Chapter 17 Standard Management of Cardiac Surgery Patients
Many of the physiologic changes, potential problems, and objectives that occur following routine cardiac surgery are common to all patients and as such are suitable for protocol-based management. In this chapter the standard management of cardiac surgery patients is reviewed. To a certain extent the management strategies outlined here reflect our own institutional practice, which may differ from the practices at other hospitals. Furthermore, the responsibility for various aspects of patient management differs among countries and institutions. The guidelines serve to highlight the areas of patient care that are amenable to protocolized management; the actual details of the protocol and who performs a particular aspect of care are relatively unimportant. Some of the issues that arise following discharge to the ward are dealt with in this chapter, but the emphasis is on early care in the intensive care unit (ICU) following uneventful surgery. The management of postoperative variances, complications, and prolonged ICU care is not addressed in this chapter.
CLINICAL PATHWAYS
Over the past decade many units have introduced clinical pathways for adult cardiac (and thoracic) surgery patients. Clinical pathways are integrated management plans that provide time-specific goals for all aspects of a patient’s care during the entire hospital stay, including the ICU period.1 Deviations from the expected postoperative course are listed as variances. Important variances, for instance failure to be discharged from the ICU on postoperative day (POD) 1, may then be audited and used for the purposes of research and continuous quality improvement. Patients requiring prolonged ICU stay (>48 hours) are typically removed from the pathway.
Potential advantages of clinical pathways include: (1) selecting “best practice” methods; (2) avoiding omissions, variations, and duplications of tests and treatments; (3) providing a common “game plan” for all staff members; (4) providing data for continuous quality improvement; (5) decreasing nursing and physician documentation; (6) potentially reducing length of hospital stay and thereby minimizing costs. In some institutions, clinical pathways are recorded electronically, which has the advantage of improving the accuracy and efficiency of data collection. Despite the widespread use of clinical pathways in cardiac surgery, they have been subjected to minimal research. In one study, the introduction of a clinical pathway for coronary artery bypass graft (CABG) surgery was associated with a 9% reduction in length of hospital stay,2 but over the same time period, a similar reduction in length of stay was observed in comparison hospitals that did not use clinical pathways.
OVERVIEW OF PATIENT CARE
The goal, after uncomplicated cardiac surgery, is for a patient to be admitted to the ICU for fewer than 24 hours, with discharge to the ward or step-down unit occurring in sufficient time to allow the bed to be used by another patient on the following day. The expected time course for a patient is as follows:
Admission to the ICU
Handover of the Patient’s Details
Initial Assessment and Treatment by the Medical Team
Cardiovascular System
The patient’s heart rate, rhythm, mean arterial pressure, and central venous pressure are reviewed. If a PAC is in situ, cardiac output, mixed venous oxygen saturation (SVO2), and pulmonary artery wedge pressure (PAWP) should be measured. Clinical assessment of cardiac output is unreliable during the early postoperative period.3
If the heart is being paced, the pacemaker settings should be confirmed; asynchronous pacing (DOO, VOO, AOO) should be converted to synchronous pacing (DDD, AAI, VVI), typically at a rate of 80 to 90/min (see Chapter 21). If an intraaortic balloon pump is in situ, its timing and augmentation should be confirmed, the insertion site inspected to ensure that there is no bleeding or hematoma formation, and the limb perfusion checked.
Hemodynamic goals vary depending on the patient’s history, the operation, and institutional practice (see Chapter 20); typical values for the early postoperative period are listed in Table 17-1. Hypovolemia and modest vasodilatation are common during the early postoperative period, and a fluid challenge may be required. The doses of any vasoactive drugs should be confirmed. Vasoactive and inotropic drugs may be titrated to the patient’s hemodynamic state (mean arterial pressure, cardiac output) or, in patients with impaired ventricular function, they may be maintained at a constant level for a set period (e.g., until extubation or overnight).
Table 17-1 Standard Hemodynamic Goals During the Early Postoperative Period
| MAP | 65-90 mmHg |
| CVP | 8-12 mmHg |
| PAWP (or PAD) | 10-14 mmHg |
| Cardiac index | >2.2-2.6 l/min/m2 |
| SVO2 (or SSVCO2) | >60%-70% |
CVP, central venous pressure; MAP, mean arterial pressure; PAWP, pulmonary artery wedge pressure; SSVCO2, superior vena cava oxygen saturation; SVO2, mixed venous oxygen saturation; PAD, pulmonary artery diastolic pressure
Bedside Care
A detailed record of the patient’s clinical state is recorded in the 24-hour chart. Hemodynamic and respiratory status, fluid balance (including chest drainage), and drug infusions are recorded every half hour. Blood gases, potassium, and glucose are obtained every 2 hours initially while the patient is ventilated, and every 4 hours thereafter. If a PAC is in situ, cardiac index, SVO2, and PAWP are recorded every 2 hours, at least initially. The patient’s neurologic status (pupil size and reactivity, depth of sedation) is recorded every 2 to 4 hours. If a radial graft has been used or an intraaortic balloon pump is in situ, perfusion to the affected limb should be documented every 1 to 2 hours. Immobile patients should be turned every 4 to 6 hours, varying among supine and left and right lateral tilt positions to avoid the formation of pressure areas. However, for the many patients who are ventilated for only a few hours, this is not necessary. Prior to stopping sedation, a patient is usually sponge-washed to remove any dried blood or surgical antiseptic from the skin. Once the patient is extubated, pain and sedation scores and nausea are assessed on a regular basis (see Chapter 4).
MANAGEMENT OF SPECIFIC ISSUES
Drugs Given at Admission
Most units have a standard protocol for the drugs that are prescribed on admission. They may include electrolytes, sedatives and analgesics, antiemetics, antipla telet drugs, and prophylaxis for atrial fibrillation. Additional medications are also indicated for specific cardiac procedures, such as CABG surgery and valve surgery. The routine drugs used at our institution are shown in Table 17-2. Individual practice varies widely.
| Sedation and Analgesia | |
| Propofol | 100-300 mg/hr titrated to effect |
| Morphine | IV: 1-2 mg as required; PO: 10-20 mg of a short-acting formulation as required + 10-20 mg of a long-acting formulation twice daily for 3 days |
| Acetaminophen | 1 g PO or PR 6 hourly |
| Antiemetics | |
| Ondansetron | 4 mg 6 hourly PO or IV as required |
| Prochlorperazine | 12.5-25 mg 8 hourly PO or PR as required |
| Dexamethasone | 8 mg daily IV as required |
| Atrial Fibrillation Prophylaxis | |
| Amiodarone | 400 mg PO 3 times daily for 2 days beginning on the first postoperative day; thereafter 200 mg twice daily for 1 week |
| Other Drugs | |
| Potassium chloride | 10-20 mmol IV slowly to maintain K+ 4.5-5 mmol/l |
| Furosemide | 20-40 mg IV daily starting on POD-1 and continuing until back to preoperative weight |
Note: these are the drugs used at the authors’ institution. Additional drugs are given for specific cardiac operations.
IV, intravenous; PO, per os; POD, postoperative day; PR, per rectum.
Analgesic Medications
In intubated patients, analgesia may be provided by intermittent intravenous administration of an opioid such as morphine combined with intravenous or rectal acetaminophen (see Chapter 4). Once the patient is extubated and awake, intravenous opioid therapy may be given by means of a patient-controlled analgesia system in combination with oral or rectal acetaminophen. Nonsteroidal antiinflammatory drugs may be used in patients who are without contraindications but are best avoided until postoperative renal function is known.
Drugs for Patients After CABG Surgery
Aspirin is administered following CABG surgery once bleeding has settled (see Chapter 9). Some surgeons also prescribe clopidogrel following OPCAB surgery. Intravenous nitroglycerin or a calcium channel blocker may be administered to help prevent spasm in coronary grafts. If a radial graft has been used, an oral calcium channel blocker may be commenced on POD-1. Many surgeons also routinely prescribe their patients β blockers and statins following CABG surgery, commencing on POD-1.
Drugs for Patients After Valve Surgery
After implantation of a mechanical valve or a mitral tissue valve, warfarin is commenced on the evening of POD-1 or −2 (see Chapter 10). A heparin infusion may also be used, beginning once bleeding has settled and continuing until warfarin has achieved therapeutic anticoagulation in the patient. For uncomplicated tissue valves in the aortic position, aspirin may be used as the sole anticoagulant, although a 6-week course of warfarin may be preferable.
Prophylaxis for Atrial Fibrillation
Many units administer routine drug prophylaxis against atrial fibrillation, most commonly involving combinations of a β blocker, amiodarone, and magnesium (see Chapter 21).
Insulin
Hyperglycemia is common following cardiac surgery and is associated with adverse outcomes. An intravenous sliding-scale insulin infusion is indicated for all diabetic patients (see Chapter 36). For nondiabetic patients, it is reasonable to commence insulin following two consecutive glucose recordings above 180 mg/dl (10 mmol/l).
Fluid Balance, Intravenous Fluids, and Diuretics
Following cardiac surgery, particularly that which involves CPB, patients are in a state of moderate overload of extracellular sodium and water (see Chapter 32). Despite this, intravascular volume depletion—due to bleeding, polyuria, and third-space losses—is common.
Maintenance fluid in the form of dextrose 5% may be administered at a low rate (e.g., 0.5 ml/kg/hr) to function as a carrier fluid for drugs and electrolytes. Resuscitation fluid is administered according to the prescribed hemodynamic goals (see Table 17-1). The choice of resuscitation fluid is not important; many units use entirely crystalloid solutions, others a combination of crystalloid and colloid. Within the first 24 hours following surgery, most patients develop a positive fluid balance of 1 to 3 l.
With conventional cardiac surgery and cardiopulmonary bypass, weight gains of 7.4% and 6.6% above preoperative weight on POD-1 and −2, respectively, have been reported, with wide individual variability.4 Furosemide is often administered on POD-1 and continued until the patient has returned to preoperative weight.
Prevention of Infection
Typical antibiotic prophylaxis (see Chapter 35) consists of a first-generation cephalosporin such as cephazolin, given intraoperatively. With the increasing prevalence of methicillin-resistant staphylococci, some units have favored the use of vancomycin for routine prophylaxis. A recent metaanalysis indicated that there is no benefit in this approach.5 Intranasal mupirocin, commenced preoperatively and continued twice daily for 5 days postoperatively, may reduce the incidence of infection by Staphylococcus aureus in patients who are nasal carriers of this organism.6
Tracheal Suctioning
Periodic tracheal suctioning of ventilated patients is essential because secretions can build up, causing blockage of small or large airways with resultant atelectasis, impaired gas exchange, and high airway pressures. However, suctioning itself can cause airway trauma, ventilator dysynchrony, hypoxemia, and hemodynamic instability and should be performed only when indicated clinically by coarse or noisy breath sounds on chest auscultation, increased airway pressure, and deteriorating gas exchange. Tracheal suctioning is commonly required at the time of admission to the ICU. Best-practice guidelines for tracheal suctioning are listed in Table 17-3.7,8 Although not indicated for routine suctioning, instillation of 0.9% saline (5 to 10 ml) and hand ventilation with a manual resuscitator are useful if there is acute ventilatory compromise due to mucus plugging.
| Suctioning should be performed only when clinically indicated. |
| Hands should be washed before and after suctioning; apron, gloves, and goggles should be worn during the procedure. |
| If the patient is awake, the procedure should be explained, and adequate analgesia and sedation should be administered prior to performing suctioning. |
| The size of the suction catheter should not occlude more than half of the internal diameter of the endotracheal tube. |
| The inspired oxygen fraction should be increased to 100% for at least 30 seconds prior to and after suctioning. |
| A ventilator should be used during the procedure, rather than a manual resuscitation bag. |
| A maximum of two suction passes should be performed, each lasting no longer than 10-15 sec. |
| The suction catheter should be inserted to the carina and then withdrawn 1 cm before a suction pressure of 10-20 kPa is applied. |
| The routine instillation of 0.9% saline prior to suctioning is not recommended. |
From Day T, Farnell S, Wilson-Barnett J: Suctioning: a review of current research recommendations. Intens Crit Care Nurs 18:79-89, 2002; and Thompson L: Suctioning of adults with an artificial airway. Systematic Review 9. The Joanna Briggs Institute of Evidence-Based Nursing and Midwifery, 2000. http://www.joannabriggs.edu.au.
Waking and Extubation
Once specific criteria have been met, sedation is stopped, ventilatory support is reduced, and the patient is extubated. This process may be performed safely and rapidly according to protocol by the nurse and respiratory therapist, without specific involvement of the ICU physician.9,10
Criteria for Stopping Sedation
Sedation can be stopped once the following criteria have been met:
Technique of Extubation
Once the criteria for extubation have been met, the patient is placed in a sitting position. If there are significant tracheal secretions, they should be suctioned prior to extubation. The patient is asked to inspire fully, the endotracheal tube cuff is deflated, and the tube is rapidly withdrawn. SpO2, ECG, and blood pressure should be monitored continuously during the procedure.
Bleeding and Blood Products
Excessive bleeding following cardiac surgery is common and may result from inadequate surgical hemostasis, abnormal blood clotting, or residual heparin (see Chapter 30). The expected volume of postoperative blood loss depends on the duration and nature of the surgery (it is higher for complex aortic surgery than for routine CABG surgery); whether CPB was used; and the preoperative use of antiplatelet or anticoagulant therapy. However, ongoing chest drain loss of more than 150 ml/hr after the first hour is abnormal and warrants further investigation and intervention.
Red Cell Transfusion
The transfusion threshold for an individual patient depends on the particular risk for developing complications involving inadequate tissue oxygenation and the rapidity of blood loss. A suggested regime is provided in Table 17-4.
| Clinical Situation | Transfusion Trigger |
|---|---|
| Patient stable on minimal inotropic support without significant bleeding | Hb <65-70 g/l or HCT <20-22 |
| Patient stable but on moderate-dose inotropic support and clinically important bleeding occurring | Hb <70-80 g/l or HCT <22-25 |
| Patient unstable on high inotropic support with evidence of inadequate oxygen delivery (acidosis, low venous oxygen saturation) and/or major bleeding | Hb <90 g/l or HCT <27 |
Hb, hemoglobin; HCT, hematocrit.
Blood Component Therapy
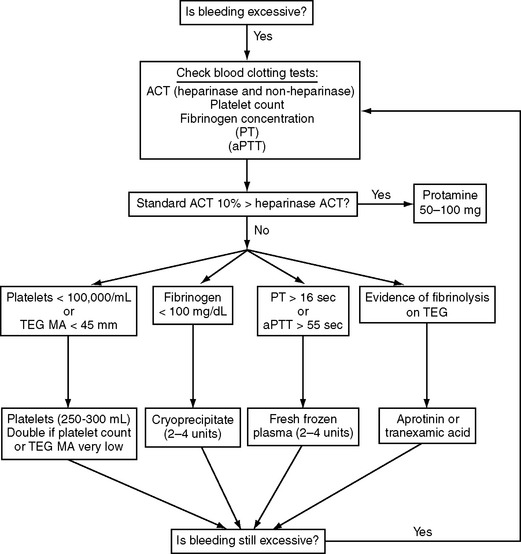
Blood component therapy (platelets, fresh-frozen plasma, cryoprecipitate) is indicated for active bleeding in the presence of documented abnormal blood clotting but not for abnormal clotting in the absence of excessive bleeding. The administration of blood component therapy should be protocol-driven so as to avoid unnecessary transfusion and reduce costs. An algorithm based on published protocols and our own practice is shown in Figure 17-1.
Pacing and Pacing Wires
Atrial (AAI) or dual-chamber pacing (DDD) is useful to augment hemodynamics and reduce the likelihood of developing atrial fibrillation (see Chapter 21).12 Some surgeons place epicardial pacing wires in all patients, others do so only when it is specifically indicated. Atrial and ventricular wires may be color-coded or may be distinguished by attaching a suture to one of the chamber’s wires. Atrial wires are typically brought out to the right of the ventricular wires.
Pacing wires are typically removed on POD-3 to −6. If a patient is receiving intravenous heparin, it should be discontinued for 4 hours before pacing wires are removed. If a patient is receiving warfarin, pacing wires should be removed before the international normalized ratio (INR) exceeds 2.5 (see Chapter 30). Even without anticoagulant therapy, there is a small but real risk for tamponade following removal of pacing wires; thus, patients should be closely observed for 4 hours following their removal.
Monitoring and Investigations
While patients are intubated, standard monitoring includes ECG, ST segment analysis, SpO2, central venous pressure, invasive arterial pressure, and temperature (see Chapter 8). Many monitors also record exhaled carbon dioxide (capnography) and respiratory rate. From the ventilator, standard parameters include FIO2, exhaled minute volume, peak and plateau airway pressures, and dynamic lung mechanics. If a PAC is in place, pulmonary artery pressures must be continuously displayed so that inadvertent wedging can be detected and the catheter withdrawn. PAWP, cardiac output, and SVO2 should be recorded every 2 to 4 hours. In the absence of a PAC, SSVCO2 may be obtained from the proximal port of a standard central venous catheter in place of SVO2. If SSVCO2 is used as a monitoring tool, it may be measured prior to critical events such as extubation, in response to hemodynamic instability, or routinely every 2 to 4 hours.
Reintroduction of Preoperative Medications
Cardiovascular Medications
If there are no contraindications, β blockers should be reintroduced on POD-1 to prevent β-blocker withdrawal syndrome and as prophylaxis against atrial fibrillation. Modest bradycardia and AAI pacing are not contraindications to restarting β blockers. In patients with a history of heart failure, β blockers should be reintroduced very carefully, usually at a reduced dosage and only once cardiac function is clearly satisfactory. Abrupt withdrawal of calcium channel blockers has been associated with coronary artery spasm following CABG surgery,13 so these drugs should be reintroduced on POD-1 or once the patient has been weaned from all inotropic support.
Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and spironolactone can cause problems in patients with low cardiac output and renal dysfunction (see Chapter 3); thus, their reintroduction should be delayed until the patient has been weaned from all inotropic support and postoperative renal function has returned to baseline. Digoxin may be reintroduced on POD-1, assuming renal function is relatively normal, but the dosage should be reduced in patients being treated with amiodarone. Aspirin, clopidogrel, enoxaparin, and warfarin can all be recommenced on POD-1, assuming bleeding is not a concern.
Central Nervous System Medications
Failure to administer anti-Parkinson medications can lead to acute rigidity, tremor, and even neuroleptic malignant syndrome.14 Thus, anti-Parkinson medications should be recommenced on POD-1. If extubation has been delayed, a nasogastric tube should be inserted and the medication given by this route.
In general, psychiatric medications can be reintroduced on POD-1 but caution is required in two circumstances. First, the combination of psychiatric drugs that prolongs the QT interval and class III antiarrhythmic drugs; and second, the combination of selective serotonin reuptake inhibitors and other drugs that affect serotonin levels (e.g., tramadol, ondansetron) because of the risk for serotonin syndrome (see Chapter 4).
Long-term Steroid Treatment
Patients on long-term corticosteroid treatment require temporary intravenous supplementation prior to the reestablishment of oral intake (see Chapter 36).
Analgesics
Chronic opioid therapy should be continued throughout the perioperative period so as to avoid acute withdrawal and pain-management problems. Higher dosages, sometimes much higher, of standard postoperative opioids are required in patients who are habituated to opioids. In patients with heart failure or renal dysfunction, nonsteroidal antiinflammatory drugs should be withheld until renal function is stable. COX-II inhibitors should probably be avoided in all patients after cardiac surgery (see Chapter 4).
Prophylaxis of Deep Venous Thrombosis
The incidences of DVT and pulmonary embolus after cardiac surgery are about 20% and 0.5%, respectively, but the likelihood varies considerably, depending on risk factors.15,16 Risk factors include delayed mobilization, obesity, increased age, previous history of DVT, and prothrombotic conditions (e.g., myeloproliferative disorders, antithrombin III deficiency, protein C or S deficiency, and factor V Leiden mutation). Routine DVT prophylaxis should include wearing above-knee, bilateral, graduated compression stockings from POD-1 until fully ambulant. Patients at high risk for DVT should probably also receive prophylaxis with either unfractionated or low molecular weight heparin, commencing on POD-1.17
Reasons for Delayed Discharge From the ICU
Common reasons patients cannot be discharged from the ICU on POD-1 are:
In addition, patients with the following problems should be considered for delayed discharge:
PRESENTING PATIENTS ON ICU ROUNDS
FAST-TRACK CARDIAC SURGERY
Fast-track techniques can be performed safely and they do result in reduced hospital costs.18,19 However, several factors must be considered. First, most cardiac surgery units no longer routinely ventilate patients overnight. Second, ICU costs are substantially reduced by early extubation only if there are facilities that increase throughput and ICU bed utilization. Most patients who are morning cases are extubated in the afternoon or evening, and most afternoon patients are extubated overnight, so it is often not possible to increase throughput unless a “hot bedding” system is used. Finally, with the increasing complexity of the cardiac surgery case mix, early extubation is not suitable for many patients.
1 Pearson SD, Goulart-Fisher D, Lee TH. Critical pathways as a strategy for improving care: problems and potential. Ann Intern Med. 1995;123:941-948.
2 Pearson SD, Kleefield SF, Soukop JR, et al. Critical pathways intervention to reduce length of hospital stay. Am J Med. 2001;110:175-180.
3 Linton RA, Linton NW, Kelly F. Is clinical assessment of the circulation reliable in postoperative cardiac surgical patients ? J Cardiothorac Vasc Anesth. 2002;16:4-7.
4 Larson SL, Schimmel CH, Shott S, et al. Influence of fast-track anesthetic technique on cardiovascular infusions and weight gain. J Cardiothorac Vasc Anesth. 1999;13:424-430.
5 Zanetti G, Platt R. Antibiotic prophylaxis for cardiac surgery: does the past predict the future ? Clin Infect Dis. 2004;38:1364-1366.
6 Perl TM, Cullen JJ, Wenzel RP, et al. Intranasal mupirocin to prevent postoperative Staphylococcus aureus infections. N Engl J Med. 2002;346:1871-1877.
7 Day T, Farnell S, Wilson-Barnett J. Suctioning: a review of current research recommendations. Intens Crit Care Nurs. 2002;18:79-89.
8 Thompson L: Suctioning of adults with an artificial airway. Systematic Review 9. The Joanna Briggs Institute of Evidence-Based Nursing and Midwifery, 2000. http://www.joannabriggs.edu.au.
9 Marelich GP, Murin S, Battistella F, et al. Protocol weaning of mechanical ventilation in medical and surgical patients by respiratory care practitioners and nurses: effect on weaning time and incidence of ventilator-associated pneumonia. Chest. 2000;118:459-467.
10 Kollef MH, Shapiro SD, Silver P, et al. A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med. 1997;25:567-574.
11 Rosenberg J, Ullstad T, Rasmussen J, et al. Time course of postoperative hypoxaemia. Eur J Surg Acta Chirurg. 1994;160:137-143.
12 Zimmer J, Pezzullo J, Choucair W, et al. Meta-analysis of antiarrhythmic therapy in the prevention of postoperative atrial fibrillation and the effect on hospital length of stay, costs, cerebrovascular accidents, and mortality in patients undergoing cardiac surgery. Am J Cardiol. 2003;91:1137-1140.
13 Engelman RM, Hadji-Rousou I, Breyer RH, et al. Rebound vasospasm after coronary revascularization in association with calcium antagonist withdrawal. Ann Thorac Surg. 1984;37:469-472.
14 Friedman JH, Feinberg SS, Feldman RG. A neuroleptic malignantlike syndrome due to levodopa therapy withdrawal. JAMA. 1985;254:2792-2795.
15 Ambrosetti M, Salerno M, Zambelli M, et al. Deep vein thrombosis among patients entering cardiac rehabilitation after coronary artery bypass surgery. Chest. 2004;125:191-196.
16 Gillinov AM, Davis EA, Alberg AJ, et al. Pulmonary embolism in the cardiac surgical patient. Ann Thorac Surg. 1992;53:988-991.
17 Shammas NW. Pulmonary embolus after coronary artery bypass surgery: a review of the literature. Clin Cardiol. 2000;23:637-644.
18 Cheng DC, Karski J, Peniston C, et al. Early tracheal extubation after coronary artery bypass graft surgery reduces costs and improves resource use: a prospective, randomized, controlled trial. Anesthesiology. 1996;85:1300-1310.
19 Cheng DC, Wall C, Djaiani G, et al. Randomized assessment of resource use in fast-track cardiac surgery 1 year after hospital discharge. Anesthesiology. 2003;98:651-657.