Chapter 33 Sports Injuries of the Elbow
Background/aetiology
Injury and reconstruction of the UCL enjoys great notoriety in the world of sports. Injury to the UCL was first described in javelin throwers in 1946.1 Perhaps due to incomplete understanding of the injury and its effect primarily on throwing athletes, little was written about the subject for the next 40 years. Interest in this injury was renewed with the first description of reconstruction of the UCL by Jobe et al in 1986.2 This was spurred in no small part by the widely publicized success of Dr Jobe’s reconstruction on major league baseball pitcher Tommy John in 1974. Known since as the ‘Tommy John surgery’, a great deal of research has furthered the understanding of UCL anatomy, the way it is injured, and the optimal treatment and rehabilitation.
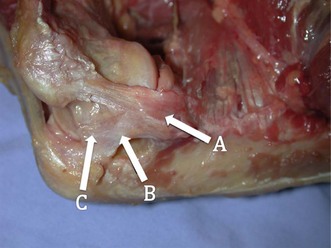
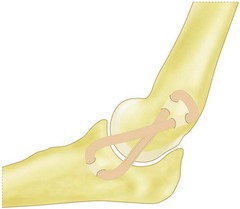
Understanding the injury and treatment of UCL tears in overhead throwers begins with an understanding of the anatomy of the medial side of the elbow. The UCL is consistently made up of an anterior and posterior bundle, with a variable transverse bundle (Fig. 33.1). Within the UCL complex, the primary restraint to valgus stress is the anterior bundle, which originates on the inferior-most aspect of the medial epicondyle of the humerus and inserts on the sublimus tubercle of the ulna. The anterior bundle is further divided into an anterior band, which is tighter in extension, and a posterior band, which is tighter in flexion. An anatomical study by Callaway et al confirmed this characterization of the bands as ‘reciprocal’ in their roles of stabilization through the flexion-extension arc.3 The authors found that the anterior band was the primary stabilizer at 30°, 60° and 90°, while the anterior and posterior band were co-primary stabilizers at 120°. The posterior bundle lends little to the valgus stability as long as the anterior bundle remains intact, except at 30° of flexion, where it is a secondary stabilizer.
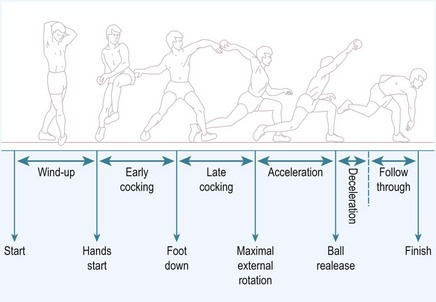
Injury to the UCL occurs as either a single traumatic event or from cumulative injury and repetitive loads experienced by the elbow during athletic activities. With overhead throwing, the UCL is most at risk during the late cocking and early acceleration phases of pitching, with the upper extremity achieving a peak angular velocity in the range of 2300–5000°/sec3 (Fig. 33.2). This finding is supported clinically by the fact that severe pain during the throwing motion occurs most commonly during the acceleration phase.4 Further, biomechanical studies have demonstrated the ultimate load to failure of the native anterior band of the UCL in resisting valgus torque to be 34 Nm,5 while the estimated demand on the UCL during pitching has been demonstrated to be 35 Nm.6 With estimated demand so near to the load to failure, it is not surprising that overhead athletes, especially baseball pitchers, are prone to an injury of this ligament.
Repetitive throwing can also lead to a spectrum of pathology involving the UCL, ranging from attenuation to partial or complete tears. As UCL insufficiency progresses, coincident pathology can also develop, including radiocapitellar arthritis, ulnar neuropathy, and valgus extension overload syndrome (VEOLS). VEOLS results from the valgus force on the elbow during the early acceleration phase of throwing and from the compression of the posteromedial olecranon against the olecranon fossa as the elbow is extended in the ‘follow through’ phase. Patients present with posteromedial elbow pain along the border of the olecranon.7 These elevated loads on the elbow may lead to the formation of osteophytes at the posterior and posteromedial tip of the olecranon with associated cartilage wear and loose bodies (Fig. 33.3).
Similar symptomatology and pathology can also develop in young pitchers. In one series, 28% of adolescent pitchers experienced elbow pain over a course of 2 years.8 However, unlike adults, the presence of physeal plates in the youth elbow leads to a different pattern of injury.9 Whereas valgus torque may lead to injury to the UCL in adults, similar stresses lead to medial epicondylar apophysitis or avulsion fractures in children and adolescents.10 Sabick et al studied the valgus torque on the elbow in adolescent baseball pitchers based upon video data.9 Controlling for height and weight and taking only kinematic variables, they found maximum shoulder external rotation to be the most significant variable in the generation of elbow valgus torque. Thus, a pitching style that eliminates exaggerated shoulder external rotation may help to prevent elbow injury.
Other authors have suggested guidelines to help diminish the risk to young pitchers such as limiting pitch counts per game and per season.8 At greater than 75 pitches per game, the odds of elbow pain increases by 50%. Pitch counts of greater than 600 per year also correlated with increased risk of elbow pain. In addition, proper instruction in mechanics and avoidance of pitching while fatigued are important. Specific pitch types, such as the slider and curveball, and a sidearm delivery have also been reported to increase the risk of elbow pain.4 Thus, it is important for coaches and leagues to adhere to pitch count guidelines based upon the athlete’s age, instruct in proper mechanics from a young age, and monitor their players’ development of these specific pitches (Table 33.1).
| League age (years) | Pitchers per day |
|---|---|
| 13–16 | 95 |
| 11–12 | 85 |
| 9–10 | 75 |
| Under 8 | 50 |
| Pitch count | Days of rest |
|---|---|
| 61 pitches per day or more | 4 |
| 41–60 pitches | 3 |
| 21–40 pitches | 2 |
| 1–20 pitches | 0 |
Presentation, investigation and treatment options
Injuries to the UCL in the overhead athlete may present with acute or insidious symptoms during throwing. Athletes may recount a history of acute medial-sided elbow pain during a pitch together with a popping sensation. More commonly, pitchers describe a gradual onset of pain, accompanied by loss of velocity or ball control.11 Pain is usually greatest during the late cocking and early acceleration phases of the throwing cycle. In addition, patients with valgus extension overload syndrome may experience posteromedial pain during deceleration due to osteophyte impingement. Occasionally, patients may also describe a sensation of instability or ‘opening’ in the elbow while throwing.12 Finally it is important to question the athlete about ulnar nerve symptoms during competition or at rest.
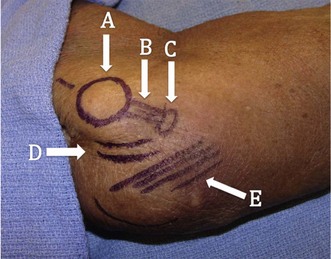
Physical examination of these patients begins with a basic evaluation of the joint. Patients will often exhibit tenderness to palpation over the UCL most often at the distal insertion site at the sublime tubercle12 (Fig. 33.4). Additionally, patients may display tenderness over posterior or posteromedial osteophytes and pain with forced extension of the elbow. In the setting of arthritis, loose bodies, or osteophytes, range of motion may be reduced.7 A complete neurovascular examination should be performed with special attention paid to ulnar nerve. This should include both an examination for a Tinel’s sign and inspection for subluxation of the nerve over the medial epicondyle during flexion and extension. The presence of both or either of these findings will guide intraoperative decisions regarding handling of the nerve.
Various specific physical examination manoeuvres have been described to assess valgus instability. Positioning the elbow in 20–30° of flexion unlocks the olecranon from the fossa, decreasing osseous stability and more accurately testing the anterior band of the UCL. By applying a valgus stress while palpating the ligament and comparing to the contralateral side, the examiner may be able to detect instability.12 Additionally, a valgus stress can be applied by placing the patient’s shoulder at 90° of abduction and maximal external rotation locks the shoulder. By pulling on the thumb in this position, a valgus load is applied to the elbow.
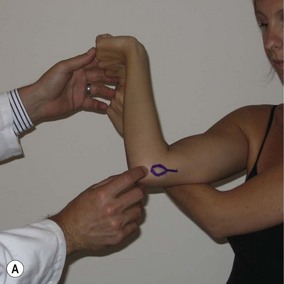
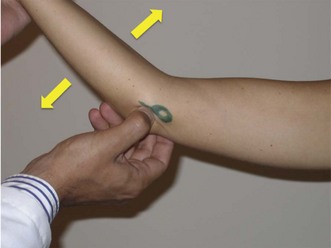
The milking manoeuvre (Fig. 33.5A) is a provocative test designed to preferentially stress the posterior portion of the anterior bundle of the UCL. The test involves the patient grasping the thumb of the affected arm while the affected elbow is supported with the other forearm.13 With traction applied to the thumb, the elbow is forced into valgus. Additionally, the examiner can palpate the ligament during this manoeuvre. Safran et al have described a modification of this milking maneuver.14 Once again, the patient stabilizes the affected elbow in supination and 90° of flexion, but the examiner pulls the affected thumb. Elicitation of pain is a positive finding (Fig. 33.5B).

(B) Modified milking manoeuvre. The patient supports the affected elbow while the examiner pulls on the patient’s thumb to create a valgus force.
(Reprinted with permission from Meyers A, Palmer B, Baratz ME. Ulnar collateral ligament reconstruction. Hand Clin 2008; 24(1):53–67.)
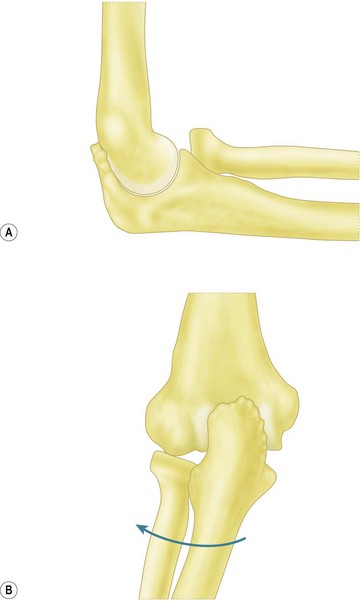
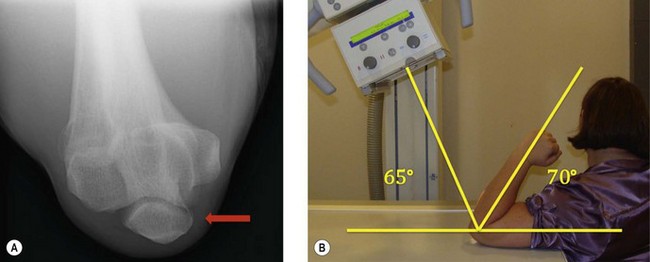
O’Driscoll et al have described the ‘moving valgus stress test’ and reported a 100% sensitivity and 75% specificity.15 To perform this test, the patient is positioned upright with the shoulder abducted to 90°. A modest valgus torque is placed on the forearm until the shoulder reaches its maximum external rotation. Starting with the elbow in maximal flexion, the elbow is rapidly extended to 30° while maintaining a constant valgus force. Two components comprise a positive result. First, the test must elicit pain similar to that during the act of throwing. Secondly, the maximal pain must occur between the position of late cocking (120°) and early acceleration (30°) of elbow flexion (Fig. 33.6).
Imaging studies are also an important aid in the diagnosis of UCL tears. Plain radiographs of the affected elbow should be obtained to determine any radiocapitellar pathology, loose bodies, or calcification within the ulnar collateral ligament. Oblique radiographs as well as an axial view of the olecranon may help to identify posteromedial osteophytes7 (Fig. 33.7). Previously stress radiographs were also undertaken and found to be of some additional diagnostic value. However, these have now largely fallen from favour due to a high incidence of false positives and a lack of reproducibility. Ellenbecker et al compared the findings of stress radiographs between dominant and non-dominant elbows in 40 asymptomatic high-level baseball players.16 The authors found a significantly greater difference in joint space opening between the stressed and unstressed states in the dominant elbow, showing a natural tendency to increased laxity. In addition, Azar et al found positive results with stress radiographs in only 46% of athletes whose clinical findings led to operative treatment for a UCL injury.17
Magnetic resonance imaging with and without arthrography is a powerful diagnostic tool for evaluating the UCL. Calcification seen within the substance of the UCL on MRI has been associated with chronic partial or complete rupture of the ligament.18 Timmerman et al compared the findings of MRI without arthrography to intraoperative findings in 25 baseball players with a history and examination suggestive of UCL tears.19 The authors reported a 57% sensitivity and 100% specificity. Specifically, MRI without arthrography was sensitive in detecting full thickness tears, but only detected partial tears in 14% of cases. The addition of arthrography to MRI improved the sensitivity of the test to 86% in one series.20 The addition of contrast allows for improved visualization of the undersurface of the UCL, allowing better identification of partial tears. In addition, contrast allows visualization of the UCL recesses, which are potential spaces between the ligament and the epicondyle proximally and the ulna distally. The distal insertion of the ligament should be visualized within 1 mm of the articular margin of the coronoid process. Extension of contrast beyond these recesses is indicative of a tear UCL. Thus, whilst injection of contrast adds to morbidity and patient discomfort, it is now the study of choice for most clinicians. However, it is important to recognize that a negative study does not preclude a tear.20
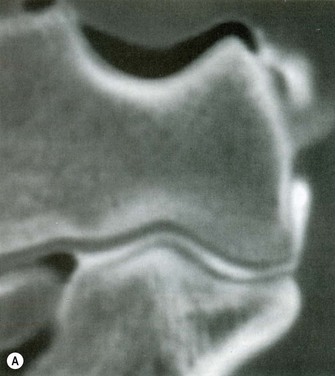
Additional studies include CT arthrogram. Timmerman et al reported a sensitivity of 86% and specificity of 91% in a population of baseball players.19 In addition, the authors described a radiographic ‘ T-sign’ which represents an undersurface tear of the UCL. The undersurface tear of the ligament allows dye to leak around the detached insertion but remains ultimately contained within the superficial layer of the ligament and capsule (Fig. 33.8).
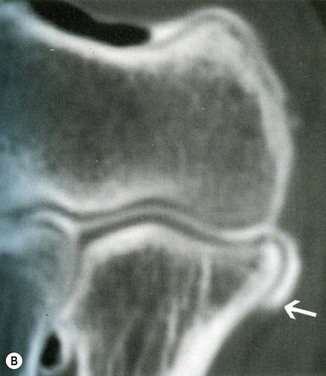
(B) CT arthrogram ‘T-sign’. Positive finding of dye extravasation along the proximal ulna beyond the insertion of the ligament (arrow).
Once the diagnosis is established, treatment must be individualized to the patient based upon their level of impairment, functional demands, desire to continue in their sport, and willingness to undergo the lengthy process of surgery and rehabilitation. Dysfunction from UCL tears usually occurs only in overhead athletes when throwing or performing equivalent activities such as spiking a volleyball. Thus, patients who are able to avoid these activities in their sport can usually be treated without surgery.21,33
Non-operative management generally includes rest from throwing for 2–4 weeks with the application of physical therapy modalities such as phonophoresis, iontophoresis, or electrical stimulation.12 Patients progress to a programme promoting elbow range of motion, flexor-pronator strengthening, and a progressive throwing programme at approximately 3 months.12,42 One study has reported success with nighttime splinting and progressive physical therapy in 42% of throwing athletes.21 Patients who fail a thorough attempt at non-operative treatment and wish to return to their sport are candidates for surgical treatment.
Contraindications to surgical management include patients with asymptomatic tears of the UCL.33 Given the sensitivity of magnetic resonance imaging (MRI) with arthrogram, pathology of the UCL may be detected that is not clinically relevant. Although, the natural history of asymptomatic partial tears has not been established, surgery is not indicated in such patients. In addition, certain athletes may be able to modify their activities to lessen valgus load on the elbow, making surgery unnecessary. A relative contraindication to ligament reconstruction is concomitant ulnohumeral or radiocapitellar arthritis.23 With reconstruction, such patients may experience worsened joint pain.
Surgical techniques and rehabilitation
Various modifications and refinements of UCL reconstruction have been made since Dr Jobe’s first description of the procedure in 1986.2 However, the goal of the procedure remains the same: to stabilize the elbow against the extreme valgus forces encountered during the late cocking and early acceleration phases of the throwing cycle.
Although, not performed on a routine basis, arthroscopy is an important tool in treating patients with UCL rupture. Field et al has described an arthroscopic valgus instability test of the elbow.22 Viewed from the anterolateral portal, a valgus stress is applied to the elbow, which is flexed between 60° and 75° with the forearm in pronation. A positive result corresponds with a visible opening in the ulnohumeral articulation of 1 mm or more.
In addition to diagnosis of instability, arthroscopy can be important in diagnosing and treating concomitant intra-articular pathology. In one report, routine arthroscopy was performed prior to reconstruction of the UCL. The authors removed osteophytes from either the posteromedial olecranon or the coronoid process in 29% of cases, performed microfracture on cartilaginous defects in 9%, and removed loose bodies in 7%.11 While some surgeons may prefer to forgo routine arthroscopy due to challenges in positioning and additional time added to the procedure, it may augment treatment of patients with valgus extension overload syndrome (VEOLS), especially those with intra-articular symptoms.
It is our practice to perform arthroscopic resection of posteromedial osteophytes in patients with posteromedial pain from VEOLS. Failure to identify and treat this related problem leads to continued pain from impingement of these osteophytes against the articular wall of the olecranon fossa and possible need for reoperation.7 However, there continues to be some concern that olecranon resection may lead to a loss of ulnohumeral constraint and as a consequence increasing strain on the UCL. Andrews et al found that 25% of professional athletes who underwent debridement of posteromedial osteophytes developed valgus instability requiring UCL reconstruction.25 Reports vary on the amount of olecranon that can be safely resected, ranging from 3 to 8 mm.24,26
After any intra-articular pathologies are addressed, attention can be turned to reconstruction of the UCL. If the surgeon has elected to position the patient in a lateral decubitus position for elbow arthroscopy, the patient is then turned to a supine position with the arm abducted and supported on a table. A tourniquet should be used to control bleeding. A longitudinal 6 cm curvilinear skin incision is made centred over the medial epicondyle. Care must be taken to protect branches of the medial antebrachial cutaneous nerve as they cross the distal aspect of the incision. In his original description of the procedure, Dr Jobe described detachment of the flexor-pronator mass.2 However, in an effort to limit damage to this structure, which is important in throwers, most authors now utilize a muscle-splitting approach to expose the UCL.11,17,27–30 Thompson et al described this approach as a splitting of the fascia over the muscle fibres beginning at the anterior portion of the anterior band of the UCL.30 An intramuscular interval is developed bluntly between the two heads of the flexor carpi ulnaris (FCU). With anterior retraction of the humeral head of the FCU and careful posterior retraction of the ulnar nerve, the remnant of the UCL, distal surface of the medial epicondyle, and the medial cortex of the ulna, including the sublime tubercle, are exposed (Fig. 33.9). An additional benefit of the muscle-splitting technique is that it obviates transposition of the ulnar nerve. Obviously the surgeon must be aware of the course of the nerve and be careful to protect it from compression or direct damage during the procedure; however, the choice of transposition can be made by the surgeon based upon surgeon preference and preoperative symptoms. Current reports reflect differing opinions on transposition, as some authors routinely transpose the ulnar nerve, while others only do so in the setting of preoperative ulnar nerve complaints.11,17,25–29 When performed, most authors prefer subcutaneous transposition of the nerve via fascial slings.
The graft can be procured from a multitude of locations depending on surgeon preference. Common sources of graft include ipsilateral and contralateral palmaris longus tendons, toe extensors, and the plantaris. Use of a gracilis autograft has also been described.25 We prefer to use ipsilateral palmaris longus tendon when the graft is available.
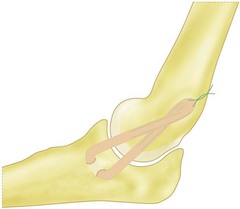
Multiple methods of graft fixation have been described in both the clinical and biomechanical setting, including transosseus figure-of-eight reconstruction, docking technique, docking technique employing additional strands, interference screw fixation, and EndoButton fixation.2,5,25,26,30 The two most common methods of fixation include the Jobe transosseus figure-of-eight reconstruction and the docking procedure.2,27 In the figure-of-eight reconstruction, two 3.2 mm humeral bone tunnels are drilled from a proximal direction to converge at a single exit point at the isometric point of the ligament on the medial epicondyle.2 Two additional drill holes are made to form an ulnar tunnel at the insertion of the UCL. The graft is then passed through these tunnels so that it forms a figure-of-eight. To reduce the elbow, the joint is positioned in 60° of flexion, the forearm is supinated, and a varus force is applied.33 The graft is pulled taut and sutured upon itself, with any extra graft forming a reinforcing limb to the construct (Fig. 33.10).
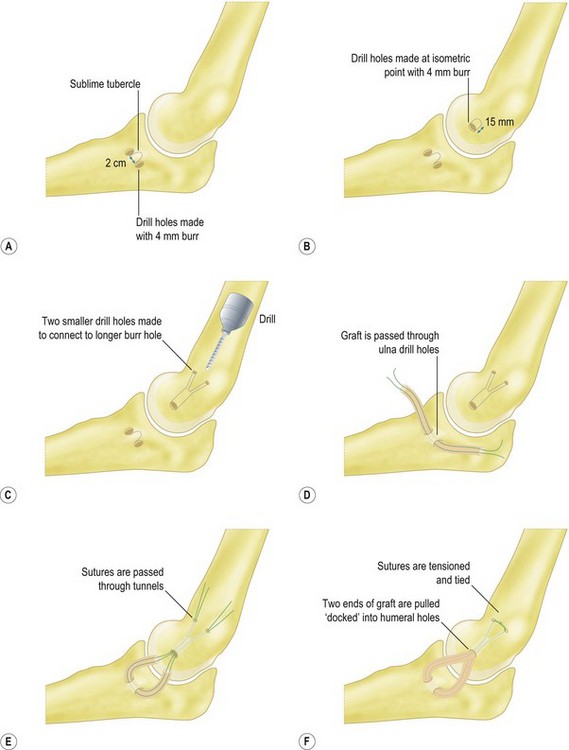
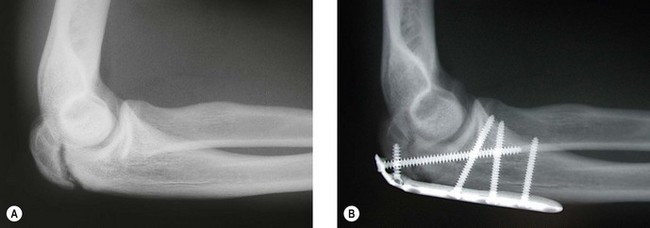
In the docking technique,25 the ulnar tunnel is created with the use of a 4 mm burr to create the openings of the tunnel anterior and posterior to the sublime tubercle leaving a 2 cm bone bridge (Fig. 33.11A). The openings are connected with a curved curette or towel clamp. The humeral tunnel is positioned at the anterior portion of the existing UCL remnant. A 4 mm burr is used to create a single longitudinal tunnel 15 mm deep (Fig. 33.11B). Two smaller exit tunnels are then created with a smaller drill or dental burr to allow for suture passage (Fig. 33.11C). The graft is passed through the ulnar tunnel (Fig. 33.11D). The two ends of the graft are then brought to the isometric point and placed under tension with the elbow reduced. The two ends are marked at a point just short of the depth of the humeral tunnel. This ensures that the graft will not ‘bottom out’ against the ceiling of the humeral tunnel. The graft is cut at this length and the two ends are sutured with a Krakow type stitch or a loop stitch (Fig. 33.11E). The sutures’ ends are passed through the two tunnels, tensioned with the elbow reduced and the forearm supinated, and tied over the bony bridge of the humeral epicondyle (Fig. 33.11F).
Rehabilitation protocols vary, but all maintain the principles of early range of motion, strengthening, throwing progression, and return to competition. The protocol is generally divided into four phases: immediate postoperative, intermediate, advanced strengthening, and return-to-activity.32 Average time to competitive play for baseball pitchers is one year.
During phase three (weeks 9–13), the patient increases strength, power and endurance, with a gradual return to sports-specific rehabilitation and activities. Azar et al initiate progression of isotonic strengthening to the shoulder, elbow, and wrist.17 At week 12, a plyometric sports-specific programme prepares the athlete for a return to throwing. Drills include chest passes, soccer throws, and side throws. Plyometric exercises are designed to train a muscle to perform quick, explosive movements through a specific sequence of movements. This involves rapid lengthening of the muscle (eccentric phase), a short resting phase (amortization phase), and rapid contraction of the muscle (concentric phase).
In phase four (week 14 to return to play), the goal is gradual return to sport. During this phase, a throwing programme is initiated. Throwing is begun at 45 ft and progressed to a 180 ft long toss. Pitchers then progress to an ‘off the mound’ interval programme in which they gradually progress to the point that they are allowed to throw from the mound, with gradual return to more stressful pitches.28 Throwing is undertaken every other day and should be pain-free. If, however, the player does experience some discomfort, then he or she should go back one step in the rehabilitation programme until they are pain free. On completion of the programme, the player is allowed to return to competitive play; although, the authors prefer to wait for at least 1 year before allowing competitive pitching.
Clinical Pearl 33.1 Docking procedure
Outcome including literature review
The primary outcome measure after UCL reconstruction is return to competitive sport. Whilst this does not allow for other reasons why an athlete may not return to his or her sport, the tremendous valgus forces that are placed upon the elbow during the act of throwing are the ultimate test of any reconstruction. By the Conway classification system, an excellent outcome is return of the athlete to the same or higher level of competition prior to the injury for at least 12 months.29 Results were graded as good if the patient was able to return to a lower level of play or to throw in daily batting practice. Results were graded as fair with return to a recreational level and poor if there is an inability to return to play at all.
Outcome after UCL reconstruction has generally improved as the various techniques for reconstruction have evolved. In the original description, Jobe et al routinely performed elevation of the common flexor origin and most of the pronator teres to expose the UCL.2 Furthermore, the ulnar nerve was routinely mobilized and transposed. Using either the palmaris longus or a strip of Achilles tendon, a figure-of-eight reconstruction was performed. In this original report, 10/16 (62%) patients returned to their sports activity at the same level of competition, while five patients retired for reasons unrelated to their surgery.
More recent studies have modified this original technique and have reported excellent outcomes in 81–95% of patients.11,17,27–29 In the largest series to our knowledge, Dodson et al reported on their experience with UCL reconstruction in 100 consecutive patients.11 The patient population consisted of 96 baseball players, 91 of whom were pitchers, 2 football quarterbacks, and 2 tennis players. Patients underwent routine arthroscopic examination, a muscle-splitting approach, intramuscular ulnar nerve transposition only in the setting of preoperative ulnar nerve symptoms (22 patients, 22%), and reconstruction via a docking technique. The authors felt that routine arthroscopy was indicated as this allowed them to confirm the diagnosis. Furthermore, they reported removal of posteromedial olecranon osteophytes in 22 (22%), microfracture of cartilaginous defects in 9 (9%), and removal of loose bodies in 7 patients (7%). With a mean follow-up of 36 months, 90 patients (90%) were able to perform at the same or higher level of competition and 7 patients (7%) were able to compete at a lower level. This result is comparable to the 92% good results from an earlier study by the same group in which they first described the docking technique.27 Additional studies have also reported on subsequent modifications of the docking technique. Koh et al have reported results of a three-strand technique and Paletta et al have reported on a four-strand variation of the docking technique with 95% and 92% excellent results, respectively.29,30
In a comparable cohort size, Azar et al reported their results in 91 patients with UCL pathology at a mean follow up of 35.4 months.17 Patients underwent routine arthroscopy, subcutaneous transposition of the ulna nerve, and a muscle-splitting approach. Seventy-eight patients underwent reconstruction via figure-of-eight technique and 13 underwent partial ligament repairs. While the authors do not report outcomes separately for reconstruction versus repair, their overall rate of excellent outcomes was 79%. Having reviewed their results with repair, the authors noted a correlation to other studies29 specifically inferior results when compared to reconstruction. As a consequence, in cases amenable to repair, they recommended augmentation with an autograft.
Certain advances in surgical techniques have had a clear effect on improving clinical outcomes. However, to date there are still no randomized studies comparing the various reconstruction techniques. Vitale et al systematically reviewed all published reports in an attempt to determine which surgical techniques were associated with improved outcomes.34 The authors reported an improvement in results with a transition from detachment of the flexor-pronator mass to a muscle-splitting approach. The muscle-splitting approach appears beneficial for multiple reasons. Firstly, it diminishes surgical trauma to the flexor-pronator mass. In addition, it obviates obligatory ulnar nerve transposition. Minimizing handling of the ulnar nerve may play a major role in improved outcomes. While postoperative ulnar neuropathy was the most common complication, a shift away from obligatory transposition was associated with improved outcomes. It must be recognized, however, that simple comparisons do not tell the whole story, as different methods of ulnar nerve transposition, submuscular and subcutaneous fascial slings, were employed in various studies.2,17
Controversy continues to exist regarding the superiority of figure-of-eight versus docking procedure reconstructions. Certainly, surgeon preference and experience may prove to be the most important factor in improved outcomes for the various techniques. However, based upon the review by Vitale et al,34 the docking procedure is associated with improved results over the figure-of-eight reconstruction (90% vs. 76% excellent results, respectively). It should be remembered, however, that most of the figure-of-eight reconstruction studies pre-date the docking articles by at least two decades.11,23,26
Several biomechanical studies have attempted to evaluate various reconstruction techniques. Ahmad et al explored the novel method of graft fixation with a tenodesis screw.5 Rationale for the use of tenodesis screws include technical ease of fixation and tensioning, reduction of tunnel drilling, reconstruction of the central fibres of the UCL to maximize graft isometry, diminished tissue dissection and less risk of ulnar nerve injury. The authors in this study found average stiffness to be significantly greater in intact as against reconstructed elbows. However they also concluded that fixation with tenodesis screws was adequate and could be considered in the clinical setting. However in a direct biomechanical comparison, Large et al compared figure-of-eight with tenodesis screw fixation in a cadaver model and found the figure-of-eight fixation to be significantly stronger.34 They also found that tenodesis fixation failed via graft slippage in 70% of specimens.
Armstrong et al compared four methods of reconstruction (docking, figure-of-eight, EndoButton, interference screw) in a cadaver model.30 Consistent with other biomechanical studies, the strength of the intact ligament was greater than any of the methods of reconstruction.5,34,35 Docking and figure-of-eight reconstructions were performed in the standard manner. Reconstruction with a 5 mm tenodesis screw was performed with a doubled palmaris tendon graft, with one end of the graft anchored at the sublime tubercle of the ulna and the other at the isometric point of the medial epicondyle of the humerus. The EndoButton reconstruction was performed by extending the ulnar drill hole through the near and far cortices of the ulna and fixing a looped graft through an EndoButton. The proximal end was fixed in a manner similar to the standard docking procedure. The authors found a mean peak to failure of 142.5 ± 39.4 N for intact ligaments and no statistically significant difference between the docking (53.0 ± 9.5 N) or EndoButton (52.5 ± 10.4 N) reconstructions. The docking and EndoButton reconstructions were stronger than both the figure-of-eight (33.3 ± 7.1 N) and interference screw (41.0 ± 16.0 N) reconstructions. These findings were supported by another study comparing a modified docking procedure, in which the extra length of graft was used to increase the number of strands crossing the joint to four.35
In addition to reconstruction, several authors have reported their results with direct repair of the UCL. Both Azar et al and Conway et al have contrasted their results of repair versus reconstruction.17,29 Both reports show inferior results with attempts at directly repairing the ligament. This has led many authors to advocate reconstruction over repair, or in the rare event that a ligament is reconstructable, that augmentation of the ligament be employed.17,33 However, in both of these reports, there is limited discussion regarding indications for direct repair of the ligament. Savioe et al have more recently attempted to refine possible indications for direct repair.37 In their study, indications for repair relied upon evaluation of the ligament intraoperatively. If the ligament was deemed to be of ‘normal’ quality with the injury isolated to the origin or insertion of the ligament, a direct repair was performed. Ligaments with midsubstance tears or evidence of damage to the ligament were reconstructed. Of note, the average age of patients in this cohort was 17.2 years, lower than any other comparable series. In 60 patients who underwent direct repair, 56/60 (93%) returned to sport at the same or higher level of competition. Furthermore, these patients returned to sport at an average of 6.0 months; a significantly shorter time period than for most other series. Thus, careful selection of patients may spare them a more invasive procedure and hasten their return to sport.
Complications of treatment
Complications following reconstruction of the UCL vary from mild postoperative ulnar nerve paraesthesias and wound issues to more major complications such as ulnar nerve neurapraxia, tunnel fracture, arthrofibrosis and complex regional pain syndrome. The complication rate following reconstruction of the UCL has significantly decreased since Jobe et al first reported their technique and complication rate of 31.25% in 1986.2 More recent authors, however, have not consistently delineated between minor and major complications, but have reported a complication rate between 3% and 15.7%.11,17,27–29
Fracture of the bony tunnels is rare. Rohrbough et al and Paletta et al each reported one case of fracture of the ulnar tunnel.27,29 In one instance the fracture was treated with rest and a bone stimulator, while the other was treated with revision surgery. Both patients returned to competition. Generally, graft harvest is a relatively benign procedure, although Azar et al did report a 4.4% complication of wound infection or stiffness following harvest of the palmaris longus tendon.17 Other complications requiring additional surgery included residual pain from posteromedial osteophytes requiring excision and postoperative stiffness requiring arthroscopic release of adhesions.11,17,27
Additional elbow injuries
Although injury to the UCL is the most common injury among throwing athletes, additional medial pathology may be present. Ulnar neuropathy (Chapter 31) can affect athletes as well as the general population. However, the increased dynamic stresses applied to the elbows of some athletes may cause ulnar nerve symptoms from traction and compression of the cubital tunnel even if they were not otherwise predisposed to the condition. Additionally, medial epicondylitis (Chapter 35) can occur with repetitive forceful contraction of the wrist flexors. Especially at risk are golfers who require considerable force at the origin of the wrist flexors when stabilizing their wrist on impact of the club head with the ground.
Multiple pathologies are associated with posterior elbow pain. Olecranon stress fractures are the most common upper extremity stress fracture seen in baseball players; they are also common in upper extremity weight-bearing athletes such as gymnasts and weightlifters. In the skeletally mature athlete, fractures occur in two locations: at the tip or in the middle third of the olecranon.38 Tip fractures generally occur in throwers. These fractures are a result of repetitive loads induced by the forceful pull of the triceps or from impaction of a hypertrophic olecranon into the olecranon fossa. Fractures at the mid-portion of the olecranon have been reported in throwing athletes, gymnasts, and weightlifters. These fractures are felt to occur from repetitive valgus force across the olecranon from impaction of the olecranon within the fossa. Throwers experience posterior elbow pain during the acceleration and follow-through phases of the throwing cycle.38
Repetitive contraction of the triceps can likewise cause a stress reaction of the olecranon in the skeletally immature athlete. The olecranon ossific nucleus first appears around 9 years of age and ossifies between the ages of 12 and 15 years. In adolescents, the apophysis has matured but the physis remains the weakest point. Repetitive tension on the olecranon epiphyseal plate can lead to a transverse growth plate fracture.38 Additionally, younger patients can develop olecranon apophysitis, manifested as disturbed ossification at the insertion of the triceps. Patients generally complain of chronic, aching posterior elbow pain particularly after bouts of activity.
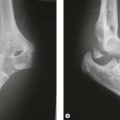
Examination reveals tenderness directly over the olecranon and commonly also reveals pain with resisted elbow extension. Plain radiographs can reveal the diagnosis; however, a high index of suspicion is necessary as commonly only a subtle finding such as a periosteal reaction may be present. In the adolescent athlete, the stress fracture appears as a persistent and often widened physis; although, displacement of the proximal fragment may also occur39 (Fig. 33.12). Often, radiographs will demonstrate fragmentation of the apophysis. In the face of normal radiographs, additional imaging such as MRI may be necessary.
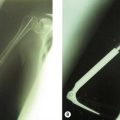
Treatment of olecranon stress fractures begins with a period of 6–8 weeks of rest from the inciting activity. At 8 weeks, patients are generally advanced to a graduated programme of sports-specific rehabilitation. Usually patients respond favourably to non-operative treatment and are able to return to their sports without limitations. However, occasionally patients fail to heal or choose a more aggressive course of treatment in an attempt to hasten their return. Surgical treatment consists of debridement of the non-union site and open reduction and internal fixation with or without bone grafting. Skeletally immature patients are treated similarly with open reduction and fixation. Rettig et al have recently reported a series of five adolescent pitchers treated with a cannulated screw across the physis and a tension band construct.39 Patients achieved radiographic union at a mean of 15.4 weeks, and all five patients were able to return to sport at a mean time of 29.4 weeks.
Pathology of the triceps tendon can also manifest as posterior elbow pain in the athletic population. Complete or partial ruptures of the triceps tendon are rare, but have been associated with anabolic steroid use, local steroid injection, renal dialysis and metabolic disease. This injury commonly occurs as a result of an eccentric load on a contracted tendon or from a direct blow. Clearly, in violent sports such as rugby and American football, the risk of rupture is heightened. Mair et al reported on distal triceps tendon ruptures in professional American football players.40 Eleven complete and 10 partial ruptures were identified, with the most common mechanism being an eccentric load on a contracted triceps. Five players had previously received at least one steroid injection. From their review, the authors concluded that near complete or complete ruptures are best treated with repair, while partial ruptures were found to heal with rest. All players in this series were able to return to their sport for at least one season.
Posterior elbow pain can also be caused by posterior or posterolateral osteophyte formation and impingement. Termed ‘boxer’s elbow’ or ‘handball goalie’s elbow,’ the unifying mechanism is repetitive high force hyperextension of the elbow. Hyperextension occurs in boxers with missed punches41 or with impact from blocking a high velocity ball in handball or similar sports. Unlike VEOLS, in which the elbow also undergoes valgus force causing posteromedial osteophytes, forced hyperextension in boxer’s elbow is not associated with instability; although, handball goalie’s elbow has been associated with medial instability.41 Valkering et al reported good to excellent results with partial resection of the posterior olecranon tip and removal of loose bodies in a series of five boxers.41
Lateral elbow pain can be caused by a number of conditions which are often difficult to distinguish clinically. More obvious causes of lateral sided pathology result from a fall onto an extended arm resulting in a fracture of the radial head or capitellum (Chapters 18 and 21). Additionally, trauma may lead to damage to the lateral collateral ligament and a spectrum of posterolateral instability (Chapter 26). More subtle sources of pain and disability may stem from lateral epicondylitis, a common overuse injury in sports such as golf (Chapter 35).
Finally, a rare cause of pain on the lateral side of the elbow is intra-articular plica syndrome. These patients describe position and motion-dependent lateral elbow pain and snapping. In addition these patients may present after having failed treatment for lateral epicondylitis. The offending structure is a band-like synovial fold running from the humerus to the radial head. This band of synovial tissue can enfold into the radiocapitellar joint causing mechanical symptoms or lead to an area of chondromalacia (or a ‘kissing lesion’) on the radial head visible on arthroscopic examination. Examination for this condition involves passively flexing and pronating the elbow in an attempt to reproduce a snap; however, physical findings are often subtle and difficult to elicit. MRI with arthrography may be useful in aiding diagnosis and eliminating other potential sources of pathology. Arthroscopy is the best method to confirm the diagnosis and treat the condition. Antuna et al reported improvement in 12 out of 14 patients with symptomatic intra-articular plica after arthroscopic debridement.42
1 Waris W. Elbow injuries of javelin throwers. Acta Chir Scand. 1946;93:563-575.
2 Jobe FW, Stark J, Lombardo SJ. Reconstruction of the ulnar collateral ligament in athletes. J Bone Joint Surg (Am). 1986;68:1158-1163.
3 Callaway GH, Field LD, Deng XH, et al. Biomechanical Evaluation of the Medial Collateral Ligament of the Elbow. J Bone Joint Surg (Am). 1997;79:1223-1231.
4 Lyman S, Fleisig GS, Andrews JR, et al. Effect of pitch type, pitch count, and pitching mechanics on risk of elbow and shoulder pain in youth baseball pitchers. Am J Sports Med. 2002;30:463-468.
5 Ahmad CS, Lee TQ, ElAttrache NS. Biomechanical evaluation of a new ulnar collateral ligament reconstruction technique with interference screw fixation. Am J Sports Med. 2003;31(3):332-337.
6 Fleisig GS, Andrews JR, Dillman CJ. Kinetics of baseball pitching with implications about injury mechanism. Am J Sports Med. 1995;23:233-239.
7 Wilson FD, Andrew JR, Blackburn TA, McCluskey G. Valgus extension overload in the pitching elbow. Am J Sports Med. 1983;11:83-88.
8 Lyman S, Fleisig GS, Waterbar JW, et al. Longitudinal study of elbow and shoulder pain in youth baseball pitchers. Med Sci Sports Exerc. 2001;33:1803-1810.
9 Sabick MB, Torry MR, Lawton RL, et al. Valgus torque in youth baseball pitchers: a biomechanical study. J Shoulder Elbow Surg. 2004;13:349-355.
10 Ireland ML, Hutchinson MR. Upper extremity injuries in young athletes. Clin Sports Med. 1995;14:533-569.
11 Dodson CC, Thomas A, Dines JS, et al. Medial ulnar collateral ligament reconstruction of the elbow in throwing athletes. Am J Sports Med. 2006;34(12):1926-1932.
12 Ciccotti MG, Jobe FW. Medial collateral ligament instability and ulnar neuritis in the athlete’s elbow. AAOS Instructional Course Lectures. 1999;48:383-391.
13 Veltri DM, O’Brien SJ, Field LD, et al. The milking maneuver: a new test to evaluate the MCL of the elbow in the throwing athlete. Presented at the 10th Open meeting of the American Shoulder and Elbow Surgeons Specialty Day, New Orleans LA, February, 1994.
14 Safran MR, Caldwell GLIII, Fu FH. Chronic instability of the elbow. In: Peimer CA, editor. Surgery of the Hand and Upper Extremity. New York, NY: McGraw-Hill; 1996:467-490.
15 O’Driscoll SW, Lawton RL, Smith AM. The moving valgus stress test for medial collateral ligament tears of the elbow. Am J Sports Med. 2005;33:231-239.
16 Ellenbecker TS, Mattalino AJ, Elam EA, et al. Medial elbow joint laxity in professional baseball pitchers. Am J Sports Med. 1998;26(3):420-424.
17 Azar FM, Andrew JR, Wilk KE, et al. Operative treatment of ulnar collateral ligament injuries of the elbow in athletes. Am J Sports Med. 2000;28(1):16-23.
18 Mulligan SA, Schwartz MI, Broussard MF, et al. Heterotopic calcification and tears of the ulnar collateral ligament: radiographic and MR imaging findings. AJR Am J Roentgenol. 2000;175:1099-1102.
19 Timmerman LA, Schwartz ML, Andrews JR. Preoperative evaluation of the ulnar collateral ligament by magnetic resonance imaging and computed tomography arthrography. Evaluation in 25 baseball players with surgical confirmation. Am J Sports Med. 1994;22:26-32.
20 Schwartz ML, Al-Zahrani S, Morwessel RM, et al. Ulnar collateral ligament injury in the throwing athlete: evaluation with saline-enhanced MR arthrography. Radiology. 1995;197:297-299.
21 Ahmad CS, ElAttrache NS. Elbow valgus instability in the throwing athlete. J Am Acad Orthop Surg. 2006;14:693-700.
22 Park MC, Ahmad CS. Dynamic contributions of the flexor-pronator mass to elbow valgus stability. J Bone Joint Surg (Am). 2004;86:2268-2274.
23 Rettig AC, Sherrill C, Snead DS, et al. Nonoperative treatment of ulnar collateral ligament injuries in throwing athletes. Am J Sports Med. 2001;29:15-17.
24 Andrews JR, Heggland EJH, Fleisig GS, et al. Relationship of ulnar collateral ligament strain to the amount of medial olecranon osteotomy. Am J Sports Med. 1995;23:233-239.
25 Andrews JR, Timmerman LA. Outcome of elbow surgery in professional baseball players. Am J Sports Med. 1995;23:407-413.
26 Kamineni S, ElAttrache NS, O’Driscoll SW, et al. Medial collateral ligament strain with partial posteromedial olecranon resection: biomechanical study. J Bone Joint Surg (Am). 2004;86:2424-2430.
27 Rohrbough JT, Altcheck DW, Hyman J, et al. Medial collateral ligament reconstruction of the elbow using the docking technique. Am J Sports Med. 2002;30:541-548.
28 Koh JL, Schafer MF, Keuter G, et al. Ulnar collateral ligament reconstruction in elite throwing athletes. Arthroscopy. 2006;22(11):1187-1191.
29 Paletta GAJr, Wright RW. The modified docking procedure for elbow ulnar collateral ligament reconstruction: 2-year follow up in elite throwers. Am J Sports Med. 2006;34(10):1594-1598.
30 Thompson WH, Jobe FW, Yocum LA, Pink MM. Ulnar collateral ligament reconstruction in athletes: muscle-splitting approach without transposition of the ulnar nerve. J Shoulder Elbow Surg. 2001;10:152-157.
31 Armstrong AD, Dunning CE, Ferreira LM, et al. A biomechanical comparison of four reconstruction techniques for the medial collateral ligament-deficient elbow. J Shoulder Elbow Surg. 2005;14:207-215.
32 Wilk KE, Reinold MM, Andrew JR. Rehabilitation of the thrower’s elbow. Clin Sports Med. 2004;23(4):765-801.
33 Conway JE, Jobe FW, Glousman RE, et al. Medial instability of the elbow in throwing athletes: treatment by repair or reconstruction of the ulnar collateral ligament. J Bone Joint Surg (Am). 1992;74:67-83.
34 Vitale MA, Ahmad CS. The outcome of elbow ulnar collateral ligament reconstruction in overhead athletes. Am J Sports Med. 2008;36:1193-1205.
35 Paletta GAJr, Klepps SJ, Difelice GS, Allen T, Brodt MD, Burns ME, Silva MJ, Wright RW. Biomechanical evaluation of 2 techniques for ulnar collateral ligament reconstruction of the elbow. Am J Sports Med. 2006;34(10):1599-1603.
36 Meyers A, Palmer B, Baratz ME. Ulnar collateral ligament reconstruction. Hand Clin. 2008;24(1):53-67.
37 Savoie FH3rd, Trenhaile SW, Roberts J, et al. Primary repair of ulnar collateral ligament injuries of the elbow in young athletes: A case series of injuries to the proximal and distal ends of the ligament. Am J Sports Med. 2008;36:1066-1072.
38 Jones GL. Upper extremity stress fractures. Clin Sports Med. 2006;25(1):159-174. xi
39 Rettig AC, Wurth TR, Mieling P. Nonunion of olecranon stress fractures in adolescent baseball pitchers. Am J Sports Med. 2006;34:653-656.
40 Mair SD, Isbell WM, Gill TJ, et al. Triceps tendon ruptures in professional football players. Am J Sports Med. 2004;32:431-434.
41 Valkering KP, van der Hoeven H, Pijnenburg BC. Posterolateral elbow impingement in professional boxers. Am J Sports Med. 2008;36(2):328-332.
42 Antuna SA. Snapping plicae associated with radiocapitellar chondromalacia. Anthropology. 2001;17:491-495.