade | H. pylori gastritis causative; usually in the stomach, less commonly in the small bowel; low grade in indolent type |
| Mantle cell | Higher grade | Any area of GI tract; typically polypoid lesions; poorer prognosis |
| AIDS-related lymphoma | High grade | Second most common malignancy in AIDS patients after Kaposi sarcoma; mainly in the stomach and small bowel; EBV related; aggressive |
| PTLD | Lower grade | EBV related; GI tract is the most common site |
| Burkitt lymphoma | High grade | EBV related; 50% curable |
| T-cell lymphomas | High grade | Subtypes peripheral, anaplastic large cell, angioimmunoblastic and cutaneous |
| EATL | High grade | Associated with celiac disease; jejunum is mostly involved |
| Mediterranean | High grade | Spectrum of alpha heavy chain disease and immunoproliferative small intestinal disease |
∗Denis Burkitt (1911-1993), Irish physician.
AIDS, Acquired immune deficiency syndrome; EATL, enteropathy-associated T-cell lymphoma; EBV, Epstein-Barr virus; GI, gastrointestinal; MALT, mucosa-associated lymphoid tissue; PTLD, posttransplant lymphoproliferative disorder.
Table 2-6
Ann Arbor Staging of Lymphoma
| Stage I | Single lymph node region (I); involvement of single extralymphatic organ/site (IA) |
| Stage II | Two or more lymph node sites on one side of diaphragm (II); involvement of single organ (IIE) |
| Stage III | Lymph nodes both sides of diaphragm (III); involvement of extralymphatic organ (IIIE); splenic disease (IIIS); both spleen and extralymphatic organ (IIISE) |
| Stage IV | Diffuse or disseminated involvement of one or more sites; extralymphatic organs |
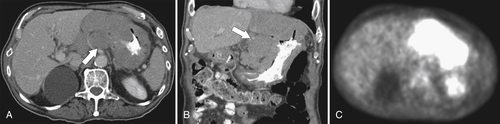
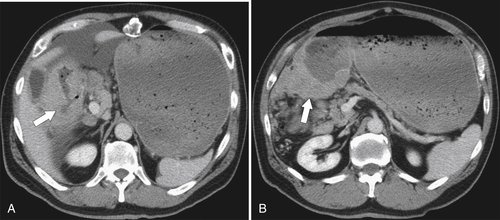
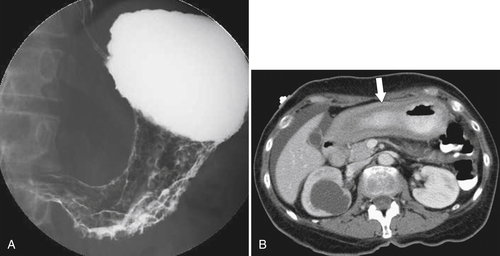
Burkitt Lymphoma
Metastases
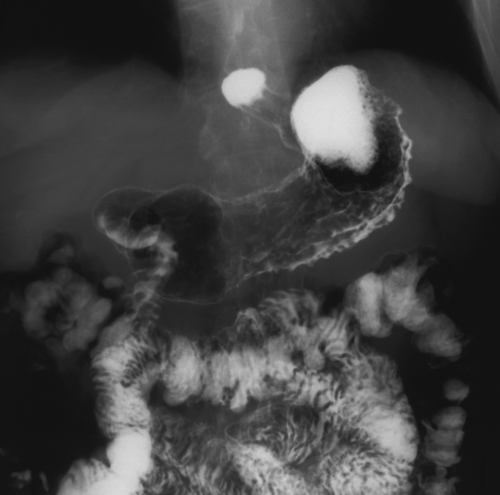
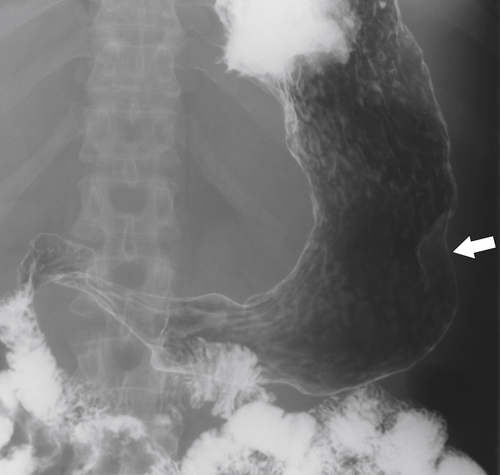
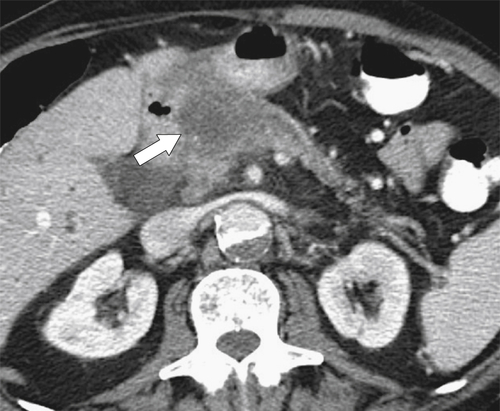
Kaposi Sarcoma
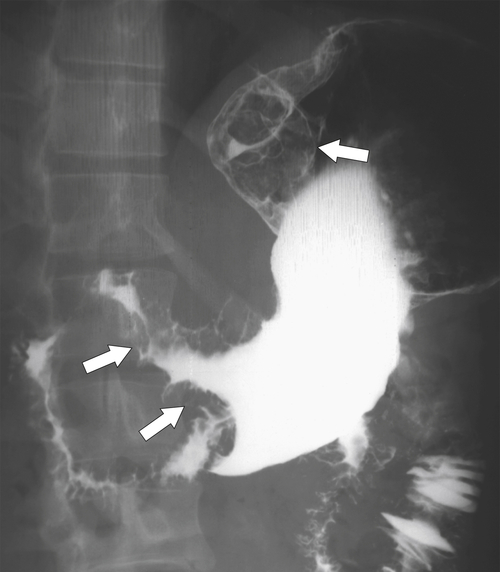
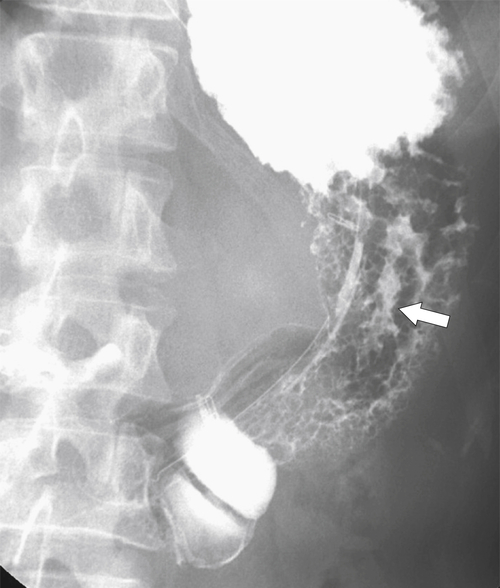
Linitis Plastica Stomach
Table 2-7
Causes of Linitis Plastica (Leather-Bottle) Stomach
| Malignant | Benign |
| Gastric carcinoma Lymphoma Metastases (breast, lung) |
Eosinophilic gastroenteritis Crohn disease Tuberculosis, syphilis Caustic ingestion Sarcoidosis Zollinger-Ellison syndrome (multiple ulcers) |
Gastric Outlet Obstruction
Table 2-8
Causes of Gastric Outlet Obstruction
| Congenital | Pyloric stenosis |
| Mechanical | Bezoar Diaphragmatic hernia Volvulus |
| Inflammatory | Peptic ulcer disease Pancreatitis Crohn disease |
| Infection | Tuberculosis Syphilis |
| Corrosives | Linitis plastica |
| Malignancy | Carcinoma Lymphoma |
| Radiation therapy | Radiation stricture |
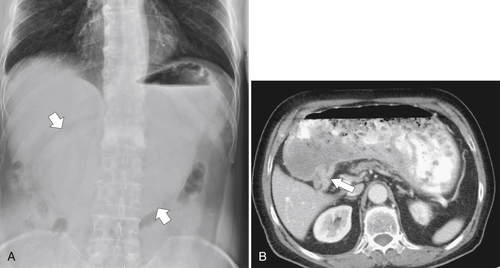
Gastric Bezoar
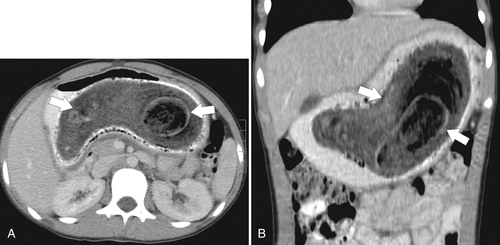
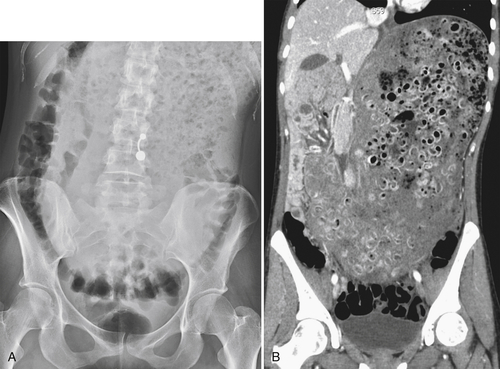
Functional Obstruction
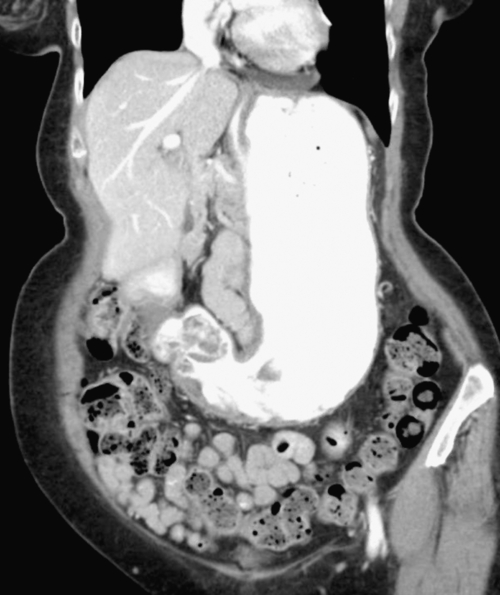
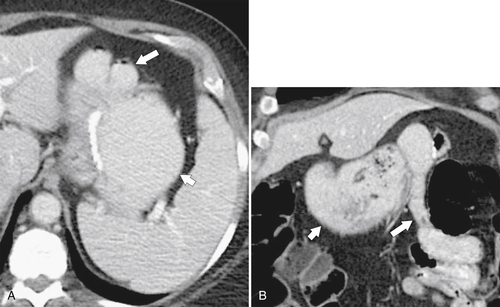
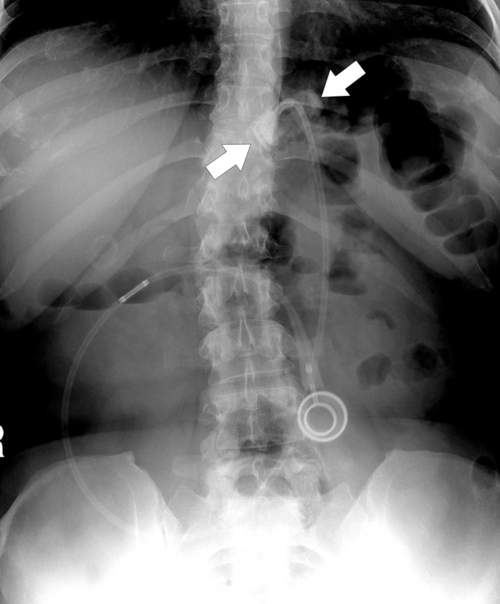
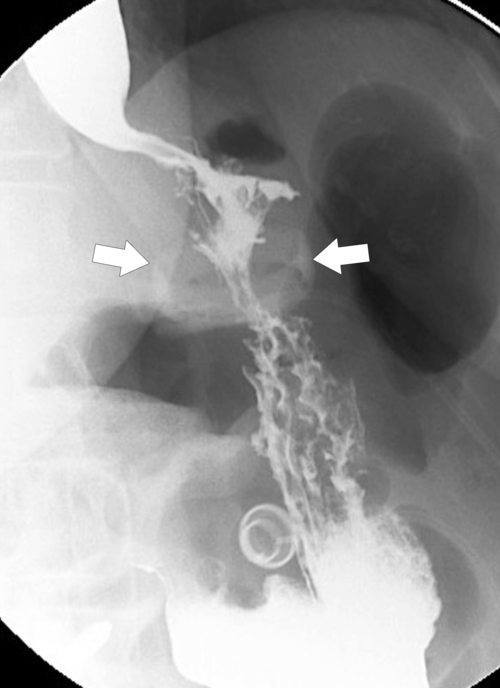
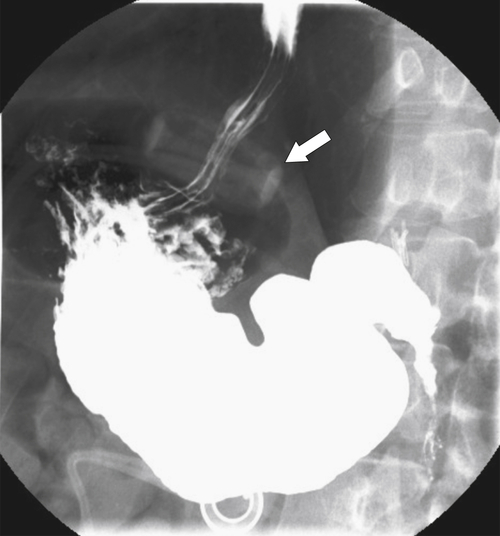
Postsurgical Stomach
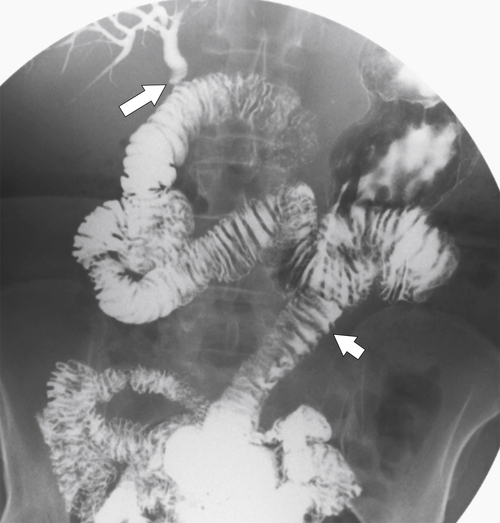
Complications of Gastric Surgical Procedures
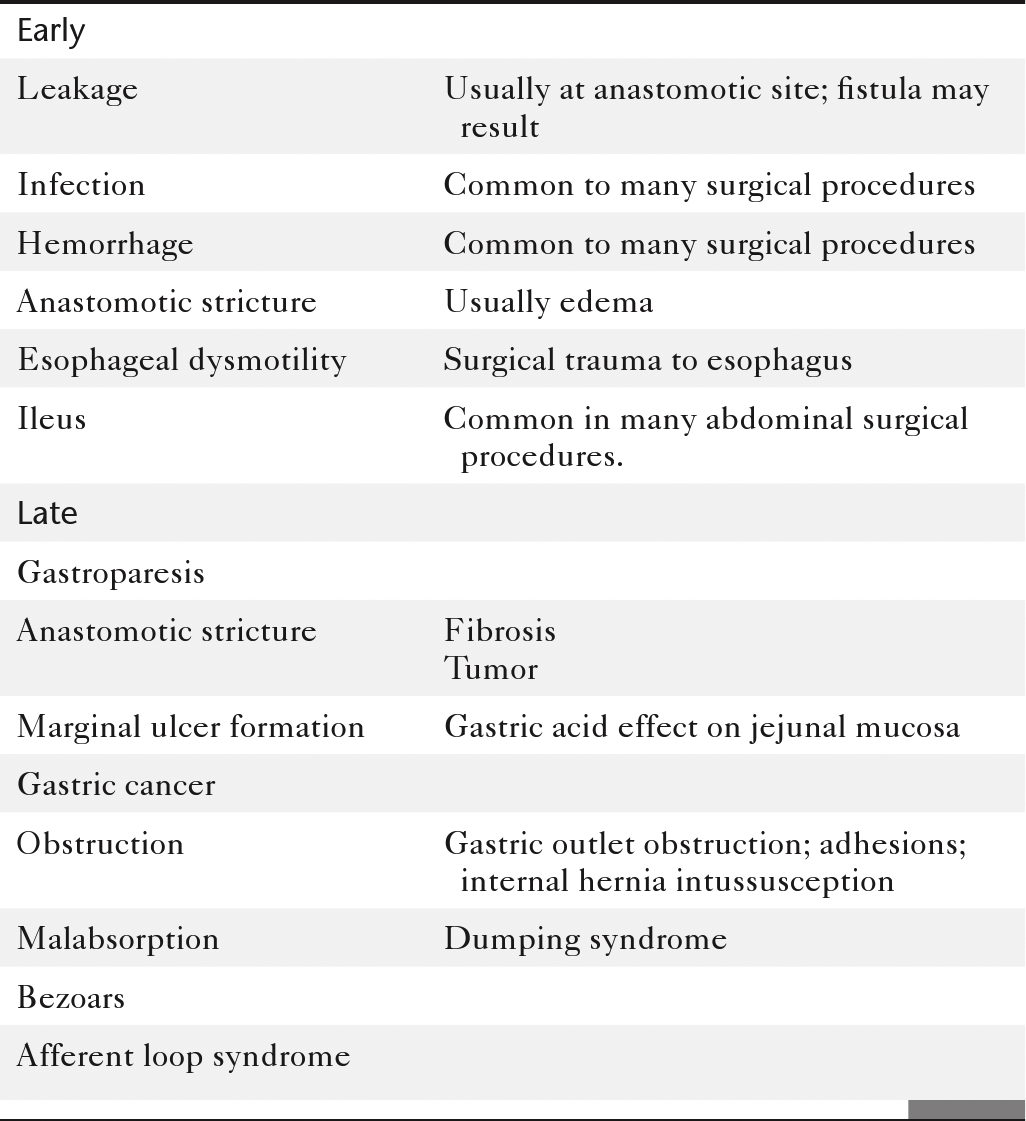
Table 2-9
Complications of Gastric Surgical Procedures
Leakage
Gastroparesis
Marginal Ulcers
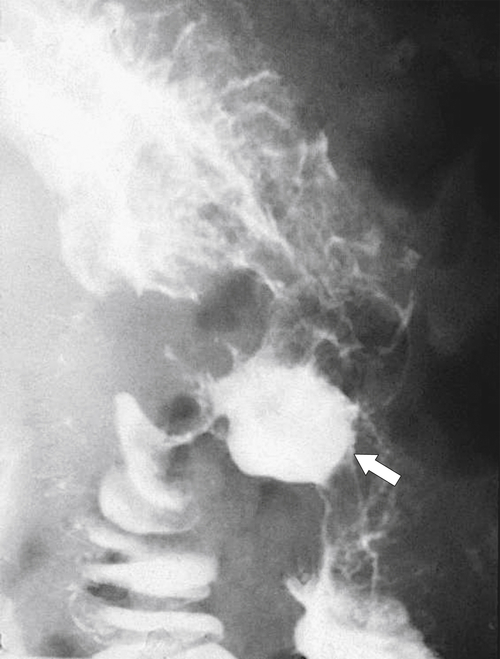
Obstruction
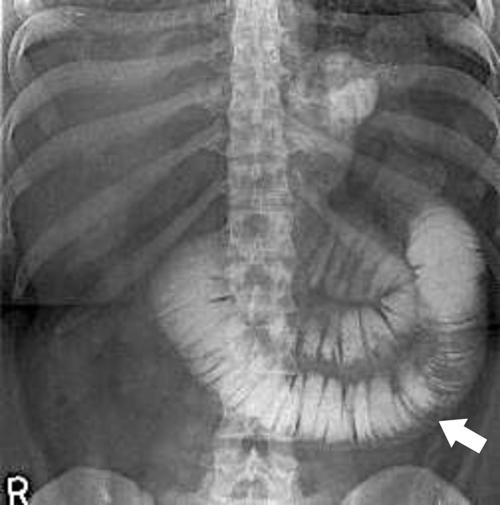
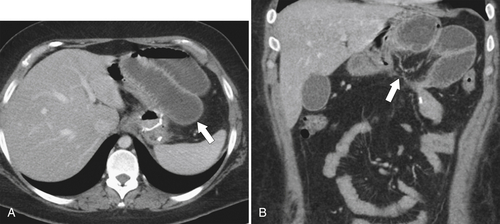
Dumping Syndrome
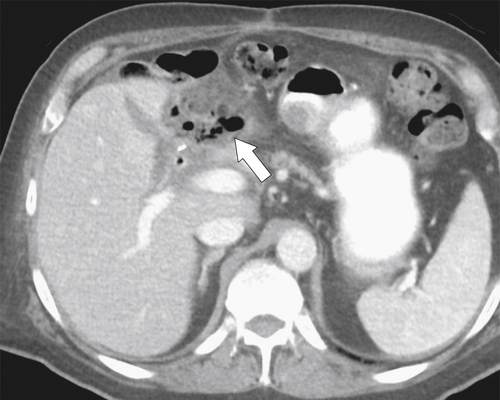
Afferent Loop Syndrome
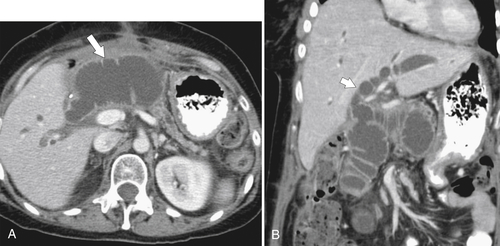
Gastric Bypass (Bariatric) Surgery
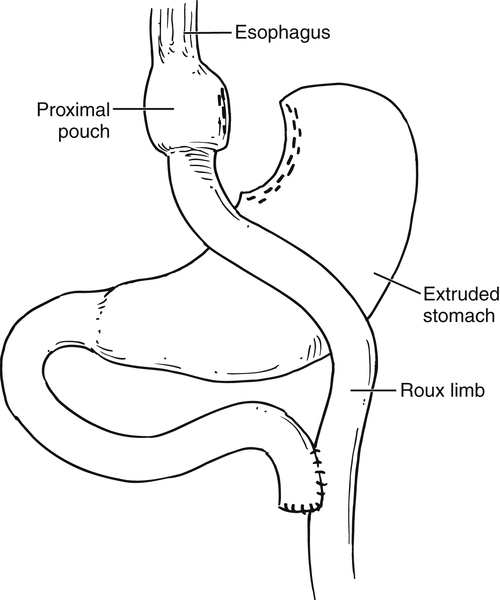
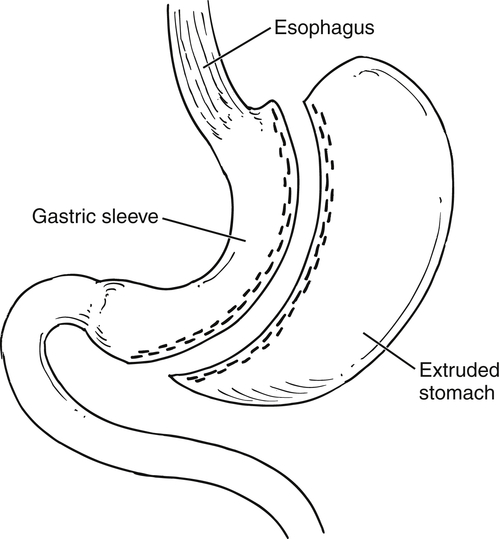
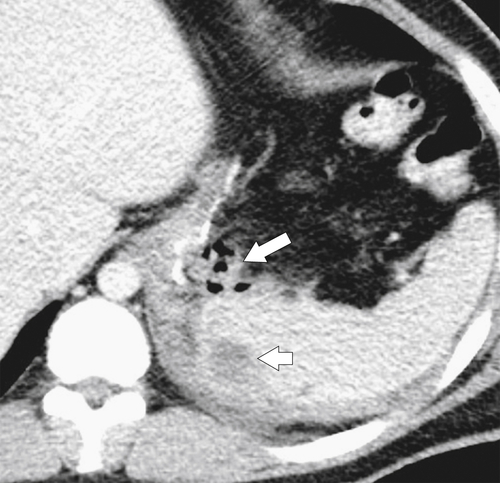
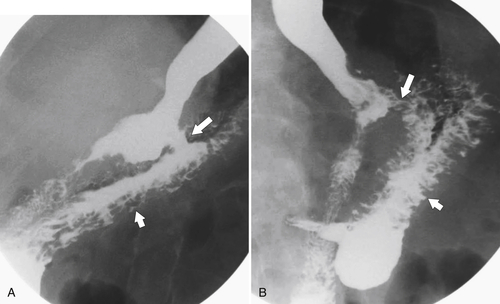
Nissen Fundoplication
∗ Friedrich Trendelenburg (1844-1924), German surgeon.
∗ Hans Christian Joachim Gram (1850-1938), Danish bacteriologist.
† Robert M. Zollinger (1903-1992), American surgeon; Edwin H. Ellison (1918-1970), American surgeon.
‡ Thomas Blizard Curling (1811-1888), British surgeon.
∗ Aubrey O. Hampton (1900-1955), American radiologist.
† Russell Daniel Carman (1875-1926), Canadian-born American radiologist.
∗ Pierre E. Ménétrier (1859-1935), French pathologist.
∗ Robert M. Zollinger (1903-1992), American surgeon; Edwin H. Ellison (1918-1970), American surgeon.
∗ Eldon J. Gardner (1909-1989), American geneticist.
† Johannes Peutz (1864-1940), Dutch physician; Harold Jeghers (1904-1990), American physician.
‡ Leonard W. Cronkhite, Jr., American pediatrician; Wilma J. Canada, American radiologist.
∗ Cowden’s disease. Named after the first described patient.
∗ Santiago Ramón y Cajal (1852-1934), Spanish pathologist.
† J Aidan Carney, American pathologist.
∗ William B. Bean (1909-1989), American physician.
∗ Friedrich Ernst Krukenberg (1871-1946), German physician.
† Thomas Hodgkin (1798-1866), British physician and pathologist.
∗ Michael A. Epstein (1921-2006), British pathologist and virologist; Yvonne Barr (1932- ), British virologist.
∗ Moritz Kaposi (1837-1902), Hungarian physician and dermatologist.
∗ Theodor Billroth (1829-1894), German surgeon.
† César Roux (1857-1934), Swiss surgeon.
∗ Rudolph Nissen (1896-1981), Swiss surgeon.