Standard Precautions and Infectious Exposure Management
Contamination of health care workers with body fluids is a frequent occurrence in the emergency department (ED). A survey of ED staff found that 54% reported contact of intact skin with body fluids and 1.5% reported contact of nonintact skin within the preceding year.1 These fluids often contain various transmissible infectious diseases because the prevalence of human immunodeficiency virus (HIV) infection, hepatitis, and other communicable diseases is high in ED patient populations.1,2 In a prospective study of penetrating trauma patients admitted to an urban trauma center, 9% tested positive for anti-HIV, hepatitis B surface antigen (HBsAg), or anti–hepatitis C virus (HCV). Patients were infrequently aware of their seropositive status.2 In the ED, patient characteristics were found to be poor predictors of hepatitis positivity, thus making it more difficult to identify patients who pose a risk to health care workers.3 These factors make widespread use of universal precautions in the ED essential.
Compliance with standard precautions, formerly known as universal precautions, is far from universal.4–6 In a video-taped observational study of 88 ED trauma resuscitations, 33.4% had major breaks in standard precautions. The most common major break was failure to wear a mask (32.2% of procedures), followed by inadequate eyewear (22.2%), no gown (5.6%), and no gloves (3.0%).6 Henry and colleagues showed that ED personnel significantly overestimate their use of standard precautions.7
In 1985, the combination of high-risk illness with low-compliance barrier use prompted the Centers for Disease Control and Prevention (CDC) to recommend guidelines for the protection of health care workers.8 In 1991, these recommendations were enacted into law by mandate of the Occupational Safety and Health Administration (OSHA).9 The primary focus of the CDC guidelines is to reduce mucocutaneous exposure to body fluids by encouraging hand washing and barrier protection. These measures do little to protect from percutaneous exposure, which is the most efficient method of transmission of hepatitis and HIV.10,11 The current strategy for risk reduction in the ED includes immunization against hepatitis B virus (HBV), use of standard precautions (including reengineered safety products), and prompt initiation of postexposure prophylaxis (PEP) when appropriate.
Guidelines for Standard Precautions
Barrier Precautions
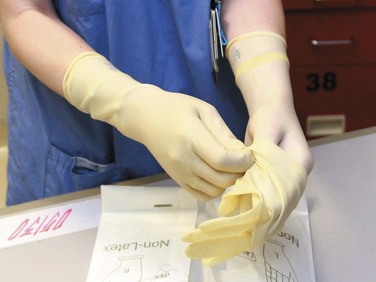
1. Use gloves for any patient contact with a risk for exposure to body fluids (Fig. 68-1). Both cutaneous and percutaneous exposure can be reduced by the use of gloves. Gloves have been shown to reduce disease transmission in needlestick injuries, with greater reduction seen with double gloving in animal models and in a case crossover study of health care workers.12 Mansouri and associates compared the biomechanical performance of single and double latex and nitrile examination gloves. Transmission of red blood cells was less with nitrile gloves than with a single layer of latex gloves despite being thinner than the latex examination gloves. A double layer of latex gloves provided the best protection in this study.13
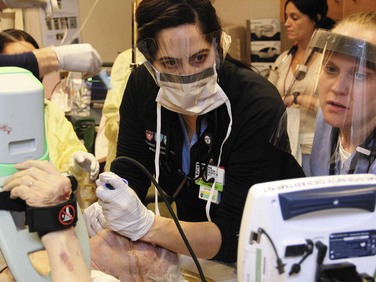
2. Wear a mask and protective eyewear when exposure to body fluid aerosols is possible (e.g., wound irrigation, traumatic chest wound) (Fig. 68-2).
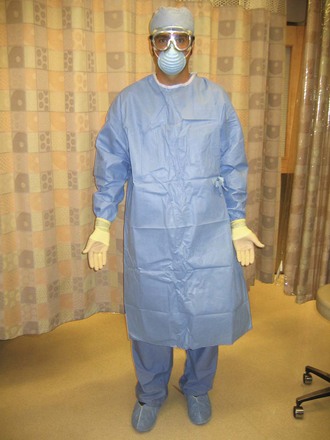
3. Wear a gown and shoe covers when there is the risk for large volumes of splashed body fluids (e.g., chest tube, thoracotomy) (Fig. 68-3).
Sharps Precautions
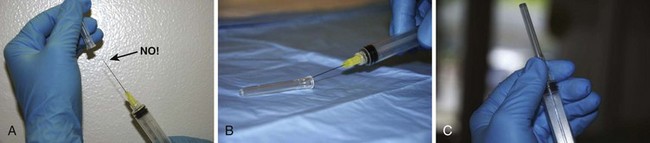
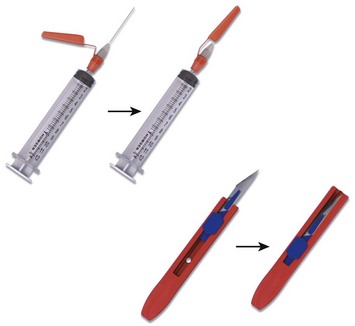
Most importantly, sharps precautions mean no recapping, bending, or breaking of needles. If needle recapping is deemed necessary, use a single-handed technique (Fig. 68-4). A safer alternative is to immediately dispose of the needle in an approved sharps container without recapping. In an observational study of ED employees, the rate of needle recapping was 34%, with most practitioners using a two-handed technique.14 Various reengineered products are available for use in the ED, including retracting scalpels, auto-capping needles, and needleless intravenous systems (Fig. 68-5). Using such devices decreases percutaneous injury rates among health care workers.15,16 A survey of infection control professionals at Iowa and Virginia hospitals found that implementation of such devices was the most common action taken to decrease percutaneous injuries.17 U.S. federal law now requires the use of safety-engineered sharps devices to protect health care workers. In brief, the Needle-stick Safety and Prevention Act of 2000 required the use of safety-engineered sharps devices to protect health care workers from needlestick injuries.18 A review of data from the Exposure Prevention Information Network (EPINet) sharps injury surveillance program estimated that needlestick injuries have decreased by 34% overall, with a 51% decline in nurses since the implementation of federal legislation.16
Respiratory Precautions
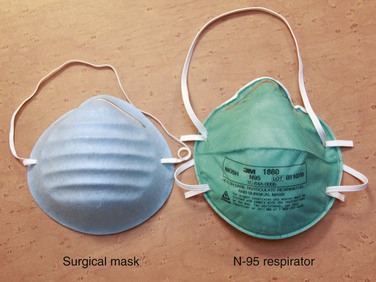
During contact with patients who have suspected or confirmed pulmonary tuberculosis (TB), wear a National Institute for Occupational Safety and Health (NIOSH)–approved N-95 particulate respirator (Fig. 68-6). These masks are designed to efficiently filter 1- to 5-µm particles and are less costly and more comfortable than high-efficiency particulate air (HEPA)–filtered masks. In addition, place such patients in a respiratory isolation room with negative pressure, high circulation (optimally at least 12 air changes per hour), and external exhaust. While in the ED, avoid procedures resulting in increased release of infectious droplets, such as sputum induction. Make sure that all potentially infectious patients wear a surgical-type mask, especially during transportation outside the respiratory isolation room (e.g., to radiology).19 Implementation of the CDC guidelines is variable. A study of three California hospitals in which CDC guidelines and hospital procedure were compared with actual practice found that 19% of patients with TB were not in negative pressure rooms. Of the 62 health care workers observed using a respirator for TB, 65% did not use it properly.20
Hand Washing
Immediately wash any skin surface coming in contact with body fluids with soap and water. If performed properly, both soap and water and alcohol-based products are generally efficacious in removing bacteria.21 Soap and water perform better in removing Clostridium difficile spores,22 but alcohol-based products are preferred and may be more viricidal.21,23 In most health care settings, hand-washing rates remain low.24–26
Occupational Disease Exposure
HBV
HBV is a well-recognized occupational risk for health care providers, and multiple studies have documented the high prevalence of hepatitis in ED patients.3,27–29 An estimated 100 to 200 health care workers have died annually during the past decade because of the chronic consequences of HBV infection.30 Despite the attention focused on transmission of HIV, the infectivity of HBV is significantly higher. HBV is a more virulent organism and requires a relatively small inoculum for transmission.31 Percutaneous injuries are the most efficient mode of HBV transmission, but many infected health care workers do not recall a specific injury.32 Many body fluids other than blood contain HBsAg, but levels of infectious HBV particles in blood-free body fluids are 100 to 1000 times lower than in blood itself. Although human saliva alone does not appear to pose a significant risk for transmission of disease, human bites have been associated with transmission of HBV.33 Implementation of the CDC’s standard precautions, along with the OSHA regulations for barrier protection and preexposure vaccination, has led to a decrease in the incidence of HBV transmission.34
To understand the risk of HBV transmission resulting from occupational exposure, an understanding of a few key serologic markers for HBV is essential. HBsAg is a marker of active infection in the source patient. From a practical standpoint, HBV can be transmitted when HBsAg is present, and it is not generally transmissible when this marker is absent. Hepatitis B surface antibody (HBsAb) is a protective antibody against HBV. In vaccinating health care workers, the goal is to stimulate the immune system to produce a sufficient quantity of this antibody. Hepatitis B e antigen (HBeAg) can be found in the bloodstream of HBV-infected individuals during times of peak virus replication. When a source patient is positive for HBeAg, the bloodstream contains a much larger number of infectious HBV particles. If a nonimmune individual sustains a needlestick from an HBsAg-positive patient, the risk for HBV transmission depends on the HBeAg status of the source. The risk for clinical hepatitis is approximately 2% (range, 1% to 6%) if HBeAg is absent as opposed to a risk of 22% to 31% if HBeAg is present.35
Postexposure Management
PEP following exposure to an HBsAg-positive source may require hepatitis B vaccine, hepatitis B immunoglobulin (HBIG), both, or neither (Table 68-1). This depends on the vaccination and antibody response status of the exposed health care worker. HBIG is derived from pooled human plasma and provides passive immunization for nonimmune exposed individuals. This preparation is very safe and not known to transmit disease.34,35 When HBIG is used for PEP, give it ideally within 24 hours after exposure, and note that it is of questionable value beyond 7 days.36 PEP for HBV is remarkably effective, and infection is unlikely to develop in individuals who receive PEP.30 Hepatitis B vaccine may also be given with PEP. Individuals who have not previously been vaccinated or who have not demonstrated an adequate response should receive the hepatitis B vaccine. Adverse reactions to the hepatitis B vaccine are generally quite mild, and it is safe to give during pregnancy. For primary immunization, give an initial intramuscular injection, followed by subsequent intramuscular vaccinations at 1 and 6 months. Check antibody levels (HBsAb) at 4 to 6 weeks after the series is completed to confirm that the desired titer of at least 10 mIU/mL has been attained. Vaccinated individuals who achieve this antibody level are referred to as “responders” and are believed to be immune for life. Although 25% to 50% of vaccine responders demonstrate a decline in HBsAb levels to below 10 mIU/mL within 5 to 7 years, these individuals are still protected against clinical disease because of a robust immune system memory or anamnestic response.37 There is no need to provide vaccination or to check titers in individuals who have previously had an adequate titer.30 PEP with these agents is not contraindicated during pregnancy or lactation. Health care workers who have previously been infected with HBV are immune to reinfection, so PEP is not indicated in such individuals.
TABLE 68-1
Recommendations for Hepatitis B Prophylaxis after Percutaneous or Permucosal Exposure
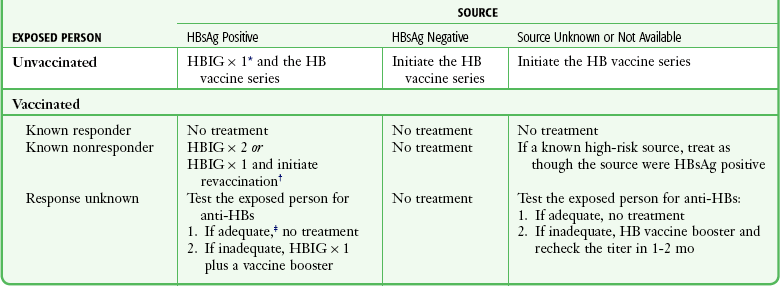
HB, hepatitis B; HBIG, hepatitis B immunoglobulin; HBsAg, hepatitis B surface antigen.
*HBIG dose = 0.06 mL/kg intramuscularly.
†The option of giving one dose of HBIG and reinitiating the vaccine series is preferred for nonresponders who have not completed a second three-dose vaccine series. For persons who previously completed a second vaccine series but failed to respond, two doses of HBIG are preferred.
‡Adequate anti-HBs = 10 mIU/mL.
Adapted from Panlillio AL, Cardo DM, Grohskopf LA, et al. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54(RR-9):1-17.
HCV
Approximately 1.6% of Americans (4.1 million) are infected with HCV,38 and many individuals are unaware of their infection. ED patients have a higher prevalence than the general population. In one study the prevalence of HCV antibody was 4.0% as compared with a prevalence in the overall U.S. population of 1.8%.27 HCV is often acquired from injection drug use. It was once commonly transmitted by blood transfusion but is fortunately rare now with modern screening. Although HCV can be transmitted sexually, this a minor route. Mucous membrane transmission of HCV is possible but much less common. Percutaneous transmission is the most efficient route. The incidence of seroconversion after an HCV-positive needlestick is about 1.8% (estimates range from 0% to 7%).35 It is useful to remember that the risk for transmission of HCV after a needlestick is similar to that for transmission of HBV when the source is HBeAg negative. When seroconversion does occur, 80% of patients will demonstrate antibodies at 15 weeks and 97% at 6 months after exposure. Although the clinical course of HCV is often asymptomatic or mild, chronic hepatitis will develop in approximately 85% of patients, cirrhosis in 10% to 20%, and hepatocellular carcinoma in 1% to 5%.39–41
HIV
According to the CDC, 57 cases of occupational HIV transmission to health care workers occurred in the United States through 2010. In addition, another 143 health care workers demonstrated HIV seroconversion that may have been occupationally related.43 The risk for contracting HIV from working in the ED depends on the prevalence of HIV in the local patient population. Wears and coworkers44 estimated the cumulative career risk of contracting HIV from occupational exposure in a high-prevalence ED to be as high as 1.4%. The overall risk for HIV seroconversion is about 1 in 300 (0.3%) after a needlestick and less than 1 in 1000 for mucous membrane exposure. Cardo and colleagues45 demonstrated that the risk for HIV seroconversion after needlestick injuries is not uniform. Seroconversion was found to be more likely for deep injuries (odds ratio [OR] = 15), if blood was visible on the device (OR = 6.2), if the needle had been used in a source patient’s artery or vein (OR = 4.3), or if the source patient suffered from terminal acquired immunodeficiency syndrome (AIDS; OR = 5.6). It is essential to gather information regarding the nature of the injury to “risk-stratify” the exposure. Exposure of intact skin to contaminated blood has not been identified as a risk for transmission of HIV.46,47
When seroconversion occurs, HIV antibodies can be detected as early as 3 weeks after exposure and are almost always present by 6 months. Seroconversion at 6 to 12 months is rare but has been reported in individuals co-infected with HIV and HCV. Follow-up HIV testing is recommended for 12 months for health care workers who become infected with HCV after dual exposure to both HCV and HIV.46 Acute retroviral syndrome is a clinical manifestation of HIV seroconversion that occurs in approximately 80% of newly infected individuals at a median of 25 days after exposure. The signs and symptoms of acute retroviral syndrome are similar to those of mononucleosis and consist of fever, lymphadenopathy, and rash.
Postexposure Management46
Evidence Supporting PEP: In 1998 the U.S. Public Health Service recommended using PEP for selected HIV exposures.40 These recommendations were based on a single case-control study.45 Currently, a large placebo-controlled trial would be considered unethical. The CDC-sponsored case-control study, undertaken in the United States, France, the United Kingdom, and Italy, compared 33 health care workers who seroconverted after exposure to HIV with 665 control health care workers who did not seroconvert after exposure to HIV. About 90% of the patients in this study were exposed via hollow-bore needles.45 When postexposure zidovudine (azidothymidine [AZT]) was used, the risk for HIV infection was reduced by 81% (95% confidence interval, 48% to 94%). Although the study methodology was limited by its retrospective design and the potential for recall bias, these results strongly support the efficacy of AZT for PEP. In a human study of vertical transmission, the use of antiretroviral agents during pregnancy decreased perinatal HIV transmission by 67%.48 Of children born to HIV-positive mothers who were given HIV PEP within 48 hours of birth, HIV transmission was also decreased.49 Although perinatal exposure is different from occupational needlestick, this evidence supports the concept of a “window of opportunity” during which PEP may prevent transmission of HIV to an exposed individual.
Selecting Patients for PEP: In both 2001 and 2005, the U.S. Public Health Service published updated recommendations regarding the use of HIV PEP.35,46 In general, the decision to use PEP depends on the type of exposure and the source’s HIV status. The first step in determining whether PEP is indicated is to assess the severity of exposure. Percutaneous exposure can be categorized as “less severe” or “more severe.” A less severe exposure involves a solid needle, a superficial injury, and no blood visible on the device. All other percutaneous injuries are categorized as more severe. Exposure to mucous membranes and nonintact skin is categorized as either “small volume” (a few drops of blood) or “large volume” (a major blood splash). There are no reported cases of HIV seroconversion after exposure of intact skin to blood.35,46,47 After assessing the severity of exposure, determine the potential infectivity of the source. Consider PEP only for exposure to blood and body fluids from a source known or likely to be HIV positive. Exposure from an HIV-negative source does not require PEP. Do not test sharp instruments for HIV because this is not reliable or recommended. Categorize HIV-positive source patients as either “lower risk” (class 1) or “higher risk” (class 2). Class 1 patients have asymptomatic HIV infection and a low viral load (<1500 RNA copies/mL). Higher-risk patients include those with symptomatic HIV, AIDS, acute seroconversion, or a high viral load. Once the severity of exposure and source HIV status are determined, use Table 68-2 and Table 68-3 to guide the proper PEP regimen. Note that drug development is ongoing and recommendations change rapidly. The clinician is urged to periodically check for updates. The most reliable source is the CDC website or the Morbidity and Mortality Weekly Report journals, most easily found via an Internet search under various topics. For exposure of skin and mucous membranes, choose a PEP regimen from three general categories. For small-volume exposure to an HIV class 1 source, consider using the basic regimen (two drugs). If either the exposure is of large volume or the source is HIV class 2, recommend the basic regimen. In cases in which there is both a large-volume exposure and an HIV class 2 source, recommend the expanded regimen (at least three drugs). For most percutaneous exposures, recommend the expanded regimen. For less severe percutaneous exposures from an HIV class 1 source, recommend the basic regimen.
TABLE 68-2
Recommended HIV PEP for Percutaneous Injuries
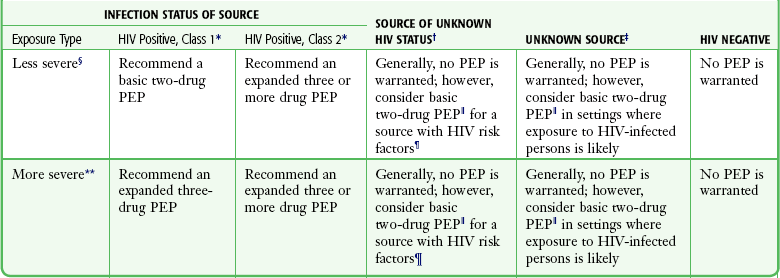
*HIV positive, class 1: asymptomatic HIV infection or known low viral load (e.g., <1500 RNA copies/mL); HIV-positive, class 2: symptomatic HIV infection, AIDS, acute seroconversion, or known high viral load. If drug resistance is a concern, obtain expert consultation. Initiation of PEP should not be delayed pending expert consultation, and because expert consultation alone cannot substitute for face-to-face counseling, resources should be available to provide immediate evaluation and follow-up care for all exposures.
†Source of unknown HIV status (e.g., deceased source person with no samples available for HIV testing).
‡Unknown source (e.g., a needle from a sharps disposal container).
§Less severe (e.g., solid needle or superficial injury).
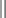 The designation “consider PEP” indicates that PEP is optional and should be based on an individualized discussion between the exposed person and the treating clinician regarding the risks versus benefits of PEP.
The designation “consider PEP” indicates that PEP is optional and should be based on an individualized discussion between the exposed person and the treating clinician regarding the risks versus benefits of PEP.
¶If PEP is offered and administered and the source is later determined to be HIV negative, PEP should be discontinued.
**More severe (e.g., large-bore hollow needle, deep puncture, blood visible on the device, or needle used in the patient’s artery or vein).
Adapted from Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54(RR-9):3.
TABLE 68-3
Recommended HIV PEP for Mucous Membrane Exposure and Nonintact Skin* Exposure
HIV, human immunodeficiency virus; PEP, postexposure prophylaxis.
*For skin exposure, follow-up is indicated only if there is evidence of compromised skin integrity (e.g., dermatitis, abrasion, or an open wound).
†HIV positive, class 1: asymptomatic HIV infection or known low viral load (e.g., <1500 RNA copies/mL); HIV-positive, class 2: symptomatic HIV infection, AIDS, acute seroconversion, or known high viral load. If drug resistance is a concern, obtain expert consultation. Initiation of PEP should not be delayed pending expert consultation, and because expert consultation alone cannot substitute for face-to-face counseling, resources should be available to provide immediate evaluation and follow-up care for all exposures.
‡Source of unknown HIV status (e.g., deceased source person with no samples available for HIV testing).
§unknown source (e.g., splash from inappropriately disposed blood).
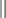 Small volume (e.g., a few drops); large volume (e.g., major blood splash).
Small volume (e.g., a few drops); large volume (e.g., major blood splash).
¶The designation “consider PEP” indicates that PEP is optional and should be based on an individualized discussion between the exposed person and the treating clinician regarding the risks versus benefits of PEP.
**If PEP is offered and administered and the source is later determined to be HIV negative, PEP should be discontinued.
Adapted from Updated U.S. Public Health service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54(RR-9):3.
PEP and antiretroviral use are considered safe and recommended during pregnancy50 and in pediatric patients for occupational and nonoccupational exposure.51
Choice of PEP Medications: When HIV PEP is administered, a minimum of two drugs is recommended. Although there is no direct evidence that combination PEP regimens are beneficial, concerns about antiretroviral resistance and the synergistic effects of antiviral medications when treating patients with AIDS support such an approach. As discussed earlier, the U.S. Public Health Service recommends using a basic (two-drug) PEP regimen for lower-risk HIV exposure and an expanded (three- and even four-drug) PEP regimen for higher-risk exposure (Fig. 68-7). The basic PEP regimen consists of either two nucleoside reverse transcriptase inhibitors or a nucleoside reverse transcriptase inhibitor plus a nonnucleoside reverse transcriptase inhibitor. The traditional regimen is zidovudine (ZDV) plus lamivudine (3TC), available as Combivir. Alternative basic regimens include ZDV plus emtricitabine (FTC), tenofovir (TDF) plus 3TC or FTC, and stavudine (d4T) plus 3TC or FTC. When using the expanded PEP regimen, add a protease inhibitor to the basic regimen. In the currently preferred expanded regimen, lopinavir/ritonavir is added to the basic regimen. Acceptable alternative protease inhibitors include atazanavir, fosamprenavir, indinavir, saquinavir, and nelfinavir.
A number of second-line and alternative agents may be chosen for HIV PEP. Regional variation in PEP recommendations exist. New York state guidelines recommend that PEP always consist of three nucleoside analogue transcriptase inhibitors.52 Expert consultation is recommended, especially if antiretroviral resistance is suspected. An important resource for emergency clinicians is the National Clinicians’ Postexposure Prophylaxis Hotline at UCSF/San Francisco General Hospital. Expert consultation can be obtained at www.nccc.ucsf.edu or by calling 888-448-4911
Timing, Duration, and Side Effects of PEP: HIV exposure should be considered a true emergency. Administer PEP as soon as possible after exposure, ideally within 1 hour. Animal studies indicate that the efficacy of PEP diminishes with a delay in initiation.53 HIV PEP regimens consist of a 4-week course of therapy. In the ED, patients can be prescribed the first 3 days of medications as long as outpatient follow-up is arranged (see Fig. 68-7). Side effects from the medications used for HIV PEP are not insignificant. Side effects are experienced by about 50% of health care workers taking PEP and cause approximately 33% of health care workers to discontinue therapy prematurely.54 Depending on the choice of PEP medications, patients should also be prescribed antiemetics and antidiarrheal agents when PEP is initiated.
TB
During the mid-1980s the United States experienced a resurgence in TB, especially among HIV-positive patients. Although the incidence of TB cases in the United States has since declined, more than 11,000 cases were reported in 2010.55 TB continues to pose a serious risk to both public health and health care workers. Baussano and colleagues estimated that the annual risk for TB infection in health care workers, attributable to occupational exposure, is between 3.8% and 8.4%, depending on local prevalence.56 TB is transmitted by infectious droplets 1 to 5 µm in size. Primary infection occurs when one to three organisms are inhaled into the alveoli, where they begin to replicate. Host defenses usually stop infection within 2 to 10 weeks, and the patient enters the latent period. During this time, patients are asymptomatic and not contagious. Reactivation occurs when cell-mediated immunity wanes, and patients are again contagious. This can be due to advancing age, HIV infection, steroid use, malignancy, malnutrition, or other causes of suppression of the immune system. The lifetime risk for reactivation is 5% to 10%, with about half this risk occurring in the first few years after primary infection. Patients with increased infectivity include those with pulmonary or laryngeal TB, an active cough, positive sputum smears for acid-fast bacilli, cavitary lesions on chest radiographs, and inadequate therapy. Overall, children are less contagious than adults but can still transmit the disease. Extrapulmonary TB is contagious only in cases of an open skin lesion or involvement of the oral cavity.19
Depending on the patient population and geographic location, ED personnel can be at high risk for occupational TB infection. In a 1993 study at a county hospital in Los Angeles it was reported that 31% of ED workers became positive for purified protein derivative (PPD) during employment, including 20% of attendings, 32% of nurses, and 33% of residents.57 The risk for PPD conversion was found to be 6% after 1 year of ED employment, 14% after 2 years, and 27% after 4 years. EDs typically care for higher-risk patients—those who are homeless, foreign born, recently incarcerated, or chronically debilitated. Overcrowding can lead to extended waiting periods and delays in admission. The clinical manifestation of TB in ED patients is often atypical, which can lead to a delay in diagnosis.58,59 This is especially true for HIV-infected patients, in whom the symptoms may mimic Pneumocystis jiroveci pneumonia, skin tests are often negative, findings on chest radiographs are commonly atypical, and sputum tests may be less sensitive.60–66 In one study of ED patients with TB, the mean time from ED registration to respiratory isolation was 6.5 hours, and 46% of patients were first isolated on the hospital ward.67 A 14-year review of the U.S. National Tuberculosis Surveillance System showed that rates of advanced pulmonary TB are on the rise, especially in populations without traditional risk factors.68 These data suggest that substantial delays in diagnosis and misdiagnosis of pulmonary TB may be increasing.
Preventing exposure to TB requires a multifaceted approach.19 Proper ED ventilation plays a key role; inadequate ventilation has been a contributing factor in many nosocomial outbreaks. Ideally, install single-pass airflow from waiting rooms to the outside. Within the ED, make sure that air flows from clean areas to less clean areas, not vice versa. If patients with TB are seen frequently, provide at least one true respiratory isolation room in the ED. Make sure that such rooms have at least 12 air changes per hour and that they are “negative pressure” rooms in which air flows into the room from other ED areas. Other engineering approaches to control of TB infection include using HEPA filters and upper room ultraviolet light irradiation.
Familiarize all ED personnel with the appropriate use of respiratory protection against TB. Provide surgical masks (e.g., string-tie masks) for source control. Place these masks on potentially contagious patients to decrease the passage of infectious droplets into the air. Because air can leak around such masks, they are not optimal for protection of health care workers. In late 1995, NIOSH certified a new class of masks known as N-95 particulate respirators.69 These masks filter particles 1-µm in size with at least 95% efficiency and are generally the preferred mask for health care workers (Fig. 68-8). In some circumstances, such as when patients are undergoing cough-inducing or aerosol-generating procedures, health care workers need better protection. N-95 masks are usually the appropriate choice for ED use, but these masks should be thought of as the minimum required level of respiratory protection against TB.19
Initiate early respiratory isolation of patients with suspected pulmonary TB as soon as possible in the ED, ideally at triage. Screening protocols at triage can detect patients with more classic signs and symptoms of TB, but reported protocols are only moderately sensitive and somewhat cumbersome.58,59 Consider immediate respiratory isolation for patients with high-risk chief complaints and those with hemoptysis, weight loss, or a prolonged cough. Also be aware of patients with HIV, with a history of TB, or from high-risk populations who have a cough or fever.70 The best guideline is to initiate respiratory isolation as soon as TB is considered to be a possible diagnosis. Place masks on such patients before obtaining chest radiographs. Consider isolating patients with chest radiograph findings of an apical infiltrate, cavitary lesions, extensive hilar or mediastinal lymphadenopathy, or a miliary pattern.70–73
Postexposure Management
Health care workers in whom PPD converts to positive after an exposure should undergo chest radiography to screen for active pulmonary TB.19 If active disease is present, initiate treatment with at least four antituberculous medications. In PPD converters who do not have active disease, consider chemoprophylaxis. When deciding whether to initiate chemoprophylaxis, balance the potential benefit of TB prevention with the risk for medication-associated hepatitis. In general, give chemoprophylaxis in the case of a recent (≤2 years) PPD conversion, a known TB contact, a patient who is medically predisposed to TB, HIV-infected, intravenous drug users, or those younger than 35 years. For occupationally exposed health care workers who are PPD converters, give chemoprophylaxis, regardless of age. The preferred regimen for HIV-negative persons is daily isoniazid (INH) for 9 months, although acceptable alternative regimens can be considered.74 Some health care workers may become exposed to strains of TB that are resistant to INH because of the continued emergence of multidrug-resistant and extensively drug-resistant strains of TB.55,75 For exposure to multidrug-resistant TB, consult an expert when selecting an individualized chemoprophylaxis regimen. Baseline and serial liver function testing is not necessary for administration of chemoprophylaxis in most cases, but monitor closely for clinical symptoms suggestive of hepatotoxicity.
References
1. Jagger, J, Powers, RD, Day, JS, et al. Epidemiology and prevention of blood and body fluid exposures among emergency department staff. J Emerg Med. 1994;12:753–765.
2. Seamon, MJ, Ginwalla, R, Kulp, H, et al. HIV and hepatitis in an urban penetrating trauma population: unrecognized and untreated. J Trauma. 2011;71:306–311.
3. Kelen, GD, Green, GB, Purcell, RH, et al. Hepatitis B and hepatitis C in emergency department patients. N Engl J Med. 1992;326:1399.
4. Clock, SA, Cohen, B, Behta, M, et al. Contact precautions for multidrug-resistant organisms: current recommendations and actual practice. Am J Infect Control. 2010;38:105–111.
5. Parmeggiani, C, Abbate, R, Marinelli, P, et al. Healthcare workers and health care–associated infections: knowledge, attitudes, and behavior in emergency departments in Italy. BMC Infect Dis. 2010;23:35.
6. Evanoff, B, Kim, L, Mutha, S, et al. Compliance with universal precautions among emergency department personnel caring for trauma patients. Ann Emerg Med. 1999;33:160–165.
7. Henry, K, Campbell, S, Maki, M. A comparison of observed and self-reported compliance with universal precautions among emergency department personnel at a Minnesota public teaching hospital: implications for assessing infection control programs. Ann Emerg Med. 1992;21:940–946.
8. Centers for Disease Control and Prevention (CDC). Recommendations for preventing transmission of infection with human T-lymphotropic virus type III/lymphadenopathy-associated virus in the workplace. MMWR Morb Mortal Wkly Rep. 1985;34(45):681–698. [691-696].
9. Occupational Safety and Health Administration. Occupational exposure to bloodborne pathogens: final rule. Fed Regist. 1991;1030(29 CFR Pt 1910):64004.
10. Ng, YW, Hassim, IN. Needlestick injury among medical personnel in accident and emergency department of two teaching hospitals. Med J Malaysia. 2007;62:9–12.
11. Zhang, M, Wang, H, Miao, J, et al. Occupational exposure to blood and body fluids among health care workers in a general hospital, China. Am J Ind Med. 2009;52:89–98.
12. Kimlin, LM, Mittleman, MA, Harris, AD, et al. Use of gloves and reduction of risk of injury caused by needles or sharp medical devices in healthcare workers: results from a case-crossover study. Infect Control Hosp Epidemiol. 2010;31:908–917.
13. Mansouri, M, Tidley, M, Sanati, KA, et al. Comparison of blood transmission through latex and nitrile glove materials. Occup Med. 2010;60:205–210.
14. Henry, K, Campbell, S, Collier, P, et al. Compliance with universal precautions and needle handling and disposal practices among emergency department staff at two community hospitals. Am J Infect Control. 1994;22:129.
15. Azar-Cavanagh, M, Burdt, P, Green-McKenzie, J. Effect of the introduction of an engineered sharps injury prevention device on the percutaneous injury rate in healthcare workers. Infect Control Hosp Epidemiol. 2007;28:165.
16. Jagger, J, Perry, J, Gomaa, A, et al. The impact of US policies to prevent bloodborne pathogens: the critical role of safety engineered devices. J Infect Public Health. 2008;1:62–71.
17. Beekmann, SE, Vaughn, TE, McCoy, KD, et al. Hospital bloodborne pathogens programs: program characteristics and blood and body fluid exposure rates. Infect Control Hosp Epidemiol. 2001;22:73.
18. HR 5178. Needlestick Safety and Prevention Act of 2000. Publication No. 106-430, 114 Stat. 1901; November 6, 2000. Available at http://www.govtrack.us/congress/billtext.xpd?bill=h106-5178.
19. Jensen, PA, Lambert, LA, Iademarco, MF, et al. Centers for Disease Control and Prevention. Guidelines for preventing the transmission of Mycobacterium tuberculosis in health-care settings. MMWR Recomm Rep. 2005;54(RR-17):1–141.
20. Sutton, PM, Nicas, M, Harrison, RJ. Tuberculosis isolation: comparison of written procedures and actual practices in three California hospitals. Infect Control Hosp Epidemiol. 2000;21:28–32.
21. WHO Guidelines on Hand Hygiene in Health Care. WHO 1-262, 2009. Available at http://www.who.int/gpsc/5may/tools/9789241597906/en/index.html.
22. Jabbar, U, Leischerner, J, Kasper, D, et al. Effectiveness of alcohol-based hand rubs for removal of Clostridium difficile spores from hands. Infect Control Hosp Epidemiol. 2010;31:565–570.
23. Tschudin-Sutter, S, Pargger, H, Widmer, AF. Hand hygiene in the intensive care unit. Crit Care Med. 2010;38(8 suppl):S299–S305.
24. di Martion, P, Ban, KM, Bartonloni, A, et al. Assessing the sustainability of hand hygiene adherence prior to patient contact in the emergency department: a 1-year postintervention evaluation. Am J Infect Control. 2011;39:14–18.
25. Gilbert, K, Stafford, C, Crosby, K, et al. Does hand hygiene compliance among health care workers change when patient are in contact precaution rooms in ICUs? Am J Infect Control. 2010;38:515–517.
26. Haas, JP, Larson, EL. Impact of wearable alcohol gel dispensers on hand hygiene in an emergency department. Acad Emerg Med. 2008;15:393–396.
27. Hall, MR, Ray, D, Payne, JA. Prevalence of hepatitis C, hepatitis B, and human immunodeficiency virus in a Grand Rapids, Michigan emergency department. J Emerg Med. 2010;38:401–405.
28. Coughlin, C, Craft, A. Hepatitis C—the silent epidemic. J Emerg Med Serv. 2000;25:114.
29. Kim, LE, Evanoff, BA, Parks, RL, et al. Compliance with universal precautions among emergency department personnel: implications for prevention programs. Am J Infect Control. 1999;27:453.
30. Centers for Disease Control and Prevention. Immunization of health-care workers: recommendations of the Advisory Committee on Immunization Practices (ACIP) and the Hospital Infection Control Practices Advisory Committee (HICPAC). MMWR Recomm Rep. 1997;46(RR-18):1–42.
31. Friedland, LR. Universal precautions and safety devices that reduce the risk of occupational exposure to bloodborne pathogens: a review for emergency health care workers. Pediatr Emerg Care. 1991;7:356.
32. Smellie, MK, Carman, WF, Elder, S, et al. Hospital transmission of hepatitis B virus in the absence of exposure prone procedures. Epidemiol Infect. 2006;134:259–263.
33. Hui, AY, Hung, LC, Tse, PC, et al. Transmission of hepatitis B by human bite—confirmation by detection of virus in saliva and full genome sequencing. J Clin Virol. 2005;33:254–256.
34. Mast, EE, Weinbaum, CM, Flore, AE, et al. for the Advisory Committee on Immunization Practices (ACIP), Centers for Disease Control and Prevention (CDC). A comprehensive immunization strategy to eliminate transmission of hepatitis B virus in the United States. MMWR Recomm Rep. 2006;55(RR-16):1–33.
35. U.S. Public Health Service. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2001;50(RR-11):1–52.
36. Protection against viral hepatitis. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep. 1990;39(RR-2):1–26.
37. Kane, M, Banatvala, J, DaVilla, G, et al. Are booster immunizations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet. 2000;355:561.
38. Armstrong, GL, Wasley, A, Simard, EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705.
39. Centers for Disease Control and Prevention (CDC). Recommendations for follow-up of health-care workers after occupational exposure to hepatitis C virus. MMWR Morb Mortal Wkly Rep. 1997;46(26):603–609.
40. Public Health Service guidelines for the management of health-care worker exposures to HIV and recommendations for postexposure prophylaxis. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47(RR-7):1–33.
41. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1998;47(RR-19):1–39.
42. Corey, KE, Servoss, JC, Casson, DR, et al. Pilot study of postexposure prophylaxis for hepatitis C virus in healthcare workers. Infect Control Hosp Epidemiol. 2009;30:10.
43. Centers for Disease Control and Prevention. Surveillance of Occupationally Acquired HIV/AIDS in Healthcare Personnel. Atlanta: CDC; 2010.
44. Wears, RL, Vukich, DJ, Winton, CN, et al. An analysis of emergency physicians’ cumulative career risk of HIV infection. Ann Emerg Med. 1991;20:749.
45. Cardo, DM, Culver, DH, Ciesielski, CA, et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337:1485.
46. Panlillio, AL, Cardo, DM, Grohskopf, LA, et al. Updated U.S. Public Health Service guidelines for the management of occupational exposures to HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2005;54(RR-9):1–17.
47. Gerberding, JL. Clinical practice. Postexposure prophylaxis for HIV infection. N Engl J Med. 2003;348:826–833.
48. Connor, EM, Sperling, RS, Gelber, R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. Pediatric AIDS Clinical Trials Group Protocol 076 Study Group. N Engl J Med. 1994;331:1173.
49. Wade, NA, Birkhead, GS, Warren, BL, et al. Abbreviated regimens of zidovudine prophylaxis and perinatal transmission of the human immunodeficiency virus. N Engl J Med. 1998;339:1409.
50. Sturt, AS, Read, JS. Antiretroviral use during pregnancy for treatment or prophylaxis. Expert Opin Pharmacother. 2011;12:1875–1885.
51. Merchant, RC, Keshavarz, R. Human immunodeficiency virus postexposure prophylaxis for adolescents and children. Pediatrics. 2001;108(2):E38.
52. New York State Department of Health AIDS Institute. HIV Prophylaxis following Occupational Exposure. http://www.ceiwidget.com, May 2010. [Available at].
53. Shih, CC, Kaneshima, H, Rabin, L, et al. Postexposure prophylaxis with zidovudine suppresses human immunodeficiency virus type 1 infection in SCID-hu mice in a time-dependent manner. J Infect Dis. 1991;163:625.
54. Wang, SA, Panlilio, AL, Doi, PA, et al. Experience of healthcare workers taking postexposure prophylaxis after occupational HIV exposures: findings of the HIV Postexposure Prophylaxis Registry. Infect Control Hosp Epidemiol. 2000;21:780.
55. Centers for Disease Control and Prevention. Trends in tuberculosis incidence—United States. MMWR Morb Mortal Wkly Rep. 2010;60(11):333–337.
56. Baussano, I, Nunn, P, Williams, B, et al. Tuberculosis among health care workers. Emerg Infect Dis. 2011;17:488–494.
57. Sokolove, PE, Mackey, D, Wiles, J, et al. Exposure of emergency department personnel to tuberculosis: PPD testing during an epidemic in the community. Ann Emerg Med. 1994;24:418.
58. Sokolove, PE, Lee, BS, Krawczyk, JA, et al. Implementation of an emergency department triage procedure for the detection and isolation of patients with active pulmonary tuberculosis. Ann Emerg Med. 2000;35:327.
59. Sokolove, PE, Rossman, L, Cohen, SH. The emergency department presentation of patients with active pulmonary tuberculosis. Acad Emerg Med. 2000;7:105–106.
60. Asimos, AW, Ehrhardt, J. Radiographic presentation of pulmonary tuberculosis in severely immunosuppressed HIV-seropositive patients. Am J Emerg Med. 1996;14:359.
61. Haramati, LB, Jenny-Avital, ER, Alterman, DD. Effect of HIV status on chest radiographic and CT findings in patients with tuberculosis. Clin Radiol. 1997;52:31.
62. Klein, NC, Duncanson, FP, Lenox, TH, 3rd., et al. Use of mycobacterial smears in the diagnosis of pulmonary tuberculosis in AIDS/ARC patients. Chest. 1989;95:1190.
63. Perlman, DC, el-Sadr, WM, Nelson, ET, et al. Variation of chest radiographic patterns in pulmonary tuberculosis by degree of human immunodeficiency virus–related immunosuppression. The Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA). The AIDS Clinical Trials Group (ACTG). Clin Infect Dis. 1997;25:242.
64. Pierce, JR, Jr., Sims, SL, Holman, GH. Transmission of tuberculosis to hospital workers by a patient with AIDS. Chest. 1992;101:581.
65. Pitchenik, AE, Rubinson, HA. The radiographic appearance of tuberculosis in patients with the acquired immune deficiency syndrome (AIDS) and pre-AIDS. Am Rev Respir Dis. 1985;131:393.
66. Samb, B, Sow, PS, Kony, S, et al. Risk factors for negative sputum acid-fast bacilli smears in pulmonary tuberculosis: results from Dakar, Senegal, a city with low HIV-seroprevalence. Int J Tuberc Lung Dis. 1999;3:330.
67. Moran, GJ. Delayed recognition and infection control for tuberculosis patients in the emergency department. Ann Emerg Med. 1995;26:290.
68. Wallace, RM, Kammerer, JS, Iademarco, MF, et al. Increasing proportions of advanced pulmonary tuberculosis reported in the United States: are delays in diagnosis on the rise? Am J Respir Crit Care Med. 2009;180:1016–1022.
69. Rosenstock, L. 42 CFR Part 84: respiratory protective devices: implications for tuberculosis protection. Infect Control Hosp Epidemiol. 1995;16:529.
70. Moran, GJ, Barrett, TW, Mower, WR, et al. Decision instrument for the isolation of pneumonia patients with suspected pulmonary tuberculosis admitted through US emergency departments. Ann Emerg Med. 2009;53:625–632.
71. Kunimoto, D, Long, R. Tuberculosis: still overlooked as a cause of community-acquired pneumonia—how not to miss it. Respir Care Clin N Am. 2005;11:25–34.
72. Liam, CK, Pang, YK, Poosparajah, S. Pulmonary tuberculosis presenting as CAP. Respirology. 2006;11:786–792.
73. Nyamande, K, Lalloo, UG, John, M. TB presenting as community acquired pneumonia in a setting of high TB incidence and high HIV prevalence. Int J Tuberc Lung Dis. 2007;11:1308–1313.
74. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49(RR-6):1–51.
75. Migliori, GB, D’Arcy Richardson, M, Sotgiu, G, et al. Multidrug-resistant and extensively drug-resistant tuberculosis in the West. Europe and United States: epidemiology, surveillance, and control. Clin Chest Med. 2009;30:637–665.