Chapter 174 Staphylococcus
174.1 Staphylococcus aureus
Etiology
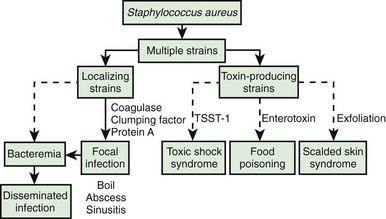
Many strains of S. aureus release 1 or more exotoxins. Exfoliatins A and B are serologically distinct proteins that produce localized (bullous impetigo) or generalized (scalded skin syndrome, staphylococcal scarlet fever) dermatologic complications (Chapter 651). Exfoliatins produce skin separation by splitting the desmosome and altering the intracellular matrix in the stratum granulosum.
Pathogenesis
The development of staphylococcal disease is related to resistance of the host to infection and to virulence of the organism (Fig. 174-1). The intact skin and mucous membranes serve as barriers to invasion by staphylococci. Defects in the mucocutaneous barriers produced by trauma, surgery, foreign surfaces (sutures, shunts, intravascular catheters), and burns increase the risk for infection.
Clinical Manifestations
Respiratory Tract
Infections of the upper respiratory tract due to S. aureus are rare, in particular considering the frequency with which the anterior nares are colonized. In normal hosts, otitis media (Chapter 632) and sinusitis (Chapter 372) are rarely caused by S. aureus. S. aureus sinusitis is relatively common in children with cystic fibrosis or defects in leukocyte function and may be the only focus of infection in some children with toxic shock syndrome. Suppurative parotitis is a rare infection, but S. aureus is a common cause. A membranous tracheitis that complicates viral croup may be due to infection with S. aureus, but other organisms are also possible. Patients typically have high fever, leukocytosis, and evidence of severe upper airway obstruction. Direct laryngoscopy or bronchoscopy shows a normal epiglottis with subglottic narrowing and thick, purulent secretions within the trachea. Treatment requires careful airway management and appropriate antibiotic therapy.
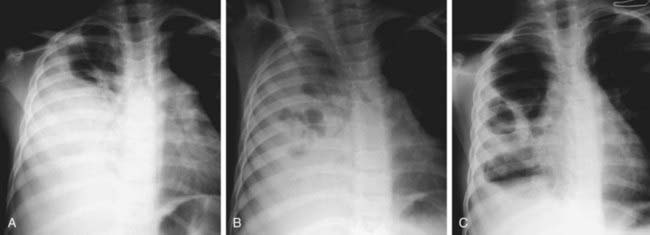
Pneumonia (Chapter 392) due to S. aureus may be primary (hematogenous) or secondary after a viral infection such as influenza. Hematogenous pneumonia may be secondary to septic emboli from right-sided endocarditis or septic thrombophlebitis, with or without the presence of intravascular devices. Inhalation pneumonia is caused by alteration of mucociliary clearance (see cystic fibrosis, Chapter 395), leukocyte dysfunction, or bacterial adherence initiated by a viral infection. Common symptoms and signs include high fever, abdominal pain, tachypnea, dyspnea, and localized or diffuse bronchopneumonia or lobar disease. S. aureus often causes a necrotizing pneumonitis that may be associated with development of empyema, pneumatoceles, pyopneumothorax, and bronchopleural fistulas.
Bones and Joints
S. aureus is the most common cause of osteomyelitis and suppurative arthritis in children (Chapters 676 and 677).
Central Nervous System
Meningitis (Chapter 595.1) caused by S. aureus is not common; it is associated with penetrating cranial trauma and neurosurgical procedures (craniotomy, cerebrospinal fluid [CSF] shunt placement) and less frequently with endocarditis, parameningeal foci (epidural or brain abscess), diabetes mellitus, or malignancy. The CSF profile of S. aureus meningitis is indistinguishable from that in other forms of bacterial meningitis.
Heart
S. aureus is a common cause of acute endocarditis (Chapter 431) on native valves. Perforation of heart valves, myocardial abscesses, heart failure, conduction disturbances, acute hemopericardium, purulent pericarditis, and sudden death may ensue.
Kidney
S. aureus is a common cause of renal and perinephric abscess (Chapter 532), usually of hematogenous origin. Pyelonephritis and cystitis due to S. aureus are unusual.
Toxic Shock Syndrome (TSS)
S. aureus is the principal cause of TSS (Chapter 174.2), which should be suspected in anyone with fever, shock, and/or a scarlet fever-like rash.
Intestinal Tract
Food poisoning (Chapter 332) may be caused by ingestion of preformed enterotoxins produced by staphylococci in contaminated foods. Approximately 2-7 hr after ingestion of the toxin, sudden, severe vomiting begins. Watery diarrhea may develop, but fever is absent or low. Symptoms rarely persist longer than 12-24 hr. Rarely, shock and death may occur.
Diagnosis
Differential Diagnosis
Skin lesions due to S. aureus may be indistinguishable from those due to group A streptococci; the former usually expand slowly, while the latter are more prone to spread rapidly. S. aureus pneumonia can be suspected on the basis of chest roentgenograms that reveal pneumatoceles, pyopneumothorax, or lung abscess (Fig. 174-2). Fluctuant skin and soft tissue lesions also can be caused by other organisms, including Mycobacterium tuberculosis, atypical mycobacteria, Bartonella henselae (cat-scratch disease), Francisella tularensis, and various fungi, among others.
Treatment
The antibiotic used as well as the dose, route, and duration of treatment depend on the site of infection, the response of the patient to treatment, and the susceptibility of the organisms recovered from blood or from local sites of infection. For most patients with serious S. aureus infection, intravenous treatment is recommended until the patient has become afebrile and other signs of infection have improved. Oral therapy is often continued for a period of time, especially in patients with chronic infection or underlying host defense problems. Treatment of S. aureus osteomyelitis (Chapter 676), meningitis (Chapter 595.1), and endocarditis (Chapter 431) are discussed in their respective chapters.
Initial treatment for serious infections thought to be due to methicillin-susceptible S. aureus (MSSA) should include semisynthetic penicillin (e.g., nafcillin, oxacillin) or less often a 1st generation cephalosporin (e.g., cefazolin). MRSA is both an important hospital and community-acquired pathogen. Community-associated MRSA infections are common throughout the USA, even in children without pre-existing risk factors. Resistance to semisynthetic penicillins and cephalosporins is related to a novel penicillin-binding protein (PB2A) that is relatively insensitive to antibiotics containing a β-lactam ring. MRSA strains appear to be at least as virulent as their methicillin-sensitive counterparts. Vancomycin (40-60 mg/kg/24 hr divided every 6 hr IV) can be used as the initial treatment for penicillin-allergic individuals and those with suspected serious S. aureus infections that might be due to MRSA. Serum levels of vancomycin should be monitored, with trough concentrations of 10-20 µg/mL, depending on the case. MRSA is also resistant to cephalosporins and carbapenems and is unreliably susceptible to the quinolones. Linezolid, daptomycin, quinupristin-dalfopristin, vancomycin with linezolid and gentamicin, and vancomycin with trimethoprim-sulfamethoxazole may be useful for serious S. aureus infections highly resistant to other antibiotics (Table 174-1).
Table 174-1 PARENTERAL ANTIMICROBIAL AGENT(S) FOR TREATMENT OF BACTEREMIA AND OTHER SERIOUS STAPHYLOCOCCUS AUREUS INFECTIONS
| SUSCEPTIBILITY | ANTIMICROBIAL AGENTS | COMMENTS |
|---|---|---|
| I. INITIAL EMPIRIC THERAPY (ORGANISM OF UNKNOWN SUSCEPTIBILITY) | ||
| Drugs of choice: | Vancomycin ± gentamicin or rifampin | For life-threatening infections (i.e., septicemia, endocarditis, CNS infection); linezolid could be substituted if the patient has received several recent courses of vancomycin |
| Nafcillin or oxacillin* | For non–life-threatening infection without signs of sepsis (e.g., skin infection, cellulitis, osteomyelitis, pyarthrosis) when rates of MRSA colonization and infection in the community are low | |
| Clindamycin | For non–life-threatening infection without signs of sepsis when rates of MRSA colonization and infection in the community are substantial and prevalence of clindamycin resistance is low | |
| Vancomycin | For non–life-threatening, hospital-acquired infections | |
| II. METHICILLIN-SUSCEPTIBLE, PENICILLIN-RESISTANT S. AUREUS (MSSA) | ||
| Drugs of choice: | Nafcillin or oxacillin*,† | |
| Alternatives (depending on susceptibility results): | Cefazolin* | |
| Clindamycin | ||
| Vancomycin | Only for penicillin- and cephalosporin-allergic patients | |
| Ampicillin + sulbactam | ||
| III. MRSA | ||
| A. Health Care–Associated (Multidrug-Resistant) | ||
| Drugs of choice: | Vancomycin ± gentamicin or ± rifampin† | |
| Alternatives: susceptibility testing results available before alternative drugs are used | ||
| Trimethoprim-sulfamethoxazole | ||
| Linezolid‡ | ||
| Quinupristin-dalfopristin‡ | ||
| Fluoroquinolones | Not recommended for people younger than 18 yr of age or as monotherapy | |
| B. Community (Not Multidrug-Resistant) | ||
| Drugs of choice: | Vancomycin† | For life-threatening infections |
| Vancomycin ± gentamicin (or ± rifampin†) | For pneumonia, septic arthritis, osteomyelitis, skin or soft tissue infections | |
| For skin or soft tissue infections | ||
| Alternatives: | Clindamycin (if strain susceptible by “D test) | |
| Trimethoprim-sulfamethoxazole | ||
| IV. VANCOMYCIN INTERMEDIATELY SUSCEPTIBLE OR VANCOMYCIN-RESISTANT S. AUREUS† | ||
| Drugs of choice: | Optimal therapy is not known | Dependent on in vitro susceptibility test results |
| Linezolid‡ | ||
| Daptomycin§ | ||
| Quinupristin-dalfopristin‡ | ||
| Alternatives: | Vancomycin + linezolid ± gentamicin | |
| Vancomycin + trimethoprim-sulfamethoxazole† | ||
CNS, central nervous system; MRSA, methicillin-resistant S. aureus.
* Penicillin- and cephalosporin-allergic patients should receive vancomycin as initial therapy for serious infections.
† One of the adjunctive agents, gentamicin or rifampin, should be added to the therapeutic regimen for life-threatening infections such as endocarditis or CNS infection or infections with a vancomycin-intermediate or vancomycin-resistant S. aureus strain. Consultation with an infectious diseases specialist should be considered to determine which agent to use and duration of use.
‡ Linezolid and quinupristin-dalfopristin are 2 agents with activity in vitro and efficacy in adults with multidrug-resistant, gram-positive organisms, including S. aureus. Because experience with these agents in children is limited, consultation with an infectious diseases specialist should be considered before use.
§ Daptomycin is active in vitro against multidrug-resistant, gram-positive organisms, including S. aureus, but has not been used often in children. Daptomycin is approved by the U.S. Food and Drug Administration only for the treatment of complicated skin and skin structure infections in patients 18 yr of age and older.
From the American Academy of Pediatrics: Red book: 2009 report of the Committee on Infectious Diseases, ed 28, Elk Grove Village, IL, 2009, American Academy of Pediatrics, pp 610–611.
Prevention
S. aureus infection is transmitted primarily by direct contact. Strict attention to handwashing techniques is the most effective measure for preventing the spread of staphylococci from one individual to another (Chapter 166). Use of a hand wash containing chlorhexidine or alcohol is recommended. In hospitals or other institutional settings, all persons with acute S. aureus infections should be isolated until they have been treated adequately. There should be constant surveillance for nosocomial S. aureus infections within hospitals. When MRSA is recovered, strict isolation of affected patients has been shown to be the most effective method for preventing nosocomial spread of infection. Thereafter, control measures should be directed toward identification of new isolates and strict isolation of newly colonized or infected patients. Clusters of cases may be defined by molecular typing. If associated with a singular molecular strain, it may also be necessary to identify colonized hospital personnel and eradicate carriage in affected individuals.
Food poisoning (Chapter 332) may be prevented by excluding individuals with S. aureus infections of the skin from the preparation and handling of food. Prepared foods should be eaten immediately or refrigerated appropriately to prevent multiplication of S. aureus with which the food may have been contaminated.
Appelbaum PC. Microbiology of antibiotic resistance in Staphylococcus aureus. Clin Infect Dis. 2007;45:S165-S170.
Baranwal AK, Singhi SC, Jayashree M. A 5-year PICU experience of disseminated staphylococcal disease, part 2: management, critical care needs and outcome. J Trop Pediatr. 2007;53:252-258.
Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus skin infections from and elephant calf—San Diego, California, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:194-198.
Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus among players on a high school football team—New York City, 2007. MMWR Morb Mortal Wkly Rep. 2009;58:52-55.
Climo MW, Sepkowitz KA, Zuccotti G, et al. The effect of daily bathing with chlorhexidine on the acquisition of methicillin-resistant Staphylococcus aureus, vancomycin-resistant Enterococcus, and healthcare-associated bloodstream infections: results of a quasi-experimental multicenter trial. Crit Care Med. 2009;37:1858-1865.
Creech CB, Beekmann SE, Chen Y, et al. Variability among pediatric infectious diseases specialists in the treatment and prevention of methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2008;27:270-272.
Day MD, Gauvreau K, Shulman S, et al. Characteristics of children hospitalized with infective endocarditis. Circulation. 2009;119:865-870.
DeLeo FR, Otto M, Kreiswirth B, et al. Community-associated methicillin-resistant Staphylococcus aureus. Lancet. 2010;375:1557-1566.
Duong M, Markwell S, Peter J, et al. Randomized, controlled trial of antibiotics on the management of community-acquired skin abscesses in the pediatric patient. Ann Emerg Med. 2010;55:401-407.
Fisher RG, Chain RL, Hair PS, et al. Hypochlorite killing of community-associated methicillin-resistant Staphylococcus aureus. Pediatr Infect Dis J. 2008;27:934-935.
Fontanilla JM, Kirkland KB, Talbot EA, et al. Outbreak of skin infections in college football team members due to an unusual strain of community-acquired methicillin-susceptible Staphylococcus aureus. J Clin Microbiol. 2010;48:609-611.
Fortunov RM, Hulten KG, Hammerman WA, et al. Community-acquired Staphylococcus aureus infections in term and near-term previously healthy neonates. Pediatrics. 2006;118:874-881.
Frederiksen MS, Espersen F, Frimodt-Moller N, et al. Changing epidemiology of pediatric Staphylococcus aureus bacteremia in Denmark from 1971 through 2000. Pediatr Infect Dis J. 2007;26:398-405.
Frymoyer A, Hersh AL, Benet LZ, et al. Current recommended dosing of vancomycin for children with invasive methicillin-resistant Staphylococcus aureus infections is inadequate. Pediatr Infect Dis J. 2009;28:398-402.
Gerber JS, Coffin SE, Smathers SA, et al. Trends in the incidence of methicillin-resistant Staphylococcus aureus infection in children’s hospitals in the United States. Clin Infect Dis. 2009;49:65-71.
Gonzalez BE, Teruya J, Mahoney DHJr, et al. Venous thrombosis associated with staphylococcal osteomyelitis in children. Pediatrics. 2006;117:1673-1679.
Hanses F, Spaeth C, Ehrenstein BP, et al. Risk factors associated with long-term prognosis of patients with Staphylococcus aureus bacteremia. Infection. 2010;38:465-470.
Haque NZ, Zuniga LC, Peyrani P, et al. Relationship of vancomycin minimum inhibitory concentrations to mortality in patients with methicillin-resistant Staphylococcus aureus hospital-acquired, ventilator-associated, or health-care-associated pneumonia. Chest. 2010;138(6):1356-1362.
Hulten KG, Kaplan SL, Gonzalez BE, et al. Three-year surveillance of community onset health care-associated Staphylococcus aureus infections in children. Pediatr Infect Dis J. 2006;25:349-353.
Hyun DY, Mason EO, Forbes A, et al. Trimethoprim-sulfamethozazole or clindamycin for treatment of community-acquired methicillin-resistant Staphylococcus aureus skin and soft tissue infections. Pediatr Infect Dis J. 2009;28:57-58.
Jacqueline C, Amador G, Caillon J, et al. Efficacy of the new cephalosporin ceftaroline in the treatment of experimental methicillin-resistant Staphylococcus aureus acute osteomyelitis. J Antimicrob Chemother. 2010;65:1749-1752.
Jang HC, Kim SH, Kim KH, et al. Salvage treatment for persistent methicillin-resistant Staphylococcus aureus bacteremia: efficacy of linezolid with or without carbapenem. Clin Infect Dis. 2009;49:395-401.
Jung YJ, Koh Y, Hong SB, et al. Effect of vancomycin plus rifampicin in the treatment of nosocomial methicillin-resistant Staphylococcus aureus pneumonia. Crit Care Med. 2010;38:175-180.
Liu C. The bundled approach to MRSA surgical site infection prevention. Arch Intern Med. 2011;171(1):73-74.
Liu C, Bayer A, Cosgrove SE, et al. Clinical practice guideline by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52(3):285-292.
Lo WT, Lin WJ, Tseng MH, et al. Risk factors and molecular analysis of panton-valentine leukocidin-positive methicillin-resistant Staphylococcus aureus colonization in healthy children. Pediatr Infect Dis J. 2008;27:713-718.
The Medical Letter. Ceftaroline fosamil (Teflaro)—a new IV cephalosporin. Med Lett Drugs Ther. 2011;53(1356):5-6.
Morales G, Picazo JJ, Baos E, et al. Resistance to linezolid is mediated by the cfr gene in the first report of an outbreak of linezolid-resistant Staphylococcus aureus. Clin Infect Dis. 2010;50:821-825.
Naseri I, Jerris RC, Sobol SE. Nationwide trends in pediatric Staphylococcus aureus head and neck infections. Arch Otolaryngol Head Neck Surg. 2009;135:14-16.
Pannaraj PS, Hulten KG, Gonzalez BE, et al. Infective pyomyositis and myositis in children in the era of community-acquired, methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2006;43:953-960.
Reed C, Kallen AJ, Patton M, et al. Infection with community-onset Staphylococcus aureus and influenza virus in hospitalized children. Pediatr Infect Dis J. 2009;28:572-576.
Ross AC, Toltzis P, O’Riordan MA, et al. Frequency and risk factors for deep focus of infection in children with Staphylococcus aureus bacteremia. Pediatr Infect Dis J. 2008;27:396-399.
Tebruegge M, Pantazidou A, Thorburn K, et al. Bacterial tracheitis: a multi-centre perspective. Scand J Infect Dis. 2009:1-10.
Valente AM, Jain R, Scheurer M, et al. Frequency of infective endocarditis among infants and children with Staphylococcus aureus bacteremia. Pediatrics. 2005;115:e15-19.
Varshney AK, Martinez LR, Hamilton SM, et al. Augmented production of Panton-Valentine leukocidin toxin in methicillin-resistant and methicillin-susceptible Staphylococcus aureus is associated with worse outcome in a murine skin infection model. J Infect Dis. 2010;201:92-96.
Wolkewitz M, Frank U, Philips G, et al. Mortality associated with in-hospital bacteremia caused by Staphylococcus aureus: a multistate analysis with follow-up beyond hospital discharge. J Antimicrob Chemother. 2011;66:381-386.
Yoshinaga M, Niwa K, Niwa A, et al. Risk factors for in-hospital mortality during infective endocarditis in patients with congenital heart disease. Am J Cardiol. 2008;101:114-118.
174.2 Toxic Shock Syndrome
Clinical Manifestations
The diagnosis of TSS is based on clinical manifestations (Table 174-2). The onset is abrupt, with high fever, vomiting, and diarrhea, and is accompanied by sore throat, headache, and myalgias. A diffuse erythematous macular rash (sunburn-like or scarlatiniform) appears within 24 hr and may be associated with hyperemia of pharyngeal, conjunctival, and vaginal mucous membranes. A strawberry tongue is common. Symptoms often include alterations in the level of consciousness, oliguria, and hypotension, which in severe cases may progress to shock and disseminated intravascular coagulation. Complications, including acute respiratory distress syndrome, myocardial dysfunction, and renal failure, are commensurate with the degree of shock. Recovery occurs within 7-10 days and is associated with desquamation, particularly of palms and soles; hair and nail loss have also been observed after 1-2 mo. Many cases of apparent scarlet fever without shock may be caused by TSST-1-producing S. aureus strains.
Table 174-2 DIAGNOSTIC CRITERIA OF STAPHYLOCOCCAL TOXIC SHOCK SYNDROME
MAJOR CRITERIA (ALL REQUIRED)
MINOR CRITERIA (ANY 3 OR MORE)
EXCLUSIONARY CRITERIA
From the American Academy of Pediatrics Red book: 2009 report of the Committee on Infectious Diseases, ed 28, Elk Grove Village, IL, 2009, American Academy of Pediatrics, p 602.
Diagnosis
Differential Diagnosis
Group A streptococcus can cause a similar TSS-like illness, termed streptococcal TSS (Chapter 176), which is often associated with severe streptococcal sepsis or a focal streptococcal infection such as cellulitis, necrotizing fasciitis, or pneumonia.
Kawasaki disease closely resembles TSS clinically but is usually not as severe or rapidly progressive. Both conditions are associated with fever unresponsive to antibiotics, hyperemia of mucous membranes, and an erythematous rash with subsequent desquamation. However, many of the clinical features of TSS are usually absent or rare in Kawasaki disease, including diffuse myalgia, vomiting, abdominal pain, diarrhea, azotemia, hypotension, acute respiratory distress syndrome, and shock (Chapter 160). Kawasaki disease typically occurs in children younger than 5 yr. Scarlet fever, Rocky Mountain spotted fever, leptospirosis, toxic epidermal necrolysis, sepsis, and measles must also be considered in the differential diagnosis.
Chan KH, Kraai TL, Richter GT, et al. Toxic shock syndrome and rhinosinusitis in children. Arch Otolaryngol Head Neck Surg. 2009;135:538-542.
Lappin E, Ferguson AJ. Gram-positive toxic shock syndromes. Lancet Infect Dis. 2009;9:281-290.
Zimbelman J, Palmer A, Todd JK. Improved outcome of clindamycin compared with beta-lactam antibiotics treatment of invasive Streptococcus pyogenes infection. Pediatr Infect Dis J. 1999;18:1096-1100.
174.3 Coagulase-Negative Staphylococci
Clinical Manifestations
Endocarditis
Infection of native heart valves or the right atrial wall secondary to an infected thrombosis at the end of a central line may produce endocarditis. S. epidermidis and other CONS may rarely produce native valve subacute indolent endocarditis in previously normal patients without a central venous catheter. CONS is a common cause of prosthetic valve endocarditis, presumably due to inoculation at the time of surgery. Infection of the valve sewing ring, with abscess formation and dissection, produces valve dysfunction, dehiscence, arrhythmias, or valve obstruction (Chapter 431).
Urinary Tract Infection
S. saprophyticus is a common cause of primary urinary tract infections in sexually active females. Manifestations are similar to those characteristics of urinary tract infection due to Escherichia coli (Chapter 532). CONS also causes asymptomatic urinary tract infection in hospitalized patients with urinary catheters and after urinary tract surgery or transplantation.
Acuna M, O’Ryan M, Cofre J, et al. Differential time to positivity and quantitative cultures for noninvasive diagnosis of catheter-related blood stream infection in children. Pediatr Infect Dis J. 2008;27:681-685.
Karlowicz MG, Furigay PJ, Croitoru DP, et al. Central venous catheter removal versus in situ treatment in neonates with coagulase-negative staphylococcal bacteremia. Pediatr Infect Dis J. 2002;21:22-27.