Chapter 176 Stereotactic Radiosurgery for the Treatment of Spinal Metastases
Spinal metastases are estimated to be 20 times more common than primary spine tumors affecting the spine.1 They are reported in as many as 50% of cancer patients and can result in devastating sequelae in 5% to 14%.2–4 Patients with spinal metastases often present with disabling pain, as well as neurologic deficits, as a result of epidural spinal cord compression. Whereas the surgical goal for primary spine tumors such as chordoma and chondrosarcoma is en bloc resection for potential cure, the role of surgery in spinal metastases is generally palliative.3,5 With this in mind, treatment decisions for spinal metastases are made with the intent of resuming systemic therapy as soon as possible for overall disease control or improving quality of life in the final stages of disease.
In North America, over 200,000 new cases of spinal metastases are diagnosed each year, with 20,000 clinical cases of spinal cord compression.6–8 This number is expected to rise as patients live longer with improved response to systemic therapy. These patients have a median overall survival of 7 months, with a range of 3 to 16 months.9–11 Both early detection and appropriate intervention are essential to minimize the sequelae of spinal metastases and maximize patient function and quality of life.12
The principle treatment modalities for solid tumor spinal metastases are surgery and radiation.3,4,13–17 These treatments are generally considered palliative in nature. Typically, most patients have concurrent systemic visceral and/or bone disease at presentation with spinal metastases. Even in the presence of solitary spinal lesions, it is unclear when systemic metastases will develop, providing reason for caution when considering “curative resections” for solitary spinal metastasis.18–20 In terms of other therapeutic options, with few exceptions (e.g., multiple myeloma, lymphoma, breast, and prostate carcinoma), chemotherapy, hormonal therapy, and immunotherapy play a limited role in the treatment of metastatic spine tumors.
Characterization of Pain
Metastatic involvement of the spine can occur anywhere along its axis. These tumors, however, most frequently involve the thoracic spine (70%), followed by the lumbar spine (20%) and cervical spine (10%).5,21 Pain is the most common symptom and can be characterized as either oncologic or mechanical, or a combination of both.19 It is essential to determine which type of pain patients are experiencing as this plays a major role in determining the most effective treatment. Pain that is oncologic or biologic in nature is described as dull, constant, and responsive to steroids. It is often worse at night and in the morning but typically does not worsen during the day with activity. As such, patients often complain of pain during sleep. The diurnal variation is thought to be related to the variable secretion of endogenous steroids, which is why this type of pain is responsive to oral steroid administration in the early stages.19 Though the pathophysiology is uncertain, it is thought to be related to inflammation of tumor within the vertebral body and stretching of the periosteum with tumor growth.
Mechanical pain is associated with activity and worsens with movement.22 In the cervical spine, neck pain that worsens with flexion, extension, and rotation is common and is related to progressive bony destruction. Pain of this type is often suggestive of underlying instability, deformity, or fracture. Rotational pain is suggestive of atlantoaxial instability, whereas pain with flexion and extension is indicative of subaxial pathology. In the thoracic spine, pain is often worse with extension as patients attempt to lie on their back during sleep. Mechanical pain in the lumbar spine is also associated with flexion, extension, and axial loading. Painful radiculopathies may occur as a result of loss of vertebral height and neuroforaminal compression of exiting nerve roots due to either tumor or loss of height with axial loading.
Pain, whether oncologic or mechanical, may be severe enough to make ambulation impossible. Though these patients may have preserved strength in their legs and appear comfortable while supine or on their side, pain may make any movement unbearable. Though a full discussion of the numerous treatment options available for metastatic disease is beyond the scope of this chapter, a number of treatments, including vertebral cement augmentation, surgery, radiation therapy, or SRS, or a combination of these, may play a role in the multimodality treatment of these patients.12 Each patient requires a specifically tailored treatment plan based on clinical and radiographic findings.
Role of Surgery
To fully appreciate the role of SRS in the management of metastatic spine tumors, an understanding of the current role of surgery for metastatic spine disease is required. In 2005, Patchell et al.4 published a prospective randomized trial comparing surgery and conventional external-beam radiation therapy to radiation therapy alone for high-grade spinal cord compression. This study showed that surgery followed by conventional radiation resulted in significant advantages in terms of overall maintenance and recovery of ambulation, continence, narcotic requirements, and survival.
Patients who did not undergo surgery but had radiation upfront had worse outcomes overall than did the surgical group. Fifty-seven percent of ambulatory patients in the radiation arm maintained ambulation for only 13 days compared with 122 days in the surgical arm.4 Nonambulatory patients in the radiation group recovered ambulation in 19% (3/16); however, these patients all recovered ambulation only after crossing over to the surgical arm.4 Essentially no patient recovered ambulation without surgery due to the radiation insensitivity of solid tumor malignancies. Although the study demonstrated the superiority of surgery and conventional radiation therapy to radiation alone in terms of neurologic function, it did not address the durability of tumor control given the relatively short survival of the patients studied.
Despite the study’s limitations, the current recommendation for optimal functional outcomes in patients with high-grade spinal cord compression resulting from solid tumor malignancies or in situations where gross spinal instability exists, is that surgery should be the first-line treatment when possible.4,18
Limitations of Conventional External-Beam Radiation Therapy
Many patients with spinal metastases may have significant medical comorbidities that preclude aggressive surgical treatment when otherwise indicated. For this reason, radiation continues to be the mainstay of treatment for many of these patients. The historical benefits of conventional radiation therapy for treating spinal metastases have been demonstrated in numerous retrospective studies showing improvement or maintenance of neurologic function.11,13–16 Although the Patchell study demonstrated the superiority of surgery, radiation is essential for achieving postoperative local tumor control.23 In a recent review of the literature, Gerszten et al.23 found that the ambulatory rate after conventional radiation was 60% to 80%, pain control was achieved in 50% to 70%, and local tumor control was achieved in 20% to 89% of patients. However, though conventional radiation (usually 30 Gy, in 3 Gy/fraction) provides durable control for the hematologic malignancies and breast carcinoma, the majority of solid tumors demonstrate radioresistance or nondurable long-term tumor control when it is used as either a primary or a postoperative treatment.24 In addition, because treatment plans with conventional radiation often include a margin of one healthy vertebral body above and below the area of concern, this results in a large volume of normal tissue being irradiated.8,25
The variable responsiveness of spinal metastases to radiation was originally reported in a series by Greenberg et al.,26 comparing outcomes of surgery and radiation to radiation alone. Though the surgical approach in this series was mainly laminectomy, thus rendering the surgical results less applicable today with the facile use of advanced decompression and instrumentation techniques, the authors demonstrated marked differences in the response to radiation based on tumor histology. Patients with breast carcinoma and hematologic malignancies showed improved outcomes compared with those with radioresistant tumors such as renal and lung carcinomas.
Maranzano and Latini16 also demonstrated significant differences in the sensitivity of various tumor types to conventional radiation when used as initial treatment. In a study of 275 patients, those with radioresistant tumors such as non–small-cell lung, bladder, and renal cell carcinoma demonstrated significantly less recovery than those with typically radiosensitive tumors, such as breast and hematologic malignancies. Patients with gross instability were excluded from the study. Overall, 98% of patients maintained ambulation but only 60% recovered. Of those who regained ambulation, 70% had radiosensitive tumors. For example, breast carcinoma demonstrated an 80% response rate compared with hepatocellular carcinoma with a 20% response rate. Furthermore, the durability of the response was 10 to 16 months for radiosensitive tumors compared with 1 to 3 months in radioresistant tumors. A number of other studies have also demonstrated this variable response based on tumor histology.27–29
Limited surgical studies have evaluated the utility of conventional radiation as a postoperative adjuvant. Klekamp and Samii30 reported on 101 surgeries, of which 91% were aggressive subtotal or complete resections. All patients underwent postoperative radiation therapy. Local recurrences were 57.9% at 6 months, 69.3% at 1 year, and 96% at 4 years. The primary factor predictive of recurrence was tumor histology.
Although surgery and conventional radiation therapy are the current mainstays of treatment for spinal metastases, the major drawback to this approach is the relatively low radiation tolerance of the spinal cord.31–33 This is particularly relevant in cases of progression or recurrence of metastatic disease following standard radiation therapy. Further conventional radiation at recurrence is typically not an option, and patients who undergo surgery after having previously had radiation are known to do poorly and have increased risk of wound complications and worse functional outcomes.23,33–35
Issues related to tumor radiosensitivity and the need for higher dosing to achieve effective and durable tumor control as well as limited spinal cord radiation tolerance has fueled the development of advanced radiation delivery systems such as SRS to achieve conformal high-dose radiation delivery and durable tumor control.8,23,25,36,37
Stereotactic Radiosurgery
The failure of conventional radiation to achieve long-standing tumor control for radioresistant tumors has limited its modern day application. Though earlier studies have demonstrated success with pain reduction, local disease control, and neurologic improvement in select tumor types, these results do not apply across all histologies.2,23,27,38 As mentioned earlier, one of the main limitations to achieving local tumor control with conventional radiation therapy is the high dose required. However, the ability to deliver this dose is limited due to the adjacent spinal cord, where standard radiation tolerance is considered to be 45 to 50 Gy.31 Though clinical studies continue to improve our understanding of spinal cord tolerance to radiation, the therapeutic index of radiotherapy limits the radiation dose near the spinal cord to such an extent that tumor control is compromised.33,34
SRS has emerged as an advanced image-guided technology, allowing for more targeted and higher radiation dosing to tumors adjacent to organs at risk (OAR) such as the spinal cord. With technological advances in image guidance and radiation delivery platforms, it is now possible to deliver SRS in a highly conformal manner with a steep dose fall-off gradient either as a single fraction or as a hypofractionated regimen (two to five fractions).8,39–44 Such a gradient allows for delivering high doses of radiation within millimeters of vital OAR such as the spinal cord, kidney, and esophagus. This enables the very focused delivery of a potentially cytotoxic tumoral dose that can spare nearby normal tissue.
SRS is increasingly being applied to primary malignant and benign spine tumors; however, its application to spinal metastases represents the largest experience to date.36,42,45,46 The increased application of SRS is further redefining the term radioresistant as tumors traditionally regarded as radioresistant, such as renal cell carcinoma and melanoma, have demonstrated marked responses with durable tumor control following SRS.37 These improved tumor control rates are seen whether used as stand-alone therapy or as a postoperative adjuvant.37,47,48
The emergence and effectiveness of SRS as an instrument to achieve durable local control has also fueled the debate regarding the optimal management strategy for solitary metastatic lesions to the spine, which by some investigators have been considered ideal candidates for en bloc resection for potential cure.17,49,50
Indications and Treatment Planning
The indications for treatment with SRS are not rigidly defined in the literature and are currently evolving based on the experience of several high-volume centers.25,37,43 The most common indication for spine radiosurgery is pain, with 70% to 90% of all patients presenting with severe oncologic pain referable to a corresponding lesion involving one to three levels on imaging. Other indications include upfront treatment for radioresistant histologies, treatment after surgery for residual tumor, impending spinal cord compression, and local disease progression either during observation or after other treatment modalities such as surgery, radiation, and chemotherapy have failed.
The prescribed dose normally takes into account the point maximum dose and volume of spinal cord being irradiated as well as previous radiation exposure to normal tissue. However, there are currently no guidelines for optimal dose and dose constraints. Common dose regimens range from 14 to 24 Gy in a single fraction, or hypofractionated regimens such as 5 Gy in six fractions or 9 Gy in three fractions.25,36,38,40,44,51
Spine Radiosurgery as Monotherapy
The utilization of SRS as monotherapy has several advantages over conventional radiation therapy. With the conformal nature of dose delivery with SRS, vertebral levels adjacent to the treated target are spared from ionizing radiation. This limits the deleterious effect of radiation on whatever viable bone marrow remains. This is particularly important for patients whose hematologic cell counts often decrease as a result of systemic chemotherapy. SRS also allows patients to resume systemic treatments quickly as radiation is usually given in one fraction versus multiple fractions for conventional radiation therapy.12
In one of the largest series to date, Gerszten et al.37 reported on 500 tumors of various histologies treated with high-dose single-fraction radiation at a median dose of 20 Gy (range 12.5–25 Gy) throughout the spine. Pain and radiographic tumor control were achieved in 86% and 90% of cases, respectively. Radiographic tumor control differed based on primary pathology, with breast and lung carcinomas showing 100% radiographic tumor control, compared with renal cell tumors (87%) and melanoma (75%).
SRS allows for the delivery of tumoricidal radiation doses to tumors historically radioresistant to conventional radiation. This response appears to be histology independent. For instance, renal cell carcinoma has traditionally been considered resistant to conventional radiation therapy. Gerszten et al.46 reported 60 cases of renal cell carcinoma treated with single-fraction SRS. The majority (48/60) had progressed despite previous conventional radiation. Treatment doses ranged from 14 to 21 Gy with a mean maximum tumoral dose of 20 Gy, and the median follow-up was 37 months. Pain improved in 34/38 (89%) of patients who presented with oncologic pain, and tumor control was achieved in 7/8 patients who presented with tumor progression. Only 6/60 patients (10%) required surgery for progressive neurologic symptoms after SRS. Despite the high number of reirradiated patients undergoing single-fraction SRS for salvage, no radiation myelopathy or other toxicity was seen in the follow-up period.
Yamada et al.43 reported 101 cases treated with single-fraction SRS predominantly to radioresistant histologies, with the exception of six breast cancer patients. No patient had prior radiation or surgery to the treated area. The treatment paradigm was a dose escalation of 18 to 24 Gy, with the maximum dose to the spinal cord set to 14 Gy. At a median follow-up of 16 months, the overall radiographic control rates were 90%. Seven failures occurred at a median time of 9 months. A statistically significant dose response was demonstrated at 24 Gy compared with 18 Gy. Yamada reanalyzed the data in 248 patients receiving single-fraction radiation, and this dose response difference was maintained at 5-year follow-up. Toxicity was limited to grade 1 and 2 esophageal and skin cancers. No patient experienced myelopathy or functional radiculopathy.
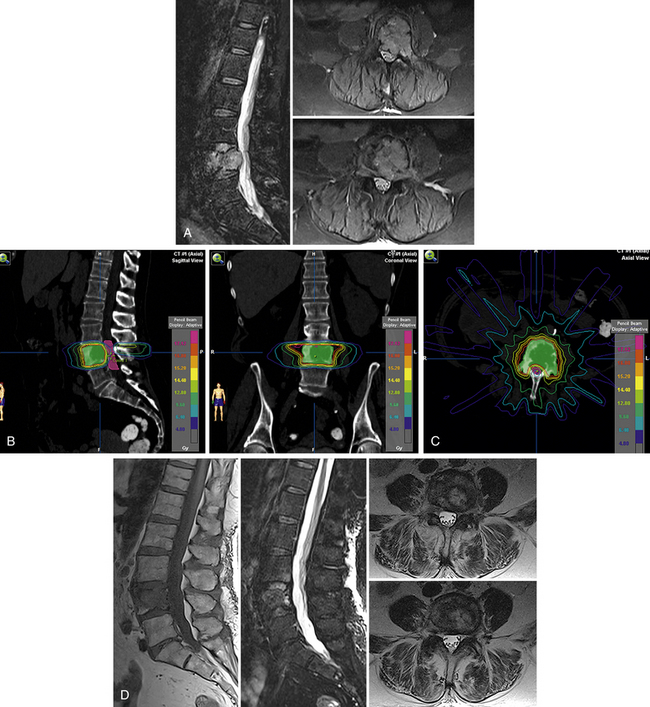
A representative case of a patient with radioresistant histology (renal cell carcinoma) from our own center is illustrated in Figure 176-1. As illustrated in this case, excellent pain control and control of tumor growth by SRS can ensure acceptable quality of life and functional performance in patients with metastases and would avoid the unnecessary morbidity and risk associated with extensive spinal surgery. SRS also allows patients to return home the same day and avoid lengthy hospitalizations.
Radiosurgery and Surgery
The decision to use SRS as part of a multimodality treatment plan needs to be tailored to the individual patient and take into consideration all of the previously discussed factors. It should be emphasized that SRS is not necessarily an alternative to surgery but is a component of a multifaceted treatment plan that may or may not include surgery at some point. Though the merits of surgery for high-grade epidural spinal cord compression and spinal instability followed by conventional radiation therapy are supported by the Patchell et al.4 study, the role of SRS as a postoperative adjunct continues to be debated and explored. It is clear that surgery cannot provide durable local tumor control and that postoperative radiation is essential for this purpose.24 Though SRS has been shown to achieve high rates of local tumor control for radioresistant pathologies, no studies to date have compared SRS versus conventional radiotherapy as a planned postoperative adjunct.
The current state of the literature is limited to three retrospective series, each with small patient numbers, limited clinical follow-up, varying treatment algorithms, and no comparative control group. Two of these report on SRS after surgery, and the third focuses on SRS after kyphoplasty.47,48,52
One challenge to this treatment paradigm is the difficulty in delineating and identifying residual tumor and the spinal cord due to imaging artifact from spine hardware.24,53 Particularly for cases in which vertebral column reconstruction has been performed using titanium cages following corpectomy, the proximity of the cage to the spinal cord often precludes appreciation of the extent of decompression due to the imaging artifact generated. Despite the use of CT and MRI fusion, this can pose significant challenges to accurately identifying key anatomic structures. For this reason, CT myelography can be used to better visualize the spinal cord in relation to the surgical hardware in these cases.
Rock et al.48 at the Henry Ford Hospital specifically evaluated the combination of open surgery followed by adjuvant SRS in a series of 18 patients for a wide variety of histopathologies, including metastases, sarcoma, multiple myeloma, and giant-cell tumor. Patients underwent a broad range of surgical interventions for epidural spinal cord compression, including laminectomy and corpectomy with instrumented fusion. SRS with a dose of 6 to 16 Gy prescribed to the 90% isodose line was delivered in a single fraction 2 to 4 weeks after surgery. Ninety-two percent of patients remained neurologically stable or improved, and only one patient became worse due to tumor progression within 1 month of treatment. There were no wound complications following either surgery or SRS. Despite the small number of patients, this is the first study that demonstrated the feasibility and safety of performing SRS postoperatively for spinal metastases.
Moulding et al.47 subsequently reviewed 21 patients who underwent surgical decompression and instrumentation for high-grade epidural spinal cord compression from metastatic tumor at the Memorial Sloan-Kettering Cancer Center followed by single-fraction SRS dosed between 18 and 24 Gy. The mean time from surgery to SRS was 43.9 days (range, 26–63 days), and none of the patients previously received radiation. Ninety-five percent of the treated tumors were radioresistant pathologies, consisting mostly of melanoma, renal cell carcinoma, and sarcoma. Tumor volume was delineated using CT myelography based on the preoperative tumor volume rather than the postoperative residual tumor.
The overall local control was 81%, with an estimated 1-year failure of 9.5%. The authors found that local control was significantly better in the group receiving 24 Gy (94%) than for those receiving less than 18 Gy (60%). Although there were no wound complications after either surgery or SRS, acute grade 1 skin reactions were observed in three patients. One patient experienced acute neuritic pain requiring hospitalization, and esophagitis was seen in three patients, one of whom eventually required surgical repair of a fistula.
Another consideration in the postoperative setting is the effect of radiation dose on instrumentation failure and fusion. Though the surgical bed in cancer patients provides a poor substrate for fusion due to previous or planned radiation, poor nutrition, and limited bone quality, the structural integrity of any instrumented construct is vital for continued pain palliation as patients undergo systemic therapies until death. In a study by Harel et al.,53 43% of patients treated with conventional radiation had evidence of instrumentation failure compared with 0% in the SRS group. Furthermore, fusion rates were 50% in the SRS group versus 17% in the conventional radiation group. Data such as these indicate that like other OARs, the surgical site, in particular the bone-screw interface, may also be subject to a dose-related effect.
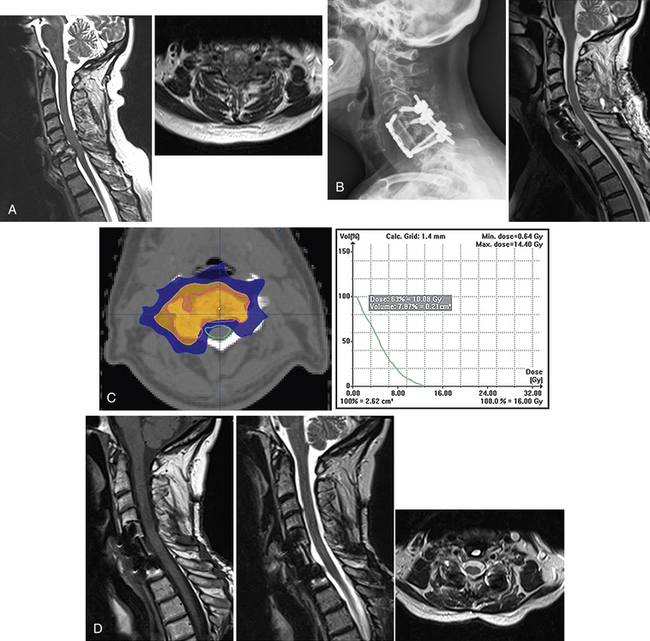
Overall, details specific to the definition of tumor treatment volume, total dose, and fractionation vary significantly among postoperative series. There are no dedicated phase I dose-escalation studies, nor are there any randomized studies testing various SRS dose schemes. Therefore, the optimal practice is unknown, making the widespread application of these principles difficult. Yamada et al.43 reported their retrospective experience in which the dose was escalated over time. Based on their institutional experience, greater rates of local control were observed with a higher single-fraction dose. This has led to their current practice of prescribing 24 Gy in a single fraction. In the three postoperative studies totaling 65 tumors treated with single-fraction SRS with doses between 14 and 24 Gy, local control rates of 81% to 94% were reported without major complications. An example of how SRS is applied in the postoperative setting is illustrated in Figure 176-2.
Concept of “Separation Surgery”
The application of SRS following surgery for spinal metastases is emerging as a new treatment paradigm that challenges previous concepts of palliative postoperative radiation.12 It is also changing how surgery is being approached for these patients. Though the tenets of decompression and stabilization have not changed, the extent of decompression and reconstruction of the vertebral column is now being redefined as the focus has shifted toward performing less morbid surgeries for metastatic disease. Though aggressive surgical strategies including en bloc resections are often utilized to manage primary spinal tumors where there is a chance for cure, surgery for spinal metastases is palliative and directed toward minimizing morbidity and improving function.
En Bloc Resection versus Radiosurgery
With data showing improved rates of tumor control for radioresistant tumors, SRS has since challenged a shift in the treatment paradigm for solitary renal cell metastases without epidural disease.9,10,17,18,49,50 Though the role of en bloc resection for solitary metastases has long been debated among surgeons, largely due to questionable long-term survival and local tumor control following these operations, the application of SRS for upfront treatment of these lesions has introduced a less morbid and arguably just as effective (if not more effective) method of treatment for solitary metastatic spinal lesions.
Because there are no accurate predictors of the propensity for systemic metastases to develop despite aggressive local treatment with either en bloc resection or SRS, a strong argument can be made for not operating on these patients because the likelihood of cure is small.18
Analysis of local tumor control rates after en bloc resection of solitary renal metastases and after SRS reveals similar results. Although there is a paucity of published outcomes reporting local control following en bloc resection, analysis of 40 patients reported in the literature reveals a 7.5% recurrence rate at a median follow-up of 16 months.18 The reported symptomatic and radiographic tumor control failures after SRS range between 6% and 13% with comparable follow-up periods.18 As en bloc tumor resection and SRS appear to have comparable tumor control rates, the less invasive and less morbid option may be the preferred upfront treatment, with surgery reserved for long-term survivors who progress despite SRS.
Complications Avoidance and Management
Despite being a noninvasive treatment, SRS has a potential for spinal cord, cauda equina, or nerve root injury, just as occurs with conventional radiation therapy. Though the incidence of damage to these neural structures is low in published series, considerable effort is made during contouring and dose planning to take advantage of the steep dose fall-off gradient and minimize radiation injury to the spinal cord.32,54 Likewise, the dose of radiation given to nearby organs such as the skin, esophagus, kidneys, and bowel must also be taken into consideration. Though the complications of radiation injury to these tissues are not different from the effect of conventional radiotherapy, the acute effect may be more severe because a larger single dose is given during SRS.
Neurologic Complications
The maximum dose constraint for the spinal cord varies in the literature and is reflective of various institutional practices. Spinal cord constraints are usually set as either a percentage of the spinal cord or a maximum dose to a single voxel on the spinal cord (Dmax). Ryu et al.32 defines spinal cord tolerance as a maximum of 10 Gy to 10% of the spinal cord, whereas Yamada43 reports safely treating to a cord Dmax of 14 Gy. At the Cleveland Clinic, spinal cord tolerance is set as a maximum of 10 Gy to 10% of the spinal cord.
In the largest published series, Gibbs et al.54 reported 6 cases of delayed radiation-induced myelopathy after SRS in 1075 patients. Six patients developed myelopathy at a mean of 6.3 months (range, 2–9 months) after SRS. Three tumors were metastatic in the mid- to upper thoracic spine, and the other three were benign cervical lesions. Two of these cases had been previously irradiated 70 and 80 months prior to SRS at doses of 50.4 and 39.6 Gy in 1.8-Gy fractions. Both of these patients also received an antiangiogenic or epidermal growth factor inhibitor–targeted therapy within 2 months of developing myelopathy, which may have had a radiosensitizing effect. Specific dosimetric factors contributing to this complication could not be identified, but all patients received spinal cord equivalent doses greater than 8 Gy, ranging from 8.5 to 29.9 Gy.
Strategies for treatment of radiation-induced myelopathy include corticosteroids, vitamin E, pentoxifylline, hyperbaric oxygen, or gabapentin.54 Of the six patients in this series, three improved after treatment, two stayed the same, and one progressed to paraplegia. All three patients who showed clinical improvement had complete radiographic resolution of their spinal cord edema on MRI.
Recent human studies have investigated spinal cord tolerance to radiation in the context of conventional radiotherapy, reirradiation, and stereotactic radiosurgery.33,34 With conventional fractionation of 2 Gy per day, total doses of 50 Gy, 60 Gy, and 69 Gy are associated with a 0.2%, 6%, and 50% rate of myelopathy, respectively.34 For reirradiation of the spinal cord after previously fractionated treatment, spinal cord tolerance appears to increase by 25% 6 months after initial treatment.34 With regard to radiosurgery, a maximum cord dose of 13 Gy in a single fraction or 20 Gy in three fractions is associated with a less than 1% risk of myelopathy.34
Organs at Risk
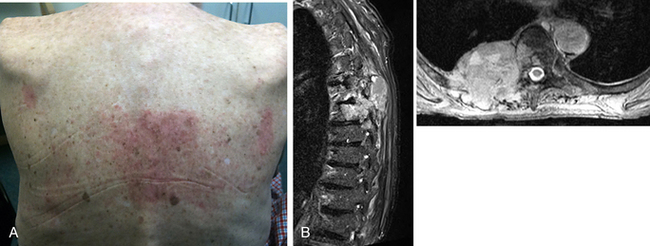
Though other organs are at risk for radiation injury, no significant toxicity has been reported other than grade 1 to 2 skin and esophageal toxicity. The skin may be susceptible to injury in thin patients whose targeted lesions involve the dorsal elements (Fig. 176-3). In previously irradiated patients or patients who recently underwent surgery, a careful examination of the skin or surgical incision is critical prior to treatment planning. Mucositis of the pharynx and esophagus may also occur after treatment of cervical or thoracic lesions. Because these structures are within millimeters of the targeted treatment area, odynophagia, dysphagia, nausea, and even emesis may occur. Most symptoms resolve within days to weeks with appropriate treatment. Renal toxicity must also be considered because careful dosing is important for those patients who have preexisting renal disease or those who have undergone nephrectomy for renal cell cancer or renal transplantation.
Vertebral Body Fracture
Another complication observed following SRS is delayed vertebral body fracture. Rose et al.55 reviewed 62 patients undergoing single-fraction SRS with the planned treatment volume receiving 18 to 24 Gy for solid tumor malignancies at 71 sites and noted a 39% delayed fracture risk of the vertebral body at a median time of 25 months. Patients with prior surgery or radiation therapy were excluded. Multivariate logistic regression analysis showed that CT appearance, lesion location, and percentage of vertebral body involvement by tumor independently predicted fracture progression. Lesions located between T10 and the sacrum were 4.6 times more likely to fracture than were lesions above T10. Lytic lesions were also 6.8 times more likely to fracture than were sclerotic and mixed lesions.
Overall, lytic disease involving more than 40% of the vertebral body and location below T10 led to a high risk of fracture. Other factors such as obesity, dorsal element involvement, bisphosphonate use, and local kyphosis were not associated with increased risk for fracture. The authors suggest that patients at high risk for fracture may benefit from prophylactic cement augmentation of the body involved, though it could be argued that augmentation should be performed following SRS only if a symptomatic fracture develops since 39% of patients in this study developed fractures, of which the number that were symptomatic with worsening pain was not provided.55
Conclusion
As the treatment of metastatic spine tumors continues to evolve with the development of new interventions such as SRS, previously published algorithms that have attempted to define which patients would potentially benefit from surgery have become outdated since none of these account for newer treatments such as vertebral augmentation and SRS.12,18 Despite these antiquated algorithms, patients with epidural compression from radioresistant tumors or spinal instability will continue to require surgery. Because high-grade epidural compression requires that the marginal dose at the spinal cord be reduced, this potentially underdoses the epidural tumor, with resultant continued compression or progression.
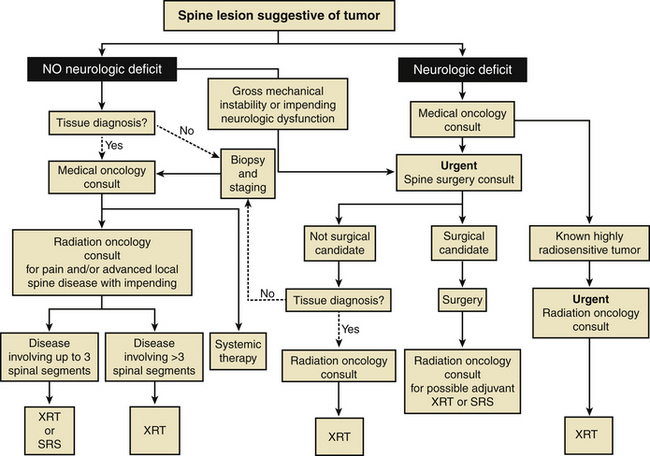
Nonetheless, the paradigm for the treatment of metastatic spine tumors has shifted, and it is imperative that further treatment algorithms and management decision schemes for metastatic disease incorporate SRS as an option for either stand-alone or postoperative treatment12 (Fig. 176-4). With a better understanding of the safety profile and clinical effectiveness of SRS, patient-specific treatment programs can then be tailored with the overall goal of minimizing morbidity, alleviating pain, and improving function and quality of life.
Bilsky M.H., Laufer I., Burch S. Shifting paradigms in the treatment of metastatic spine disease. Spine (Phila Pa 1976). 2009;34:S101-S107.
Gerszten P.C., Burton S.A., Ozhasoglu C., et al. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976). 2007;32:193-199.
Gerszten P.C., Mendel E., Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine (Phila Pa 1976). 2009;34:S78-S92.
Harel R., Angelov L. Spine metastases: current treatments and future directions. Eur J Cancer. 2010;46:2696-2707.
Moulding H.D., Elder J.B., Lis E. Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases. J Neurosurg Spine. 2010;13:87-93.
Patchell R.A., Tibbs P.A., Regine W.F., et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643-648.
Sahgal A., Bilsky M., Chang E.L., et al. Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J Neurosurg Spine. 2011;14:151-166.
1. Perrin R.G., Laxton A.W. Metastatic spine disease: epidemiology, pathophysiology, and evaluation of patients. Neurosurg Clin N Am. 2004;15:365-373.
2. Heidecke V., Rainov N.G., Burkert W. Results and outcome of neurosurgical treatment for extradural metastases in the cervical spine. Acta Neurochir (Wien). 2003;145:873-880. discussion 880–881
3. Ibrahim A., Crockard A., Antonietti P., et al. Does spinal surgery improve the quality of life for those with extradural (spinal) osseous metastases? An international multicenter prospective observational study of 223 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2007. J Neurosurg Spine. 2008;8:271-278.
4. Patchell R.A., Tibbs P.A., Regine W.F., et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643-648.
5. Steinmetz M.P., Mekhail A., Benzel E.C. Management of metastatic tumors of the spine: strategies and operative indications. Neurosurg Focus. 2001;11:e2.
6. Black P. Spinal metastasis: current status and recommended guidelines for management. Neurosurgery. 1979;5:726-746.
7. Gokaslan Z.L., York J.E., Walsh G.L., et al. Transthoracic vertebrectomy for metastatic spinal tumors. J Neurosurg. 1998;89:599-609.
8. Yamada Y., Lovelock D.M., Bilsky M.H. A review of image-guided intensity-modulated radiotherapy for spinal tumors. Neurosurgery. 2007;61:226-235. discussion 235
9. Bartels R.H., Feuth T., van der Maazen R., et al. Development of a model with which to predict the life expectancy of patients with spinal epidural metastasis. Cancer. 2007;110:2042-2049.
10. Bartels R.H., van der Linden Y.M., van der Graaf W.T. Spinal extradural metastasis: review of current treatment options. CA Cancer J Clin. 2008;58:245-259.
11. Cole J.S., Patchell R.A. Metastatic epidural spinal cord compression. Lancet Neurol. 2008;7:459-466.
12. Harel R., Angelov L. Spine metastases: current treatments and future directions. Eur J Cancer. 2010;46:2696-2707.
13. Helweg-Larsen S. Clinical outcome in metastatic spinal cord compression. A prospective study of 153 patients. Acta Neurol Scand. 1996;94:269-275.
14. Helweg-Larsen S., Sorensen P.S., Kreiner S. Prognostic factors in metastatic spinal cord compression: a prospective study using multivariate analysis of variables influencing survival and gait function in 153 patients. Int J Radiat Oncol Biol Phys. 2000;46:1163-1169.
15. Klimo P.Jr., Thompson C.J., Kestle J.R., Schmidt M.H. A meta-analysis of surgery versus conventional radiotherapy for the treatment of metastatic spinal epidural disease. Neuro-oncol. 2005;7:64-76.
16. Maranzano E., Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959-967.
17. Tomita K., Kawahara N., Kobayashi T., et al. Surgical strategy for spinal metastases. Spine (Phila Pa 1976). 2001;26:298-306.
18. Bilsky M.H., Laufer I., Burch S. Shifting paradigms in the treatment of metastatic spine disease. Spine (Phila Pa 1976). 2009;34:S101-S107.
19. Bilsky M., Smith M. Surgical approach to epidural spinal cord compression. Hematol Oncol Clin North Am. 2006;20:1307-1317.
20. Patil C.G., Patil T.S., Lad S.P., Boakye M. Complications and outcomes after spinal cord tumor resection in the United States from 1993 to 2002. Spinal Cord. 2008;46:375-379.
21. Holman P.J., Suki D., McCutcheon I., et al. Surgical management of metastatic disease of the lumbar spine: experience with 139 patients. J Neurosurg Spine. 2005;2:550-563.
22. Fisher C.G., DiPaola C.P., Ryken T.C., et al. A novel classification system for spinal instability in neoplastic disease: an evidence-based approach and expert consensus from the Spine Oncology Study Group. Spine (Phila Pa 1976). 2010;35:E1221-E1229.
23. Gerszten P.C., Mendel E., Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine (Phila Pa 1976). 2009;34:S78-S92.
24. Sahgal A., Bilsky M., Chang E.L., et al. Stereotactic body radiotherapy for spinal metastases: current status, with a focus on its application in the postoperative patient. J Neurosurg Spine. 2011;14:151-166.
25. Ryu S., Fang Yin F., Rock J., et al. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97:2013-2018.
26. Greenberg H.S., Kim J.H., Posner J.B. Epidural spinal cord compression from metastatic tumor: results with a new treatment protocol. Ann Neurol. 1980;8:361-366.
27. Katagiri H., Takahashi M., Inagaki J., et al. Clinical results of nonsurgical treatment for spinal metastases. Int J Radiat Oncol Biol Phys. 1998;42:1127-1232.
28. Maranzano E., Bellavita R., Rossi R., et al. Short-course versus split-course radiotherapy in metastatic spinal cord compression: results of a phase III, randomized, multicenter trial. J Clin Oncol. 2005;23:3358-3365.
29. Rades D., Fehlauer F., Schulte R., et al. Prognostic factors for local control and survival after radiotherapy of metastatic spinal cord compression. J Clin Oncol. 2006;24:3388-3393.
30. Klekamp J., Samii H. Surgical results for spinal metastases. Acta Neurochir (Wien). 1998;140:957-967.
31. Schultheiss T.E. The radiation dose-response of the human spinal cord. Int J Radiat Oncol Biol Phys. 2008;71:1455-1459.
32. Ryu S., Jin J.Y., Jin R., et al. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2007;109:628-636.
33. Sahgal A., Ma L., Weinberg V., et al. Reirradiation HUMAN spinal cord tolerance for stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2012;82:107-116.
34. Kirkpatrick J.P., van der Kogel A.J., Schultheiss T.E. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys. 2010;76:S42-S49.
35. Ghogawala Z., Mansfield F.L., Borges L.F. Spinal radiation before surgical decompression adversely affects outcomes of surgery for symptomatic metastatic spinal cord compression. Spine (Phila Pa 1976). 2001;26:818-824.
36. Rock J.P., Ryu S., Yin F.F. Novalis radiosurgery for metastatic spine tumors. Neurosurg Clin N Am. 2004;15:503-509.
37. Gerszten P.C., Burton S.A., Ozhasoglu C., Welch W.C. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976). 2007;32:193-199.
38. Klish M.D., Watson G.A., Shrieve D.C. Radiation and intensity-modulated radiotherapy for metastatic spine tumors. Neurosurg Clin N Am. 2004;15:481-490.
39. Bilsky M.H., Yamada Y., Yenice K.M., et al. Intensity-modulated stereotactic radiotherapy of paraspinal tumors: a preliminary report. Neurosurgery. 2004;54:823-830. discussion 830–831
40. Chang E.L., Shiu A.S., Lii M.F., et al. Phase I clinical evaluation of near-simultaneous computed tomographic image-guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2004;59:1288-1294.
41. Foote M., Letourneau D., Hyde D., et al. Technique for stereotactic body radiotherapy for spinal metastases. J Clin Neurosci. 2011;18:276-279.
42. Gerszten P.C., Burton S.A., Ozhasoglu C., et al. Radiosurgery for benign intradural spinal tumors. Neurosurgery. 2008;62:887-895. discussion 895–896
43. Yamada Y., Bilsky M.H., Lovelock D.M., et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484-490.
44. Yamada Y., Lovelock D.M., Yenice K.M., et al. Multifractionated image-guided and stereotactic intensity-modulated radiotherapy of paraspinal tumors: a preliminary report. Int J Radiat Oncol Biol Phys. 2005;62:53-61.
45. DeLaney T.F., Liebsch N.J., Pedlow F.X., et al. Phase II study of high-dose photon/proton radiotherapy in the management of spine sarcomas. Int J Radiat Oncol Biol Phys. 2009;74:732-739.
46. Gerszten P.C., Burton S.A., Ozhasoglu C., et al. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg Spine. 2005;3:288-295.
47. Moulding H.D., Elder J.B., Lis E., et al. Local disease control after decompressive surgery and adjuvant high-dose single-fraction radiosurgery for spine metastases. J Neurosurg Spine. 2010;13:87-93.
48. Rock J.P., Ryu S., Shukairy M.S., et al. Postoperative radiosurgery for malignant spinal tumors. Neurosurgery. 2006;58:891-898. discussion 898
49. Tokuhashi Y., Ajiro Y., Umezawa N. Outcome of treatment for spinal metastases using scoring system for preoperative evaluation of prognosis. Spine (Phila Pa 1976). 2009;34:69-73.
50. Tokuhashi Y., Matsuzaki H., Toriyama S., et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 1990;15:1110-1113.
51. Chang E.L., Shiu A.S., Mendel E., et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7:151-160.
52. Gerszten P.C., Germanwala A., Burton S.A., et al. Combination kyphoplasty and spinal radiosurgery: a new treatment paradigm for pathological fractures. J Neurosurg Spine. 2005;3:296-301.
53. Harel R., Chao S., Krishnaney A., et al. Spine instrumentation failure after spine tumor resection and radiation: comparing conventional radiotherapy with stereotactic radiosurgery outcomes. World Neurosurg. 2010;74:517-522.
54. Gibbs I.C., Patil C., Gerszten P.C., et al. Delayed radiation-induced myelopathy after spinal radiosurgery. Neurosurgery. 2009;64:A67-A72.
55. Rose P.S., Laufer I., Boland P.J., et al. Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases. J Clin Oncol. 2009;27:5075-5079.