Chapter 72 Stiffness
Prevention and Treatment
Anterior cruciate ligament (ACL) reconstruction has evolved into a highly successful procedure, with recent studies reporting good outcomes in more than 90% of the patients.1–3 Although the patellar tendon continues to be the most popular type of graft in North America, the quadruple hamstrings graft is emerging as the “other gold standard.” Multiple comparison studies document no difference in the outcomes between the two types of graft.4–6 Perhaps the two main reasons for the equal success rates are advances in the fixation methods of tendon grafts as well as an increase in our understanding of the biology of healing of ACL grafts.
However, complications following ACL reconstruction do occur, and motion loss is one of the most common. The reported incidence is between 2% and 11%,7,8 and its management can be quite frustrating for both the patient and the surgeon. In this chapter we will discuss factors that are associated with development of arthrofibrosis after ACL reconstruction, propose strategies to avoid this complication, and present treatment options.
Etiology
Genetic Predisposition
The tendency of certain patients to develop excessive scarring following trauma or surgery is well known. A history of arthrofibrosis from previous surgery or trauma should alert the surgeon. The exact reason behind this excessive connective tissue proliferation is not known. Several mechanisms have been proposed. Two cytokines, the platelet-derived growth factor-ß (PDGF-ß) and the transforming growth factor-ß (TGF-ß), have a central role in the healing process. Over-expression of TGF-ß has been associated with unresolved inflammation and fibrotic events.9 In animal models, neutralization of its isoforms (TGF-ß-1 and TGF-ß-2) has reduced scarring.10 Interestingly, exogenous addition of the isoform TGF-ß-3 achieved the same effect. More recently, a possible association between arthrofibrosis and certain human leukocyte antigen (HLA) types has been suggested. Skutek et al11 performed HLA typing in a pool of patients who developed primary arthrofibrosis following ACL reconstruction. Patients with secondary reasons for arthrofibrosis, such as prolonged immobilization, infection, or other surgical complications, were excluded. In their patient group, the phenotype HLA-Cw*08 was detected significantly more often compared with the control group. Additionally, in the same group of patients the phenotypes HLA-Cw*07 and HLA-DQB1 were detected significantly less frequently compared with the control group. The importance of the fat pad in the fibrotic process following surgical trauma to the knee has long been recognized.12 Adipose tissue is capable of releasing cytokines in an endocrine, autocrine, and paracrine manner.13 Ushiyama et al14 demonstrated that the infrapatellar fat pad produces a variety of growth factors and pro-inflammatory cytokines such as basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), tumor necrosis factor alpha (TNF-α) and interleukin (IL)-6, much like an endocrine organ. Additionally, Murakami et al15 found elevated concentrations of PDGF-ß and TGF-ß in the fat pad after ACL reconstruction. From this research, it is evident that the knee fat pad is capable of mounting a response much like the articular synovium and enhances the inflammatory reaction to the surgical insult. Preservation and minimal disturbance of this structure during ACL reconstruction may minimize excessive scarring and motion loss.
Surgical Factors
The timing of surgery following acute ACL tear and its association with motion loss have been the subject of many studies and an issue of controversy. Although some authors believe that early reconstruction (i.e., within 2 weeks from the injury) does not affect the ultimate knee range of motion (ROM) that the patient achieves,16–18 it seems that the majority of surgeons favor a delay varying from 1 to 3 weeks to allow resolution of the acute inflammation and restoration of ROM.7,19–21 Furthermore, even in studies where no motion complications were documented after early intervention, no advantage in terms of the outcome of the reconstruction was identified. Shelbourne et al22 found that patients who had delayed ACL reconstruction at a mean of 40 days after injury had earlier return of quadriceps strength and were able to progress to sport-specific rehabilitation sooner than patients who had their knee reconstructed early at a mean of 11 days after injury. It must be realized, however, that significant variability exists among patients in the intensity of the observed inflammation following acute ACL tear, and this is at least partially related to the energy of injury. Rather than relying on timetables or strictly followed protocols, we believe that the decision on surgical timing should be based on clinical observation of subsidence of the posttraumatic inflammation, restoration of ROM, and normalization of gait. The patient is advised to follow a classic RICE (rest, ice, compression, and elevation) regimen and is referred to physical therapy (i.e., prehab). This allows the patient to become familiar with the physical therapist and the exercises and equipment that will be used after the surgery, become emotionally prepared, and make other necessary arrangements for the upcoming surgery. These are all factors that contribute to correct patient education and, in our opinion, enhance compliance and chances for a successful outcome. The issue of timing becomes even more important when the ACL tear is combined with other ligament injuries. Associated medial collateral ligament (MCL) injury has been recognized as a combination that is particularly prone to loss of motion. The location of the MCL tear influences the return of motion, and patients with proximal (above the joint line) injury are more likely to experience motion loss postoperatively.23 Low-grade injuries are successfully treated with an initial period of functional bracing to allow healing of the MCL. This waiting period is used to prehab the knee before the ACL reconstruction. In cases of associated severe grade III MCL or multi-ligamentous injury, priority is generally given to early restoration of knee stability. Attention during surgery is given to anatomical repair of the MCL, especially in injuries where the ligament is avulsed from its tibial attachment. Fixation of the superficial MCL near the joint line will prevent the normal posterior displacement of the ligament during knee flexion and will result in postoperative loss of flexion. Generally, in cases of multi-ligament reconstruction, slow return of motion should be anticipated and treated aggressively in the postoperative period.
Graft malpositioning due to nonanatomical tunnel placement either in the tibia or the femur is one of the most common errors in the surgical technique of ACL reconstruction and is believed to be responsible for a high percentage of graft failures. Noyes and Barber-Westin24 reviewed a series of 114 consecutive ACL revisions and found that 30% involved cases of improper graft placement. Errant tunnel placement subjects the graft to excessive strains and, depending on its stiffness, can lead either to loss of motion or plastic deformation and elongation of the graft. The normal ACL has a complex anatomy, which the current, essentially cylindrical grafts are unable to reproduce. In theory, during reconstruction, an attempt is made to place the graft in an isometric position and at the same time avoid impingement of the graft on the surrounding anatomical structures. Hefzy et al25 found that of the two fixation points of the graft, the one that most affects graft isometry is the femoral. They identified a zone near the center of the femoral insertion of the normal ACL that was the most isometric, as defined by a length change of 2 mm or less. The axis of this zone has a nearly vertical orientation with the knee extended. Its width varies from 3 to 5 mm and tapers from proximal to distal. Anterior placement of the femoral tunnel was one of the most common errors during ACL reconstruction. Placing the graft too far anteriorly results in a graft that is lax in extension and tight in flexion. Often the end result is a joint with limited postoperative flexion. Attempts to regain full flexion in such a knee, as during postoperative rehabilitation, will subject the graft to excessive strains and compromise its integrity. Recognizing the problems associated with anterior femoral tunnel placement, some surgeons use the over-the-top position. Attachment of the graft in the over-the-top position essentially reverses the situation and results in a graft that is tight in extension but lax in flexion. Currently most surgeons prefer to place the entrance of the femoral tunnel high in the notch at the 10- or 2–o’clock position, at the proximal end of the zone described by Hefzy et al,25 where this zone is wider. Depending on the graft choice, a 1- to 2-mm back wall is left to allow safe fixation of the graft. We use three methods to identify the center of the femoral tunnel and place the femoral guide pin. This point may be selected using freehand technique, femoral over-the-top offset guides, and (less frequently) an isometer.
In the study of Hefzy et al,25 altering the tibial attachment site had a smaller effect on isometry of the graft. Nevertheless, the tibial attachment site is important if one is to avoid impingement of the graft on the intercondylar roof or the posterior cruciate ligament (PCL). Sapega et al26 noted that even in the normal ACL, only a few fibers are truly isometric (length change of 1 mm or less) over the full ROM of the knee. In their cadaveric study the fibers of the anteromedial bundle of the ACL demonstrated the least deviation from isometry. Many authors27–30 shared the same view and placed the tibial tunnel in the anteromedial footprint of the tibial ACL insertion in an attempt to re-create these fibers. However, anterior placement of the tibial tunnel anterior to an imaginary line and tangential to the intercondylar roof (Blumensaat’s line) with the knee in full extension results in roof impingement of the graft and loss of extension. Howell and Taylor31 found poor results in patients with impinged grafts in terms of extension loss and graft failure. They reported a 100% failure rate in the severely impinged grafts where the entire articular opening of the tibial tunnel was anterior to the slope of the intercondylar roof. The tibial insertion of the native ACL has an eccentric anterior extension32 that cannot be re-created without impingement of the graft in the intercondylar roof. Jackson and Schaefer33 reported a series of patients who presented with loss of extension following ACL reconstruction with patellar tendon autograft. Second-look arthroscopy revealed the presence of a fibrous nodule, reminiscent of a “cyclops,” in front of the ACL graft, which blocked extension. Excision of the nodule resulted in improvement of knee extension in all patients. Marzo et al34 described the same pathology in a group of patients following ACL reconstruction with patellar tendon or hamstring autograft. In both instances it was believed that the fibrous nodule was the result of anterior tibial tunnel placement and impingement of the graft in the intercondylar roof. Romano et al35 studied the effect of tibial tunnel placement on ROM. They found that placement of a portion of the tunnel medial to the medial tibial spine was associated with loss of flexion. Interestingly, in the same study, it was found that anterior placement of the tunnel was associated with loss of both extension and flexion. Lateral placement of the tibial tunnel can cause attrition of the graft on the lateral wall of the intercondylar notch and recurrent synovitis.36 A very posterior positioning of the tunnel in the tibia should be avoided because it is the least isometric and can cause excessive graft tension in extension, thus risking flexion contracture. Another cause of postoperative loss of flexion may be impingement of the graft on the PCL. This is determined by the angle of the tibial tunnel in the coronal plane. A tibial tunnel with an angle greater than 75 degrees with respect to the medial joint line will place the femoral tunnel close to the 12-o’clock position and cause impingement of the ACL graft on the PCL during knee flexion.37 Among the different landmarks that can guide tibial tunnel placement (i.e., medial tibial eminence, the PCL, the “over-the-back” position, the true posterior border of the tibia, and the posterior border of the anterior horn of the lateral meniscus), it appears that the PCL is the most reproducible.38 In an attempt to ameliorate errors in tunnel placement, avoid roof impingement of the graft, and account for individual anatomy, Howell39 introduced the one-step tibial guide that allows the surgeon to customize the sagittal and coronal position of the tibial tunnel to the specific combination of roof angle and extension (or hyperextension) in the reconstructed knee. Other options that may help correct placement of the tunnels and avoidance of roof impingement are the use of impingement rods or intraoperative fluoroscopy. It is our opinion that there is no foolproof method and the surgeon should use a combination of all of these methods to avoid nonanatomical graft placement and postoperative motion loss.
Tensioning of the graft is another area that deserves special attention to prevent motion loss following ACL reconstruction. The optimal force for graft tensioning as well as the angle of knee flexion that such force should be applied are unknown. Clinically, the risk of undertensioning the graft and therefore not correcting the posttraumatic laxity should be balanced with the risk of overconstraining the knee. It has been suggested that the magnitude of tension should be graft specific and that hamstring grafts need higher initial tension.40 However, the surgeon has to keep in mind that an equally tensioned four-strand hamstring tendon graft has higher stiffness than a 10-mm bone–patellar tendon–bone (BPTB) graft.41 As the stiffness of new fixation devices for tendon grafts continues to improve, the risk of capturing the knee by overtensioning the quadruple hamstrings graft is very real. In a prospective clinical randomized study, Heis and Paulos42 compared laxity and flexion results using initial graft tensions of 68N and 88N. At the latest follow-up, the average side-to-side laxity measured 1.7 mm in the 68N group and 2.8 mm in the 88N group. Flexion angles at 4 weeks showed statistically significant difference between the two groups with average flexion of 109 degrees in the 68N and 88 degrees in the 88N group. In terms of overconstraining the knee, the flexion angle at which the tension is applied may have a greater impact than the applied force itself. Bylski-Austrow et al43 noted greater increases in force applied to the graft and greater posterior shifts in tibial position by changing the flexion angle at tensioning from 0 to 30 degrees than by increasing the initial tension from 22N to 44N. From a practical standpoint, in order to avoid motion loss postoperatively, we prefer to tension the graft at 20 to 30 degrees of flexion and neutral rotation with 12 to 15 pounds of tension unless the graft shortens more than 3 mm or more than 10% of its length between the two fixation points when the knee is brought into full extension. If such shortening is observed, tensioning is performed in extension.
Infrapatellar Contracture Syndrome
Infrapatellar contracture syndrome represents an abnormal fibrosclerotic healing response through the anterior retinaculum, patellomeniscal ligaments, and fat pad tissues, which entraps the patella and leads to loss of extension and flexion of the knee and, in advanced stages, to patella baja and patellofemoral arthrosis. The syndrome can develop after knee injury or surgery but more often is seen after ACL reconstruction due to errors in the surgical technique or rehabilitation.12 The syndrome has three stages each, with distinctive characteristics. In stage I (2–8 weeks) the patient presents with periarticular inflammation and edema, immobility of the knee, and quadriceps weakness and lag. Later, in stage II (weeks 6–20), the knee demonstrates limited patellar mobility and an inferior tilt of the patella (shelf sign), and the quadriceps lag “disappears” but the patient ambulates with a bent knee gait. In stage III, generally from the eighth month onward, patellar mobility is somewhat improved but the knee develops patella baja and degenerative changes in the patellofemoral joint. The fat pad appears to have a central role in the pathogenesis of this syndrome; therefore every effort should be made to disturb this structure as little as possible during ACL reconstruction. Early recognition of this process and avoidance of forced motion are extremely important so as not to further propagate inflammation and fibrosis.
Rehabilitation
A well-delineated rehabilitation program is critical to avoid motion complications following ACL reconstruction. It has long been recognized that prolonged immobilization adversely affects the results of the procedure and increases the risk of arthrofibrosis.44 Immobilization of the knee in flexion after surgery has been abandoned, and today the operated knee is braced in extension. This practice has reduced the incidence of postoperative knee flexion contracture. Accelerated rehabilitation programs are in widespread use and have minimized motion complications. However, a common error is a strict adherence to timetables, which at times can be counterproductive and have the opposite from the desired effect. We use a “criteria-based rehabilitation protocol” that respects the individual response to the surgical insult and the ensuing healing process. The patient is advanced through the various phases of the rehabilitative protocol when certain criteria have been met and the knee is physiologically ready. The initial phase of this program focuses on reversal of the physiological imbalance of the knee. The main goals early are to decrease the postoperative swelling and pain (RICE), reverse the muscular inhibition of the quadriceps (electrical stimulation, biofeedback), preserve patellar mobility (glides and tilts), achieve early unassisted ambulation, and restore knee motion with emphasis in extension (quadriceps isometric sets; slow, nonmanual passive range of motion exercises; opposite-leg active assisted knee extension exercises). Once the patient has knee flexion of at least 110 degrees, is able to perform straight leg raises with no quad lag, has full passive knee extension, has minimal swelling and pain, and has good or improving patellar mobility, he or she can be advanced to the next phase(s), where the focus is shifted to strengthening and progressive neuromuscular challenging of the knee. It is important that these therapeutic interventions are nonpainful and do not further inflame the knee. Failure to recognize such responses can lead to patellar entrapment and arthrofibrosis. Close follow-up of the patient in the early postoperative period and good communication with the physical therapist are important for early detection of motion complications, which in the majority of cases can resolve easily with appropriate intervention and avoidance of forceful and painful range of motion exercises.
Other Causes
Another, less common cause of postoperative stiffness is reflex sympathetic dystrophy (RSD) or complex regional pain syndrome. It represents an exaggerated sympathetic response after trauma or surgery. Clinical manifestations include pain out of proportion after injury or surgery, decreased skin temperature and mottling, hypersensitivity to touch, atrophic skin changes, osteopenia, and restricted ROM. The syndrome has been divided in three stages, and the best treatment is prevention. Preemptive analgesia in the form of local anesthetics and preoperative administration of nonsteroidal antiinflammatory medications and/or opioids can help decrease the incidence of this complication. The theoretical basis of preemptive analgesia is that it blocks nociceptive inputs generated during surgery that can trigger a state of hyperexcitability in the central nervous system. We routinely preinject the knee before the procedure with a solution of bupivacaine (Marcaine) and morphine. Poehling et al,45 in a study of patients with clinically significant RSD, found evidence of injury to the infrapatellar branch of the saphenous nerve in all of the patients. The saphenous nerve may give multiple infrapatellar branches that frequently cross over extensively into the lateral side of the knee and upper leg. Incisional neuromas of these branches after ACL reconstruction have received little attention in the literature but can be a source of significant morbidity extending beyond numbness or dysesthesia. Injury to the infrapatellar branch or the saphenous nerve itself may happen at various stages of the reconstructive procedure, such as during the superficial dissection, semitendinosus or gracilis tendon harvesting, and medial meniscus repair.
Another cause of soft tissue irritation and restricted motion after ACL reconstruction is protruding hardware. Suspension devices used for femoral fixation of tendon grafts in the femur have been reported as a source of postoperative arthrofibrosis.46 The surgeon must ensure adequate seating within the bone of such implants.
Treatment
Whether motion loss following ACL reconstruction is due to genetic predisposition, errors in surgical timing or technique, the expression of a developing infrapatellar contracture syndrome, or merely the result of lack of appropriate rehabilitation, early recognition of this complication is crucial. It is our experience as well as that of others47 that early treatment yields better results. The clinical presentation is nearly identical in every case: an inappropriately painful and swollen knee, with significant periarticular inflammation, quadriceps inhibition and lag, inability to gain extension, limited passive patellar mobility, and failure to make progress during rehabilitation.
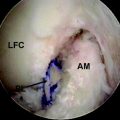
In most cases, a careful history and physical examination focused on the previously discussed factors can point to the cause of the postoperative stiffness. It is useful to review operative records and obtain orthogonal x-rays of the knee with the joint in extension to evaluate tunnel placement and hardware position. Occasionally, a knee magnetic resonance image (MRI) may give further insight. It allows evaluation of the graft and its position as well as the fat pad. An impinged graft will demonstrate increased signal in the distal two-thirds and deflection under the roof.48 In cases of suspected “Cyclops” lesion, an MRI is the study of choice (Fig. 72-1). The images should be scrutinized for other missed pathology, such as meniscal tears, failed meniscal repairs, and so on, that can hinder knee motion. Other laboratory workup such as complete blood count with differential, erythrocyte sedimentation rate (ESR) and C reactive protein (CRP) knee aspiration, and synovial fluid analysis and cultures may be indicated in cases of suspected infection.
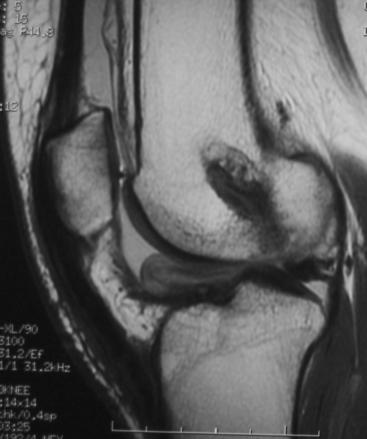
Fig. 72-1 In cases of suspected “Cyclops” lesion, magnetic resonance imaging (MRI) is the study of choice.
In cases where an anatomical block to motion is identified (e.g., graft impingement, Cyclops lesion) surgical treatment is advocated. In the early period following ACL reconstruction, loss of extension is due to pathology between the graft and the fat pad, and loss of flexion is usually due to adhesion formation in the suprapatellar pouch and medial and lateral gutters. Arthroscopic débridement of fibrotic nodules in front of the graft, roofplasty, or lysis of adhesions in the suprapatellar pouch and gutters can successfully address postoperative loss of motion.33,49 In more severe cases of global knee arthrofibrosis or advanced stages of infrapatellar contracture syndrome, open débridement is necessary. Delay for several months to allow subsidence of the inflammation of the knee may be necessary. Attempts to mobilize an inflamed knee usually prove unsuccessful, and therefore it is preferable to work on muscle strength through the available ROM while waiting for resolution of the inflammation. A thorough débridement of scar tissue is performed through a lateral and/or limited medial arthrotomy, with special attention paid to the anterior compartment of the knee. A malpositioned, overtensioned, or impinged graft that blocks motion is excised. Protruding hardware is removed or trimmed. In long-standing cases, posterior capsular releases may rarely be required to correct a flexion contracture.
1 Aglietti P, Giron F, Buzzi R, et al. Anterior cruciate ligament reconstruction: bone-patellar tendon-bone compared with double semitendinosus and gracilis tendon grafts. A prospective, randomized clinical trial. J Bone Joint Surg. 2004;86A:2143-2155.
2 Corry IS, Webb JM, Clingeleffer AJ, et al. Arthroscopic reconstruction of the anterior cruciate ligament: a comparison of patellar tendon autograft and four-strand hamstring tendon autograft. Am J Sports Med. 1999;27:444-454.
3 Williams RJ, Hyman J, Petrigliano F, et al. Anterior cruciate ligament reconstruction with a four-strand hamstring tendon autograft. J Bone Joint Surg. 2004;86A:225-232.
4 Eriksson K, Anderberg P, Hamberg P, et al. A comparison of quadruple semitendinosus and patellar tendon grafts in reconstruction of the anterior cruciate ligament. J Bone Joint Surg. 2001;83A:348-354.
5 Pinczewski LA, Deehan DJ, Salmon LJ. A five-year comparison of patellar tendon versus four-strand hamstring tendon autograft for arthroscopic reconstruction of anterior cruciate ligament. Am J Sports Med. 2002;30:523-536.
6 Shaieb MD, Kan DM, Chang SK, et al. A prospective randomized comparison of patellar tendon versus semitendinosus and gracilis tendon autografts for anterior cruciate ligament reconstruction. Am J Sports Med. 2002;30:214-220.
7 Harner CD, Irrgang JJ, Paul J, et al. Loss of motion after anterior cruciate ligament reconstruction. Am J Sports Med. 1992;20:499-506.
8 Shelbourne KD, Patel DV, Martini DJ. Classification and management of arthrofibrosis of the knee after anterior cruciate ligament reconstruction. Am J Sports Med. 1996;24:857-862.
9 Wahl SM. Transforming growth factor beta: the good, the bad, and the ugly. J Exp Med. 1994;180:1587-1590.
10 Shah M, Foreman D, Ferguson M. Neutralization of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108:985-1002.
11 Skutek M, Elsner HA, Slateva K, et al. Screening for arthrofibrosis after anterior cruciate ligament reconstruction: analysis of association with human leukocyte antigen. Arthroscopy. 2004;20:469-473.
12 Paulos LE, Rosenberg TD, Drawbert J, et al. Infrapatellar contracture syndrome: an unrecognized cause of knee stiffness with patella entrapment and patella infera. Am J Sports Med. 1987;15:331-341.
13 Mohamed-Ali V, Pinkney JH, Coppack SW. Adipose tissue as an endocrine and paracrine organ. Int J Obesity. 1998;22:1145-1158.
14 Ushiyama T, Chano T, Inoue K, et al. Cytokine production in the infrapatellar fat pad: another source of cytokines in knee synovial fluids. Ann Rheum Dis. 2003;62:108-112.
15 Murakami S, Muneta T, Ezura Y, et al. Quantitative analysis of synovial fibrosis in the infrapatellar fat pad before and after anterior cruciate ligament reconstruction. Am J Sports Med. 1997;25:29-34.
16 Hunter RE, Mastrangelo J, Freeman JR, et al. The impact of surgical timing on postoperative motion and stability following anterior cruciate ligament reconstruction. Arthroscopy. 1996;12:667-674.
17 Majors RA, Woodfin B. Achieving full range of motion after anterior cruciate ligament reconstruction. Am J Sports Med. 1996;24:350-355.
18 Marcacci M, Zaffagnini S, Iacono F, et al. Early versus late reconstruction for anterior cruciate ligament rupture. Results after five years of follow-up. Am J Sports Med. 1995;23:690-693.
19 Shelbourne KD, Wilckens JH, Mollabashy A, et al. Arthrofibrosis in acute anterior cruciate ligament reconstruction. The effect of timing of reconstruction and rehabilitation. Am J Sports Med. 1991;19:332-336.
20 Strum GM, Friedman MJ, Fox JM, et al. Acute anterior cruciate ligament reconstruction: analysis of complications. Clin Orthop Relat Res. 1990:184-189. Apr
21 Meighan AA, Keating JF, Will E. Outcome after reconstruction of the anterior cruciate ligament in athletic patients. A comparison of early versus delayed surgery. J Bone Joint Surg. 2003;85B:521-524.
22 Shelbourne KD, Foulk DA. Timing of surgery in acute anterior cruciate ligament tears on the return of quadriceps muscle strength after reconstruction using an autogenous patellar tendon graft. Am J Sports Med. 1995;23:686-689.
23 Robins AJ, Newman AP, Burks RT. Postoperative return of motion in anterior cruciate ligament and medial collateral ligament injuries. The effect of medial collateral ligament rupture location. Am J Sports Med. 1993;21:20-25.
24 Noyes FR, Barber-Westin SD. Revision anterior cruciate ligament reconstruction: report of 11-year experience and results in 114 consecutive patients. Instr Course Lect. 2001;50:451-461.
25 Hefzy MS, Grood ES, Noyes FR. Factors affecting the region of most isometric femoral attachments. Part II: the anterior cruciate ligament. Am J Sports Med. 1989;17:208-216.
26 Sapega AA, Moyer RA, Scheck C, et al. Testing for isometry during reconstruction of the anterior cruciate ligament. Anatomical and biomechanical considerations. J Bone Joint Surg. 1990;72A:259-267.
27 Graf B. Isometric placement of substitutes for the anterior cruciate ligament. In: Jackson DW, Drez D, editors. The anterior cruciate deficient knee. St Louis: Mosby; 1987:102-113.
28 Fleming BC, Beynnon BD, Nichols CE, et al. An in vivo comparison between intraoperative isometric measurement and local elongation of the graft after reconstruction of the anterior cruciate ligament. J Bone Joint Surg. 1994;76A:511-519.
29 Clancy WG, Ray JM, Zoltan DJ. Acute tears of the anterior cruciate ligament. Surgical versus conservative treatment. J Bone Joint Surg. 1988;70A:1483-1488.
30 O’Meara PM, O’Brien WR, Henning CE. Anterior cruciate ligament reconstruction stability with continuous passive motion. The role of isometric graft placement. Clin Orthop Relat Res. 1992:201-209. Apr
31 Howell SM, Taylor MA. Failure of reconstruction of the anterior cruciate ligament due to impingement by the intercondylar roof. J Bone Joint Surg. 1993;75A:1044-1055.
32 Girgis FG, Marshall JL, Monajem A. The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clin Orthop Relat Res. 1975;106:216-231.
33 Jackson DW, Schaefer RK. Cyclops syndrome: loss of extension following intra-articular anterior cruciate ligament reconstruction. Arthroscopy. 1990;6:171-178.
34 Marzo JM, Bowen MK, Warren RF, et al. Intraarticular fibrous nodule as a cause of loss of extension following anterior cruciate ligament reconstruction. Arthroscopy. 1992;8:10-18.
35 Romano VM, Graf BK, Keene JS, et al. Anterior cruciate ligament reconstruction. The effect of tibial tunnel placement on range of motion. Am J Sports Med. 1993;21:415-418.
36 Muneta T, Yamamoto H, Ishibashi T, et al. The effects of tibial tunnel placement and roofplasty on reconstructed anterior cruciate ligament knees. Arthroscopy. 1995;11:57-62.
37 Howell SM, Gittins ME, Gottlieb JE, et al. The relationship between the angle of the tibial tunnel in the coronal plane and loss of flexion and anterior laxity after anterior cruciate ligament reconstruction. Am J Sports Med. 2001;29:567-574.
38 Hutchinson MR, Bae TS. Reproducibility of anatomic tibial landmarks for anterior cruciate ligament reconstructions. Am J Sports Med. 2001;29:777-780.
39 Howell SM. Principles for placing the tibial tunnel and avoiding roof impingement during reconstruction of a torn anterior cruciate ligament. Knee Surg Sports Traumatol Arthrosc. 1998;6:S49-S55.
40 Yasuda K, Tsujino J, Tanabe Y, et al. Effects of initial graft tension on clinical outcome after anterior cruciate ligament reconstruction. Autogenous doubled hamstring tendons connected in series with polyester tapes. Am J Sports Med. 1997;25:99-106.
41 Hamner DL, Brown CHJr, Steiner ME, et al. Hamstring tendon grafts for reconstruction of the anterior cruciate ligament: biomechanical evaluation of the use of multiple strands and tensioning techniques. J Bone Joint Surg. 1999;81A:549-557.
42 Heis FT, Paulos LE. Tensioning of the anterior cruciate ligament graft. Orthop Clin North Am. 2002;33:697-700.
43 Bylski-Austrow DI, Grood ES, Hefzy MS, et al. Anterior cruciate ligament replacements: a mechanical study of femoral attachment location, flexion angle at tensioning, and initial tension. J Orthop Res. 1990;8:522-531.
44 Paulos LE, Noyes FR, Grood ES. Knee rehabilitation after anterior ligament reconstruction and repair. Am J Sports Med. 1981;9:140-149.
45 Poehling GG, Pollock FEJr, Koman LA. Reflex sympathetic dystrophy of the knee after sensory nerve injury. Arthroscopy. 1988;4:31-35.
46 Misra R, Strover A, El-Shazly M. Intra-articular protrusion of malpositioned Transfix implant following anterior cruciate ligament reconstruction. Arthroscopy. 2006;22:226.e1-226.e4.
47 Millett PJ, Wickiewicz TL, Warren RF. Motion loss after ligament injuries to the knee: part II: prevention and treatment. Am J Sports Med. 2001;29:822-828.
48 Howell SM, Berns GS, Farley TE. Unimpinged and impinged anterior cruciate ligament grafts: MR signal intensity measurements. Radiology. 1991;179:639-643.
49 Fisher SE, Shelbourne KD. Arthroscopic treatment of symptomatic extension block complicating anterior cruciate ligament reconstruction. Am J Sports Med. 1993;21:558-564.