CHAPTER 262 Stereotactic Radiosurgery for the Treatment of Spinal Metastases
Metastatic spine disease represents a significant source of morbidity in the cancer population. Therapy is aimed at reducing pain, maintaining or improving neurological status, stabilizing the spine, and achieving local tumor control. Traditionally, the principal modalities used to treat spinal metastases are radiation therapy and surgery, although hormonal therapy, immunotherapy, and chemotherapy are playing increasingly large roles for selected tumors. The wide application of technologic advances in both surgery and radiation therapy over the past 5 years has improved patient outcomes. Surgical advances include the application of posterolateral approaches to the spine,1 pedicle screw fixation, and percutaneous augmentation of the vertebral body with cement.2,3 However, the biggest advance has been the development of high-dose conformal photon radiation therapy, which can deliver cytotoxic tumoral doses while sparing normal tissue tolerance, particularly the spinal cord, kidney, and bowel. Because of the steep dose gradient, radiation techniques such as image-guided intensity modulation radiotherapy can deliver high-dose radiation within millimeters of the spinal cord. For spinal metastases, radiation can be delivered as stereotactic radiosurgery (SRS), which is given as a single fraction in doses commonly 10 to 24 Gy.4–6 Hypofractionated radiation therapy or stereotactic body radiation therapy (SBRT) is often delivered in doses of 24 to 30 Gy at 6 to 9 Gy per fraction.7,8 The greatest utility of SRS and SBRT is their ability to treat tumors that are traditionally considered resistant to conventional external beam radiation therapy (cEBRT), such as 24 to 50 Gy at 1.8 to 3 Gy per fraction. A number of published clinical series have shown long-term, durable local tumor control in excess of 15 months for radioresistant tumors when using SRS or SBRT as primary treatment, for reirradiation after failed cEBRT, or as adjuvant therapy postoperatively or after percutaneous cement augmentation.4,5,9–12 Delivery of high-dose photon therapy represents a major advance in the treatment of metastatic spinal tumors in terms of both local tumor and pain control. Additional advantages include shorter treatment times and less soft tissue toxicity than seen with cEBRT.
Conventional External Beam Radiation Therapy
Traditionally, spine tumors have been irradiated with cEBRT at doses of 30 to 50 Gy in fractions of 1.8 to 3 Gy. cEBRT provides a safe therapeutic window to irradiate the spinal cord and surrounding radiosensitive structures, but the response of the tumor is dependent on histology. Conventional fractionation schemes provide durable tumor and pain control for hematologic malignancies such as multiple myeloma and lymphoma and for solid tumors such as breast and prostate carcinoma, but they often fail to control radioresistant solid tumors such as sarcoma, melanoma, renal cell carcinoma, non–small cell lung cancer, and colon carcinoma. Maranzano and Latini presented a prospective trial of 275 consecutive patients undergoing 30-Gy radiation with two different conventional fractionation schedules for metastatic epidural spinal cord compression (ESCC).13 Twenty patients underwent initial surgery for gross spinal instability followed by radiation therapy, and 255 underwent radiation therapy alone. Patients were excluded from analysis for early death (10%) or failure to initiate steroids at the time of radiation therapy (8%) for a total of 205 evaluable patients. Significant relief of biologic pain was achieved in only 70% of patients. Overall, 89% of pretreatment ambulatory patients maintained ambulation, whereas just 60% regained ambulation. In the group that recovered ambulation, 70% had radiosensitive tumor histologies, but radioresistant histologies responded poorly. For example, breast carcinoma had an 80% response rate versus a 20% rate for hepatocellular carcinoma. Additionally, patients with favorable tumor histology had a more durable response of 10 to 16 months, in contrast to unfavorable tumors, which had a response lasting just 1 to 3 months. Similarly, a retrospective analysis from Nagoya University reported the result of 101 patients treated for spinal metastases; 95% of patients received 40-Gy cEBRT at 2 Gy per fraction.14 The cumulative survival rate was 45% at 1 year, but only 20% showed durable pain relief.
Patchell and colleagues also demonstrated lack of response to cEBRT in a prospective cohort study comparing surgery followed by cEBRT with cEBRT alone in myelopathic patients with solid tumor metastases.15 Patients with hematologic malignancies were excluded because of the known radiosensitivity. Surgery followed by cEBRT was significantly better in terms of maintenance and recovery of ambulation, continence, narcotic requirements, and survival. Of the patients who recovered ambulation, 9 of 16 in the surgery group did so but only 3 of 16 in the cEBRT group. All 3 who recovered in the radiation arm crossed over to the surgical arm in keeping with the intention-to-treat paradigm. In this study, no patient regained ambulation with radiation therapy alone. In patients who can tolerate surgery and have a predicted meaningful survival, surgery is the treatment of choice for solid tumors causing high-grade ESCC or myelopathy (or both). This is predicated on the failure of solid tumors to respond to cEBRT.
Stereotactic Radiosurgery
Classic radiobiology teaches that ionizing radiation kills tumor by creating double-stranded breaks in DNA.16 Mitotic cell death is dependent on the biologic effective dose, which is predicted by the linear quadratic model and is dependent on the dose per fraction, number of fractions, and response to therapy denoted as the α/β ratio. Based on the linear quadratic equation, radioresistant tumors are predicted to respond better to radiation given at higher dose per fraction. Although cell killing is primarily dependent on disruption of mitosis, additional factors may affect tumor response, such as apoptosis and damage to stromal cells. Experimental evidence suggests that high-dose single-fraction radiation therapy greater than 8 to 10 Gy activates the acid sphingomyelinase pathway and causes endothelial apoptosis and disruption of blood vessels.17
From a radiation biology standpoint, increasing the dose per fraction increases the biologic effective dose, particularly in the treatment of radioresistant tumors. This observation is underscored in the treatment of intracranial metastases with SRS (18 to 24 Gy), which has demonstrated 85% local control rates at 1 year.18 A large experience exists in treatment of intracranial metastases with SRS, but recent technologic advances have enabled the delivery of SRS to large, irregularly shaped spinal tumors within millimeters of the spinal cord. Photon delivery of cytotoxic tumoral doses within normal tissue tolerance is accomplished by using micromultileaf collimation with inverse treatment planning to deliver image-guided intensity modulation radiotherapy or by using robotic technology to guide the photon beams. A number of devices have been developed to immobilize patients and provide image-guided patient setup and isocenter verification.
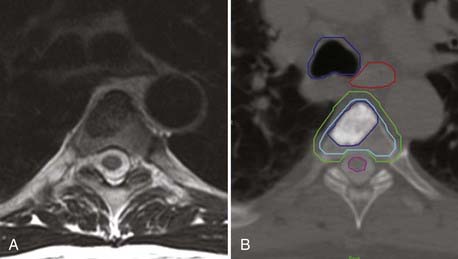
In terms of tumor delineation, the gross tumor volume (GTV) is contoured to precisely identify the tumor that is visualized on MRI and CT. The best images to identify bone tumors are the T1-weighted and T2-STIR (short tau inversion recovery) sagittal images, in which bone tumor is hypointense or hyperintense, respectively. Tumor has variable intensity on T2-weighted images, which is not useful for tumor delineation; however, T2-weighted axial images provide the best assessment of spinal canal impingement and spinal cord compression. From the GTV contour, a clinical target volume (CTV) is drawn to account for microscopic disease outside the defined GTV. As opposed to brain metastases, vertebral body tumors are thought to have an infiltrative penumbra through the entire bone. If the GTV involves a small portion of the vertebral body, the CTV will be defined as the entire vertebral body. The CTV is then extended to define the planning target volume (PTV) to account for uncertainties in setup and delivery and is the actual volume that receives treatment. The PTV is a 2- to 4-mm expansion of the CTV with care taken to not transgress the spinal cord contour based on CT myelography. The PTV is the volume that is treated, although by using dose painting, one can give an additional boost to the GTV or CTV (Fig. 262-1).
Dose escalation to treat spinal metastases has been limited by knowledge of spinal cord tolerance. Spinal cord toxicity is related to the absolute dose, fractionation schedule, and length of spinal cord irradiated. Although not well defined, the toxic dose at which myelopathy develops in 5% of patients at 5 years (TD5/5) is thought to be 50 Gy for a 5- to 10-cm length of spinal cord with conventional fractionation of 1.8 to 2 Gy.19 The Northeast Proton facility reported acceptable toxicity when delivering 60 Gy to the surface of the spinal cord and 56 Gy to the central area via mixed photon and proton beam therapy in conventional fractions.20
The TD5/5 with SRS or SBRT is unknown, but careful dose escalation has provided safe parameters.4 Hypofractionated radiation therapy consisting of 10.4 to 24 Gy at 3.7 to 7 Gy per fraction with treatment of a 1.6-cm3 volume of the spinal cord caused no morbidity at 2 years’ follow-up.19,21 For high-dose single-fraction radiation therapy, the dose to the spinal cord has been defined either as the maximal dose to a single voxel on the spinal cord (cord Dmax) or as the percentage of spinal cord irradiated. Cord Dmax is currently defined at Memorial Sloan-Kettering Cancer Center as 14 Gy to the spinal cord or 16 Gy to the cauda equina. No toxicity was encountered at this dose.4 Ryu and coauthors reported a median dose of 9.8 Gy to 10% of the spinal cord and encountered 1 case of radiation myelopathy in 230 lesions treated.22
Stereotactic Radiosurgery in Current Treatment Paradigms
High-dose single-fraction radiation therapy has been very effective in providing palliation for metastatic spinal tumors, especially radioresistant tumors, either as initial therapy or after failure of cEBRT. In most series, exclusion criteria for SRS include high-grade ESCC from radioresistant tumors and spinal instability.23 The potential morbidity from treating high-grade ESCC with SRS relates to spinal cord tolerance. To avoid radiation myelopathy, one would have to underdose at the margin of the spinal cord and thereby risk progression at the site demanding the highest degree of tumor control. Conversely, delivering a cytotoxic dose to the margin of the dura risks spinal cord injury. For this reason, in accordance with data from Patchell and colleagues,15 resection of high-grade ESCC and myelopathy in solid tumors is recommended rather than treating with SRS or SBRT. The change in treatment paradigms involves the use of SRS as a postoperative adjuvant to gain local control with radioresistant tumors, as opposed to cEBRT.
The second surgical indication is spinal instability. This is broadly defined as movement-related pain, in contradistinction to biologic pain, which is night or morning pain that resolves with steroids and frequently with radiation therapy. Symptoms of spinal instability are dependent on the level of spinal involvement.23 Patients manifest cervical spine instability in flexion-extension. Patients with occipitocervical tumors additionally demonstrate pain with lateral rotation of the head, often in association with occipital neuralgia. Counterintuitively, thoracic instability is often worse in recumbency because patients straighten an unstable kyphosis. Finally, lumbar instability is often manifested as mechanical radiculopathy or severe radicular pain on axial loading. Radiographic criteria in the occipitocervical spine include fracture subluxation greater than 5 mm or 3.5-mm subluxation with 11-degree angulation. In the subaxial cervical and thoracic spine, most instability is seen with a burst or compression fracture and extension into a unilateral joint. Finally, lumbar mechanical radiculopathy is seen with a burst or compression fracture and extension into the neural foramen or joint. Although these patients often require surgery, percutaneous cement augmentation (i.e., vertebroplasty or kyphoplasty) is now being used successfully in patients with burst or compression fractures who have axial load pain in the absence of gross instability.
Outcomes of Stereotactic Radiosurgery
Gerszten and associates reported the outcomes of 500 spinal metastases treated with SRS at 12 to 25 Gy (maximum intratumoral dose).5 The majority of patients (87%) underwent reirradiation after failure of cEBRT. Control of symptoms was achieved in 86% and local durable control in 90%. No treatment-related complications were identified, most notably an absence of radiation-induced myelopathy or plexopathy.
Gerszten and coauthors reported a series of 60 renal cell carcinomas treated with SRS, 48 (80%) of which had previously been treated with cEBRT.9 This series represents a single histology considered to be markedly radioresistant at SRS doses ranging between 14 and 21 Gy. At a median follow-up of 37 months, pain control was reportedly achieved in 89% of patients with pain. Of note, all the failures occurred in the low-dose 14-Gy cohort.
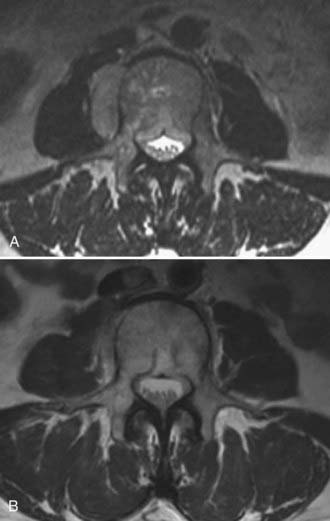
Yamada and coworkers reported the treatment of 103 spinal metastases in 93 patients as initial therapy between 2003 and 2006.4 With the exception of 6 breast carcinomas, all patients were considered to have tumors resistant to cEBRT and were oligo-metastatic. No patient had previously undergone irradiation, and patients with high-grade ESCC or myelopathy (or both) or with gross spinal instability were excluded. Patients received 18- to 24-Gy single-fraction image-guided intensity modulation therapy, with a median dose of 24 Gy. Cord Dmax was less than 14 Gy, and cauda equina Dmax was less than 16 Gy. Images were prospectively obtained at 3- to 4-month intervals or for the development of symptoms during the intervals. At a median time of 15 months, the actuarial control rate was 90%, with 7 local failures identified at a median of 9 months. Tumor responses were independent of histology. A dose-response relationship was seen in which patients who received 24 Gy had a significantly better response than did those who received less than 24 Gy. A second failure analysis showed that all 7 tumors that progressed received less than 15 Gy to a portion of the PTV, particularly at the dural margin, to remain within spinal cord tolerance parameters (Fig. 262-2).
Wowra and associates reported treatment of 134 spinal metastases in 102 patients with SRS.10 The most common tumors were breast cancer (23%), renal cell carcinoma (20%), and gastrointestinal cancers (12%). Patients with spinal cord compression, myelopathy, or instability were excluded. Of note, the median survival in this population from the initial diagnosis of cancer was unusually long at 18 years, probably reflective of the large number of breast cancer patients. Median survival after treatment was 1.4 years, with the only statistical risk factor being the Karnofsky Performance Scale score. At a median follow-up of 15 months, 98% of the tumors showed radiographic control based on the criteria of no interval growth. The two failures occurred in patients with melanoma and a malignant peripheral nerve sheath tumor at 4 and 19 months, respectively. Tumor response was independent of histology. Of the 51 patients with pain, the pretreatment visual analog score was 7, which was reduced to 1 within 1 week after treatment.
Stereotactic Radiosurgery as Postoperative Adjuvant Treatment
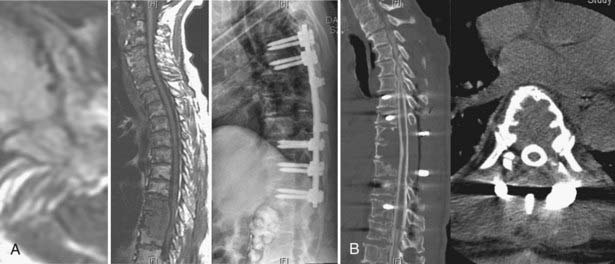
SRS is being effectively used as a postoperative adjuvant to gain local tumor control after surgery to decompress and instrument patients with high-grade spinal cord compression or spinal instability. The theoretical rationale is that one can potentially perform less aggressive tumor resection with the expectation that local tumor control can be achieved with high-dose radiation therapy. This is particularly relevant for radioresistant tumors such as renal cell carcinoma, for which gross total resection or even attempted en bloc resection of the tumor was traditionally thought to be essential for achieving local tumor control. Currently, tumor resection is less aggressive and aimed at epidural decompression and instrumentation to provide stabilization. The need to resect gross disease, including large paraspinal masses, in an attempt to gain local tumor control may be reduced with the use of SRS or SBRT (Fig. 262-3).
One benefit of radiosurgery is the lack of soft tissue injury. Re-exploration after SRS often shows no signs of fibrosis, in contradistinction to the situation after cEBRT. On this basis, SRS is offered in the early postoperative period, as early as 1 week after open surgery. Rock and associates retrospectively reported 18 patients treated with SRS for residual spinal tumors after surgery, including metastatic carcinoma, sarcoma, multiple myeloma/plasmacytoma, and giant cell tumors.11 SRS was offered 2 to 4 weeks postoperatively. The 90% isodose line received a mean of 11.4 Gy (range, 6.6 to 17.6 Gy). Overall, 92% of patients were neurologically stable or improved after the combination of surgery and radiation therapy. The one failure demonstrated neurological deterioration at 1 month, most likely from tumor progression and not radiation-induced myelopathy.
Unlike proton beam therapy, photons can be delivered with titanium or polyetheretherketone (PEEK) carbon fiber hardware adjacent to the treatment area. Pekmezci and colleagues examined the dose distributions of photon radiation with the use of various combinations of anterior and posterior titanium implants in a sawbones model.24 When compared with sawbones alone, posterior instrumentation resulted in a 5% to 7% decrease in radiation dose to the spinal canal, and anterior constructs resulted in a 1% decrease. High-dose photon radiation may be delivered safely in the presence of titanium hardware, although dose perturbation characteristics should be accounted for.
Stereotactic Radiosurgery after Percutaneous Cement Augmentation
Percutaneous cement augmentation has shown efficacy in the treatment of pathologic burst or compression fractures. A number of centers have reported excellent, durable pain relief in more than 80% of patients. Gerszten and coauthors reported the combined use of kyphoplasty and SRS to treat pathologic fractures from solid tumors of the thoracic and lumbar spine in 26 patients.12 Treatment volumes and contours were determined before cement augmentation, and SRS was performed an average of 12 days after the procedure. The tumor dose was maintained between 16 and 20 Gy to the 80% isodose line. Pain relief after kyphoplasty alone was achieved in 25 of 26 (96%) patients and long-term (11 to 24 months) relief after kyphoplasty and SRS in 24 of 26 (92%) patients. SRS is effective after percutaneous cement augmentation and should be used to achieve local tumor control.
Stereotactic Radiosurgery for High-Grade Spinal Cord Compression
Treatment of myelopathy or high-grade ESCC is somewhat controversial. The dose constraints of the spinal cord present significant challenges in delivering cytotoxic doses to the spinal cord despite the steep dose gradient provided by conformal photon delivery systems. Chang and coworkers reported patterns of failure seen when using a hypofractionated prescription dose of three fractions of 900 cGy in 63 patients.7 Seventeen recurrences occurred either at adjacent segments or in the epidural space. Epidural progression was a result of underdosing tumor at the dural margin (10 Gy) because of concern regarding spinal cord toxicity.
In the outcome study of 500 patients presented by Gerszten and colleagues, 35 had progressive neurological deficits.5 Thirty (85%) patients improved after radiation therapy. All 5 failures had progressed through previous cEBRT. No toxicity was noted even with high-dose, single-fraction radiotherapy. Better definitions of spinal cord tolerance may ultimately facilitate treatment of high-grade ESCC and thereby obviate the need for surgical decompression in these patients. However, current recommendations are that epidural disease be decompressed before delivering SRS. This recommendation is based primarily on concern for spinal cord toxicity. Additionally, responses to SRS are cytotoxic but often do not result in a reduction in tumor size. Thus, successful tumor killing may not decompress the spinal cord.
Reirradiation
A great advantage of highly conformal photon radiation therapy is the ability to reirradiate patients after failed cEBRT within spinal cord tolerance. Many centers have used SBRT to reirradiate spine metastases. Rades and coauthors reported a series of 124 patients who underwent reirradiation with a variety of fractionation schemes.25 A cumulative biologic effective dose of less than 120 Gy to the spinal cord resulted in no spinal cord injuries. Wright and associates presented a series of 37 patients reirradiated with 20 Gy at 4 Gy per fraction or with 30 Gy at 5 Gy per fraction.26 The median spinal cord or cauda equina dose was 9.9 Gy, and the cumulative dose was 42 Gy. The median time between initial cEBRT and hypofractionated reirradiation was 13 months. The probability of local control was 60% at a median follow-up of 8 months. No patient experienced radiation-induced myelopathy.
Although the results of hypofractionated image-guided intensity modulation radiotherapy are encouraging, Gerszten and colleagues have proved that SRS can be administered safely within the constraints of spinal cord tolerance and achieve control rates of 90% at 36 months’ follow-up.9 The ultimate goal in reirradiation is high-dose single-fraction treatment, which may provide better local control rates than hypofractionated schedules.
Complications
The most common complications from spine SRS have been minor grade 1 and 2 skin and esophageal toxicity. In 1 patient, a tracheoesophageal fistula thought to be related to doxorubicin (Adriamycin) recall developed. Nausea, diarrhea, and trismus have also been reported in up to 9% of patients.7,10 One patient has been reported with an intratumoral hemorrhage. To date, there have been 4 reported cases of myelopathy in the metastatic population.8,11 Vertebral body fractures are commonly seen after SRS, and these are now considered to be an organ at risk. In a review of 62 patients treated at 71 sites, 27 (38%) had progressive, vertebral body fractures, only 7 (11%) of which were associated with disease progression. Risk for fracture after SRS has been associated with lytic bone disease (6.8-fold) and thoracolumbar or lumbar tumor location. Additionally, patients with 40% to 80% lytic destruction of the vertebral body had an 85% (11/13) chance of progressive fracture. Bisphosphonate therapy may reduce the risk for fracture, although the mechanism of fracture is probably osteoblast inhibition or cell death rather than osteoclast activation. A role for prophylactic vertebral body cement augmentation is being explored.
Bilsky M, Smith M. Surgical approach to epidural spinal cord compression. Hematol Oncol Clin North Am. 2006;20:1307.
Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7:151.
Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109.
Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98:21.
Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155.
Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32:193.
Gerszten PC, Burton SA, Ozhasoglu C, et al. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg Spine. 2005;3:288.
Gerszten PC, Germanwala A, Burton SA, et al. Combination kyphoplasty and spinal radiosurgery: a new treatment paradigm for pathological fractures. J Neurosurg Spine. 2005;3:296.
Gibbs IC, Kamnerdsupaphon P, Ryu MR, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol. 2007;82:185.
Hall E, Amato J. Radiobiology for the Radiologist, 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006.
Hentschel SJ, Burton AW, Fourney DR, et al. Percutaneous vertebroplasty and kyphoplasty performed at a cancer center: refuting proposed contraindications. J Neurosurg Spine. 2005;2:436.
Katagiri H, Takahashi M, Inagaki J, et al. Clinical results of nonsurgical treatment for spinal metastases. Int J Radiat Oncol Biol Phys. 1998;42:1127.
Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959.
Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643.
Pekmezci M, Dirican B, Yapici B, et al. Spinal implants and radiation therapy: the effect of various configurations of titanium implant systems in a single-level vertebral metastasis model. J Bone Joint Surg Am. 2006;88:1093.
Rades D, Rudat V, Veninga T, et al. Prognostic factors for functional outcome and survival after reirradiation for in-field recurrences of metastatic spinal cord compression. Cancer. 2008;113:1090.
Rock JP, Ryu S, Shukairy MS, et al. Postoperative radiosurgery for malignant spinal tumors. Neurosurgery. 2006;58:891.
Ryu S, Jin JY, Jin R, et al. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2007;109:628.
Ryu S, Jin R, Jin JY, et al. Pain control by image-guided radiosurgery for solitary spinal metastasis. J Pain Symptom Manage. 2008;35:292.
Wang JC, Boland P, Mitra N, et al. Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:287.
Wara WM, Phillips TL, Sheline GE, et al. Radiation tolerance of the spinal cord. Cancer. 1975;35:1558.
Weber DC, Trofimov AV, Delaney TF, et al. A treatment planning comparison of intensity modulated photon and proton therapy for paraspinal sarcomas. Int J Radiat Oncol Biol Phys. 2004;58:1596.
Wowra B, Zausinger S, Drexler C, et al. CyberKnife radiosurgery for malignant spinal tumors: characterization of well-suited patients. Spine. 2008;33:2929.
Wright JL, Lovelock DM, Bilsky MH, et al. Clinical outcomes after reirradiation of paraspinal tumors. Am J Clin Oncol. 2006;29:495.
Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484.
Yamada Y, Lovelock DM, Bilsky MH. A review of image-guided intensity-modulated radiotherapy for spinal tumors. Neurosurgery. 2007;61:226.
1 Wang JC, Boland P, Mitra N, et al. Single-stage posterolateral transpedicular approach for resection of epidural metastatic spine tumors involving the vertebral body with circumferential reconstruction: results in 140 patients. Invited submission from the Joint Section Meeting on Disorders of the Spine and Peripheral Nerves, March 2004. J Neurosurg Spine. 2004;1:287.
2 Fourney DR, Schomer DF, Nader R, et al. Percutaneous vertebroplasty and kyphoplasty for painful vertebral body fractures in cancer patients. J Neurosurg. 2003;98:21.
3 Hentschel SJ, Burton AW, Fourney DR, et al. Percutaneous vertebroplasty and kyphoplasty performed at a cancer center: refuting proposed contraindications. J Neurosurg Spine. 2005;2:436.
4 Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484.
5 Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32:193.
6 Ryu S, Jin R, Jin JY, et al. Pain control by image-guided radiosurgery for solitary spinal metastasis. J Pain Symptom Manage. 2008;35:292.
7 Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7:151.
8 Gibbs IC, Kamnerdsupaphon P, Ryu MR, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol. 2007;82:185.
9 Gerszten PC, Burton SA, Ozhasoglu C, et al. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg Spine. 2005;3:288.
10 Wowra B, Zausinger S, Drexler C, et al. CyberKnife radiosurgery for malignant spinal tumors: characterization of well-suited patients. Spine. 2008;33:2929.
11 Rock JP, Ryu S, Shukairy MS, et al. Postoperative radiosurgery for malignant spinal tumors. Neurosurgery. 2006;58:891.
12 Gerszten PC, Germanwala A, Burton SA, et al. Combination kyphoplasty and spinal radiosurgery: a new treatment paradigm for pathological fractures. J Neurosurg Spine. 2005;3:296.
13 Maranzano E, Latini P. Effectiveness of radiation therapy without surgery in metastatic spinal cord compression: final results from a prospective trial. Int J Radiat Oncol Biol Phys. 1995;32:959.
14 Katagiri H, Takahashi M, Inagaki J, et al. Clinical results of nonsurgical treatment for spinal metastases. Int J Radiat Oncol Biol Phys. 1998;42:1127.
15 Patchell RA, Tibbs PA, Regine WF, et al. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomised trial. Lancet. 2005;366:643.
16 Hall E, Amato J. Radiobiology for the Radiologist, 6th ed. Philadelphia: Lippincott Williams & Wilkins; 2006.
17 Garcia-Barros M, Paris F, Cordon-Cardo C, et al. Tumor response to radiotherapy regulated by endothelial cell apoptosis. Science. 2003;300:1155.
18 Yamada Y, Lovelock DM, Bilsky MH. A review of image-guided intensity-modulated radiotherapy for spinal tumors. Neurosurgery. 2007;61:226.
19 Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109.
20 Weber DC, Trofimov AV, Delaney TF, et al. A treatment planning comparison of intensity modulated photon and proton therapy for paraspinal sarcomas. Int J Radiat Oncol Biol Phys. 2004;58:1596.
21 Wara WM, Phillips TL, Sheline GE, et al. Radiation tolerance of the spinal cord. Cancer. 1975;35:1558.
22 Ryu S, Jin JY, Jin R, et al. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2007;109:628.
23 Bilsky M, Smith M. Surgical approach to epidural spinal cord compression. Hematol Oncol Clin North Am. 2006;20:1307.
24 Pekmezci M, Dirican B, Yapici B, et al. Spinal implants and radiation therapy: the effect of various configurations of titanium implant systems in a single-level vertebral metastasis model. J Bone Joint Surg Am. 2006;88:1093.
25 Rades D, Rudat V, Veninga T, et al. Prognostic factors for functional outcome and survival after reirradiation for in-field recurrences of metastatic spinal cord compression. Cancer. 2008;113:1090.
26 Wright JL, Lovelock DM, Bilsky MH, et al. Clinical outcomes after reirradiation of paraspinal tumors. Am J Clin Oncol. 2006;29:495.