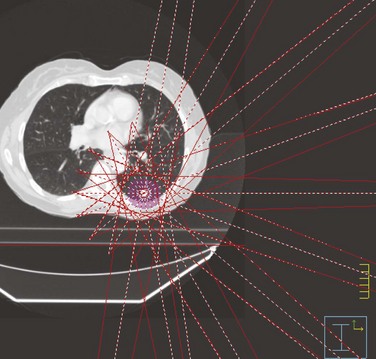
Chapter 17 Stereotactic Irradiation
Linear Accelerator and Gamma Knife
Radiosurgery was first described in 1951 by Lars Leksell,1 a neurosurgeon at the Karolinska Institute in Stockholm, Sweden. The term radiosurgery was selected because of the similarity of this technique to stereotactic neurosurgery. The initial work by Leksell first involved the treatment of patients with functional disorders and benign conditions such as chronic pain and arteriovenous malformations but later included benign and malignant tumors. The first patient was treated with the Gamma Knife in 1968.2 The limited availability, skepticism regarding the technology, and prohibitive costs initially limited the spread of Gamma Knife technology. By the 1980s, linear accelerator-based radiosurgery had evolved and further renewed the interest in stereotactic radiosurgery.
Basic Principles Of Radiosurgery
Radiosurgery can be performed using various devices, including the multicobalt unit known commercially as the Gamma Knife (GK), particle beam devices, or modified linear accelerators. With technologic advances in software and hardware, a clear superiority of one technology over another has disappeared and mature clinical results demonstrate no outcome differences based on technology.3 A comparison of some of the features of the Gamma Knife and linear accelerator techniques is presented in Table 17-1. Because the linear accelerator-based units can also treat non-radiosurgery patients during “downtimes,” cost and volume considerations are key features that determine equipment selection.3
TABLE 17-1 Comparing Gamma Knife and Linear Accelerator Radiosurgery Characteristics
| Gamma Knife | Linac Radiosurgery | |
|---|---|---|
| Clinical experience | Over three decades | Over two decades |
| Accuracy | Submillimeter | Submillimeter |
| QA | Fewer QA checks | More QA checks |
| Machine use | Dedicated machine | Usually not a dedicated machine |
| Functional disorders | Longer experience | Shorter experience |
| Can be used for extracranial radiosurgery | No | Yes |
| Price | High; must replace source every 5-7 years | Less expensive; no source replacement |
| Tumor location | Difficult to radiate peripheral lesions | Can treat peripheral lesions |
| Fractionated treatments | Not practical | Can treat with fractionated regimens |
| Treatment time | About equal | About equal |
QA, quality assurance.
Radiosurgical and neurosurgical approaches are often complementary, but there are key differences. Radiosurgery does not require a craniotomy or general anesthesia and is performed on an outpatient basis. In a cost-effectiveness/cost-utility analysis by Mehta and colleagues4 in the 1990s, the authors showed that the average cost per week of survival for a single brain metastasis was $310 for radiotherapy, $524 for resection plus irradiation, and only $270 for radiosurgery plus whole-brain irradiation. With the advent of shorter hospital stays following neurosurgical procedures, these numbers have probably changed somewhat. Other advantages include lower postoperative risks for bleeding or infection and rapid recovery times. Patients who are working before treatment often can return to work within 1 or 2 days. More importantly, neurosurgically inaccessible lesions and patients deemed unfit for surgery or anesthesia can often be treated with radiosurgery.
The clinical applications of radiosurgery have grown substantially over the past decade. There are multiple disease processes where the use of stereotactic radiosurgery has a well-established and defined role. Current indications, discussed later in greater detail, include arteriovenous malformations, benign brain tumors, malignant brain tumors, and functional disorders (Table 17-2). With improved ability to localize targets throughout the body, the principles of radiosurgery have expanded to include extracranial applications, with emerging roles in the management of lung, hepatic, and spinal tumors, among others. Some concepts of the radiosurgery paradigm have been incorporated into a strategy employing fractionated treatments; this approach, referred to as fractionated stereotactic radiotherapy (FSRT), is applied to both intracranial and extracranial lesions.
Radiosurgery is an important tool available to the neurosurgeon and radiation oncologist. Proper implementation of the treatment requires collaboration among neurosurgeons, radiation oncologists, and medical physicists. This allows for thoughtful coordination of care, improved quality assurance, reduction in practice variation, a strategic marketing advantage, and improved patient satisfaction. Each specialty brings unique skills that are required for a successful radiosurgery program.5
Physics Of Radiosurgery
Stereotactic radiosurgery (SRS) involves the use of numerous beamlets of radiation aimed precisely at an immobilized target to deliver a single session of high-dose radiation. Though no single beamlet carries significant weight, a large dose is deposited at the intersection of these beamlets with a steep dose fall-off outside the target. As tumor size increases, this fall-off becomes shallower6; typically, radiosurgery becomes prohibitive at sizes in excess of 4 to 5 cm.
Physics Fundamentals
Multicobalt units containing cobalt-60 (60Co) sources include devices such as the Gamma Knife and the Rotating Gamma System (developed in China) (Figs. 17-1 and 17-2). 60Co decays with a half-life of 5.26 years, requiring that sources be replaced every 5 to 7 years. For new sources, total activity is approximately 6000 Ci with a dose rate of 400 cGy/minute. Radioactive decay releases two gamma rays with an average energy of approximately 1.25 MeV. A gamma ray and an x ray cannot be discriminated and differ only in source of origin. Gamma rays result from radioactive decay, whereas x rays are generated by accelerating electrons (in a linear accelerator) and colliding them into a tungsten target.
Components of a Radiosurgery Unit
Both multicobalt and linear accelerator units have at their heart a source of radiation (cobalt for the Gamma Knife [GK] and the linear accelerator for linac-based units), a couch, a stereotactic helmet or frame system to ensure precise localization and immobilization, and sophisticated treatment planning software. When properly calibrated, an isocenter alignment to better than 1 mm can be achieved with either the GK or the linac radiosurgery system, although it is important to recognize that image resolution, which depends on the slice thickness, is usually of a cruder magnitude.7
Pretreatment Quality Assurance
An in-depth description of the various linac radiosurgery systems is beyond the scope of this chapter, and the interested reader is referred to Task Group Report 54 of the American Association of Physicists in Medicine.7 In general, linac radiosurgery systems can be classified into three groups. First, there are couch-mounted systems that use the treatment lasers or implanted fiducial markers as their target verification system; these systems are probably the least accurate because they have an isocenter stability of at best ±2 mm for any couch and gantry angle. Next, there are the so-called floor stand–mounted systems that connect to the treatment couch and allow the user to correct for inaccuracies in the rotation of the couch during treatment. However, these systems do not allow for corrections of inaccuracies in gantry rotation and, therefore, such systems have at best an isocenter stability of ±1 mm for any couch and gantry angle. The most accurate linac radiosurgery system uses a floor stand with a high-precision bearing that is indexed to the linac but does not connect to the treatment couch. In this system, the floor stand is decoupled from the linac treatment couch; this allows for a high accuracy of couch rotation, on the order of a few hundredths of a millimeter. Furthermore, this system has a separate arm rotating around a high-precision bearing that holds the collimator, which is coupled to the linac gantry using a gimbal bearing. Because all translational and rotational degrees of freedom are decoupled from the linac, this system can be calibrated to have an isocenter stability that is equal to that of a GK of ±0.25 mm for any gantry and couch angle.8
Radiobiologic Considerations
Radiobiologically, the use of a single fraction to treat a small volume differs significantly from conventional fractionated external beam irradiation (EBRT) procedures. This poses unique challenges in that the increased biologic effect on normal tissues is more pronounced than it is on tumors9 (Fig. 17-3). The most important factor influencing the risk of developing late side effects is fraction size. Therefore single-fraction radiosurgery needs to be exquisitely accurate and must minimize the dose to normal tissue.
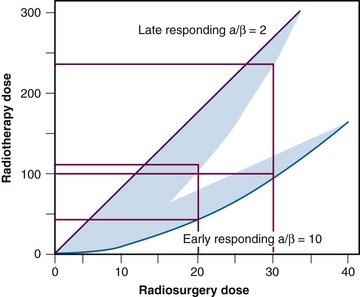
Figure 17-3 Representation of radiosurgery versus radiotherapy dose for both early- and late-responding tissues.
From Mehta MP: The physical, biologic, and clinical basis of radiosurgery, Curr Probl Cancer 19(5):265-329, 1995.
Despite these theoretical disadvantages of single-fraction radiation, efficacy with minimal toxicity has been shown in clinical trials in a multitude of disease sites. Radiosurgery, by treating in a single-fraction regimen, minimizes the deleterious effects of repopulation (tumor regrowth between fractions). Further, the high doses of radiation could potentially overcome the disadvantages of single-fraction delivery. Recent observations suggest the DNA-centric model of classical radiobiology is more complex than initial experiments appreciated, including effects on membrane-bound signaling pathways.10 It appears that radiation effects on the tumor microvasculature are also an important component of tumor response and control.11 Higher ablative doses of radiation have been shown to induce marked endothelial apoptosis with substantial downstream effects on the competency of tumor microvasculature.11 At doses of more than 8 Gy per fraction, the linear quadratic (LQ) model does not accurately predict cell kill or toxicity.12 This poses significant clinical challenges in developing dose-volume normal tissue constraints for SRS and stereotactic body radiotherapy (SBRT), as the LQ model is often used to account for differences in dose per fraction when extrapolating from toxicity analyses obtained using conventional regimens.
Treatment Planning
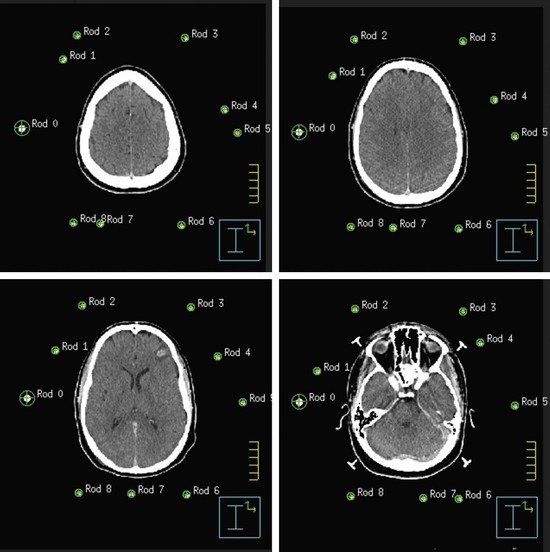
Both GK and conventional linac-based SRS treatment planning procedures begin with the placement of the stereotactic head frame on the patient. The head frame is used for precise localization and rigid immobilization to guarantee that the patient’s position from the time of imaging to the time of treatment does not change. Before the stereotactic CT is acquired, a stereotactic localizer ring is attached to the head frame. For linac radiosurgery, the most commonly used independent stereotactic localization system is the Brown-Roberts-Wells (BRW) system (Fig. 17-4). The BRW localizer has an inner diameter of 29 cm and a height of 16 cm and consists of nine carbon fiber rods that are arranged into three N-shaped structures between two aluminum rings, which are placed at 120-degree angles around the rings. Because three N-shaped fiducials are used, one obtains a system of linear equations that allow for definition of an independent, absolute coordinate system. Therefore one does not have to QA the table indexing of the CT scanner, because the slice position of each scan can be uniquely determined.
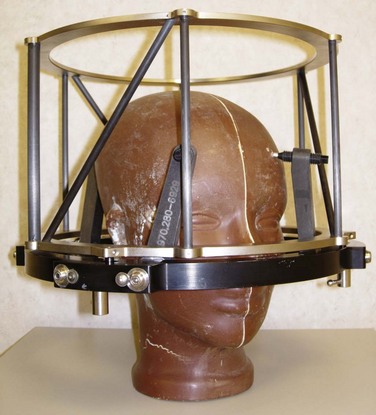
Figure 17-4 Phantom with head ring as well as the Brown-Roberts-Wells (BRW) coordinate system in place.
The origin of the BRW system lies within the localizer and therefore divides the brain into eight distinct quadrants. Any point within this geometric configuration can be uniquely defined by specifying anterior-posterior, lateral, and axial coordinates. During the stereotactic CT scan, portions of these N-shaped structures are included in each image, which allows for their identification during treatment planning. To reduce the error in localization, the slice thickness of the CT scan should be the smallest attainable on the scanner. Once the stereotactic CT scan has been acquired, it is transferred to the treatment planning system and the BRW coordinate system is defined by localizing each of the nine rods in each of the CT slices (Fig. 17-5).
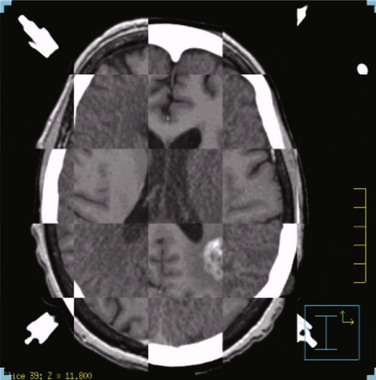
Next, the target volume, or gross tumor volume (GTV), is outlined. Because magnetic resonance imaging (MRI) often provides better tumor visualization than CT scanning, the GTV is often delineated directly from MRI studies. MRI alone does not provide a sufficient database for stereotactic treatment planning, because magnetic field nonuniformity, gradient field nonlinearity, eddy current effects, and susceptibility artifacts at air/tissue interfaces can introduce significant geometric image distortions that affect the accuracy of treatment plans generated using MRI alone. A three-dimensional volumetric MRI is acquired either before or after head ring placement. After acquiring the stereotactic CT scan, the MRI dataset (which may or may not be stereotactically generated) is fused onto the CT image space through correlation of anatomic landmarks in both image sets. During treatment planning, the regions of interest and dose distribution may be displayed on either the CT image or the fused MRI images, but dosimetric calculations are ideally performed on the underlying CT database (Fig. 17-6). If the MRI is to be obtained after head frame placement, magnetic resonance–compatible head rings need to be used.
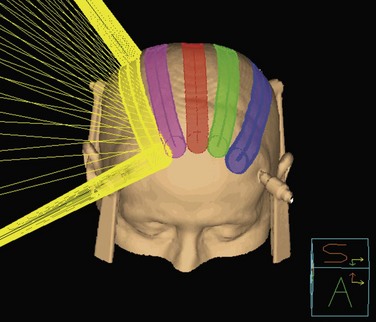
Conventional linac radiosurgery uses multiple non-coplanar arc sets to treat the target volume through a variety of collimator diameters ranging in size from 4 mm to 50 mm, depending on the system (Fig. 17-7). For irregularly shaped lesions such as vestibular schwannomas and arteriovenous malformations (AVMs), arcs delivered through a single circular collimator would lead to the inclusion of a large amount of normal brain tissue, which would yield inferior conformality. In these cases, it is advantageous to use multiple isocenters to conform the prescription isodose shell to the GTV (Fig. 17-8). The dose is typically prescribed to the 70% to 90% isodose line. Whenever multiple isocenters are used, homogeneity is sacrificed for conformality. Ideally, a desirable conformality index (prescribed isodose volume divided by target volume) should be 2 or less and the heterogeneity index (maximum dose divided by prescribed dose) should also be 2 or less.
Clinical Applications
Both the GK and linac-based forms of radiosurgery have been used in a variety of benign and malignant diseases. Table 17-2 summarizes the most common clinical applications for radiosurgery. In this section we outline some of the basic applications of radiosurgery and results of therapy. Specifics pertaining to each disease are discussed in greater detail in its respective chapter.
Benign Tumors
Meningiomas
Two of the largest series that have examined results of SRS are from the Mayo Clinic and the University of Pittsburgh, both of which have shown local control in more than 90% for benign meningiomas at 5 years.13,14,15 The Mayo Clinic series had a significant complication rate of 13% versus 7% for the University of Pittsburgh series.
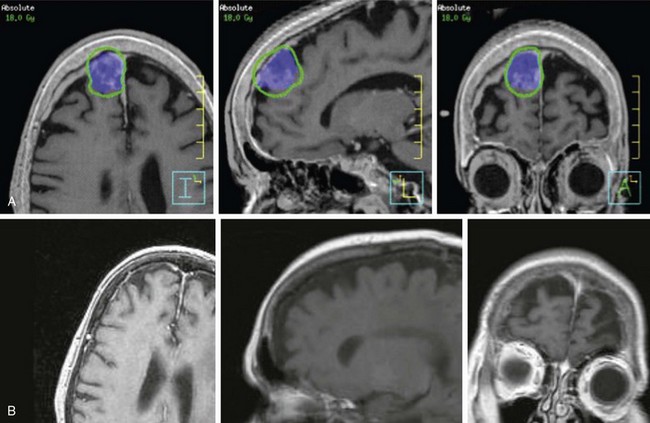
Based on published reports, the optimal dose to the margin of the tumor appears to be 12 to 16 Gy. A recent report by Kollova and colleagues16 showed that doses above 16 Gy increased the risk of treatment-related edema without a corresponding improvement in tumor control. Inclusion of the dural tail within the treatment volume remains a topic of controversy. Limited histopathologic analysis has shown that microscopic tumor extension into the dural tail is limited to 2 mm.17 The most common toxicities include cranial nerve deficits for basal tumors and peritumoral edema for nonbasal tumors. The risk of optic neuropathy is very low, with maximum dose constraints to the optic nerves and chiasm of between 8 and 10 Gy.13,18 In addition to the dose, a treatment volume of more than 5 cm3, a tumor-brain contact interface of 1 cm2 or more, the presence of pretreatment edema, and parasagittal location each increase the risk of peritumoral edema.16,19,20,21 These factors should be considered when deciding whether or not to include none of the dural tail or only a portion of it within the clinical target volume (CTV), which for all practical purposes is the same as the GTV. With SRS, radiologic response is seen in 50% to 60% of patients, and the remainder of those without progression demonstrated tumor stability.16,22
Based on these data, it is reasonable to conclude that small meningiomas can be controlled with radiosurgery in the majority of patients, with initial results comparable to those of complete resection (Fig. 17-9). It must, however, be borne in mind that most cases in which this approach has been used have had relatively short follow-up studies and that long-term results (more than 10 years of follow-up studies for each patient) are indeed very sparse. The University of Pittsburgh recently updated their 18-year experience in a cohort of 972 patients. Local control rates in grade 1 meningioma or lesions without histology were 91% and 95%, respectively, in 75 patients with a minimum follow up of 10 years.
Vestibular Schwannomas
Treatment options include observation for appropriately selected patients, microsurgical resection, SRS, or fractionated radiotherapy. Based on single-institution experiences, all three approaches appear to result in a tumor control probability of more than 90%. Microsurgery is preferred for lesions larger than 3 cm.24 There are no randomized trials directly comparing different methods. The goals of treatment include maximizing tumor control while preserving hearing and facial nerve and trigeminal nerve function. Treatment recommendations are influenced by age, comorbidities, tumor size, presenting symptoms, proximity to the brainstem or cochlea, and patient preference.
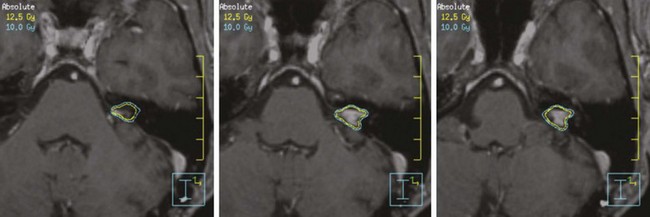
When SRS is chosen as the treatment option, the recommended dose is 12 to 13 Gy in a single fraction.25,26,27 Initial experiences with doses in the range of 16 to 20 Gy resulted in a higher rate of treatment-related toxicity. A recent review of the literature reveals that with modern SRS techniques and doses, hearing preservation is achieved in 50% to 89% of patients, with a risk of less than 5% for facial or trigeminal neuropathy.28,29 The risk of sensorineural hearing loss is related to the dose of radiation delivered to cranial nerve VIII, the cochlea, and the ventral cochlear nucleus.30,31
There are three prospective nonrandomized studies in the literature comparing SRS and microsurgery, all of which showed improved serviceable hearing preservation and reduced facial nerve toxicity in patients treated with SRS.32–34 Two retrospective, single-institution, matched-pair analyses have reported similar findings.35,36 The prospective series by Regis and associates33 reported a statistically lower incidence of trigeminal nerve deficits with SRS. The risk of cranial nerve injury with microsurgical resection is highly dependent on tumor size, operative approach (retrosigmoid, middle cranial fossa, or translabyrinthine), and the surgeon’s skill and experience. Nonetheless, SRS may be the preferred treatment in properly selected patients with vestibular schwannomas less than 3 cm in size.
Published nonrandomized data support comparable efficacy between SRS and fractionated stereotactic radiotherapy (FSRT). Common regimens include conventionally fractionated doses of 45 to 50 Gy at 1.8 Gy/fraction or abbreviated-course, hypofractionated regimens, such as 20 to 25 Gy at 4 to 5 Gy/fraction. The best series to date is a trial done by Meijer and colleagues37 in the Netherlands. Their group prospectively evaluated 129 patients with vestibular schwannomas. Patients were assigned to receive either 20 to 25 Gy in four to five fractions (80 dentate patients) or 10 to 12.5 Gy in a single fraction (49 edentate patients). Local control rates, hearing preservation, and cranial nerve VII complications were not statistically different. There was an increase in cranial nerve V complications with single-fraction radiosurgery (8% vs. 2%).
A retrospective series from Thomas Jefferson University reported high rates of hearing preservation in the range of 80% to 85% using conventional FSRT, a rate that was 2.5-fold higher than rates for SRS.38 A more recent analysis showed no difference in tumor control or cranial nerve deficits between SRS at 13 Gy or less and conventional FSRT.39 Neither SRS nor fractionated approaches have been tested in prospective, controlled clinical trials for this disease.
Pituitary Adenomas
Ten to fifteen percent of intracranial neoplasms are pituitary adenomas. Irradiation is generally reserved for patients who have incompletely resected tumors or recurrent disease. Based on the size and location, either SRS or conformal EBRT may be considered. SRS is typically contraindicated for tumors within 3 to 5 mm of the optic chiasm, respecting a maximum dose constant of 10 Gy or less to the optic nerves and chiasm.40 Typical single-fraction SRS doses to the pituitary have ranged from 10 to 30 Gy, but doses in the range of 13 to 14 Gy have been found to be equivalent to higher doses.41 Local control using SRS is generally in excess of 90% for nonsecretory tumors. Radiation-induced hypopituitarism occurs in more than 50% of patients and is the most common late toxicity.42
For secretory tumors, SRS appears to result in a shorter time to hormone normalization than fractionated radiotherapy.43,44 Though tumor control remains high, hormonal remission is seen in 25% to 75% of patients and depends on the tumor subtype (i.e., prolactinoma, Cushing’s disease, Nelson’s syndrome, or acromegaly). Cytostatic medical management of secreting pituitary adenomas is often employed. Patients receiving octreotide or dopamine agonists have had markedly inferior control rates with radiotherapy in several series.45,46 If symptoms allow, these medications should be discontinued in advance of treatment.
Malignant Tumors
Brain Metastasis
Dose selection parameters for treating brain metastases with SRS were defined by a phase I Radiation Therapy Oncology Group (RTOG) trial. Properly selected parameters result in a rate of less than 20% of grade 3 or higher neurotoxicity when they are combined with whole-brain radiotherapy (WBRT).47 Recommended doses for brain metastasis are based on a maximal diameter of 21 to 24 Gy for lesions of 2 cm or less, 18 Gy for lesions of 2 to less than 3 cm, and 15 Gy for lesions of 3 to 4 cm. RTOG 95-08 investigated WBRT (37.5 Gy/15 fractions) with or without SRS in a randomized design. The trial showed a survival benefit for patients with a single brain metastasis. Additionally, post-hoc subgroup analysis suggests an improved median survival with up to three brain metastases in patients younger than 50 years, those who have non–small cell lung cancer, or those with recursive partitioning analysis (RPA) class I disease.48
Both surgery and SRS have a proven survival benefit in the management of a single brain metastasis.48,49 Typically, surgery is preferred in patients with good performance status, large lesions (>3 cm), or symptomatic tumors with substantial vasogenic edema.50 In patients who are good candidates for either surgery or SRS, there are no randomized data currently available to indicate which is the preferred treatment modality. Multiple phase III trials are currently ongoing. Rades and colleagues51 recently reported a matched-pair analysis of WBRT plus surgery or SRS in 104 patients with one to three brain metastases. At 1 year, this study found improved rates of overall survival, intracerebral control, and local control with SRS.51 However, the value of surgery for multiple brain metastases has not been established.
Currently, eliminating WBRT in SRS patients is controversial. Initial retrospective series have suggested that eliminating WBRT increases the likelihood of central nervous system relapse but does not necessarily affect survival rates.52,53,54 This was confirmed in a randomized trial by the Japanese Radiation Oncology Study Group (JROSG 99-11).55 The preservation of neurologic function is a fine balance between the toxicity of brain irradiation and the negative consequences of tumor relapse.56 A recent randomized trial from the M. D. Anderson Cancer Center showed that the addition of WBRT to SRS is associated with a significant decline in learning and memory at 4 months.57 However, this trial and others have consistently shown higher rates of intracranial relapse when WBRT is excluded and the preservation of neurocognitive function is strongly correlated with tumor control with more extended follow-up.58
Gliomas
Because at least 80% of gliomas fail within 2 cm of the primary tumor, high-dose local irradiation has been hypothesized as a means of improving tumor control rates. In an effort to delineate the role of SRS, the RTOG conducted a randomized trial of 203 patients with glioblastoma multiforme who received either 60 Gy of EBRT at 2 Gy/fraction with BCNU or SRS prior to EBRT and BCNU.59 Radiosurgery did not add to the overall survival rates, quality of life, or neurologic function as performed in this trial. Malignant gliomas are most commonly large, diffusively infiltrative tumors with substantial surrounding edema known to harbor microscopic disease, reducing the likelihood of success of SRS. Based upon this trial, there is currently no well-defined role for SRS in the adjuvant setting for glioblastoma multiforme and potentially for all gliomas.
Despite a lack of proven benefit for primary gliomas, there is considerable interest in SRS for treatment of recurrent malignant gliomas. Multiple institutional series have been reported, some of which suggest a survival benefit in comparison with historical controls.60 However, such reports are particularly susceptible to patient selection bias, and no prospective studies have adequately defined a role for SRS at present. Patients with small-volume, focal, or nodular recurrent disease may be candidates for this modality. Current limitations of this strategy include adequately defining target volumes and a high risk of radiation-related complications. SRS dose selection is based upon volume and was defined by RTOG 90-05 (see the previous section on brain metastases) because approximately 40% of patients included in this phase I trial had had previously irradiated primary brain tumors. Several analyses have suggested that younger patient age, higher performance status, smaller tumor volume, unifocal recurrence, and hemispheric location are factors that affect the outcome following SRS for recurrent disease.61,62
Vascular Lesions
Arteriovenous Malformations
The high dose of radiation presumably unleashes a cytokine cascade that induces fibrointimal reaction, thrombosis, and eventual obliteration of the AVM nidus over 1 to 3 years. Typical doses used are in the range of 15 to 30 Gy. Target volume definition is a multidisciplinary process using a combination of angiography, CT angiography, and MRI/MRA of the brain. Large or diffuse AVMs can be approached with initial embolization to reduce the volume of nidus before SRS. Sequential SRS to larger volumes has also been reported.63
Complete obliteration of the nidus is observed in 75% to 90% of AVMs treated with radiosurgery. Both smaller nidus volume and radiation dose are predictors of successful obliteration.64,65,66 However, it may take up to 3 years for complete obliteration. During the latency period, there is evidence for a reduced risk of hemorrhage despite radiologic persistence.67,68 Following obliteration, the risk of hemorrhage is reduced by approximately 90%, but it is not eliminated.68
Functional Disorders
Trigeminal Neuralgia
For patients refractory to medications, SRS is the least invasive procedure. Typical doses are 70 to 90 Gy in a single fraction directed at the dorsal root entry zone of cranial nerve V into the pons (Fig. 17-10). Initial SRS directed at the gasserian ganglion produced inferior results. It is thought that delivering high doses of radiation to this region will induce a block of the emphatic transmission through the pain fibers only. Pain relief is experienced with a latency of approximately 1 month. Paresthesia is the most common side effect.
Results from the University of Pittsburgh series indicate that approximately 70% of patients have some pain relief, with about 40% experiencing complete pain relief and no longer needing any medications.69 Patients with typical symptoms (i.e., sharp, electric shock–like pain as opposed to dull aching or burning pain) have superior results compared with those with atypical symptoms. Also, patients with no previous surgical manipulations have a higher likelihood of obtaining complete pain relief. Similar results using linac-based SRS have been reported by Bradley and colleagues70 from the University of Wisconsin. The response rate is durable for approximately 50% of patients.71,72
Flickinger and associates73 randomized 87 patients with trigeminal neuralgia to receive either a single isocenter (sphere) or two isocenters (elliptical treatment volume) set up using the GK. A maximal dose of 75 Gy was delivered with 4-mm–diameter collimators. The actuarial rate of obtaining complete pain relief was 67.7% ±5.1%, with pain relief similar for one- and two-isocenter SRS. Pain recurred in 30 of 72 responding patients. Numbness and paresthesias correlated with the length of nerve irradiated. Therefore the authors concluded that calibrating the treatment volume to include a longer nerve length for trigeminal neuralgia SRS does not significantly improve pain relief but may increase complications.
Attempts for retreatment with SRS have also been reported. The University of Pittsburgh series reports rates of approximately 50% for complete pain relief and 13% for numbness following retreatment, whereas the Mayo Clinic series reports rates of 90% for pain relief and minor numbness in all patients with retreatment.74,75 Similar results have been reported by others.76
Other Functional Disorders
Medical management is generally the first line of treatment for cluster headaches. If patients are refractory to medical or surgical techniques, SRS can be considered. The target, as in trigeminal neuralgia, is the root entry zone of cranial nerve V. Typical doses are 70 to 80 Gy to the 100% isodose line. Initial results from small series are conflicting on the efficacy of radiosurgery in the management of intractable cluster headaches.77,78
Stereotactic Body Radiotherapy
With improvements in hardware, image guidance, and immobilization, the principles of radiosurgery are now being applied outside of the brain. As with SRS, stereotactic body radiotherapy (SBRT) was developed at the Karolinska Institute in Sweden in the 1990s, using a body frame for immobilization and the principles of stereotaxis for target localization.79 It involves the precise delivery of high doses of radiation over one to five fractions using sophisticated treatment planning to generate steep dose gradients for maximal normal tissue sparing, often aided by various methods of image guidance and motion management. Over the last decade, these techniques have been refined and more widely implemented in the treatment of many cancers. Broadly, SBRT is useful as aggressive local therapy for oligometastatic disease and ablation of clinically localized, early-stage malignant tumors. It is currently being investigated for early-stage non–small cell lung cancer and lung metastases, prostate cancer, bone and spinal metastases, liver metastases, hepatocellular carcinoma, pancreatic cancer, renal cell carcinoma, and other abdominal tumors. Here we briefly introduce some of the concepts of SBRT.
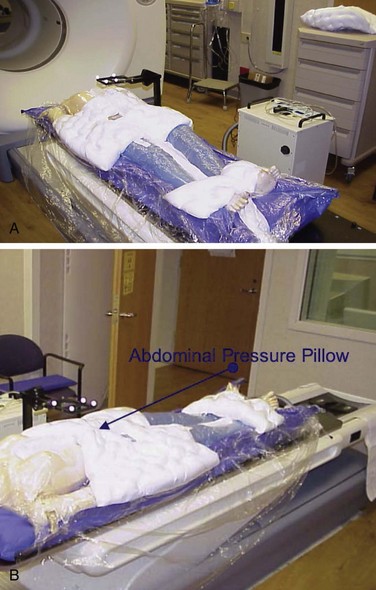
During the CT simulation process, immobilization is necessary; a whole body vacuum mold, which also serves to reduce internal motion, may be needed (see Fig. 17-11A). There are multiple reproducible systems that provide the necessary immobilization—many of which are institution specific—such as body frames, vacuum molds, abdominal compression techniques, and thermoplastic devices. Such immobilization strategies limit the effects of respiratory motion by restricting chest wall rise and diaphragmatic excursion. At the University of Wisconsin, chest wall motion can be reduced to 2 mm by using a vacuum system with an abdominal pressure pillow (Fig. 17-11B). Patients with poor pulmonary reserve, however, must be monitored closely. Alternatively, respiratory gating can be used to track the patient’s range of motion throughout respiration and deliver treatment only during the chosen phase of the respiratory cycle. In the management of lung and liver tumors, four-dimensional CT scans or fluoroscopic motion studies are conducted to provide accurate planning target volume (PTV) measurements. For tumors with avidity for positron emission tomography (PET), FDG-PET imaging is often performed with the patient in a body mold to improve the accuracy of delineating the tumor. Because the PET image is acquired over an extended period, motion is already accounted for in the fusion of the PET image to the four-dimensional CT image.
The goal of dependable and reproducible immobilization, motion management, and image guidance is to restrict necessary PTV margins yet ensure the desired coverage of the CTV. There is no consensus on required PTV margins, which are typically institution specific and depend on an understanding of interfractional and intrafractional variability for a given delivery system and disease site. Treatment planning usually involves three-dimensional conformal radiotherapy (3DCRT) with multiple beam arrangement, often non-coplanar, or intensity-modulated radiotherapy (IMRT) to produce distributions with a very rapid fall-off (Fig. 17-12). Typically, when using 3DCRT, dosimetric margins around the PTV are selected so that the 80% isodose line encompasses 100% of the PTV. When inverse planning is employed, dose homogeneity within the PTV is important, given the high dose per fraction. For all techniques, the goal is to limit the volume of normal tissue receiving the prescription dose.
Early-Stage Lung Cancer
Most of the experience with SBRT to date is in the treatment of early-stage non–small cell lung cancer (NSCLC). The standard of care for medically operable, stage I NSCLC is lobar resection. Traditional EBRT produced inferior results.80,81 With modern techniques, however, dose-per-fraction escalation using SBRT is possible and offers the potential for improved results.
Single-institution experiences have reported improved outcomes with SBRT in comparison with historical controls.82,83,84,85–87 The initial phase I dose escalation protocol from Timmerman and colleagues88 failed to reach the maximum tolerated dose for a three-fraction regimen for peripheral lesions, reaching 20 Gy per fraction for medically inoperable patients. Multiple fractionation schemes have been employed, ranging from 24 to 60 Gy in one to five fractions. A Japanese multi-institutional analysis showed that, regardless of the chosen hypofractionated regimen, a biologically effective dose (BED) of more than 100 Gy was associated with a statistically significant improvement in local control.84
The first North American multicenter phase II trial (RTOG 0236) in medically inoperable, peripherally located, early-stage NSCLC patients with tumors of less than 5 cm was recently completed. It accrued 59 patients treated to 60 Gy in three fractions of 20 Gy per fraction separated by at least 40 hours. The 3-year primary tumor and lobar control rates were 97.6% and 90.6%, respectively.89
Future directions include evaluating the role of SBRT in the definitive management of operable patients. RTOG 0618, a single-arm phase II trial with the same fractionation, is currently enrolling operable early stage patients. A recent case-matched analysis comparing SBRT with sublobar resection for borderline resectable T1-2N0 NSCLC suggested similar disease control rates.90 A randomized trial is currently ongoing in the Netherlands comparing SBRT with definitive resection.
At present, SBRT for NSCLC is limited to peripheral lesions, based on excessive rates of grade 3 to 5 toxicity when treating lesions within 2 cm of the proximal bronchial tree with doses of 60 Gy in three fractions.91 A report from the M. D. Anderson Cancer Center showed acceptable toxicity when treating central lesions using image-guided IMRT to a dose of 50 Gy in four fractions.92 The RTOG is currently conducting a phase I/II dose escalation study to evaluate the optimal fractionation schedule and associated toxicity and disease control rates when treating centrally located early-stage tumors.
Metastatic Disease; Miscellaneous Indications
In addition to primary lung tumors, SBRT has been used for lung and liver metastasis. Excellent local control rates ranging from 75% to 100% have been reported.93,94,95–98
SBRT is also a developing modality in the palliative treatment of spinal metastases. Multiple series using single-fraction regimens with doses ranging from 10 to 25 Gy have shown high rates of local control and pain relief without substantial late neurologic toxicity.99,100,101
7 Schell M, Bova F, Larson V. AAPM Report 54. Stereotactic radiosurgery. Am Assoc Physicist Med. 1995. Available at www.acr.org/SecondaryMainMenuCategories/quality_safety/guidelines/ro/stereotactic_radiosurgery.aspx
10 Prise KM, Schettino G, Folkard M, Held KD. New insights on cell death from radiation exposure. Lancet Oncol. 2005;6(7):520-528.
11 Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8(2):89-91.
13 Stafford SL, Pollock BE, Foote RL, et al. Meningioma radiosurgery. Tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49(5):1029-1037. discussion 1037-1038
15 Lee JY, Niranjan A, McInerney J, et al. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg. 2002;97(1):65-72.
16 Kollova A, Liscak R, Novotny JJr, et al. Gamma Knife surgery for benign meningioma. J Neurosurg. 2007;107(2):325-336.
17 Nagele T, Petersen D, Klose U, et al. The “dural tail” adjacent to meningiomas studied by dynamic contrast-enhanced MRI. A comparison with histopathology. Neuroradiology. 1994;36(4):303-307.
18 Leber KA, Bergloff J, Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88(1):43-50.
19 Cai R, Barnett GH, Novak E, et al. Principal risk of peritumoral edema after stereotactic radiosurgery for intracranial meningioma is tumor-brain contact interface area. Neurosurgery. 2010;66(3):513-522.
21 Patil CG, Hoang S, Borchers DJ3rd, et al. Predictors of peritumoral edema after stereotactic radiosurgery of supratentorial meningiomas. Neurosurgery. 2008;63(3):435-440. discussion 440-442
23 Kondziolka D, Mathieu D, Lunsford LD, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62(1):53-58. discussion 58-60
25 Flickinger JC, Kondziolka D, Niranjan A, et al. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2004;60(1):225-230.
31 Linskey ME. Hearing preservation in vestibular schwannoma stereotactic radiosurgery. What really matters? J Neurosurg. 2008;109(Suppl):129-136.
32 Pollock BE, Driscoll CL, Foote RL, et al. Patient outcomes after vestibular schwannoma management. A prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery. 2006;59(1):77-85.
33 Regis J, Pellet W, Delsanti C, et al. Functional outcome after gamma knife surgery or microsurgery for vestibular schwannomas. J Neurosurg. 2002;97(5):1091-1100.
34 Myrseth E, Moller P, Pedersen PH, Lund-Johansen M. Vestibular schwannoma. Surgery or gamma knife radiosurgery? A prospective, nonrandomized study. Neurosurgery. 2009;64(4):654-661. discussion 661-663
37 Meijer OW, Vandertop WP, Baayen JC, Slotman BJ. Single-fraction vs. fractionated linac-based stereotactic radiosurgery for vestibular schwannoma. A single-institution study. Int J Radiat Oncol Biol Phys. 2003;56(5):1390-1396.
38 Andrews DW, Suarez O, Goldman HW, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas. Comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys. 2001;50(5):1265-1278.
39 Combs SE, Welzel T, Schulz-Ertner D, et al. Differences in clinical results after LINAC-based single-dose radiosurgery versus fractionated stereotactic radiotherapy for patients with vestibular schwannomas. Int J Radiat Oncol Biol Phys. 2010;76(1):193-200.
41 Sheehan JP, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for residual or recurrent nonfunctioning pituitary adenoma. J Neurosurg. 2002;97(5 Suppl):408-414.
44 Landolt AM, Haller D, Lomax N, et al. Stereotactic radiosurgery for recurrent surgically treated acromegaly. Comparison with fractionated radiotherapy. J Neurosurg. 1998;88(6):1002-1008.
46 Pollock BE, Nippoldt TB, Stafford SL, et al. Results of stereotactic radiosurgery in patients with hormone-producing pituitary adenomas. Factors associated with endocrine normalization. J Neurosurg. 2002;97(3):525-530.
47 Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases. Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291-298.
48 Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases. Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
49 Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494-500.
51 Rades D, Kueter JD, Veninga T, et al. Whole brain radiotherapy plus stereotactic radiosurgery (WBRT+SRS) versus surgery plus whole brain radiotherapy (OP+WBRT) for 1-3 brain metastases. Results of a matched pair analysis. Eur J Cancer. 2009;45(3):400-404.
52 Sneed PK, Suh JH, Goetsch SJ, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002;53(3):519-526.
55 Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases. A randomized controlled trial. JAMA. 2006;295(21):2483-2491.
57 Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation. A randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
58 Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25(10):1260-1266.
59 Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme. Report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60(3):853-860.
62 Larson DA, Gutin PH, McDermott M, et al. Gamma knife for glioma. Selection factors and survival. Int J Radiat Oncol Biol Phys. 1996;36(5):1045-1053.
64 Flickinger JC, Pollock BE, Kondziolka D, Lunsford LD. A dose-response analysis of arteriovenous malformation obliteration after radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36(4):873-879.
65 Karlsson B, Lindquist C, Steiner L. Prediction of obliteration after gamma knife surgery for cerebral arteriovenous malformations. Neurosurgery. 1997;40(3):425-430. discussion 30-31
67 Karlsson B, Lax I, Soderman M. Risk for hemorrhage during the 2-year latency period following gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2001;49(4):1045-1051.
68 Maruyama K, Kawahara N, Shin M, et al. The risk of hemorrhage after radiosurgery for cerebral arteriovenous malformations. N Engl J Med. 2005;352(2):146-153.
69 Maesawa S, Salame C, Flickinger JC, et al. Clinical outcomes after stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2001;94(1):14-20.
72 Riesenburger RI, Hwang SW, Schirmer CM, et al. Outcomes following single-treatment Gamma Knife surgery for trigeminal neuralgia with a minimum 3-year follow-up. J Neurosurg. 2010;112(4):766-771.
73 Flickinger JC, Pollock BE, Kondziolka D, et al. Does increased nerve length within the treatment volume improve trigeminal neuralgia radiosurgery? A prospective double-blind, randomized study. Int J Radiat Oncol Biol Phys. 2001;51(2):449-454.
75 Pollock BE, Foote RL, Stafford SL, et al. Results of repeated gamma knife radiosurgery for medically unresponsive trigeminal neuralgia. J Neurosurg. 2000;93(Suppl 3):162-164.
77 Kano H, Kondziolka D, Mathieu D, et al. Stereotactic radiosurgery for intractable cluster headache. An initial report from the North American Gamma Knife Consortium. J Neurosurg. Apr 30, 2010. [Epub ahead of print]
82 Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27(20):3290-3296.
84 Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non–small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7 Suppl 3):S94-S100.
88 Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation. Results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124(5):1946-1955.
89 Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070-1076.
90 Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. 2010;28(6):928-935.
91 Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833-4839.
92 Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;72(4):967-971.
93 Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27(10):1579-1584.
94 Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27(10):1572-1578.
100 Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases. Clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976). 2007;32(2):193-199.
1 Leksell L. The stereotaxic method and radiosurgery of the brain. Acta Chir Scand. 1951;102(4):316-319.
2 Leksell L. [Trigeminal neuralgia. Some neurophysiologic aspects and a new method of therapy.]. Lakartidningen. 1971;68(45):5145-5148.
3 Stieber VW, Bourland JD, Tome WA, Mehta MP. Gentlemen (and ladies), choose your weapons. Gamma knife vs. linear accelerator radiosurgery. Technol Cancer Res Treat. 2003;2(2):79-86.
4 Mehta M, Noyes W, Craig B, et al. A cost-effectiveness and cost-utility analysis of radiosurgery vs. resection for single-brain metastases. Int J Radiat Oncol Biol Phys. 1997;39(2):445-454.
5 Larson DA, Bova F, Eisert D, et al. Current radiosurgery practice. Results of an ASTRO survey. Task Force on Stereotactic Radiosurgery, American Society for Therapeutic Radiology and Oncology. Int J Radiat Oncol Biol Phys. 1994;28(2):523-526.
6 Kubsad SS, Mackie TR, Gehring MA, et al. Monte Carlo and convolution dosimetry for stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 1990;19(4):1027-1035.
7 Schell M, Bova F, Larson V. AAPM Report 54. Stereotactic radiosurgery. Am Assoc Physicist Med. 1995.
8 Friedman WA, Bova FJ. The University of Florida radiosurgery system. Surg Neurol. 1989;32(5):334-342.
9 Mehta MP. The physical, biologic, and clinical basis of radiosurgery. Curr Probl Cancer. 1995;19(5):265-329.
10 Prise KM, Schettino G, Folkard M, Held KD. New insights on cell death from radiation exposure. Lancet Oncol. 2005;6(7):520-528.
11 Fuks Z, Kolesnick R. Engaging the vascular component of the tumor response. Cancer Cell. 2005;8(2):89-91.
12 Park C, Papiez L, Zhang S, et al. Universal survival curve and single fraction equivalent dose. Useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70(3):847-852.
13 Stafford SL, Pollock BE, Foote RL, et al. Meningioma radiosurgery. Tumor control, outcomes, and complications among 190 consecutive patients. Neurosurgery. 2001;49(5):1029-1037. discussion 1037-1038
14 Kondziolka D, Niranjan A, Lunsford LD, Flickinger JC. Stereotactic radiosurgery for meningiomas. Neurosurg Clin North Am. 1999;10(2):317-325.
15 Lee JY, Niranjan A, McInerney J, et al. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J Neurosurg. 2002;97(1):65-72.
16 Kollova A, Liscak R, Novotny JJr, et al. Gamma Knife surgery for benign meningioma. J Neurosurg. 2007;107(2):325-336.
17 Nagele T, Petersen D, Klose U, et al. The “dural tail” adjacent to meningiomas studied by dynamic contrast-enhanced MRI. A comparison with histopathology. Neuroradiology. 1994;36(4):303-307.
18 Leber KA, Bergloff J, Pendl G. Dose-response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J Neurosurg. 1998;88(1):43-50.
19 Cai R, Barnett GH, Novak E, et al. Principal risk of peritumoral edema after stereotactic radiosurgery for intracranial meningioma is tumor-brain contact interface area. Neurosurgery. 2010;66(3):513-522.
20 Kondziolka D, Flickinger JC, Perez B. Judicious resection and/or radiosurgery for parasagittal meningiomas. Outcomes from a multicenter review. Gamma Knife Meningioma Study Group. Neurosurgery. 1998;43(3):405-413. discussion 413-414
21 Patil CG, Hoang S, Borchers DJ3rd, et al. Predictors of peritumoral edema after stereotactic radiosurgery of supratentorial meningiomas. Neurosurgery. 2008;63(3):435-440. discussion 440-442
22 Kreil W, Luggin J, Fuchs I, et al. Long term experience of gamma knife radiosurgery for benign skull base meningiomas. J Neurol Neurosurg Psychiatry. 2005;76(10):1425-1430.
23 Kondziolka D, Mathieu D, Lunsford LD, et al. Radiosurgery as definitive management of intracranial meningiomas. Neurosurgery. 2008;62(1):53-58. discussion 58-60
24 Nikolopoulos TP, O’Donoghue GM. Acoustic neuroma management. An evidence-based medicine approach. Otol Neurotol. 2002;23(4):534-541.
25 Flickinger JC, Kondziolka D, Niranjan A, et al. Acoustic neuroma radiosurgery with marginal tumor doses of 12 to 13 Gy. Int J Radiat Oncol Biol Phys. 2004;60(1):225-230.
26 Bhandare N, Jackson A, Eisbruch A, et al. Radiation therapy and hearing loss. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S50-S57.
27 Yang I, Sughrue ME, Han SJ, et al. A comprehensive analysis of hearing preservation after radiosurgery for vestibular schwannoma. J Neurosurg. 2010;112(4):851-859.
28 Myrseth E, Pedersen PH, Moller P, Lund-Johansen M. Treatment of vestibular schwannomas. Why, when and how? Acta Neurochir (Wien). 2007;149(7):647-660. discussion 660
29 Yang I, Sughrue ME, Han SJ, et al. Facial nerve preservation after vestibular schwannoma Gamma Knife radiosurgery. J Neurooncol. 2009;93(1):41-48.
30 Paek SH, Chung HT, Jeong SS, et al. Hearing preservation after gamma knife stereotactic radiosurgery of vestibular schwannoma. Cancer. 2005;104(3):580-590.
31 Linskey ME. Hearing preservation in vestibular schwannoma stereotactic radiosurgery. What really matters? J Neurosurg. 2008;109(Suppl):129-136.
32 Pollock BE, Driscoll CL, Foote RL, et al. Patient outcomes after vestibular schwannoma management. A prospective comparison of microsurgical resection and stereotactic radiosurgery. Neurosurgery. 2006;59(1):77-85.
33 Regis J, Pellet W, Delsanti C, et al. Functional outcome after gamma knife surgery or microsurgery for vestibular schwannomas. J Neurosurg. 2002;97(5):1091-1100.
34 Myrseth E, Moller P, Pedersen PH, Lund-Johansen M. Vestibular schwannoma. Surgery or gamma knife radiosurgery? A prospective, nonrandomized study. Neurosurgery. 2009;64(4):654-661. discussion 661-663
35 Pollock BE, Lunsford LD, Kondziolka D, et al. Outcome analysis of acoustic neuroma management. A comparison of microsurgery and stereotactic radiosurgery. Neurosurgery. 1995;36(1):215-224. discussion 224-229
36 Karpinos M, Teh BS, Zeck O, et al. Treatment of acoustic neuroma. Stereotactic radiosurgery vs. microsurgery. Int J Radiat Oncol Biol Phys. 2002;54(5):1410-1421.
37 Meijer OW, Vandertop WP, Baayen JC, Slotman BJ. Single-fraction vs. fractionated linac-based stereotactic radiosurgery for vestibular schwannoma. A single-institution study. Int J Radiat Oncol Biol Phys. 2003;56(5):1390-1396.
38 Andrews DW, Suarez O, Goldman HW, et al. Stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of acoustic schwannomas. Comparative observations of 125 patients treated at one institution. Int J Radiat Oncol Biol Phys. 2001;50(5):1265-1278.
39 Combs SE, Welzel T, Schulz-Ertner D, et al. Differences in clinical results after LINAC-based single-dose radiosurgery versus fractionated stereotactic radiotherapy for patients with vestibular schwannomas. Int J Radiat Oncol Biol Phys. 2010;76(1):193-200.
40 Mayo C, Martel MK, Marks LB, et al. Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S28-S35.
41 Sheehan JP, Kondziolka D, Flickinger J, Lunsford LD. Radiosurgery for residual or recurrent nonfunctioning pituitary adenoma. J Neurosurg. 2002;97(5 Suppl):408-414.
42 Fernandez A, Brada M, Zabuliene L, et al. Radiation-induced hypopituitarism. Endocr Relat Cancer. 2009;16(3):733-772.
43 Tinnel BA, Henderson MA, Witt TC, et al. Endocrine response after gamma knife-based stereotactic radiosurgery for secretory pituitary adenoma. Stereotact Funct Neurosurg. 2008;86(5):292-296.
44 Landolt AM, Haller D, Lomax N, et al. Stereotactic radiosurgery for recurrent surgically treated acromegaly. Comparison with fractionated radiotherapy. J Neurosurg. 1998;88(6):1002-1008.
45 Landolt AM, Lomax N. Gamma knife radiosurgery for prolactinomas. J Neurosurg. 2000;93(Suppl 3):14-18.
46 Pollock BE, Nippoldt TB, Stafford SL, et al. Results of stereotactic radiosurgery in patients with hormone-producing pituitary adenomas. Factors associated with endocrine normalization. J Neurosurg. 2002;97(3):525-530.
47 Shaw E, Scott C, Souhami L, et al. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases. Final report of RTOG protocol 90-05. Int J Radiat Oncol Biol Phys. 2000;47(2):291-298.
48 Andrews DW, Scott CB, Sperduto PW, et al. Whole brain radiation therapy with or without stereotactic radiosurgery boost for patients with one to three brain metastases. Phase III results of the RTOG 9508 randomised trial. Lancet. 2004;363(9422):1665-1672.
49 Patchell RA, Tibbs PA, Walsh JW, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322(8):494-500.
50 Kalkanis SN, Kondziolka D, Gaspar LE, et al. The role of surgical resection in the management of newly diagnosed brain metastases. A systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96(1):33-43.
51 Rades D, Kueter JD, Veninga T, et al. Whole brain radiotherapy plus stereotactic radiosurgery (WBRT+SRS) versus surgery plus whole brain radiotherapy (OP+WBRT) for 1-3 brain metastases. Results of a matched pair analysis. Eur J Cancer. 2009;45(3):400-404.
52 Sneed PK, Suh JH, Goetsch SJ, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002;53(3):519-526.
53 Chidel MA, Suh JH, Reddy CA, et al. Application of recursive partitioning analysis and evaluation of the use of whole brain radiation among patients treated with stereotactic radiosurgery for newly diagnosed brain metastases. Int J Radiat Oncol Biol Phys. 2000;47(4):993-999.
54 Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases. Is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43(3):549-558.
55 Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases. A randomized controlled trial. JAMA. 2006;295(21):2483-2491.
56 Regine WF, Huhn JL, Patchell RA, et al. Risk of symptomatic brain tumor recurrence and neurologic deficit after radiosurgery alone in patients with newly diagnosed brain metastases. Results and implications. Int J Radiat Oncol Biol Phys. 2002;52(2):333-338.
57 Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation. A randomised controlled trial. Lancet Oncol. 2009;10(11):1037-1044.
58 Li J, Bentzen SM, Renschler M, Mehta MP. Regression after whole-brain radiation therapy for brain metastases correlates with survival and improved neurocognitive function. J Clin Oncol. 2007;25(10):1260-1266.
59 Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme. Report of Radiation Therapy Oncology Group 93-05 protocol. Int J Radiat Oncol Biol Phys. 2004;60(3):853-860.
60 Romanelli P, Conti A, Pontoriero A, et al. Role of stereotactic radiosurgery and fractionated stereotactic radiotherapy for the treatment of recurrent glioblastoma multiforme. Neurosurg Focus. 2009;27(6):E8.
61 Hsieh PC, Chandler JP, Bhangoo S, et al. Adjuvant gamma knife stereotactic radiosurgery at the time of tumor progression potentially improves survival for patients with glioblastoma multiforme. Neurosurgery. 2005;57(4):684-692. discussion 692
62 Larson DA, Gutin PH, McDermott M, et al. Gamma knife for glioma. Selection factors and survival. Int J Radiat Oncol Biol Phys. 1996;36(5):1045-1053.
63 Sirin S, Kondziolka D, Niranjan A, et al. Prospective staged volume radiosurgery for large arteriovenous malformations. Indications and outcomes in otherwise untreatable patients. Neurosurgery. 2008;62(Suppl 2):744-754.
64 Flickinger JC, Pollock BE, Kondziolka D, Lunsford LD. A dose-response analysis of arteriovenous malformation obliteration after radiosurgery. Int J Radiat Oncol Biol Phys. 1996;36(4):873-879.
65 Karlsson B, Lindquist C, Steiner L. Prediction of obliteration after gamma knife surgery for cerebral arteriovenous malformations. Neurosurgery. 1997;40(3):425-430. discussion 30-31
66 Lunsford LD, Kondziolka D, Flickinger JC, et al. Stereotactic radiosurgery for arteriovenous malformations of the brain. J Neurosurg. 1991;75(4):512-524.
67 Karlsson B, Lax I, Soderman M. Risk for hemorrhage during the 2-year latency period following gamma knife radiosurgery for arteriovenous malformations. Int J Radiat Oncol Biol Phys. 2001;49(4):1045-1051.
68 Maruyama K, Kawahara N, Shin M, et al. The risk of hemorrhage after radiosurgery for cerebral arteriovenous malformations. N Engl J Med. 2005;352(2):146-153.
69 Maesawa S, Salame C, Flickinger JC, et al. Clinical outcomes after stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2001;94(1):14-20.
70 Bradley K, Tome W, Resnick D, Mehta M. Treatment of trigeminal neuralgia using linear accelerator-based radiosugery. Basel: Karger; 2004.
71 Lopez BC, Hamlyn PJ, Zakrzewska JM. Stereotactic radiosurgery for primary trigeminal neuralgia. State of the evidence and recommendations for future reports. J Neurol Neurosurg Psychiatry. 2004;75(7):1019-1024.
72 Riesenburger RI, Hwang SW, Schirmer CM, et al. Outcomes following single-treatment Gamma Knife surgery for trigeminal neuralgia with a minimum 3-year follow-up. J Neurosurg. 2010;112(4):766-771.
73 Flickinger JC, Pollock BE, Kondziolka D, et al. Does increased nerve length within the treatment volume improve trigeminal neuralgia radiosurgery? A prospective double-blind, randomized study. Int J Radiat Oncol Biol Phys. 2001;51(2):449-454.
74 Hasegawa T, Kondziolka D, Spiro R, et al. Repeat radiosurgery for refractory trigeminal neuralgia. Neurosurgery. 2002;50(3):494-500. discussion 552
75 Pollock BE, Foote RL, Stafford SL, et al. Results of repeated gamma knife radiosurgery for medically unresponsive trigeminal neuralgia. J Neurosurg. 2000;93(Suppl 3):162-164.
76 Cheuk AV, Chin LS, Petit JH, et al. Gamma knife surgery for trigeminal neuralgia. Outcome, imaging, and brainstem correlates. Int J Radiat Oncol Biol Phys. 2004;60(2):537-541.
77 Kano H, Kondziolka D, Mathieu D, et al. Stereotactic radiosurgery for intractable cluster headache. An initial report from the North American Gamma Knife Consortium. J Neurosurg. Apr 30, 2010. [Epub ahead of print]
78 McClelland S3rd, Tendulkar RD, Barnett GH, et al. Long-term results of radiosurgery for refractory cluster headache. Neurosurgery. 2006;59(6):1258-1262. discussion 1262-1263
79 Blomgren H, Lax I, Naslund I, Svanstrom R. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34(6):861-870.
80 Haffty BG, Goldberg NB, Gerstley J, et al. Results of radical radiation therapy in clinical stage I, technically operable non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 1988;15(1):69-73.
81 Wisnivesky JP, Bonomi M, Henschke C, et al. Radiation therapy for the treatment of unresected stage I-II non-small cell lung cancer. Chest. 2005;128(3):1461-1467.
82 Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non-small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27(20):3290-3296.
83 Fakiris AJ, McGarry RC, Yiannoutsos CT, et al. Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma. Four-year results of a prospective phase II study. Int J Radiat Oncol Biol Phys. 2009;75(3):677-682.
84 Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non–small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2(7 Suppl 3):S94-S100.
85 Uematsu M, Shioda A, Suda A, et al. Computed tomography-guided frameless stereotactic radiotherapy for stage I non-small cell lung cancer. A 5-year experience. Int J Radiat Oncol Biol Phys. 2001;51(3):666-670.
86 Lagerwaard FJ, Haasbeek CJ, Smit EF, et al. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;70(3):685-692.
87 Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005;63(5):1427-1431.
88 Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation. Results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124(5):1946-1955.
89 Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070-1076.
90 Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. 2010;28(6):928-935.
91 Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24(30):4833-4839.
92 Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;72(4):967-971.
93 Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27(10):1579-1584.
94 Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol. 2009;27(10):1572-1578.
95 Okunieff P, Petersen AL, Philip A, et al. Stereotactic body radiation therapy (SBRT) for lung metastases. Acta Oncol. 2006;45(7):808-817.
96 Lee MT, Kim JJ, Dinniwell R, et al. Phase I study of individualized stereotactic body radiotherapy of liver metastases. J Clin Oncol. 2009;27(10):1585-1591.
97 Katz AW, Carey-Sampson M, Muhs AG, et al. Hypofractionated stereotactic body radiation therapy (SBRT) for limited hepatic metastases. Int J Radiat Oncol Biol Phys. 2007;67(3):793-798.
98 Norihisa Y, Nagata Y, Takayama K, et al. Stereotactic body radiotherapy for oligometastatic lung tumors. Int J Radiat Oncol Biol Phys. 2008;72(2):398-403.
99 Gerszten PC, Germanwala A, Burton SA, et al. Combination kyphoplasty and spinal radiosurgery. A new treatment paradigm for pathological fractures. J Neurosurg Spine. 2005;3(4):296-301.
100 Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases. Clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976). 2007;32(2):193-199.
101 Chang EL, Shiu AS, Lii MF, et al. Phase I clinical evaluation of near-simultaneous computed tomographic image-guided stereotactic body radiotherapy for spinal metastases. Int J Radiat Oncol Biol Phys. 2004;59(5):1288-1294.