49 Stereotactic Radiosurgery for Spine Tumors
KEY POINTS
Radiosurgery
Clinical Case Examples
Case 1
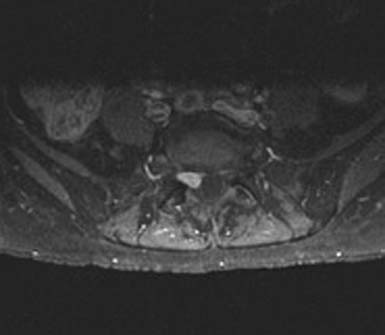
MC, a 66-year-old woman with medical contraindications to open surgery, was treated with SRS for a spinal schwannoma. She initially presented with a chronic cough and generalized weakness. Routine laboratory studies showed pancytopenia, and flow cytometry was consistent with acute lymphocytic leukemia. The diagnosis was confirmed by bone marrow biopsy. While undergoing chemotherapy in December 2008, she developed lower back pain with subjective right leg weakness. A 10-by 7-mm epidural lesion, compressing the S1 nerve root, was seen on MRI (Figure 49-1). A CT-guided biopsy was consistent with schwannoma, but definitive treatment was postponed because of her leukemia. By July 2009, the pain became intolerable. She had 4/5 gastrocnemius weakness, numbness in the S1 distribution, and loss of the ankle reflex. A repeat MRI confirmed an increase in the size of the schwannoma. Open surgery remained high risk, so in September 2009, the patient underwent CyberKnife treatment. She received 16 Gray (Gy) in a single session. Pain complaints improved, and all examination findings resolved over 2 months.
Case 2
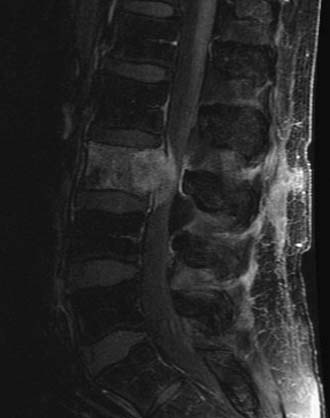
WF is a 68-year-old man with melanoma who received SRS treatment for a recurrent spinal metastasis in a previously irradiated field. The patient was initially seen with a melanoma of the back in 1999 and another of the neck in 2003. Following local resections, he remained disease-free until March 2007, when, after complaining of low back pain, he was found to have a 4 × 3 cm lesion of the L3 vertebral body. There was significant compression of the cauda equina due to epidural extension. A PET-CT showed hypermetabolic areas in the lung, bone, and brain. He received conventional radiotherapy of 37.5 Gy to the brain and 37.5 Gy to the lumbar spine. In September 2009, the patient returned with increasing back pain, associated with weakness. His strength was 4/5 in the right leg but his sensation was intact. A follow-up PET-CT and MRI showed multiple new lesions and enlargement of the previously treated L3 mass (Figure 49-2). Additional conventional radiotherapy was not an option and a surgical decompression was contraindicated based on his other medical problems. The L3 lesion was treated with CyberKnife SRS, 24 Gy in three sessions. His symptoms improved.
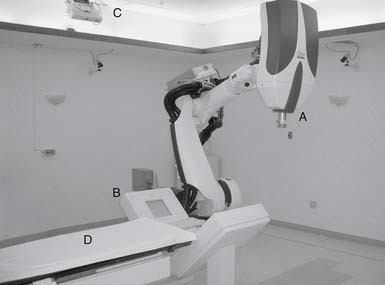
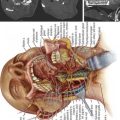
linear accelerator attached to an industrial robot (Figure 49-3). The robotic arm is unconstrained, using six degrees of freedom to deliver beams to virtually any part of the body from a wide range of angles. During treatment, real-time orthogonal images of the patient are obtained frequently, enabling the system to identify and automatically correct for small changes in patient position.
Indications for Spinal Radiosurgery
Indications for spinal SRS continue to evolve (Tables 49-1 and 49-2). The most commonly treated spinal lesions are metastatic (Table 49-3). A biopsy may not be necessary prior to treatment if the diagnosis is clear from the clinical history and imaging. Ideally, lesions should be less than 5 cm in maximal diameter, well demarcated, and clearly seen on CT and/or MRI. For most tumors, local control rates are equivalent or superior to conventional radiation and complications are generally lower than with open surgery. In some particular cases, spinal SRS may be useful for ablating the more radioresistant tumors.1 However, in those previously irradiated patients where the adjacent spinal cord has already received the maximum tolerated radiation dosage, the efficacy of spinal radiosurgery may be compromised because of the need to lower the radiosurgical dose.
| Tumors that are highly radiosensitive. |
| Post-resection cavity |
| Post-radiation therapy local irradiation |
| Recurrent disease post surgery and/or irradiation |
| Inoperable lesion |
| High-risk location of lesion |
| Slowly progressive but minimal neurological deficits |
| Patient with medical comorbidities that preclude surgery |
| Patient declines surgery. |
TABLE 49-2 Contraindications for Spinal SRS
| Spinal instability |
| Neurological deficit due to physical spinal cord or nerve root compression |
| Adjacent cord previously irradiated to the maximum dosage |
| Generalized metastatic involvement of the axial skeleton |
| Epidural carcinomatosis |
TABLE 49-3 Lesions Treatable with CyberKnife Radiosurgery
| Tumors |
| Benign |
Treatment Details
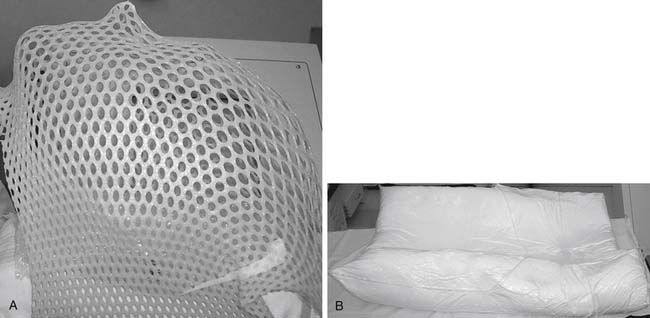
Image-guided systems do not require rigid immobilization or invasive frames. Instead, noninvasive custom masks or cradles are made for each patient and used during image acquisition and radiosurgery. These devices improve comfort, expedite alignment, and limit movement. For upper cervical lesions, a thermoplastic mask is made for each patient (Aquaplast, WFR Corp., Wyckoff, NJ; Figure 49-4A). For thoracic and lumbar lesions a custom vacuum-molded body cradle is used (AlphaCradle, Smithers Medical Products, Inc., Akron, OH; Figure 49-4B). For some cervicothoracic lesions, both devices are utilized.
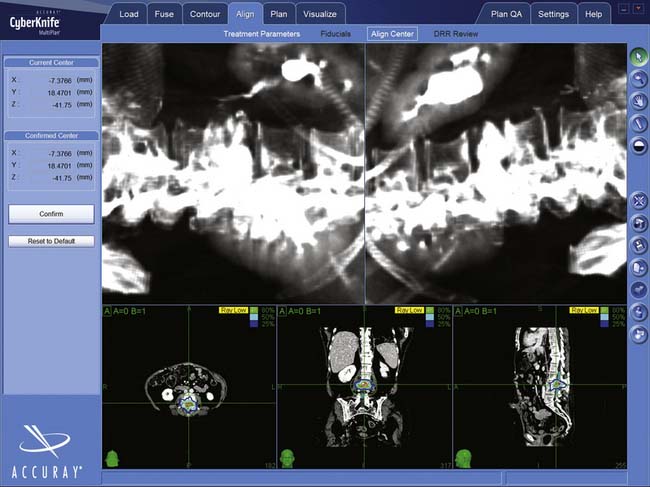
Bony landmarks of the spine are used to target cervical, thoracic, and lumbar lesions, as well as some pelvic lesions, scapular and rib head masses, and paravertebral soft tissue tumors. The presence of spinal stabilization hardware does not interfere with target localization. Digitally-reconstructed radiographs (DRRs) are created as part of the treatment plan and are used to establish the relationship of the target to regional bony landmarks. The accuracy of CyberKnife using bony landmarks approaches ±0.5 mm2.
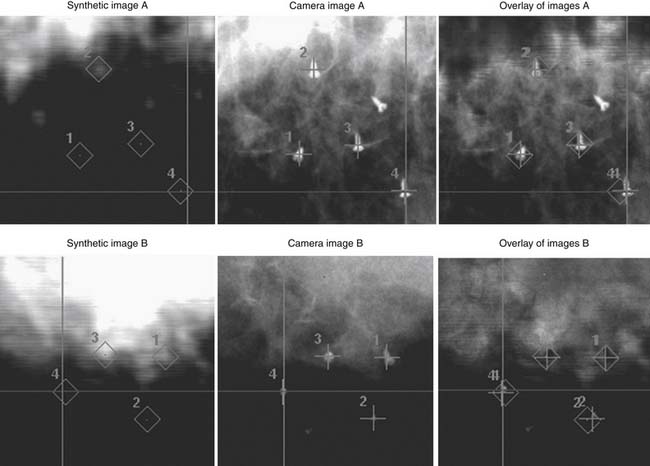
For lesions not associated with bony landmarks, or where there is severe osteoporosis, localization may be based on implanted fiducials. Stainless steel screws in adjacent bone, or “gold seeds” adjacent to or within the lesion, can be inserted prior to imaging (Figure 49-5). A minimum of three clearly visible, non-collinear fiducials is needed. Ideally, they are placed in bone or firm tissue, surround the target lesion, and do not overlap in 45° oblique images. Prior to treatment delivery, the tumor location relative to the implants or bony landmarks is established based on DRRs. The accuracy using implanted fiducials may be lower than with bony landmarks and depends on the number and location of the implants.2
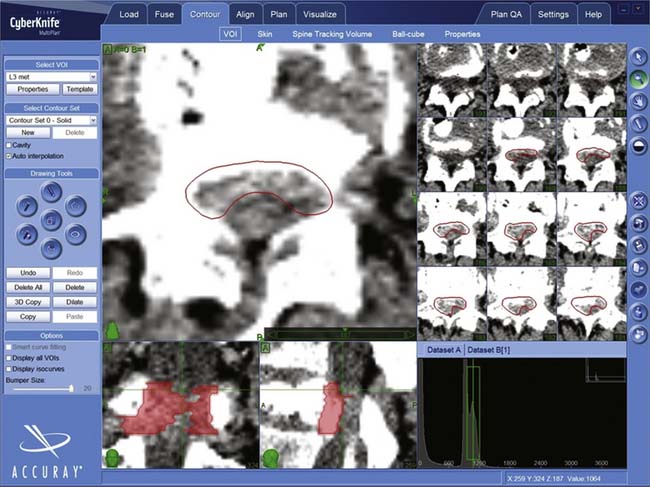
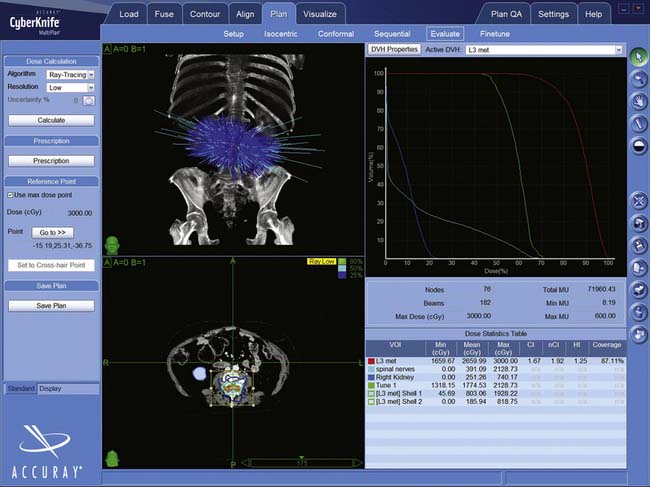
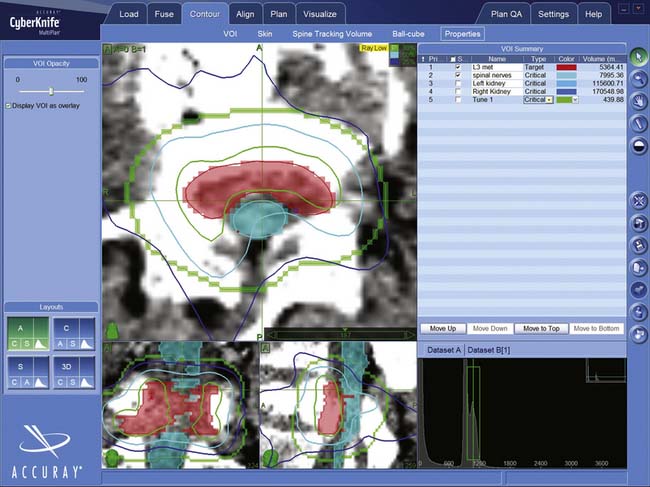
Most patients are imaged and treated supine. Treatment planning begins with a fine cut CT scan, (1.25-mm slices). The CT has the special resolution of available technologies and is required to delineate the lesion (Figure 49-6) and create the DRRs used for localization (Figure 49-7). MRIs, positron emission tomography (PET) scans, or three-dimensional (3-D) angiograms are commonly used in addition. Treatment plans for CyberKnife are designed using the Accuray Multiplan System (Figure 49-8). The various stereotactic image sets needed for target definition are transferred to the planning computer and aligned to one another using a semi-automatic process. Utilizing a graphic interface, the surgeon outlines the target lesion and adjacent radiation-sensitive structures, such as the spinal cord, esophagus, or kidneys, creating a 3-D representation of relevant anatomy (Figure 49-9). A dose and treatment schedule is specified by the surgeon and the radiation oncologist. A radiation physicist computes treatment plans, seeking an optimal dose conformation and a corresponding array of treatment beams. Physical parameters are adjusted and refined iteratively until an optimal plan is obtained. Ideally, the beams are evenly distributed over the surface of the target, the target receives at least the prescribed dose, and the dose to adjacent structures is minimized.
Treatment of Spinal Metastases
In older populations, the majority of spinal tumors are metastatic (see Case 2). Forty percent of cancer patients develop at least one spinal metastasis. SRS is perhaps the least invasive of available treatments, and can deliver much higher doses than conventional radiotherapy while limiting cord exposure. SRS generally takes 1 to 3 days, while conventional radiotherapy may require 4 to 6 weeks. Multiple lesions can be treated safely and, because of the shorter treatment schedules, the treatment of asynchronous metastases is more convenient. SRS is appropriate as an adjuvant following a debulking procedure or in conjunction with a stabilization procedure such as fusion or vertebroplasty. SRS can be a good treatment modality for those with limited life expectancies, or those undergoing other concurrent treatments. Spinal radiosurgery can be highly effective in controlling pain, such as in Case 1, with up to 100% of patients reporting relief in some series.3
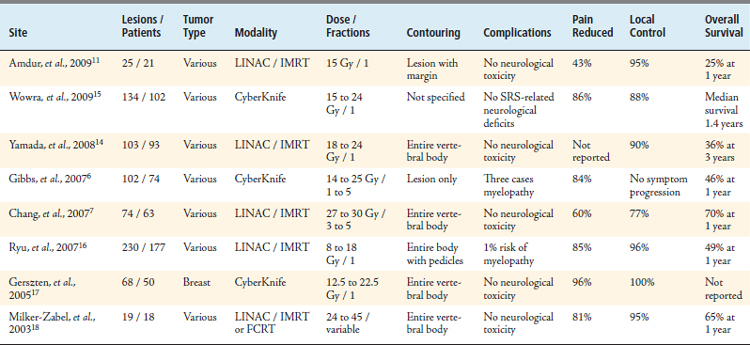
Debate continues regarding the most appropriate treatment margins. Some centers radiate only tumor seen on MRI, while others recommend treating the entire affected vertebral body including pedicles. Up to 18% of local failures are due to recurrences in the pedicles.4 Amdur et al5 advocate treating visible tumor plus a 1-cm margin in bone or a 2-mm volume beyond the cortex. We typically treat only the volume of tumor seen on CT or MRI. There are no studies that clearly demonstrate a benefit of one approach over the other. Dose recommendations are variable, with single session prescriptions ranging from 8 to 24 Gy in the published literature.5 We use 16 to 25 Gy in one to three fractions, depending on tumor type. Local control is achieved in 77% to 100% of cases, and control rates are independent of histopathology (Table 49-4).
Treatment of Intradural Extramedullary Lesions
In our institution, we have treated 110 patients with 117 lesions6 (unpublished data). Fifty-six percent of schwannomas (see Case 1) and meningiomas have stabilized after SRS and 44% have regressed radiographically. Neurofibromas did less well, with 11% enlarging, and up to 80% of patients reporting progressive neurological deficits. We have observed that most myelopathies and radiculopathies improve after SRS treatment. Two of our SRS-treated patients required open resection for tumor enlargement. Three needed surgery for persistent or progressing symptoms. One patient developed a radiation-induced myelopathy.
Treatment of Intramedullary Lesions
Sixteen of the 92 hemangioblastomas treated in our institution were spinal intramedullary tumors. These were treated with a median radiosurgical dose of 23 Gy. After a median follow-up of 34 months, 15 of the 16 spinal hemangioblastomas either decreased or remained the same in size. Intramedullary hemangioblastomas associated with significant edema or cysts might do less well, based on our experience with similar intracranial lesions.
Although the data for ependymomas are limited, a few published studies have shown SRS to be efficacious.7 We know less about SRS for spinal astrocytomas, but for those which are well circumscribed, spinal SRS may be an appropriate alternative to surgery.
Intramedullary spinal cord metastases are rarely seen. They constitute only 8.5% of central nervous system metastases,8 but their frequency will likely increase with longer patient survival and as the population ages. Wowra et al9 reported that 96% of spinal metastases were well controlled with spinal SRS and that the risk of radiation myelopathy was less than 1%.
Complications
Neurological complications of SRS are categorized by their time of onset. Acute complications occur within a month and are usually transient. They are related to edema and can be treated with steroids. Subacute complications occur 3 to 6 months after treatment and are usually secondary to demyelination. The prognosis for recovery is good. Radiation-induced myelopathy, the most feared side effect of SRS, is a late effect, occurs after 6 months, and is usually irreversible. In 1000 patients treated with CyberKnife for spinal lesions, six developed myelopathy (0.6%).10 To prevent radiation-induced myelopathy, we avoid exposing more than one cubic centimeter of spinal cord to more than 8 Gy in single session plans.
Conclusion
The successes of intracranial radiosurgery inspired the development of spinal SRS. Many spinal lesions may not be amenable to complete surgical resection. SRS is both safe and effective treatment for metastatic lesions of the spine and for some intradural extramedullary tumors. Early results in treating intramedullary lesions are encouraging. Spinal SRS, a completely noninvasive treatment, is particularly suited for older patients and those with significant concomitant medical problems.
1. Henderson F.C., McCool K., Seigle J., Jean W., Harter W., Gagnon G.J. Treatment of chordomas with CyberKnife: Georgetown University experience and treatment recommendations. Neurosurgery. 2009;64(Suppl. 2):A44-A53.
2. Ryu S., Fang Yin F., Rock J., Zhu J., Chu A., Kagan E., Rogers L., Ajlouni M., Rosenblum M., Kim J.H. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97:2013-2018.
3. Gerszten P.C., Burton S.A., Welch W.C., Brufsky A.M., Lembersky B.C., Ozhasoglu C., Vogel W.J. Single-fraction radiosurgery for the treatment of spinal breast metastases. Cancer. 2005;104:2244-2254.
4. Chang E.L., Shiu A.S., Mendel E., Mathews L.A., Mahajan A., Allen P.K., Weinberg J.S., Brown B.W., Wang X.S., Woo S.Y., Cleeland C., Maor M.H., Rhines L.D. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J. Neurosurg. Spine. 2007;7:151-160.
5. Amdur R.J., Bennett J., Olivier K., Wallace A., Morris C.G., Liu C., Mendenhall W.M. A prospective phase II study demonstrating the potential value and limitation of radiosurgery for spine metastases. Am. J. Clin. Onc.. 2009;32:1-6.
6. Dodd R.L., Ryu M.R., Kamnerdsupaphon P., Gibbs I.C., Chang S.D., Adler J.R. CyberKnife radiosurgery for benign intradural extramedullary spinal tumors. Neurosurgery. 2006;58:674-685.
7. Ryu S.I., Kim D.H., Chang S.D. Stereotactic radiosurgery for hemangiomas and ependymomas of the spinal cord. Neurosurg. Focus. 2003;15(15(5)):E10.
8. Parikh S., Heron D.E. Fractionated radiosurgical management of intramedullary spinal cord metastasis: a case report and review of the literature. Clin. Neurol. Neurosurg.. 2009;111:858-861.
9. Wowra B., Zausinger S., Drexler C., Kufeld M., Muacevic A., Staehler M., Tonn J.C. CyberKnife radiosurgery for malignant spinal tumors: characterization of well-suited patients. Spine. 2008;33:2929-2934.
10. Gibbs I.C., Patil C., Gerszten P.C., Adler J.R.Jr., Burton S.A. Delayed radiation-induced myelopathy after spinal radiosurgery. Neurosurgery. 2009;64:A67-A72.