Chapter 16 Stereotactic Radiosurgery of the Spine
INTRODUCTION
The standard radiation treatment field for spinal tumors generally includes one to two vertebral segments above and below the lesion to compensate for targeting errors and patient movement (Fig. 16-1). More importantly, the close proximity of the spinal cord to the lesion typically makes it impossible to exclude the spinal cord from the treatment field. In an effort to protect the spinal cord and other healthy tissue, conventional radiation therapy is highly fractionated to allow time for these tissues to recuperate from damage caused during treatment. However, despite such attempts, adequate therapeutic doses are often unobtainable because of the low tolerance threshold of the spinal cord to radiation.1 Consequently, relapse within the treatment field after standard radiotherapy is not uncommon.2–5
Shaping the irradiation beam to strictly conform to the target volume enables maximal dose delivery to the lesion while minimizing the risk of injury to the spinal cord. One attempt to achieve this goal was the development of intensity modulated radiation therapy (IMRT), which uses dynamic multi-leaf collimators to modify the intensity of the radiation as it is distributed to the tumor.6,7 This technology enables the shape of the treatment field to more closely resemble that of the lesion. However, most of the advantages of this technique go unrecognized because the method of radiation targeting remains relatively imprecise. During conventional radiotherapy, the radiation beams are aimed at the lesion by means of surface markers attached to the skin. The inherent nature of this process results in limited spatial accuracy and reproducibility. Consequently, even the most modern radiation therapy technique fails to address the unique demands of optimal radiation delivery to spinal tumors. Ultimately, the dilemma with all radiotherapy methods is that greater success can only be achieved at the cost of substantially increasing the risk of injury.
EVOLUTION OF SPINAL STEREOTACTIC RADIOSURGERY
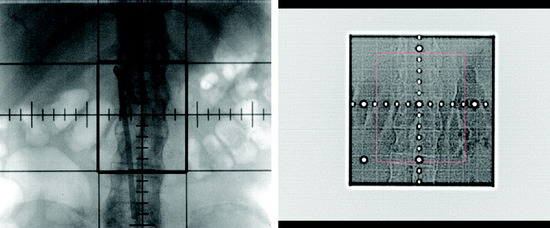
Stereotactic radiosurgery uses the principles of stereotactic localization in combination with multiple radiation beams from a highly collimated source to deliver high-dose radiation to a target while limiting exposure to healthy tissues.8 This technology traditionally has been used to treat intracranial lesions and is based on the presumed fixed relationship between the target and skull that serves as a reference for target localization. During the past several years, the success of stereotactic radiosurgery has redefined the standards of treatment for intracranial pathology and has inspired the development of similar techniques for the treatment of spinal tumors. Lesions of the spine have essentially a fixed spatial relationship to the surrounding spinal column. Therefore, similar to early intracranial radiosurgery devices such as the Gamma Knife (Elekta, Atlanta, GA), the initial development of spinal radiosurgery used the rigid bony fixation to target spinal tumors (Figs. 16-2 and 16-3).9–11 Although preliminary results were encouraging, rigid fixation was extremely cumbersome and impractical.
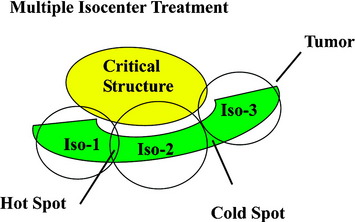
Traditional frame-based stereotactic systems use multiple, overlapping isocenters to achieve some semblance of field shaping (Fig. 16-4). This results in an inhomogeneous distribution of radiation that is often less than ideal for maintaining coverage within a target area and away from critical structures. Such inhomogeneity is particularly disadvantageous when administering very aggressive doses of radiation to non-spherical lesions immediately adjacent to the spinal cord.
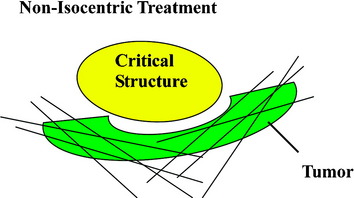
The frameless image-guided radiosurgery system, the CyberKnife, developed by Accuray Inc. (Sunnyvale, CA), overcomes many of the limitations encountered by earlier systems. Rather than use an external fixation device, targeting is based on internal radiographic features such as skeletal anatomy or implanted fiducials. The system also allows multiple images to be acquired during treatment to track and compensate for any changes in target location. Finally, instead of using fixed isocenters, the CyberKnife is capable of distributing doses in a conformal and homogeneous manner that enables the treatment of irregularly shaped lesions with unmatched precision (Fig. 16-5).
TECHNICAL OVERVIEW OF THE CYBERKNIFE SYSTEM FOR SPINAL RADIOSURGERY
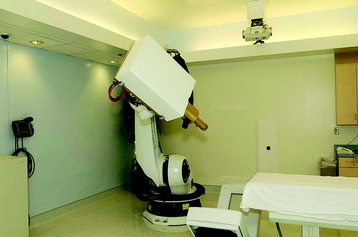
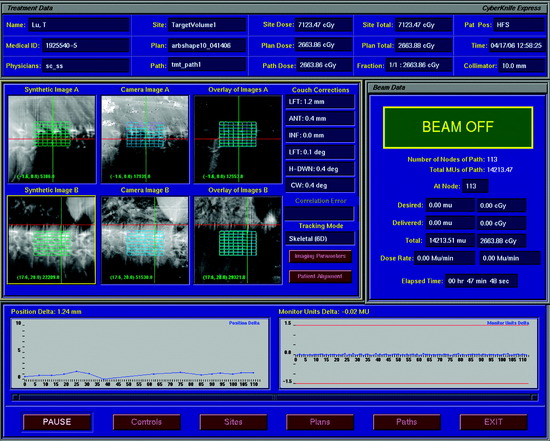
The CyberKnife radiosurgery system (Fig. 16-6) consists of a lightweight 6-MV linear accelerator mounted on a computer-controlled robotic arm. The robot receives information regarding target location via an x-ray based imaging feedback system. This system consists of two orthogonally aligned x-ray cameras that acquire radiographs of targeting landmarks during treatment. The x-ray cameras are at fixed positions within the treatment room thereby providing a stationary frame of reference for spinal localization. Once the images are referenced within the imaging system’s coordinate frame, the position of the lesion is known. Targeting is based on the assumption of a fixed relationship between the lesion and the spine. The radiographs acquired by the real-time imaging system are compared to digitally reconstructed radiographs (DRRs) derived from computed tomography (CT) scans obtained during pretreatment planning (Fig. 16-7). The CyberKnife software accounts for both translation and rotation of the patient’s anatomy by changing the position of the DRR until an exact match of the x-ray image and DRR is achieved.12 This algorithm eliminates the need to fix the orientation of the patient during treatment. Once the location of the spine is determined, the coordinates are relayed to the robotic arm, which controls the targeting of the linear accelerator (LINAC). This concept enables the system to detect and adjust to changes in target position in less than 1 second with an accuracy approaching ±0.5 mm.13,14
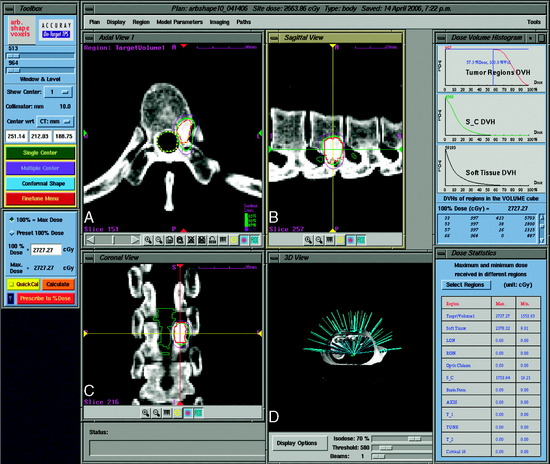
The CyberKnife targeting algorithm is designed by positioning the lesion within the center of an 80-cm3 sphere that is defined with respect to the patient’s anatomy. On the surface of the sphere, there are 100 equally spaced points called nodes. At each node the robot defines 12 beams of radiation that intersect various portions of the tumor volume. The robotic arm stops at each node where radiation beams of a specific prescribed dose are administered. Treatment plans are created from a subset of the entire constellation of radiation beams that concentrates dose to the target area while minimizing dose to the adjacent normal tissue. In practice, not all nodes are available because of objects within the treatment room that obstruct the path of certain beams or prevent the robotic arm from positioning the LINAC at a particular node. At least 50 nodes and approximately 95–200 beams are used during treatment. Since the robot can aim a beam virtually anywhere within the tumor volume, highly conformal treatment plans can be created for complex tumor morphologies in a non-isocentric manner (Fig. 16-8).12
TREATMENT PROCEDURE
SPINAL IMMOBILIZATION
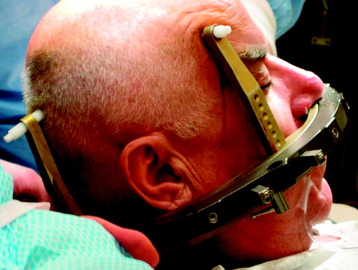
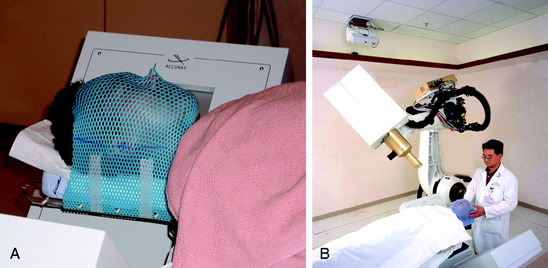
Special immobilization devices are used to provide comfort and to help restrict patient movement during pretreatment scanning and treatment delivery. For the head and cervical spine, a custom-fitted Aquaplast mask (WFR/Aquaplast Corp, Wyckoff, NJ) is constructed; the mask consists of a thermoplastic mesh that conforms to the patient’s face (Fig. 16-9). The patient’s head and neck rest on a radiolucent support pillow while the mask is fastened to the treatment table. For thoracic and lumbar spine lesions, a custom vacuum cradle is form fitted to support the body. The patient is placed supine on the vacuum cradle, which is secured to the body using large Velcro straps fastened to the treatment table (Fig. 16-10).
TREATMENT DELIVERY
Typically, all CyberKnife radiosurgery treatments are performed on an outpatient basis. During treatment delivery, patients are positioned supine on the operative table and are supported by their customized immobilization devices. Once positioned, images of the relevant spinal anatomy are obtained from the two orthogonally placed digital x-ray cameras and compared to the DRRs created from the pretreatment planning scan. Initially, the operative table is moved manually to optimally align the real time x-ray images with the synthetic DRRs. The system software then matches bony landmarks within these images that will be used for position tracking. Once the initial patient position has been manually optimized, the remaining processes are automatic.15 The robotic arm moves the LINAC sequentially through the planned beam positions or nodes, delivering a specific dose at each node. The CyberKnife system intermittently corrects for small shifts in patient position during treatment by re-establishing the location of the target and sending corrective targeting instructions to the robotic arm. Although this can be accomplished at each node, position is usually checked approximately once every minute when immobilization seems adequate and the patient is cooperative. This process of intermittent re-targeting has proven to be reliable and accurate.13
INDICATIONS AND CONTRAINDICATIONS FOR SPINAL RADIOSURGERY
The indications for spinal radiosurgery have been evolving rapidly as experience with this relatively new technology is acquired. Similar to cranial radiosurgery, appropriate spinal lesions should be reasonably well circumscribed to benefit from a steep dose gradient. The primary indications include progressive lesions with no or minimal symptoms that threaten neurological function, radiographic progression despite previous surgery or irradiation, multiple metastatic lesions, or treatment of inoperable lesions.6,7,16 Another common indication involves treatment to the postoperative resection cavity as an adjunct to surgery. Among the majority of spinal cases selected for CyberKnife treatment, lesions were either unresectable or patients had significant comorbidities that precluded them from surgery. In a small subset of patients, open surgical resection was refused. A wide spectrum of spinal pathologies have been treated, including primary and metastatic tumors, vascular malformations, and developmental anomalies.6,7
STANFORD CYBERKNIFE SPINAL RADIOSURGERY EXPERIENCE
MALIGNANT TUMORS
Metastatic spread to the spine occurs most commonly within the vertebral column (≈85%), followed by the extramedullary (≈10–15%) and intramedullary (<5%) compartments.17–20 Presenting symptoms often include back pain caused by bony involvement and/or neurological impairment from nerve root or spinal cord compression.17,21 Surgery generally is reserved for patients with a reasonable life expectancy, acute neurological decline, spinal instability, or progression after radiotherapy.22 Conventional radiotherapy may be used in conjunction with surgery or as a palliative option delivered most often as multiple (5–20) daily fractions. Even though this treatment option may provide transient pain relief, many patients develop recurrent pain, tumor progression, or neurological deterioration.23–27 The low tolerance of the spinal cord to radiation often limits the treatment dose to levels that fall short of adequate tumor control.28 With the advances in radiation targeting and beam contouring offered by stereotactic radiosurgery, it is possible to deliver higher effective radiation doses to a tumor while limiting the dose to healthy critical structures.
BENIGN TUMORS
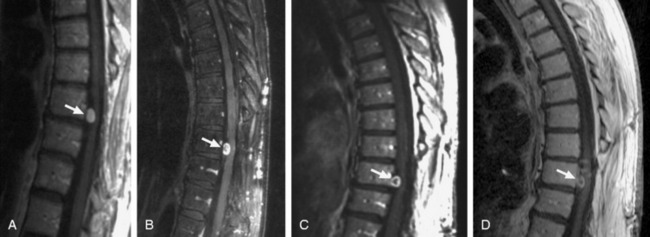
Surgical resection of benign neoplasms of the spine is generally regarded as a safe and effective treatment option. However, patients who are poor surgical candidates, such as those with significant comorbidities or those with multiple or recurrent tumors, may benefit from an alternative non-invasive treatment strategy. In a study conducted at Stanford University Medical Center, 51 patients with 55 benign spinal tumors (30 schwannomas, 9 neurofibromas, 16 meningiomas) were treated prospectively as part of an IRB-approved protocol.29 The prescribed treatment doses ranged from 16–30 Gy delivered in 1–5 fractions to tumor volumes from 0.136 to 24.6 cm3. Twenty-eight of the 51 patients had clinical and radiographic follow-up of more than 24 months. Of these 28 patients, after a mean follow-up of 36 months, all lesions were either stable (61%) or smaller (39%). Three of the 51 patients (1 meningioma, 1 schwannoma, and 1 neurofibroma) underwent surgery less than 1 year after CyberKnife treatment for removal of their tumors because of persistent or worsening symptoms; only one of these lesions was slightly larger radiographically. There was one case of radiation-induced myelopathy that appeared 8 months after radiosurgery. Although greater patient accrual and longer follow-up are needed to determine the long-term efficacy of spinal radiosurgery for these lesions, recent results indicate that CyberKnife stereotactic ablation is technically feasible and associated with low morbidity (Fig. 16-11).
INTRAMEDULLARY ARTERIOVENOUS MALFORMATIONS
Intramedullary arteriovenous malformations (AVMs) are difficult lesions to treat because of their location within the spinal cord parenchyma. Treatment traditionally has been accomplished with endovascular embolization and/or microsurgical resection when possible.30 However, definitive treatment is often not possible because of size, location, or complex AVM morphology. The success of stereotactic radiosurgery to treat intracranial AVMs has led to its application for use in the spine. Previous experience with spinal AVM radiosurgery was limited because of the initial requirement for invasive, rigid patient immobilization.7 This method of targeting also did not allow for fractionation, an important strategy used to improve the tolerance of the spinal cord to radiation.
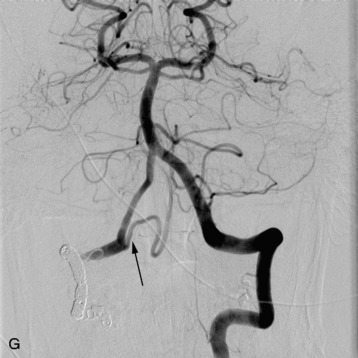
In a study conducted at Stanford University Medical Center during the period from 1997–2005, 15 patients with intramedullary AVMs were treated with the CyberKnife. Nine of the lesions were located in the cervical spinal cord, three in the thoracic cord, and three in the region of the conus medullaris.31 Radiosurgery was delivered in an average of three sessions (range 2–5) with a mean prescription dose of 20.5 Gy to the margin of the nidus. The mean target volume for the AVMs in this series was 2.36 cm3 (range 0.79–5.23 cm3). All patients received magnetic resonance imaging (MRI) examinations after an initial 6-month interval, followed by yearly evaluations. Spinal angiography was performed 3 years after treatment to document AVM obliteration. Mean follow-up was 27.9 months (range 3–59 months). One patient with a T12 conus medullaris AVM demonstrated evidence of complete angiographic obliteration 26 months after radiosurgery. Four patients demonstrated evidence of residual AVM on final post-treatment angiography, although the treated AVM volumes were significantly reduced in all patients. Three additional patients did not undergo final angiography, despite being more than 3 years from radiosurgical treatment. However, all of these patients demonstrated a significant reduction in AVM volume on interim MRI examinations. In this series, 7 of the 15 were less than 3 years from their last radiosurgical treatment and had not yet received post-treatment angiography. All of these latter patients also demonstrated evidence of significant AVM reduction on their last interim MRI examinations (Fig. 16-12).
COMPLICATIONS OF SPINAL RADIOSURGERY
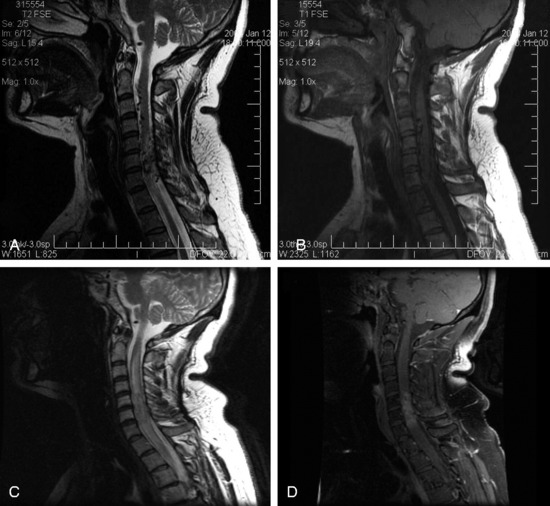
In the preliminary spinal radiosurgery studies performed at Stanford, a total of 137 patients underwent treatment with CyberKnife for 169 lesions. Four of 137 patients (2.9%) suffered a radiation-induced myelopathy. Three of these patients received treatment for a metastatic lesion located within the thoracic spine; one patient was initially asymptomatic. For the three patients in the metastatic group, the mean time to onset of signs and symptoms was 7 months (range 6 – 10 months). Classic radiographic signs of spinal cord necrosis were apparent at the onset of clinical neurological deterioration. Spinal cord edema initially appeared, followed by contrast enhancement within the cord at the level of the treated lesion. Characteristically, the edema resolved over a period of 3–6 months; however, the contrast enhancement persisted. A variety of patient characteristics and treatment parameters, including age, gender, primary site, anatomical location, anatomical level, previous treatment, total dose, dose per fraction, maximum dose, maximum spinal cord dose, and tumor volume, were analyzed by logistic regression to identify possible predictors for complication. Another possibility considered was the relative increased susceptibility of the mid-thoracic spine to radiation and other forms of injury, presumably as a result of reduced blood flow.32 In addition, the adjuvant use of certain chemotherapies may predispose to myelopathy when administered in conjunction with aggressive spinal irradiation. However, in our preliminary study, logistic regression analysis failed to identify factors that predicted complications despite the fact that all three patients had lesions located within the thoracic spine and two of the three patients received anti-angiogenic or epidermal growth factor inhibitor within 2 months before developing clinical myelopathy.
In the benign tumor series at our institution, one patient appeared to have suffered a radiation-induced spinal cord injury.29 By the standards within the study the treatment was not especially aggressive; volume of tumor, dose, and number of stages were relatively unremarkable. However, this complication did not occur in the watershed region of the mid-thoracic region. It is reasonable to speculate that the trauma from two prior surgical resections of a cervicothoracic meningioma may have predisposed this patient’s spinal cord to subsequent radiation injury. Until our knowledge is more complete, physicians need to be mindful of the potential complications of spinal radiosurgery treatment.