75 Stereotactic Body Radiation Therapy
History of Stereotactic Body Radiation Therapy
Derived from the Greek term stereo, meaning solid or three-dimensional, and the Latin term tact, meaning to touch, stereotactic radiosurgery (SRS) has evolved as a widely used radiation technique that delivers high doses of radiation with exquisite accuracy to target lesions. By cross-firing radiation beams, rapid dose fall-off at the junction of the target and adjacent tissue is achieved, which reduces radiation dose to adjacent normal tissues. Introduced by Swedish neurosurgeon Lars Leksell in 1951 using a rigidly affixed skull frame to achieve accuracy, SRS has been widely successful in the management of a variety of intracranial conditions. The broad application of this concept to areas outside of the brain and head and neck regions in the past decades was limited by the complexities of internal organ motion, as well as less sophisticated technology. However, in 1991, 40 years after Leksell coined the term stereotactic radiosurgery, Ingmar Lax and colleagues at the Karolinska Hospital in Stockholm were credited as the first to propose the idea of extending stereotactic treatment approaches outside of the head to targets in the thoracic and abdominal cavities, reporting the initial results of this treatment in 1995.1 Soon thereafter, Hamilton and colleagues at the University of Arizona published the first studies on spinal radiosurgery using a skeletal fixation device applied during an operative procedure followed by treatment on a linear accelerator under anesthesia.2 Evolving from similar concepts as SRS, stereotactic body radiotherapy (SBRT) has emerged during the past decade and a half, coincident with the availability of high-speed computing and advanced imaging to allow for improved methods of dealing with respiratory motion and less invasive treatment delivery. Currently, with several mature prospective clinical trials of SBRT completed and many more ongoing, the first decade of the 21st century has witnessed an extraordinary expansion of treatment advancements for spinal tumors, lung cancer, pancreatic cancer, and liver malignancies. Emerging data in prostate cancer and renal cancer are also promising.
Radiobiology
The linear quadratic model (LQM) was developed as a mechanistic model to describe the radiobiologic effects of cell killing and sublethal repair.3 The LQM describes the probabilities of double-strand DNA breaks (DSBs), which are considered the lethal lesion induced by radiation. This probability is governed by a linear component that represents single-track damage causing a DSB, whereas the quadratic component is derived from two separate actions on the DNA leading to DSBs. The LQM predicts the isoeffect relationships for alternative fractionation schedules for early and late normal radiation reactions. Although the early models accounted mainly for repair, modifications to the original model have been made over time to account for the effects of the Rs of radiobiology: Repopulation, Redistribution, Reoxygenation and Repair.4,5
Although the LQM has proven to be almost indispensible for clinical judgments using relatively low fractional doses, the terminal bend of the survival curve at high doses creates some ambiguity about its utility for SBRT. Using the LQM, validations against clinical data appear to overestimate cell kill and underestimate toxicity at high fractional dose ranges. Some argue that mechanistic models may not fully account for all processes that are at work and favor empirically derived models. Based on the notion that the elements of the therapeutic ratio, namely tumor control as well as normal tissue complications are ultimately related predominantly to cell kill, Brenner demonstrated that the LQM has been validated for fractional doses of up to 10 Gy per fraction. Moreover, he argues that it is probably reasonable to use the LQM for fractional doses of up to 18 Gy.6 Citing a study by Ch’ang et al. who irradiated mice with a range of single fractions and analyzed gastrointestinal stem cells, Kirkpatrick et al. argues that the validity of the LQM is in question at very high doses.7 In this study, although animals irradiated to doses of 8 to 13 Gy did not die of the gastrointestinal syndrome, there was death of stem cells by apoptosis resulting in gastrointestinal syndrome and death of the animal following single doses greater than 17 to 18 Gy.8 The suggestion is that (at least in mice), with doses greater than 17 to 18 Gy, a radiation threshold of either stem cell or vasculature cell killing had been crossed. Because the LQM does not account for these observed effects, caution should be used when applying the LQM to situations of high dose per fraction. Although this and other arguments fuel the fierce debate over its validity for SBRT hypofractionated schemes, in the absence of other suitable models, the LQM remains widely used to make quantitative estimates of radiobiologic effectiveness for SBRT.
Current Stereotactic Body Radiotherapy Technologies
By today’s standards, the first SBRT treatments may seem simplistic and less sophisticated. However, the concepts of stereotactic localization and internal motion managment have served as a foundation for expanding the application of SBRT. In 1994, Lax et al.9 first described a nonrigid stereotactic body frame that contained markers for localization and a vacuum system to maximize the body contact with the frame. To reduce internal motion caused by breathing, slight constant pressure was applied to the abdomen, reducing the measured excursion of the diaphragm from 1.5 to 2.5 cm to 0.5 to 1.0 cm.9 An array of approximately eight noncoplanar, isocentric beams, which were shaped to the beam’s-eye view, were used to achieve a conformal dose distribution around the target. Similar to the initial SBRT treatments, the most widely used systems for SBRT use linear accelerators. Unlike the early techniques, several of the newer systems for SBRT in use today are integrated image-guidance systems, which incorporate either digital x-ray or tomographic imaging to assist in localization. The most widely used systems for SBRT fall into one of the following categories:
Clinical Outcomes By Organ Site
Lung Tumors
Early Stage Non–Small Cell Lung Carcinoma
Approximately 15% to 20% of patients with non–small cell lung carcinoma (NSCLC) present with stage I or II surgically resectable disease.10 Surgical resection is still the preferred curative treatment for medically operable patients, yielding 5-year survival rates of 60% to 70%.11,12 However, the surgical approach may not be an option for patients who have poor lung function, cardiac disease, other comorbid conditions, or who refuse surgery. In the past, these patients have been treated with conventional radiation therapy achieving poorer 5-year local control rates (30%-50%) and overall survival rates (10%-30%). In an analysis of Survival, Epidemiology, and End Results data comparing the outcomes of more than 4300 non–surgically resected, early stage lung cancers treated with or without radiotherapy, Wisnivesky et al.13 showed that radiotherapy was associated with improvement in median survival from 14 months to 21 months. However, these general outcomes are still poor compared with surgery. Recent studies have suggested that better local control of the primary lesion correlated with the dose of radiation delivered. In a phase I dose escalation trial of 3-D conformal radiotherapy, escalating doses from 70.2 Gy to 84 Gy delivered in 1.8-2 Gy fractions, doses of greater than 80 Gy were associated with significantly higher local control and overall survival rates, implying a need for radiation dose intensification for improved outcomes.14 In this study, dose could not be escalated beyond 84 Gy because of unacceptable toxicity associated with irradiating excessive volumes of normal tissue. Using more sophisticated respiratory motion management and improved treatment planning techniques, SBRT has emerged as the platform to achieve dose intensification in treating early stage lung cancer.15,16
Several groups worldwide have now reported encouraging prospective and retrospective results of SBRT for NSCLC.17–21 More recently, these data have been analyzed to determine factors that may predict for improved outcome and avoidance of excessive complications. Factors that have been determined to be of significance are: (1) tumor size, (2) effective radiation dose delivered, and (3) location of the tumor. In a retrospective analysis of 257 patients with early stage NSCLC who were treated with doses ranging from 18 to 75 Gy (1-22 fractions), Onishi et al.22 demonstrated a dose response for survival and local control. In this study, the dose regimens were converted to a biologically effective dose (BED) according to the LQM. Local control was significantly improved when BED was greater than 100 Gy; in other words, local recurrence rates were 8.4% for BED larger than 100 Gy versus 42.9% for BED less than 100 Gy (P < 0.001). Moreover, the 5-year overall survival rates were also significantly improved for BED larger than 100 Gy versus less than 100 Gy, of 70.8% versus 30.2%.22
Building upon a previously reported phase I dose escalation trial of SBRT,23 Timmerman et al. reported the results of a phase II study of patients with stage I inoperable NSCLC who received 60 to 66 Gy in three fractions. With a median follow-up at 17.5 months, the 2-year local control rate was 95% and the 2-year overall survival rate was 54.7%.24 In a recent update of this series with a median follow-up of 50.2 months (range 1.4-64.8 months), 3-year local control was 88.1% and 3-year overall survival was 42.7%.25 Tumor volume (<5 cc, 5-10 cc, 10-20 cc, >20 cc) did not significantly affect survival. Although interim reporting of these data showed that the majority of the 14 of 70 patients who experienced grade 3 through 5 toxicity had centrally located tumors adjacent to the proximal bronchial tree and central chest,24 in this more recent analysis, grade 3 to 5 was not statistically different between the cohorts with peripheral lung tumors (10.4%) and central tumors (27.3%) (Fisher’s exact test, P = 0.088). Furthermore, there was no significant survival difference between patients with peripheral versus central tumors (median survival 33.2 versus 24.4 months, respectively, P = 0.697). Even in light of these emerging data, SBRT practitioners remain cautious when considering treatment of centrally located tumors.
Lung Metastases
The rationale of treating lung metastases comes from the surgical observation that showed increase survival after metastatectomy.26–28 The International Registry of Lung Metastases, established in 1991 to assess the long-term results of pulmonary metastasectomy reported 36% actuarial survival at 5 years and 26% at 10 years following complete metastasectomy among its cohort of 5206 subjects.26
Song et al.29 reported a series of 25 metastatic tumors in 17 patients treated with three daily fractions of 9 to 15 Gy per fraction. Using the normal tissue complication probability calculations with the Lyman model to guide the dose delivery, 23 of 25 tumors remained controlled at median follow-up of 13 months and two late complications were encountered.29 The majority of phase I and II studies included lung metastases as well as primary lung cancer under their protocol. More recently, however, a multi-institutional phase I/II trial of SBRT in 38 patients with one to three pulmonary metastases smaller than 7 cm in greatest dimension has confirmed efficacy and tolerability.30 In the phase I portion of the trial, the dose was safely escalated from 48 Gy to 60 Gy in three fractions. Additional patients were enrolled at 60 Gy. After a median follow-up of 15 months, 50 of the 63 lesions were assessable for evaluation, yielding 1-year and 2-year local control rates of 100% and 96%, respectively.
Constraints and Toxicities
The most important variables that may be related to increased toxicity include central tumor location, large tumor size (>5 cm), prior radiation therapy, and concurrent chemotherapy. Although many trials exclude treatment of centrally located tumors within 2 cm of the proximal bronchial tree, the final 4-year update of the Indiana University trial that first reported tumor location as a potential factor for increased toxicity failed to confirm the statistical significance of tumor location.25
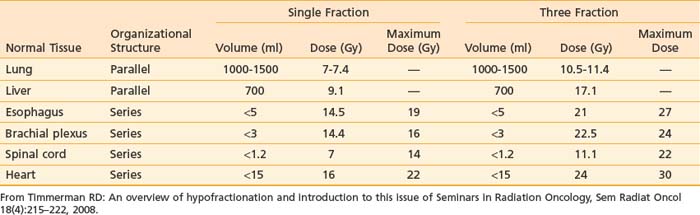
Timmerman compiled unvalidated normal tissue dose constraints for SBRT to serve as a starting point for normal tissue tolerance.31 Accordingly, the volume of serially organized tissues receiving a radiation dose above a particular threshold is recommended in addition to guidelines for a maximum dose. Critical volumes of parallel organized tissues are also recommended (Table 75-1). It is expected that in the coming years, these guidelines will be modified and replaced with data based on solid clinical outcome.
Spinal Tumors
Nearly 40% of osseous metastases involve the vertebral column, making the spine the most common site of skeletal metastases. Spinal metastases is a common problem affecting more than 100,000 patients per year and can lead to serious consequences.32 Although some patients may be asymptomatic, more than 20,000 patients per year develop epidural spinal cord compression, a medical emergency causing back pain or neurologic compromise. Treatment options may include systemic chemotherapy, surgery, or radiotherapy. Although conventional radiotherapy has been the mainstay of treatment for symptomatic lesions in an effort to achieve pain or neurologic symptom control and to decrease the chance of pathologic fracture, spinal radiosurgery has recently emerged as a useful technique of durable symptomatic management.
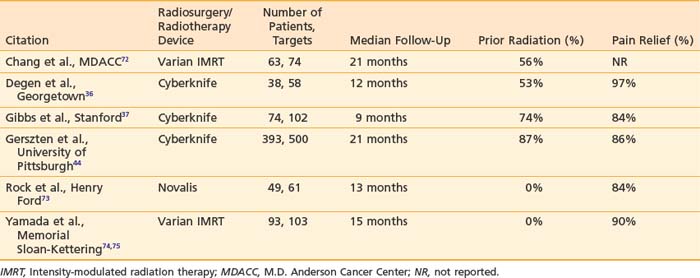
The first reports of spinal SRS in 1995, by Hamilton et al.2 and Hamilton and Lulu,33 attempted to translate traditional stereotactic principles to the treatment of spinal tumor by using an invasive stereotactic frame. Although the preliminary clinical results seemed promising, the need for general anesthesia and the invasive spinal fixation required prevented wide application of the technique. In 1994, a prototype of the Cyberknife radiosurgical system was developed at Stanford University, with the first spinal lesion treated in 1996.34 The feasibility, safety, and efficacy of spinal radiosurgery using a variety of modified linear accelerator systems has been established.35–40 The most common indication for spinal radiosurgery is spinal metastases (Table 75-2). Thus far, the largest reported single-institution series of spinal radiosurgery patients has come from Gerszten et al. of the University of Pittsburgh.41–45 In their study of 500 patients, long-term overall pain improvement was achieved in 86% of patients at up to 53 months follow-up.44
Spinal radiosurgery has also proved effective for benign spinal tumors.46–48 The largest series of radiosurgery for benign intradural spinal tumors have been reported by Dodd et al.46 and Gerszten et al.48 treating 55 lesions and 73 lesions, respectively by either single fraction (Gerszten) or multifraction (Dodd) regimens. In long-term follow-up (median >36 months), both series report excellent local control of the treated tumors. In early follow-up in the Stanford series, however, three patients required surgery because of failure to achieve symptomatic improvement. The best improvement of pain was seen in patients with meningioma or schwannoma; poorer symptomatic responses were seen with neurofibroma. Likewise, the Pittsburgh series reported poorer symptomatic response (73%) compared with local tumor control (100%). Radiation myelopathy was encountered in a single patient in the Stanford series and in three patients in the Pittsburgh series.
Reported acute, transient complications to spinal SBRT include nausea, vomiting, mucositis, dysphagia, transient flare of pain, hyperpigmentation, transient laryngitis, transient radiculitis, and esophagitis.39 Other complications include vertebral body fracture or collapse. Rose et al. of Memorial Sloan Kettering Cancer Center recently reported vertebral fracture progression in 39% (27 of 71) of vertebral metastases treated by single-fraction spinal SBRT.49 Based on the observation that increased fracture risk correlated with lytic lesions, an increased percentage of vertebral body involvement by tumor, and lesions located below T10, this group concluded that prophylactic procedures such as vertebroplasty or kyphoplasty may be considered in patients with lytic tumors involving more than 40% of the vertebral body lesions below T10. One of the most dreaded complications of spinal radiosurgery is permanent radiation-induced myelopathy. Fortunately, this complication has been only rarely reported. These have generally been represented as isolated case reports, thus limiting the ability to make general conclusions about spinal cord tolerance.37,46,50,51 In an analysis of more than 1000 patients treated by spinal SBRT for spinal metastases at Stanford University and University of Pittsburgh, six cases of radiation myelopathy were observed.52 Although definitive dosimetric correlations could not be validated, the authors recommended limiting the volume of spinal cord treated above an 8-Gy equivalent dose to less than 1 cc. Similarly, Ryu et al. recommend limiting the V10 Gy volume of the spinal cord to less than 10% (contouring the spinal cord 6 mm above and below the target lesion), or less than 0.35 cc.51
Gastrointestinal
Liver Tumors
In the United States, the most common liver tumors are colorectal liver metastases. In 2007, the incidence of colorectal cancer was estimated to be greater than 150,000, resulting in more than 52,000 annual deaths.53 The most common site of spread is metastasis to the liver and autopsy studies suggest that up to 40% of patients have metastatic disease limited to the liver.54 Worldwide, the most common liver tumor is hepatocellular carcinoma with an estimated incidence of greater than 500,000, making this the fifth most common cancer in the world.55 For selected patients with liver tumors, aggressive focal liver irradiation is indicated and SBRT is a promising technology capable of delivering highly conformal radiotherapy with great accuracy. This ablative radiotherapy approach minimizes radiation dose to surrounding normal liver as well as adjacent normal tissues. Application of this technology to appropriately selected patients may translate into high local control rates with minimal toxicity.
Herfath and colleagues56 reported one of the first studies applying single-fraction stereotactic radiation therapy for the treatment of liver tumors. These investigators initially treated patients (n = 60) with a single-fraction dose of 14 Gy and escalated the dose to 26 Gy. The median tumor size was 10 cc (range: 1-132 cc). Generally, the treatment was relatively well-tolerated, with 11 patients experiencing transient nausea or loss of appetite for 1 to 3 weeks following treatment. No patient developed radiation-induced liver disease (RILD). The overall actuarial local tumor control rates were 75% (at 6 months), 71% (at 12 months), and 67% (at 18 months). Although there was a statistically significant difference in Kaplan-Meier estimates of local tumor control between tumors treated with 14 to 20 Gy versus 22 to 26 Gy, the local control may have also been influenced by a “learning” phase. The investigators noted that the local control was improved in patients treated later in the study after establishing the proper margin expansion. In the patients treated after this phase, the actuarial local tumor control rate was 81% at 18 months. Stratification by size did not reveal a statistically significant difference in the local control rate for larger lesions (≥15 cm3) compared with smaller ones (<15 cm3) in the 22 to 26 Gy range.56 Another study examined the efficacy of treating colorectal liver metastases using a 15 Gy × 3 regimen. These authors reported a 2-year local control rate of 86%.57
Schefter and colleagues performed a dose escalation study and treated liver tumors up to 20 Gy × 3 without reaching any dose-limiting toxicity.58 These investigators treated tumors up to 6 cm in maximal dimension and limited 700 cc of normal liver to less than 15 Gy. In an expanded analysis of patients receiving 20 Gy × 3, the 18-month local control was estimated to be 93%.59 More recently, Tse and colleagues60 conducted a phase I study of SBRT for primary liver tumors using a six-fraction SBRT schedule administered over 2 weeks. The prescribed radiation dose was based on the volume of liver irradiated and estimated risk of developing liver toxicity using a normal tissue complication probability model. The dose was escalated based on a 5%, 10%, and 20% toxicity risk. The median dose for this study was 36 Gy and no RILD or grade 4 or 5 toxicity was observed within 3 months of SBRT. These investigators concluded that liver SBRT was feasible and safe for liver tumors.60
Pancreas Tumors
In 2008, there were estimated to be 37,680 new cases of pancreatic cancer in the United States with a similar number of annual deaths.61 Despite aggressive combined-modality treatments, overall survival in this disease has not changed substantially during the last 20 years. The role of radiotherapy in the management of pancreatic cancer continues to evolve as improved methods of targeting have allowed for greater precision in treating these tumors. One strategy has been to apply SRS techniques to the treatment of these tumors. Koong et al. were the first to demonstrate the feasibility of this approach in a phase I dose escalation study from Stanford University.62 Starting with a single-fraction dose of 15 Gy, these investigators increased the single fraction SBRT dose to 25 Gy without observing any significant acute gastrointestinal toxicities. In this pilot study, 100% of patients treated at the highest dose did not experience any local tumor progression from SBRT treatment until death. In a subsequent phase II study, these investigators integrated 25-Gy, single-fraction SBRT with standard gemcitabine chemotherapy.63 The 1-year survival in this group of patients with locally advanced pancreatic cancer was 50%. Acute toxicity was mild. However, late duodenal toxicity was more common, including duodenal ulcers (5/16), duodenal stenosis (1/16), and duodenal perforation (1/16).
Other investigators, however, reported disappointing results using SRS in pancreatic cancer.64 A study from Aarhus Hospital treated patients with locally advanced pancreatic cancer using a 15 Gy × 3 regimen. There was substantial toxicity in this study and only 5% of patients were alive after 1 year. Although a different fractionation scheme was used between the two studies, the major difference was that the Aarhus study used different margin expansions around the gross tumor volume, resulting in substantially greater volumes irradiated. The median volume irradiated in the Aarhus study was 136 cc (range: 38-376 cc). In contrast, the median volume irradiated in the Stanford study was 28.9 cc (range: 19.2-71.9 cc). Irradiation of larger tumor volumes likely resulted in a higher radiation dose to normal adjacent duodenum. This factor most likely accounted for the different outcomes of these studies.
Prostate
The first study to suggest the unique biology of prostate cancer quantified the sensitivity of prostate cancer to dose per fraction by comparing dose response of permanent low-dose-rate brachytherapy regimens to conventional fractionated courses.65 Using the standard LQM, Brenner and Hall65 were able to demonstrate that prostate cancer control was usually sensitive to dose per fraction with an estimated α/β of 1.5 Gy. This α/β value is unusually low compared with that of other tumor types, which typically are estimated to be 8 to 10 Gy. Since this first report in 1999, a variety of investigations have reported similar findings. In a recent review of 17 studies, it was estimated that the mean α/β was 1.85 Gy.66
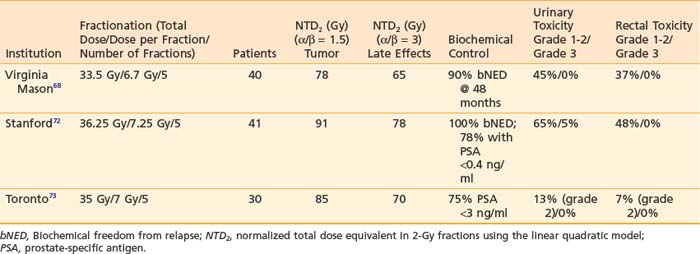
There have been both external-beam studies and high-dose-rate brachytherapy studies demonstrating the feasibility and efficacy of a hypofractionated approach. Lloyd-Davies et al. reported the safety and economic merits of hypofractionated radiotherapy for a group of 209 patients with prostate cancer treated between 1962 and 1984 with six fractions over 3 weeks.67 One of the first modern studies to report hypofractionated regimens of less than 10 fractions using techniques with improved accuracy was published by Madsen et al.68 This study of 40 patients treated with 33.5 Gy in five fractions with linear accelerator–based radiotherapy reported a 90% biochemical freedom from relapse rate at 48 months in which the PSA nadir + 2 ng/ml was the definition of biochemic failure.68 Although this study was pioneering, the effective biologic dose (if converted to a 2-Gy fraction dose equivalent) was approximately 78 Gy, which is equivalent to the common dose fractionation schemas using conventional IMRT techniques. The potential promise of SBRT is its ability to escalate the effective dose to the tumor and improve the therapeutic ratio by limiting dose and toxicity to normal structures such as the rectum and bladder. King et al.69 at Stanford University published the results of a series of 41 patients with low-risk prostate cancer treated with 36.25 Gy SBRT in five fractions delivered either daily or every other day. After a median follow-up time of only 33 months, there was no case of biochemical failure (regardless of the definition used); there were only two cases of grade 3 urinary toxicity and no grade 3 rectal toxicity. In comparison with the MD Anderson dose escalation trial of 78 Gy in 2-Gy fractions, which has a 8.7-year median follow-up time, the grade 1 and 2 urinary toxicity of the Stanford SBRT series was nearly double. In light of the finding that the MD Anderson series showed increasing rates of urinary and rectal toxicity with time, the authors expressed some caution in that longer follow-up will be needed. Current studies are ongoing for patients with low-risk prostate cancer using SBRT with similar fractionation schemes and radiation planning techniques that mimic high-dose-rate brachytherapy plans.70,71 Thus far, SBRT trials that have shown good tolerability (Table 75-3); however, given the long natural history of prostate cancer, much longer follow-up will be necessary.
1 Blomgren H, Lax I, Naslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol. 1995;34:861-870.
2 Hamilton AJ, Lulu BA, Fosmire H, et al. Preliminary clinical experience with linear accelerator-based spinal stereotactic radiosurgery. Neurosurgery. 1995;36:311-319.
3 Barendsen G. Dose fractionation, dose rate and isoeffect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys. 1982;8:1981-1997.
4 Brenner DJ, Hlatky LR, Hahnfeldt PJ, et al. A convenient extension of the linear-quadratic model to include redistribution and reoxygenation. Int J Radiat Oncol Biol Phys. 1995;32:379-390.
5 Dale RG. The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br J Radiol. 1985;58:515-528.
6 Brenner DJ. The linear-quadratic model is an appropriate methodology for determining isoeffective doses at large doses per fraction. Semin Radiat Oncol. 2008;18:234-239.
7 Kirkpatrick JP, Meyer JJ, Marks LB. The linear-quadratic model is inappropriate to model high dose per fraction effects in radiosurgery. Semin Radiat Oncol. 2008;18:240-243.
8 Ch’ang HJ, Maj JG, Paris F, et al. ATM regulates target switching to escalating doses of radiation in the intestines. Nat Med. 2005;11:484-490.
9 Lax I, Blomgren H, Naslund I, et al. Stereotactic radiotherapy of malignancies in the abdomen. Methodological aspects. Acta Oncol. 1994;33:677-683.
10 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106-130.
11 Manser R, Wright G, Hart D, et al: Surgery for early stage non-small cell lung cancer, Cochrane Database Syst Rev CD004699, 2005.
12 Raz DJ, Zell JA, Ou SH, et al. Natural history of stage I non–small cell lung cancer: implications for early detection. Chest. 2007;132:193-199.
13 Wisnivesky JP, Bonomi M, Henschke C, et al. Radiation therapy for the treatment of unresected stage I-II non–small cell lung cancer. Chest. 2005;128:1461-1467.
14 Rosenzweig KE, Fox JL, Yorke E, et al. Results of a phase I dose-escalation study using three-dimensional conformal radiotherapy in the treatment of inoperable nonsmall cell lung carcinoma. Cancer. 2005;103:2118-2127.
15 Nagata Y, Takayama K, Matsuo Y, et al. Clinical outcomes of a phase I/II study of 48 Gy of stereotactic body radiotherapy in 4 fractions for primary lung cancer using a stereotactic body frame. Int J Radiat Oncol Biol Phys. 2005;63:1427-1431.
16 Wulf J, Baier K, Mueller G, et al. Dose-response in stereotactic irradiation of lung tumors. Radiother Oncol. 2005;77:83-87.
17 Baumann P, Nyman J, Lax I, et al. Factors important for efficacy of stereotactic body radiotherapy of medically inoperable stage I lung cancer. A retrospective analysis of patients treated in the Nordic countries. Acta Oncol. 2006;45:787-795.
18 Fritz P, Kraus HJ, Blaschke T, et al. Stereotactic, high single-dose irradiation of stage I non-small cell lung cancer (NSCLC) using four-dimensional CT scans for treatment planning. Lung Cancer. 2008;60:193-199.
19 Nyman J, Johansson KA, Hulten U. Stereotactic hypofractionated radiotherapy for stage I non–small cell lung cancer—mature results for medically inoperable patients. Lung Cancer. 2006;51:97-103.
20 Zimmermann FB, Geinitz H, Schill S, et al. Stereotactic hypofractionated radiation therapy for stage I non–small cell lung cancer. Lung Cancer. 2005;48:107-114.
21 Baumann P, Nyman J, Hoyer M, et al. Outcome in a prospective phase II trial of medically inoperable stage I non–small-cell lung cancer patients treated with stereotactic body radiotherapy. J Clin Oncol. 2009;27:3290-3296.
22 Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non–small cell lung cancer: Updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol. 2007;2:S94-100.
23 McGarry RC, Papiez L, Williams M, et al. Stereotactic body radiation therapy of early-stage non–small-cell lung carcinoma: Phase I study. Int J Radiat Oncol Biol Phys. 2005;63:1010-1015.
24 Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. 2006;24:4833-4839.
25 Fakiris AJ, McGarry RC, Yiannoutsos CT, et al, Stereotactic body radiation therapy for early-stage non-small-cell lung carcinoma: four-year results of a prospective phase II study, Int J Radiat Oncol Biol Phys; 3; 2009:677-682.
26 Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. The International Registry of Lung Metastases. J Thorac Cardiovasc Surg. 1997;113:37-49.
27 Jaklitsch MT, Mery CM, Lukanich JM, et al. Sequential thoracic metastasectomy prolongs survival by re-establishing local control within the chest. J Thorac Cardiovasc Surg. 2001;121:657-667.
28 Kondo H, Okumura T, Ohde Y, et al. Surgical treatment for metastatic malignancies. Pulmonary metastasis: indications and outcomes. Int J Clin Oncol. 2005;10:81-85.
29 Song DY, Benedict SH, Cardinale RM, et al. Stereotactic body radiation therapy of lung tumors: preliminary experience using normal tissue complication probability-based dose limits. Am J Clin Oncol. 2005;28:591-596.
30 Rusthoven KE, Kavanagh BD, Burri SH, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for lung metastases. J Clin Oncol. 2009;27:1579-1584.
31 Timmerman RD. An overview of hypofractionation and introduction to this issue of seminars in radiation oncology. Semin Radiat Oncol. 2008;18:215-222.
32 Harrington KD. Orthopedic surgical management of skeletal complications of malignancy. Cancer. 1997;80:1614-1627.
33 Hamilton AJ, Lulu BA. A prototype device for linear accelerator-based extracranial radiosurgery. Acta Neurochir Suppl. 1995;63:40-43.
34 Adler JRJr, Murphy MJ, Chang SD, et al. Image-guided robotic radiosurgery. Neurosurgery. 1999;44:1299-1306.
35 Bilsky MH, Yenice K, Lovelock M, et al. Stereotactic intensity-modulation radiation therapy for vertebral body and paraspinal tumors. Neurosurg Focus. 2001;11:e7.
36 Degen JW, Gagnon GJ, Voyadzis JM, et al. CyberKnife stereotactic radiosurgical treatment of spinal tumors for pain control and quality of life. J Neurosurg Spine. 2005;2:540-549.
37 Gibbs IC, Kamnerdsupaphon P, Ryu MR, et al. Image-guided robotic radiosurgery for spinal metastases. Radiother Oncol. 2007;82:185-190.
38 Ryu S, Fang Yin F, Rock J, et al. Image-guided and intensity-modulated radiosurgery for patients with spinal metastasis. Cancer. 2003;97:2013-2018.
39 Sahgal A, Larson DA, Chang EL. Stereotactic body radiosurgery for spinal metastases: a critical review. Int J Radiat Oncol Biol Phys. 2008;71:652-665.
40 Yamada Y, Lovelock DM, Yenice KM, et al. Multifractionated image-guided and stereotactic intensity-modulated radiotherapy of paraspinal tumors: A preliminary report. Int J Radiat Oncol Biol Phys. 2005;62:53-61.
41 Gerszten PC, Burton SA, Belani CP, et al. Radiosurgery for the treatment of spinal lung metastases. Cancer. 2006;107:2653-2661.
42 Gerszten PC, Burton SA, Ozhasoglu C. CyberKnife radiosurgery for spinal neoplasms. Prog Neurol Surg. 2007;20:340-358.
43 Gerszten PC, Burton SA, Ozhasoglu C, et al. Stereotactic radiosurgery for spinal metastases from renal cell carcinoma. J Neurosurg Spine. 2005;3:288-295.
44 Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine. 2007;32:193-199.
45 Gerszten PC, Welch WC. Cyberknife radiosurgery for metastatic spine tumors. Neurosurg Clin N Am. 2004;15:491-501.
46 Dodd RL, Ryu MR, Kamnerdsupaphon P, et al. CyberKnife radiosurgery for benign intradural extramedullary spinal tumors. Neurosurgery. 2006;58:674-685.
47 Gerszten PC, Ozhasoglu C, Burton SA, et al. CyberKnife frameless single-fraction stereotactic radiosurgery for benign tumors of the spine. Neurosurg Focus. 2003;14:e16.
48 Gerszten PC, Burton SA, Ozhasoglu C, et al. Radiosurgery for benign intradural spinal tumors. Neurosurgery. 2008;62:887-895.
49 Rose PS, Laufer I, Boland PJ, et al, Risk of fracture after single fraction image-guided intensity-modulated radiation therapy to spinal metastases, J Clin Oncol; 30; 2009:5075-5079.
50 Gwak HS, Yoo HJ, Youn SM, et al. Hypofractionated stereotactic radiation therapy for skull base and upper cervical chordoma and chondrosarcoma: Preliminary results. Stereotact Funct Neurosurg. 2005;83:233-243.
51 Ryu S, Jin JY, Jin R, et al. Partial volume tolerance of the spinal cord and complications of single-dose radiosurgery. Cancer. 2007;109:628-636.
52 Gibbs IC, Patil C, Gerszten PC, et al. Delayed radiation-induced myelopathy after spinal radiosurgery. Neurosurgery. 2009;64:A67-A72.
53 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43-66.
54 Weiss L, Grundmann E, Torhorst J, et al. Haematogenous metastatic patterns in colonic carcinoma: an analysis of 1541 necropsies. J Pathol. 1986;150:195-203.
55 Bosch FX, Ribes J, Borras J. Epidemiology of primary liver cancer. Semin Liver Dis. 1999;19:271-285.
56 Herfarth KK, Debus J, Lohr F, et al. Stereotactic single-dose radiation therapy of liver tumors: results of a phase I/II trial. J Clin Oncol. 2001;19:164-170.
57 Hoyer M, Roed H, Traberg Hansen A, et al. Phase II study on stereotactic body radiotherapy of colorectal metastases. Acta Oncol. 2006;45:823-830.
58 Schefter TE, Kavanagh BD, Timmerman RD, et al. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys. 2005;62:1371-1378.
59 Kavanagh BD, Schefter TE, Cardenes HR, et al. Interim analysis of a prospective phase I/II trial of SBRT for liver metastases. Acta Oncol. 2006;45:848-855.
60 Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657-664.
61 Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96.
62 Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2004;58:1017-1021.
63 Schellenberg D, Goodman KA, Lee F, et al, Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advanced pancreatic cancer, Int J Radiat Oncol Biol Phys; 3; 2008:678-686.
64 Hoyer M, Roed H, Sengelov L, et al. Phase-II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol. 2005;76:48-53.
65 Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095-1101.
66 Dasu A. Is the alpha/beta value for prostate tumours low enough to be safely used in clinical trials? Clin Oncol (R Coll Radiol). 2007;19:289-301.
67 Lloyd-Davies RW, Collins CD, Swan AV. Carcinoma of prostate treated by radical external beam radiotherapy using hypofractionation. Twenty-two years’ experience (1962–1984). Urology. 1990;36:107-111.
68 Madsen BL, Hsi RA, Pham HT, et al. Stereotactic hypofractionated accurate radiotherapy of the prostate (SHARP), 33.5 Gy in five fractions for localized disease: First clinical trial results. Int J Radiat Oncol Biol Phys. 2007;67:1099-1105.
69 King CR, Brooks JD, Gill H, et al. Stereotactic body radiotherapy for localized prostate cancer: interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys. 2009;73:1043-1048.
70 Martinez AA, Pataki I, Edmundson G, et al. Phase II prospective study of the use of conformal high-dose-rate brachytherapy as monotherapy for the treatment of favorable stage prostate cancer: a feasibility report. Int J Radiat Oncol Biol Phys. 2001;49:61-69.
71 Fuller DB, Naitoh J, Lee C, et al. Virtual HDR CyberKnife treatment for localized prostatic carcinoma: dosimetry comparison with HDR brachytherapy and preliminary clinical observations. Int J Radiat Oncol Biol Phys. 2008;70:1588-1597.
72 Chang EL, Shiu AS, Mendel E, et al. Phase I/II study of stereotactic body radiotherapy for spinal metastasis and its pattern of failure. J Neurosurg Spine. 2007;7:151-160.
73 Rock JP, Ryu S, Yin FF, et al. The evolving role of stereotactic radiosurgery and stereotactic radiation therapy for patients with spine tumors. J Neurooncol. 2004;69:319-334.
74 Yamada Y, Bilsky MH, Lovelock DM, et al. High-dose, single-fraction image-guided intensity-modulated radiotherapy for metastatic spinal lesions. Int J Radiat Oncol Biol Phys. 2008;71:484-490.
75 Yamada Y, Lovelock M, Bilsky MH. Image-guided intensity-modulated radiation therapy of spine tumors. Curr Neurol Neurosci Rep. 2006;6:207-211.