Chapter 16 Spontaneous Intracerebral Hemorrhage
• Hemorrhagic stroke accounts for 10% of all strokes. Strokes are the third leading cause of death and the main cause of long-term disability in the United States.
• Spontaneous intracerebral hemorrhage (ICH) is most commonly caused by chronic hypertension and occurs in penetrating arteries in the basal ganglia, thalamus, pons, and cerebellum. However, other causes, such as vascular malformations, tumors, and amyloid angiopathy, should always be considered.
• The presentation of ICH depends on its location, with neurological deficits being specific to the function of the brain involved. A decreased level of consciousness can occur with large lesions that cause herniation or lesions in the posterior fossa that result in brainstem dysfunction or hydrocephalus.
• The prognosis for ICH depends on the location and size of the hemorrhage, the age of the patient, and the degree of neurological impairment at the time of presentation.
• Medical therapy can try to prevent expansion of the hematoma, but emergent surgical evacuation should be considered for patients with rapid deterioration.
Background and Epidemiology
Spontaneous intracerebral hemorrhage is defined as a nontraumatic hemorrhage into brain parenchyma. The clinical significance of intracerebral hemorrhage (ICH), also known as hemorrhagic stroke, can be more clearly understood when it is viewed as a subtype of stroke. According to the National Vital Statistics Report, stroke is the third leading cause of death in the United States, behind only heart disease and cancer, and is responsible for nearly 6% of total deaths on an annual basis.1 ICH accounts for approximately 10% of all strokes with an annual incidence between 15.9 and 32.9 per 100,000.2 A sharp increase in incidence occurs in patients more than 75 years old and the incidence is even higher in patients older than 85, with reported incidence rates as high as 309.8 per 100,000, nearly seven times greater than the rate in the general population.3 Though less frequent than ischemic stroke, the overall mortality rate of ICH is significantly greater, with 30-day mortality estimated at 44% to 52% and half of all deaths occurring within the first 2 days of hemorrhage.4–6 The national cost of first-time strokes has been estimated at $40.6 billion, with more than $6 billion accounting for aggregate lifetime cost.7 Of the $40.6 billion, only 45% of the cost was attributable to acute stroke care, with more than 47% of the total cost coming from long-term ambulatory and nursing care. Of all patients with ICH, only 20% have been noted to be functionally independent 6 months after their stroke.5 Although these statistics underscore the significant economic burden of ICH, they fail to emphasize the immeasurable emotional and social impact of ICH. It is essential to note, however, that since 1958, there has been a general annual decline in stroke deaths nationally. Between 2005 and 2006, there was a 6.4% decrease in stroke deaths.1 This decline is attributable to significant improvements in treatment of modifiable risk factors related to cardiovascular and cerebrovascular health. Still, the rate of ICH is expected to increase in the future as a result of increasing population age.
Pathophysiology
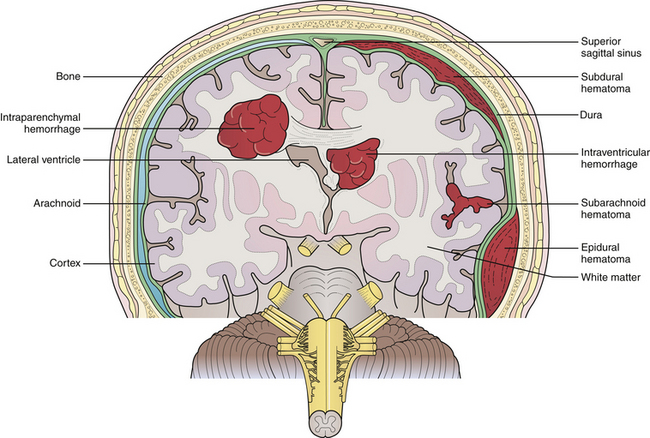
ICH may be classified as primary or secondary, depending on the etiology of the hemorrhage. Primary ICH most commonly results from chronic arterial hypertension. It occurs in small perforating arteries, typically at bifurcations from larger cerebral arteries where a pressure gradient is transmitted from the larger vessel to smaller, susceptible vessels. Small perforating vessels with diameters of 50 to 700 µm are often the offending vessels and may have multiple sites of rupture.8 Hypertension is the most important risk factor for ICH, with nearly 60% of patients with ICH in a prospective study having elevated blood pressure.9 Hypertensive bleeds are noted to occur more frequently within deep gray matter structures, most commonly the basal ganglia, followed by the thalamus, pons, and the cerebellum (Fig. 16.1). Lobar hemorrhages, however, are not infrequent. Of all ICHs, one population study reviewed the locations of cerebral bleeds and noted 49% were deep in location, 35% were lobar, 10% were in the cerebellum, and 6% were in the brainstem.10
Classically, hypertensive bleeds have been attributed to the rupture of miliary aneurysms as described initially by Charcot and Bouchard in 1868 from their postmortem analysis of hemorrhage cavities in autopsy specimens. Further research has clarified that the miliary aneurysms described by Charcot and Bouchard are in fact pseudoaneurysms or weaknesses in the perforating arteriole walls where there has been extravasated blood and accumulated fibrin.11 Pathological analysis of vessels in ICH specimens and in patients with chronic hypertension has more clearly demonstrated the process of lipohyalinosis in which chronic hypertensive damage gives rise to intimal hyperplasia, fibrin deposition, and focal accumulation of fat-filled macrophages, ultimately leading to necrosis in the vessel walls.11 These vessel changes are more commonly seen at sites of hemorrhages than miliary aneurysms and thus are believed to be the source.
After the inciting hemorrhage, blood disperses along surrounding fiber tracts in the parenchyma. In an estimated 10% to 15% of cases, blood will decompress from the parenchyma into the ventricular system, creating an intraventricular hemorrhage.12 Ultimately, the hemorrhage is self-contained by tamponade from the parenchyma adjacent to the bleed site and by activation of hemostatic pathways. Regions of brain surrounding a hemorrhage are often characterized by extensive inflammation, edema, apoptosis, and necrosis that may contribute to the neurological deficit. Moreover, increased intracranial pressure and herniation secondary to mass effect occur with particularly large-volume hemorrhages. The rate of expansion of a hemorrhagic lesion is also an important variable to consider. Rapidly expanding lesions cause greater direct injury to axons and tissues and therefore result in greater damage to adjacent neural structures than slowly expanding lesions.
Secondary ICH results from underlying lesions such as aneurysms, vascular malformations, coagulopathies, tumors, hemorrhagic conversion of ischemic infarcts, cerebral amyloid angiopathy, and drug-related hemorrhages. Common metastatic tumors that are prone to hemorrhage include melanoma, choriocarcinoma, and renal carcinoma. Lung and breast cancer should also be considered because of their high incidence in the population. Primary brain neoplasms associated with ICH include glioblastoma multiforme, oligodendroglioma, and ependymoma.13 Although secondary ICH is less common, it is important to consider these causes in the differential diagnosis and work them up appropriately because they place patients at risk for recurrent hemorrhage with the potential for extensive morbidity and even death.
Etiology
Hypertension
Normal blood pressure is currently defined as systolic blood pressure (SBP) less than 120 mm Hg and diastolic blood pressure (DBP) less than 80 mm Hg. Hypertension is currently classified as four stages: prehypertension (120-139/80-89 mm Hg), stage 1 hypertension (140-159/90-99 mm Hg), stage 2 hypertension (160-179/100-109 mm Hg), and stage 3 hypertension (≥190/≥110 mm Hg). Multiple studies have verified the dramatically increased risk of ICH as it corresponds to increases in hypertensive stage.9,14–16 One meta-analysis of 11 case-control studies reported an overall odds ratio of 3.68 for ICH in hypertensive patients. Relative risk for patients with high normal hypertension (130-139 mm Hg systolic or 85-89 mm Hg diastolic) was 2.2, 5.3 for stage 1 hypertension, 10.4 for stage 2 hypertension, and 33.3 for stage 3 hypertension, as described by Suh and colleagues.16 Individuals with stage 3 hypertension are five times more likely to experience ICH than individuals with normal blood pressure or prehypertension. The rate of ICH increases 22% for every 10 mm HG increment of SBP.9 Naturally, improvements in hypertensive management and available antihypertensive medications have aided in decreasing the incidence of intracerebral hemorrhage.17
Cerebral Amyloid Angiopathy
In the elderly population, cerebral amyloid angiopathy (CAA) is an important etiological factor for ICH, accounting for an estimated 5% to 10% of all cases.18 CAA results from the deposition of β-amyloid in the media and adventitia of cerebral vasculature, including capillaries, arterioles, and even small to medium-sized cerebral arteries in the cerebral cortex, leptomeninges, and cerebellum. CAA is commonly associated with the gene encoding apolipoprotein E, and a familial syndrome has been described accounting for CAA-related hemorrhages in younger patients.19 For every decade increase in age, there is a 1.97 relative risk increase for ICH and a three times and seven times increased incidence rate for patients aged 65 to 74 and greater than age 85, respectively.14,20 ICH due to CAA is most commonly in lobar locations, classically in the posterior parietal and occipital lobes. Special consideration of CAA should be given for bleeds in these locations, especially in elderly patients.12 Recurrent lobar hemorrhages are relatively common in CAA and account for substantial increases in morbidity and mortality rates. The 2-year recurrence rate of lobar hemorrhages in patients with known CAA is 28%.21
Anticoagulation Therapy
ICH is a common yet serious complication of anticoagulation therapy. Patients on oral anticoagulation therapy suffering from ICH typically have significant vasculopathy related to chronic hypertension or CAA.22 There is a 1% per year increase in risk for ICH while on anticoagulation therapy, seven to ten times the rate in the normal population.23 This increased risk is quite significant given the high mortality rate of ICH associated with anticoagulation therapy, which is approximately 70%.24 Moreover, patients on oral anticoagulation therapy have a significantly greater hematoma volume than patients not on anticoagulation. Prompt recognition of ICH is essential in this patient population, with particular attention paid to correcting the coagulopathy and stabilizing hematoma expansion.
Drugs and Alcohol
Certain drugs are capable of causing spontaneous ICH. Those most commonly associated with ICH are sympathomimetic agents, such amphetamines, cocaine, and pseudoephedrine. Hemorrhages associated with sympathomimetic agents are often related to transient elevations in blood pressure, leading to arterial rupture. Additionally, transient hypertensive episodes associated with substance abuse may cause hemorrhage from an otherwise asymptomatic vascular lesion such as an aneurysm or arteriovenous malformation. In younger age groups, cocaine abuse is commonly associated with ICH and subarachnoid hemorrhage.25 As such, underlying vascular pathology should still be considered in young patients with ICH.
ICH has also been associated with alcohol consumption. Juvela and colleagues demonstrated an increased risk of ICH in patients who were moderate or heavy drinkers in a dose-dependent manner. A positive CAGE questionnaire∗ was associated with increased risk of ICH.26 The exact pathophysiological mechanism by which alcohol contributes to ICH is not clear; however, alcohol-associated hypertension, impaired hemostasis, and decreased levels of clotting factors have been implicated.
Aneurysms
Saccular Aneurysms
The majority of aneurysms are saccular aneurysms whose etiology may be a combination of congenital, hereditary, and acquired factors, including smoking and hypertension. The mortality associated with subarachnoid hemorrhage from a saccular aneurysm rupture is estimated to be between 40% and 50%, making this a grave disease.27
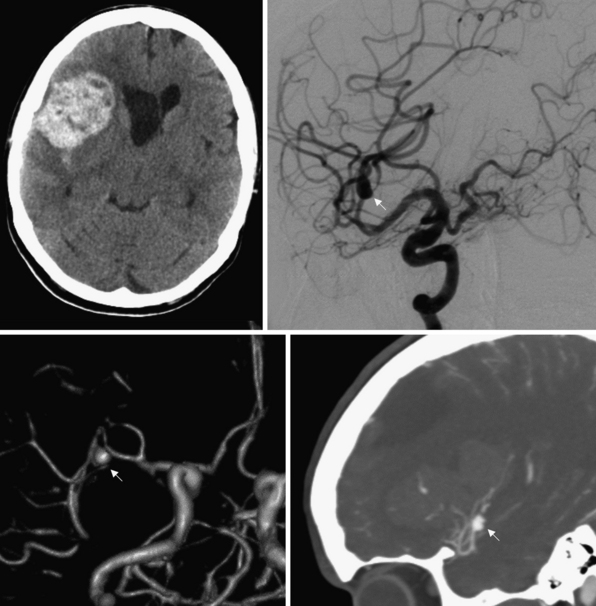
Saccular aneurysms are typically located at branch points of major cerebral arteries, usually arising from the vessels that form the circle of Willis. Saccular aneurysms may be associated with inherited conditions, including Ehlers-Danlos syndrome, adult polycystic kidney disease, and other conditions that predispose individuals to aneurysm formation because of intrinsic weaknesses in their vessel walls. In fact, 20% of patients with saccular aneurysms harbor more than just one. Additionally, lesions that alter cerebral blood flow, such as tumors or vascular malformations, may lead to aneurysm formation. Bifurcations and trifurcations are particularly susceptible areas for aneurysm formation because these points experience a greater hydrostatic pressure owing to loss of laminar blood flow and greater turbulence. Although saccular aneurysm rupture usually leads to subarachnoid hemorrhage, intraparenchymal hematomas into adjacent brain are common (Fig. 16.2). The presence of an intraparenchymal hematoma after aneurysm rupture may play an important role in the need for and timing of surgical intervention.
The mean frequency of aneurysm occurrence is 5%, with a prevalence between 10 and 15 million people in the United States.28 The annual rate of rupture of aneurysms has been quoted at between 0.3% and 1.3%, but estimates are highly variable.27,28 There does appear to be a relationship between aneurysm size and annual rate of rupture, with a rate of rupture of aneurysms 10 mm or greater being slightly less than 1% per year and an astounding 6% rate of rupture in the first year for giant aneurysms measuring greater than 25 mm.28 In an attempt to better elucidate this relationship, the International Study of Unruptured Intracranial Aneurysms Investigators (ISUIAI) performed a combined retrospective and prospective multicenter study to examine the rupture rates. They found that risk of rupture was associated with aneurysm size and location, with small aneurysms (<7 mm) in the anterior circulation having an annual rupture rate of 0.05%. The risk increased with aneurysm size from 0.5% to 3% per year in 7- to 24-mm aneurysms and 8% per year for giant aneurysms. Aneurysms in the posterior circulation had an 8 to 14 times higher relative risk of rupture.28,29 Although this study has been criticized for design, it reinforces the notion of size and location being major determinants in risk of rupture.
Mycotic Aneurysms
Mycotic aneurysms are a potentially life-threatening condition resulting from septic emboli. They commonly occur in smaller cortical arteries, where the emboli become lodged. Transmigration of infection through the arterial wall and extensive damage to the vessel media weaken the vessel wall, resulting in subsequent dilatation and rupture. Mycotic aneurysms are usually seen in the setting of bacterial endocarditis, with 30% of cases of bacterial endocarditis having neurological complications, including stroke and hemorrhage.30
Vascular Malformations
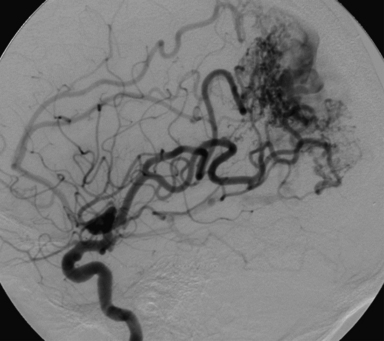
The most common and most clinically significant of the vascular malformations is the arteriovenous malformation (AVM). AVMs are congenital vascular anomalies consisting of a meshwork of vessels fed by one or more arteries that drain directly into the venous circulation (Fig. 16.3). As there is no flow through a capillary bed, the arterial pressure is transmitted directly into the venous system. AVMs carry a significant risk of bleeding. The annual risk of hemorrhage from an AVM is estimated to be 2% to 3% per year, with higher rates in patients with smaller AVMs, AVM-associated aneurysms, or deep venous drainage.31 The most common presentation of AVMs is ICH, accounting for 69% of symptomatic cases.32 Hemorrhages are most common during the second through fourth decades of life, as opposed to primary ICH, which is more likely to occur starting in the fifth decade of life. Traditionally, these lesions have been treated by open resection if the location and characteristics of the AVM are amenable. Recent advances in neurosurgery, including endovascular embolization techniques and stereotactic radiosurgery, have become useful tools in the treatment of this disease.
Signs and Symptoms
The presentation of patients with ICH is highly variable and depends on multiple factors, including the location and size of the hemorrhage. Neurological symptoms have a gradual onset over a period of minutes or hours, as opposed to the sudden onset of a subarachnoid hemorrhage or embolic event. Many of these patients may present with hemiplegia or other focal neurological deficits. Decreased level of consciousness and brainstem dysfunction are frequently seen with large hematomas, especially those located in the posterior fossa. Signs of increased intracranial pressure may include headache, nausea, and vomiting. One third of patients have seizure activity during presentation, often related to hemorrhages in lobar locations.33 The symptoms of ICH may correspond to more than one vascular territory, allowing differentiation from patients with ischemic events who typically display symptoms confined to a single vascular territory. In addition, symptoms of ICH rarely regress in the acute phase of hemorrhage as is sometimes seen during the acute phase of an ischemic or embolic event.
Intraventricular hemorrhage (IVH), hydrocephalus, and herniation syndromes are of particular importance in ICH as they may be lethal to patients. Intraventricular extension of hemorrhage occurs in approximately 40% of patients with ICH and is associated with a mortality rate ranging from 45% to 80%.12,10,34 Hydrocephalus can result either from the obstruction of the ventricular system by IVH (noncommunicating hydrocephalus) or blockage of cerebrospinal fluid (CSF) resorption at the arachnoid granulations by blood in the CSF (communicating hydrocephalus). Occasionally, mass effect in the cerebellum from a hemorrhage and associated edema may efface the fourth ventricle, causing an obstructive hydrocephalus without IVH. Patients with a decreased level of consciousness and severe neurological deficits out of proportion to the size of the bleed during and after their hospitalizations should be evaluated for IVH and hydrocephalus. Increased intracranial pressure due to hydrocephalus or from mass effect resulting from a rapid and large hematoma is a true neurosurgical emergency. In this clinical setting, immediate extraventricular drainage and surgical decompression may be indicated.
Natural History
It has been estimated by two population studies that between 20% and 40% of hemorrhages undergo volume expansion after the initial bleed.35,36 Moreover, hematoma expansion has been shown to carry a fivefold increased risk of clinical deterioration and was associated with ICH-related death.36 Brott and colleagues demonstrated that the majority of the hematoma growth occurred within 3 to 4 hours after hemorrhage onset and was similarly associated with neurological deterioration. Kazui and colleagues found that, in patients who had hematoma expansion, 36% had growth within 3 hours of onset, 16% had expansion between 3 and 6 hours after onset, 15% had growth between 6 and 12 hours, and only 6% had growth between 12 and 24 hours. Of note, no patients in the study had hematoma expansion between 24 and 48 hours after hemorrhage onset. The most rapid phase of hemorrhage, however, is still felt to occur within the first hour. It is important to note that 35% to 46% of patients with neurological deterioration did not have hematoma expansion and their deterioration was likely related to mass effect associated with edema, IVH, or hydrocephalus.35,36 Nonetheless, clot stabilization is a priority of initial management of ICH, as hematoma expansion is associated with worse outcome.
As previously mentioned, the 30-day mortality rate of ICH is estimated at 44% to 52%.4,5 Given the high mortality rate, many studies have investigated predictors of poor outcome in this patient population. Independent predictors of poor outcome include low Glasgow Coma Scale (GCS) scores at the time of presentation, age 80 or older, infratentorial origin, ICH volume, and the presence of IVH. Volume of intracerebral hemorrhage alone is a very strong predictor of the 30-day mortality rate.37 Hemorrhages with volumes greater than 60 cm3 resulted in 30-day mortality rate of 93% for deep hemorrhages and 71% for lobar. Lethal volume of hemorrhage is variable and dependent on the location of the hemorrhage, with hemorrhages in the pons and cerebellum having significant mortality rates in hemorrhages greater than 5 cm3 and 30 cm3, respectively.4
In an effort to provide a more accurate determination of prognosis, mortality risk, and clinical course for patients with ICH, Hemphill and colleagues have devised the ICH score. The scale utilizes GCS scores, ICH volume, presence of IVH, infratentorial origin, and age older than 80 years at the time of presentation to stratify patient mortality risks. The scale was found to be an accurate tool for assessing 30-day mortality risk. In the study, no patient with an ICH score of 0 died, though the mortality rate was 100% for patients with a score of 5.38
Diagnosis
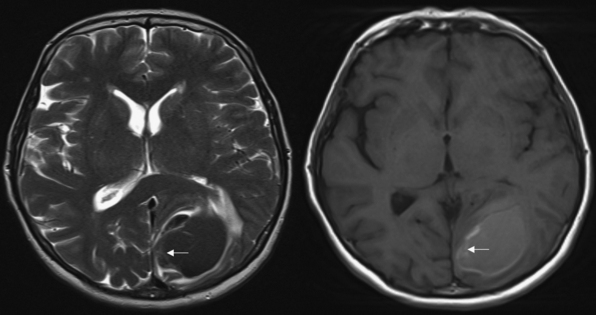
Magnetic resonance imaging (MRI) is more sensitive than CT but takes significantly longer to obtain. Therefore, it is not a good tool for screening but should be reserved for situations in which a patient has been deemed clinically stable. The signal characteristics of ICH are complex. They are determined by the molecular constituents of the blood clot as they undergo catabolism from oxyhemoglobin to deoxyhemoglobin, intracellular methemoglobin, and extracellular methemoglobin (Fig. 16.4). The administration of intravenous contrast agents may increase the sensitivity of MRI for underlying tumors or vascular malformations. It should be noted, however, that in many instances the hematoma can obscure the underlying anomaly. Waiting 3 to 4 weeks after the hemorrhage often allows enough resorption of the clot to visualize the lesion.
Treatment
Medical Management
The purpose of medical therapy in ICH patients is to stabilize the patient and eliminate any factors that may exacerbate the hemorrhage or its sequelae, including blood pressure control, reversal of coagulopathies, and treatment of intracranial hypertension. The medical care of patients with ICH is complex. Because of the potential for rapid neurological deterioration, these patients require admission to an intensive care unit (ICU). Being treated in an ICU and by physicians that specialize in neurological conditions has been shown to decrease the morbidity and mortality rates of ICH.39
 If SBP is 200 mm Hg or mean arterial pressure (MAP) is 150 mm Hg, then consider aggressive reduction of blood pressure with continuous intravenous infusion, with frequent blood pressure monitoring every 5 minutes.
If SBP is 200 mm Hg or mean arterial pressure (MAP) is 150 mm Hg, then consider aggressive reduction of blood pressure with continuous intravenous infusion, with frequent blood pressure monitoring every 5 minutes.
 If SBP is 180 mm Hg or MAP is 130 mm Hg and there is evidence of or suspicion of elevated intracranial pressure (ICP), then consider monitoring ICP and reducing blood pressure using intermittent or continuous intravenous medications to keep cerebral perfusion pressure 60 to 80 mm Hg.
If SBP is 180 mm Hg or MAP is 130 mm Hg and there is evidence of or suspicion of elevated intracranial pressure (ICP), then consider monitoring ICP and reducing blood pressure using intermittent or continuous intravenous medications to keep cerebral perfusion pressure 60 to 80 mm Hg.
 If SBP is 180 mm Hg or MAP is 130 mm Hg and there is no evidence or suspicion of elevated ICP, then consider a modest reduction of blood pressure (e.g., MAP of 110 mm Hg or target blood pressure of 160/90 mm Hg) using intermittent or continuous intravenous medications to control blood pressure, and clinically reexamine the patient every 15 minutes.37
If SBP is 180 mm Hg or MAP is 130 mm Hg and there is no evidence or suspicion of elevated ICP, then consider a modest reduction of blood pressure (e.g., MAP of 110 mm Hg or target blood pressure of 160/90 mm Hg) using intermittent or continuous intravenous medications to control blood pressure, and clinically reexamine the patient every 15 minutes.37
Several recent studies have examined whether or not stricter control is warranted. The Intensive Blood Pressure Reduction in Acute Cerebral Haemorrhage Trial (INTERACT) randomized 276 patients with spontaneous ICH, without an underlying tumor or vascular abnormality, to the parameters suggested by the 1999 AHA guidelines or a more intensively controlled group with SBP less than 140 mm Hg. The investigators found that the more intensively controlled group had less hematoma growth than the guideline group (11.9%, 8.3%, and 9.8% less at 24 hours, 72 hours, and over 72 hours, respectively).40 Additionally, the Antihypertensive Treatment of Acute Cerebral Hemorrhage (ATACH) study sought to evaluate the safety and effectiveness of aggressive blood pressure reduction and identify an appropriate blood pressure target. Sixty patients were randomized into three groups (SBP targeted to 170-200 mm Hg, 140-170 mm Hg, and 110-140 mm Hg), and 3-month mortality rate was recorded. Although the study was not powered to compare the mortality rates between the three groups, all had lower than expected mortality rates.41
Although ICH itself can lead to devastating neurological injuries, it is worsened by the presence of coagulopathy. A number of etiologies exist for coagulopathy, but the most common is the use of anticoagulation and antiplatelet medications. Though they do not cause hemorrhages by themselves, they can significantly exacerbate the bleeding that occurs. Without the normal ability to clot, hematomas may continue to grow, worsening local tissue injury and ICP. The reversal of warfarin-induced anticoagulation may be achieved by administration of plasma, which contains the factors depleted by the medication. This is not without risk, however. Transfusion reactions and systemic volume overload are common. Although plasma is an effective means of restoring clotting factors, its usefulness may be limited by the time it takes to administer multiple units to a patient with severe coagulopathy who has evidence of significant mass effect from the hematoma. Studies are currently being undertaken to evaluate exogenous activated factor VII, a substrate whose endogenous counterpart allows the conversion of prothrombin to thrombin, almost immediately reversing the coagulopathy. Of note, activated factor VII has been studied in noncoagulopathic patients with ICH in a large randomized trial.42 This study demonstrated that activated factor VII was able to decrease hematoma growth in a dose-dependent fashion but did not alter clinical outcome at 6 months.
Surgical Management
Surgical management of ICH has traditionally been reserved for patients with ongoing neurological deterioration that require emergent relief of intracranial hypertension or decompression of vital structures. The extent to which clot evacuation ameliorates injury outside these conditions is not well understood. Numerous trials have looked at craniotomy as a treatment for ICH. The largest of these was the Surgical Trial in Intracerebral Hemorrhage (STICH) that randomized 1033 patients with supratentorial hemorrhages to early surgery (<72 hours) or medical management alone. No difference was found between the groups in the rate of mortality or good outcome at 6 months.43 Trends were seen for improved outcome in younger patients, GCS scores at presentation, more superficial hemorrhages, and absence of intraventricular extension. These factors did not reach significance, though. Unlike supratentorial hemorrhages, patients with cerebellar hemorrhages clearly benefit from surgery. Because of the proximity of these hemorrhages to the brainstem and the fourth ventricle, any significant hematoma in the cerebellum should be evacuated emergently.
Anderson C.S., Huang Y., Arima H., et al. Effects of early blood pressure-lowering treatment on growth of hematoma and perihematoma edema in acute intracerebral hemorrhage. Stroke. 2010;41:307-312.
Broderick J.P., Brott T.G., Duldner J.E., et al. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24(7):987-993.
Hemphill J.C.III, Bonovich D.C., Besmertis L., et al. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32(4):891-897.
Kazui S., Naritomi H., Yamamoto H., et al. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27(10):1783-1787.
Mendelow A.D., Gregson B.A., Fernandes H.M., et al. Early surgery versus conservative medical management in patients with spontaneous supratentorial intracerebral hemorrhage in the International Surgical Trial in Intracerebral Hemorrhage (STICH): a randomized trial. Lancet. 2005;365(9457):387-397.
Please go to expertconsult.com to view the complete list of references.
1. Heron M., Hoyert D.L., Murphy S.L., et al. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57(14):1-134.
2. Lloyd-Jones D., Adams R., Carnethon M., et al. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:e21-e181.
3. Sacco S., Marini C., Toni D., et al. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. 2009;40:394.
4. Broderick J.P., Brott T., Tomsick T., et al. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg. 1993;78(2):188-191.
5. Counsell C., Boonyakarnkul S., Dennis M., et al. Primary intracerebral haemorrhage in Oxfordshire Community Stroke Project 2: prognosis. Cerebrovasc Dis. 1995;5:26-34.
6. Broderick J.P., Brott T.G., Duldner J.E., et al. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24(7):987-993.
7. Taylor T., Davis P., Torner J., et al. Lifetime cost of stroke in the United States. Stroke. 1996;27:1459-1466.
8. Qureshi A.I., Mendelow A.D., Hanley D.F. Intracerebral haemorrhage. Lancet. 2009;373(9675):1632-1644.
9. Sturgeon J.D., Folsom A.R., Longstreth W.T.Jr., et al. Risk factors for intracerebral hemorrhage in a pooled prospective study. Stroke. 2007;38(10):2718-2725.
10. Broderick J., Connolly S., Feldmann E., et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults. 2007 update: a guideline from the American Heart Association/American Stroke Association Stroke Council, High Blood Pressure Research Council, and the Quality of Care and Outcomes in Research Interdisciplinary Working Group. Stroke. 2007;38(6):2001-2023.
11. Fisher C.M. Cerebral miliary aneurysms in hypertension. Am J Pathol. 1972;66:313.
12. Nyquist P. Management of acute intracranial and intraventricular hemorrhage. Crit Care Med. 2010;38(3):946-953.
13. Little J.R., Dial B., Bélanger G., et al. Brain hemorrhage from intracranial tumor. Stroke. 1979;10(3):283-288.
14. Ariesen M.J., Claus S.P., Rinkel G.J., et al. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003;34(8):2060-2065.
15. Iribarren C., Jacobs D.R., Sadler M., et al. Low total serum cholesterol and intracerebral hemorrhagic stroke: is the association confined to elderly men? The Kaiser Permanente Medical Care Program. Stroke. 1996;27(11):1993-1998.
16. Suh I., Jee S.H., Kim H.C., et al. Low serum cholesterol and haemorrhagic stroke in men: Korea Medical Insurance Corporation Study. Lancet. 2001;357(9260):922-925.
17. Furlan A.J., Whisnant J.P., Elveback L.R. The decreasing incidence of primary intracerebral hemorrhage: a population study. Ann Neurol. 1979;5:367-373.
18. Vinters H.V. Cerebral amyloid angiopathy: a critical review. Stroke. 1987;18:311-324.
19. Rost N.S., Greenberg S.M., Rosand J. The genetic architecture of intracerebral hemorrhage. Stroke. 2008;39:2166-2173.
20. Sacco S., Marini C., Toni D., et al. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. 2009;40:394.
21. O’Donnell H.C., Rosand J., Knudsen K.A., et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med. 2000;342(4):240-245.
22. Hart R.G. What causes intracerebral hemorrhage during warfarin therapy? Neurology. 2000;55:907-908.
23. Hart R.G., Boop B.S., Anderson D.C. Oral anticoagulants and intracranial hemorrhage. Facts and hypotheses. Stroke. 1995;26(8):1471-1477.
24. Franke C.L., de Jonge J., van Swieten J.C., et al. Intracerebral hematomas during anticoagulant treatment. Stroke. 1990;21(5):726-730.
25. Levine S.R., Brust J.C., Futrell N., et al. Cerebrovascular complications of the use of the “crack” form of alkaloidal cocaine. N Engl J Med. 1990;323:699-704.
26. Juvela S., Hillbom M., Palomäki H. Risk factors for spontaneous intracerebral hemorrhage. Stroke. 1995;26(9):1558-1564.
27. Juvela S., Porras M., Poussa K. Natural history of unruptured intracranial aneurysms: probability of and risk factors for aneurysm rupture. Neurosurg Focus. 8(5), 2000. Preview 1
28. Wiebers D.O. Unruptured intracranial aneurysms—risk of rupture and risks of surgical intervention. International Study of Unruptured Intracranial Aneurysms Investigators. N Engl J Med. 1998;339(24):1725-1733.
29. Wiebers D.O., Whisnant J.P., Huston J.3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110.
30. Peters P.J., Harrison T., Lennox J.L. A dangerous dilemma: management of infectious intracranial aneurysms complicating endocarditis. Lancet Infect Dis. 2006;6(11):742-748.
31. Brown R.D.Jr., Wiebers D.O., Torner J.C., et al. Frequency of intracranial hemorrhage as a presenting symptom and subtype analysis: a population-based study of intracranial vascular malformations in Olmsted County, Minnesota. J Neurosurg. 1996;85(1):29-32.
32. Brown R.D.Jr., Weibers D.O., Forbes G., et al. The natural history of unruptured intracranial arteriovenous malformations. J Neurosurg. 1998;68(3):352-357.
33. Vespa P.M., O’Phelan K., Shah M., et al. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60(9):1441-1446.
34. Broderick J.P., Adams H.P.Jr., Barsan W., et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 1999;30(4):905-915.
35. Brott T., Broderick J., Kothari R., et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke. 1997;28(1):1-5.
36. Kazui S., Naritomi H., Yamamoto H., et al. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke. 1996;27(10):1783-1787.
37. Broderick J., Sander C., Feldmann E., et al. Guidelines for the management of spontaneous intracerebral hemorrhage in adults: 2007 update. Stroke. 2007;38:2001-2023.
38. Hemphill J.C.III, Bonovich D.C., Besmertis L., et al. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32(4):891-897.
39. Diringer M.N., Edwards D. Admission to a neurologic/neurosurgical ICU is associated with reduced mortality after intracerebral hemorrhage. Crit Care Med. 2001;29:635-640.
40. Anderson C.S., Huang Y., Arima H., et al. Effects of early blood pressure-lowering treatment on growth of hematoma and perihematoma edema in acute intracerebral hemorrhage. Stroke. 2010;41:307-312.
41. Antihypertensive Treatment of Acute Intracerebral Hemorrhage Investigators. Antihypertensive treatment of acute intracerebral hemorrhage. Crit Care Med. 2010;38(2):637-648.
42. Mayer S.A., Brun N.C., Begtrup K., et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med. 2008;358(20):2127-2137.
43. Mendelow A.D., Gregson B.A., Fernandes H.M., et al. Early surgery versus conservative medical management in patients with spontaneous supratentorial intracerebral hemorrhage in the International Surgical Trial in Intracerebral Hemorrhage (STICH): a randomized trial. Lancet. 2005;365(9457):387-397.