CHAPTER 99 Spondylolisthesis: Medical Rehabilitation and Interventional Spine Techniques
INTRODUCTION
Patients presenting with lumbar spine complaints who have radiographs showing spondylolisthesis may experience painful or neurologic symptoms from a variety of clinical entities. The evaluation and treatment prescription should evolve from a biopsychosocial model that has been suggested for low back pain.1–8 Radiographic features, neuromuscular function, psychological, social factors and medical comorbities must be considered as a part of the overall disease when formulating the treatment plan. The most commonly used classification system for spondylolisthesis is based on Wiltse, Newman, and Macnab (Table 99.1).9
Table 99.1 The most commonly used classification system for spondylolisthesis
| Type 1 Dysplastic |
Based on Wiltse, Newman, and Macnab.9
PATHOANATOMIC CONSIDERATIONS
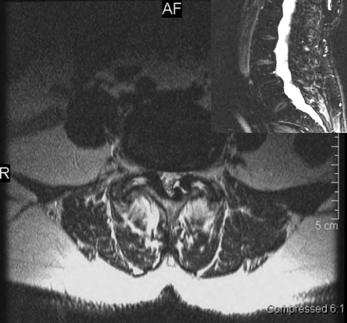
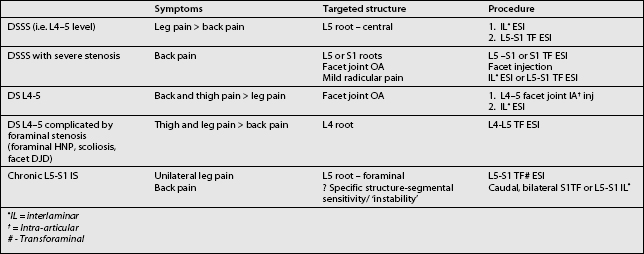
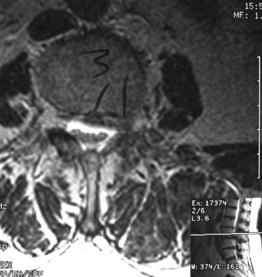
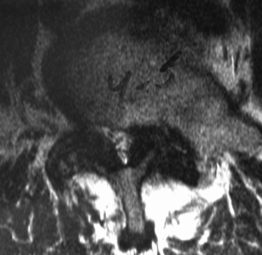
Degenerative spondylolisthesis (DS) is a segmental degenerative process characterized by facet joint arthrosis and segmental subluxation (Fig. 99.1).
Multiple causes may initiate the process of subluxation but facets with a sagittal alignment are probably an important contributing factor.10–12 As the posterior elements move forward with the vertebral body,13,14 the inferior articulating processes eventually can move forward enough to abut the superior endplate of the caudal vertebral body. This may explain why the degree of degenerative spondylolisthesis is usually only grade 1 or 2. Intervertebral disc space narrowing may occur simultaneously or later in the overall process.11,15 It remains unclear if the degree of subluxation has any correlation to the severity and presence of back pain or diability. Matsunaga et al. followed 145 patients with degenerative spondylolisthesis for a minimum of 10 years and observed no correlation between changes in clinical symptoms and progression of spondylolisthesis.16,17 The occurrence of increased translation or angular motion on flexion radiographs can be noted, although the relevance to different treatment outcomes is unclear
Those with DS may initially present with the insidious onset of low back pain with or without thigh pain that could be attributed to facet joint disease.16–19 Leg pain, which may occur in early stages, in the absence of significant spinal stenosis, is often quickly responsive to treatment. In the later stages both lateral and central spinal stenosis may develop secondary to zygapophyseal joint hypertrophy, ligamentum flavum buckling, and/or disc protrusion. Neurogenic claudication secondary to spinal stenosis causes referred pain into the buttocks and lower limbs while walking, and is relieved when the patient sits or bends forward. Degenerative spondylolisthesis with spinal stenosis represents a more severe anatomic disease.20–24 There is evidence that patients presenting with severe back and leg pain who have radiographic findings of severe central stenosis will respond poorly to medical rehabilitation and interventional spine treatment.25,26 In addition to symptoms caused by central stenosis, entrapment of nerve roots within the lateral recess and intervetebral foramen may cause radicular symptoms. For example, a superimposed foraminal disc protrusion may cause symptomatic L4 radiculopathy. A posterolateral disc protrusion at the L4–5 or asymmetric facet joint arthrosis may cause unilateral radiculopathies affecting the L5 nerve root.
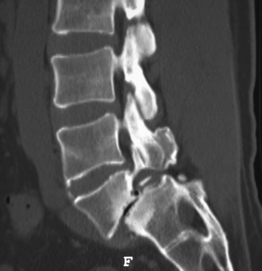
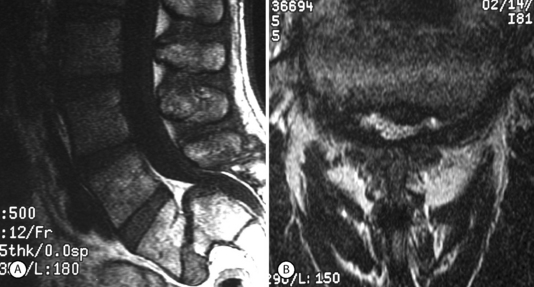
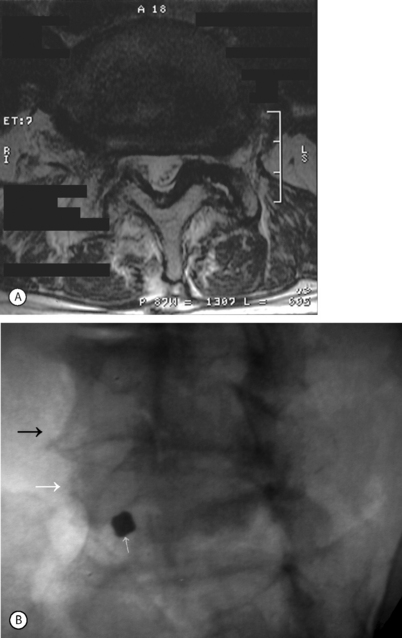
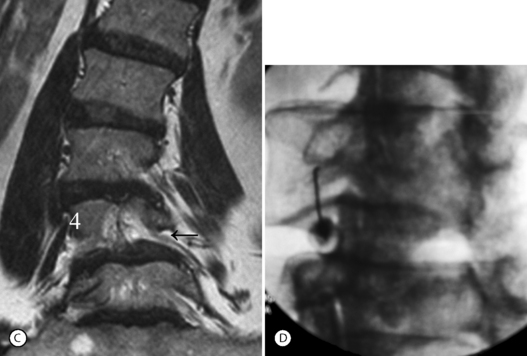
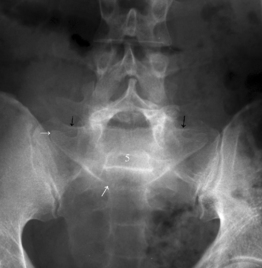
Isthmic spondylolisthesis (IS) is caused by a defect in the pars interarticularis which allows anterior translation to occur in the absence of facet joint arthrosis. The distortion of the foramina by the proximal pars stump may cause dynamic or static compression of the exiting nerve roots (Figs 99.2, 99.3).27–31
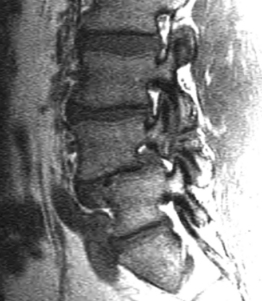
Although foraminal stenosis is common, central canal stenosis usually is absent in low-grade IS. In large measure, this is due to the fact that the most common level afflicted with IS is L5–S1; the most capacious portion of the spinal canal in the lumbar spine (Figs 99.4, 99.5). Clinical studies suggest that the amount of slip will not significantly progress in adult IS.32–38 It is also unclear whether isolated IS is a primary cause of chronic low back pain. A thorough evaluation of other causes of low back pain should be conducted in all patients who present with low back pain and IS.39,40 Recalcitrant episodes of unilateral leg pain, or back pain associated with leg or thigh radiation, may be attributable to foraminal radiculopathy. Symptoms unresponsive to oral medications and physical therapy will often respond to transforaminal epidural steroid injections.
MEDICAL REHABILITATION AND INTERVENTIONAL SPINE PROGRAM
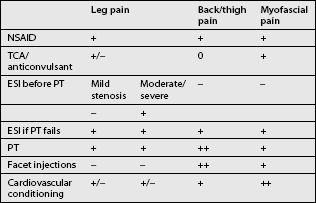
A step-wise approach to treatment can be divided conceptually into three independent but overlapping phases: pain control, stabilization, and a conditioning phase. Treatment interventions can include oral medications, behavioral modifications, physical therapeutics and therapeutic exercise, bracing, and a variety of injection procedures. Therapeutic decisions should be made on the basis of the clinical and radiographic evaluation. Categorizing patient complaints into back pain, leg pain, and myofascial pain can help organize treatment options (Table 99.2). Leg pain not directly attributable to radiculopathy may be caused by somatic referral, hip bursitis, osteoarthritis of the hip or knee, or myofascial pain.41
Oral medications
Medications for the treatment of various forms of lumbar spondylolisthesis have not been studied. In a large meta-analysis of low back pain,42 there is good evidence supporting the use of nonsteroidal antiinflammatory medications and acetaminophen in the treatment of acute pain, and fair evidence for the use of muscle relaxants. Using these medications may help allow the patient to continue in a physical therapy program. The efficacy of drug therapy for chronic low back pain is less clear. Current controversy surrounds the use of narcotic medication for chronic low back pain.43–46 Patients with significant radiculopathy can be treated with a course of oral corticosteroids and nonsteroidal antiinflammatory medication for 1 week. Although unproven, NSAIDs may be more effective in the treatment of DS because of the presence of facet arthrosis.
Anticonvulsant and antidepressant medications should be also be considered for all patients with myofascial pain and for patients presenting with dysesthetic leg pain, especially if the leg pain is nocturnal. Gabapentin has become one of the off-label anticonvulsant drugs most commonly prescribed in the treatment of spinal pain, and although the mechanism is unknown, a recent study suggests that gabapentin may be useful in the treatment of patients with neuropathic or myofascial pain.25 A newer variation of gabapentin, pregabelin, may prove to be an even more effective agent. Currently, it is approved in the US for the treatment of neuropathic pain developing from postherpetic neuralgia or diabetic peripheral neuropathy. The mechanism of pain relief for tricyclic medications may be independent from their effect on depression. The positive effects seem to depend on two mechanisms: the blockade of norepinephrine uptake, and its antagonist action on the N-methyl D-aspartate receptor.8, 22, 27
Physical therapy
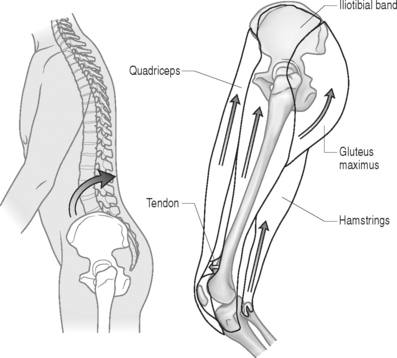
The initial step in prescribing a physical therapy program depends upon a detailed musculoskeletal assessment. Evaluation of biomechanical relationships is an integral part of this process. Patients who present with concomitant osteoarthritis of the lower extremities (hip, knee, ankle), causing restricted and painful joint range of motion (ROM), will require special consideration. Lower extremity flexibility should be assessed in all patients. Short hamstring and iliopsoas muscles may increase the stress on lumbar lordosis and the lumbopelvic motion relationship. Patients with significant restrictions may greatly benefit from physical therapeutic stretching. A kyphotic posture, adopted to reduce pincer effect of lordosis in spinal stenosis, will secondarily lead to shortening of the hip flexors (Fig. 99.6). Chronically shortened hip flexors will aggravate the condition by forcing the patient to effectively increase lordosis when attempting to stand erect.
Studies on low back pain suggest that strong secondary psychologic benefits are derived from positive self-efficacy concepts, and confrontational rather than avoidance behavioral strategies.9, 19, 32 These strategies are reinforced by the reasonable return to activity program and independent daily exercise program. The benefits of this positive health belief system may be more important than the physiologic counterpart of exercise. Despite this fact, many patients who do not tolerate the transition to exercise may receive benefit from limited passive treatments such as therapeutic modalities of heat, ultrasound, and soft tissue mobilization.
When a significant amelioration of painful symptoms occurs, a vigorous cardiovascular conditioning exercise program is typically introduced. While maintaining flexibility and some degree of trunk strength through a program of daily exercise seems to make sense, only fitness has been implied as preventive for lower back pain.1 Patients may be advised to partake in regular and slowly progressive amounts of safe aerobic exercise (exercise bicycle, swimming pool exercise). For cardiovascular conditioning, the authors have found that the upright stationary bicycle is well tolerated. Other patients with a more extensive history of back pain may perform better on recumbent bicycle. Patients are encouraged to increase their tolerance to treadmill in order to develop upright endurance. More functionally advanced patients may be able to use stair climbers or a stairmaster, but these activities present more obvious difficulties to many elderly patients whose balance and coordination may be affected or whom may have hip or knee osteoarthritis. Most patients will be able to exercise on the elliptical machines that build lower extremity endurance and balance without the same degree of axial impact loading.47 Many patients with lytic or degenerative spondylolisthesis of varying severity may be able to successfully return to most sport activities. High-grade spondylolisthesis should be a contraindication to participation in contact sports. Patients can be encouraged to perform exercise and noncontact sports to tolerance. Many can engage in running and tennis even after having experienced significant episodes of radiculopathy (Fig. 99.7).
The hypothetical rationale for assessment-based physical therapy requires an understanding of the pathoanatomic process associated with each type of spondylolisthesis. For example, in DSSS, a flexion-biased movement and exercise program is preferable. There are relatively few specific studies confirming the outcome effects of specific therapy.15,47
Sinaki et al.15 reported on the outcomes of 48 patients with symptomatic back pain secondary to spondylolisthesis treated conservatively for 3 years. They compared the outcomes of flexion-biased and extension back strengthening exercise programs. The patients were divided into two groups: those performing flexion, and those performing extension back strengthening exercises. Flexion exercise consisted of partial sit-ups, pelvic tilts, and chest-to-thigh exercises. Extension exercises consisted of upper back extension from the prone position and hip extension from the prone position. At 3 years, overall recovery rate was 62% for the flexion group and 0% for the extension group. O’Sullivan et al.47 prospectively compared the effect of a spine stabilization exercise program with non-specific exercise therapy in 44 patients with spondylolisthesis or spondylolysis. Subjects were randomly assigned to one of the two treatments. At 30 months, the stabilization exercise group experienced significant benefits while those in the non-specific exercise therapy group did not. While the majority of the patients, whose average age was 30 years, appeared to experience chronic low back pain it is unclear how the authors established that symptoms were specifically related to chronic IS.
Bracing
Bracing may play a role in early protection of a painful segment while the patient begins a comprehensive stabilization program. In addition to support of the area, both the warmth and proprioception48 gained by the brace may lead to patient comfort. Bracing may be the best option, particularly for debilitated patients when physical therapy would be either difficult or simply pose a health risk. The role of bracing has been studied most extensively in acute IS presenting in young or adolescent patients and is discussed in detail by Frank Lagatutta (see Ch. 129). There is little or no literature investigating the clinical effect of bracing in the conservative management of degenerative spondylolisthesis or spinal stenosis.49 Some authors have advocated a 4–6 week trial of brace management. We have found that soft or semirigid lumbar supportive braces are sufficient for patient comfort and ease of use. Braces will generally be tolerated better by thin than obese patients. Many patients with uncomplicated spondylolisthesis may find that a properly fit spinal orthotic provides comfort and pain relief. Bracing may be indispensable in patients suffering with spondylolisthesis complicated by additional spinal alignment and mechanical problems such as scoliosis, uncompensated scoliosis, lateral listhesis, or hyperlordosisis. The most important aspect of bracing is whether the patient will actually use the device. The authors’ experience is that patients rarely tolerate rigid bracing, whether custom made or the soft spinal variety. Since these orthotics are costly, a realistic appraisal of probable usage should be undertaken prior to providing a prescription for their purchase.
INTERVENTIONAL SPINE TECHNIQUES FOR SPONDYLOLISTHESIS
Rationale for use: hypothetical mechanisms
Incorporating spinal injection procedures into the rehabilitation program of patients with spondylolisthesis can be an effective tool. When other medical rehabilitation fails (e.g. physical therapy, oral medications) interventional treatment is well recognized as a potent therapeutic aide. As with the development of the medical rehabilitation component of treatment the anticipated success of an injection procedure will depend upon a careful clinical and radiographic assessment of the patient. Ultimately, this allows the spine clinician to determine which spinal structures are pertinent to symptomatic complaints, choosing a logical sequence of treatment interventions, and proper execution of what often are technically demanding procedures (Table 99.3). A detailed evaluation of this nature will maximize the potential benefit of this therapeutic modality. The extent to which it is used in specific clinical scenarios is a matter of greater controversy and the subject of ongoing investigations. Their effectiveness in maintaining reasonable outcomes in patients with more severe presentations of disease who are otherwise healthy candidates for surgery is one area of debate, whereas treatment is more widely supported for mild to moderate disease.24,50
There is a hypothetical rationale for the use of a spinal injection using glucocorticoids. The pathophysiology explanation for this hormonal therapy stems from evidence that biochemical and neurochemical inflammatory mediators play a role in the occurrence of lumbar radiculopathy.51–57 Inflammatory mediators may also play a role in the pathophysiology of some low back pain when factors including facet joint degenerative arthritis and lumbar radiculopathy are at play.58,59 As previously stated, one should remember that the severity of symptoms is unrelated to the pathologic abnormalities observed with imaging studies. As well, radiographic studies of degenerative spondylolisthesis and spinal stenosis have revealed that radiographic severity imperfectly correlates with symptoms.60–62
Scientific data supporting the use of injections in spondylolisthesis
Several reports are available reporting on outcomes of lumbar spinal stenosis treated with epidural steroids.26,63–68 None of these has contained sufficient data to analyze an effect on subgroups with spondylolisthesis. No data are available on the effect of lumbar facet injections and DS. Facet joint injections have questionable efficacy when used on facet joint low back pain in general when defined by response to anesthetic facet block.69–74 Despite this fact, the case for use of facet injections in degenerative spondylolisthesis is intuitively apparent because of the specific aberrant pathomechanics of the disease.13,19
The beneficial effect of epidural steroid injections on patients with DSSS is suggested by several studies assessing those patients with symptoms of lumbar spinal stenosis. Botwin et al. prospectively evaluated 34 stenosis patients for 1 year treated with transforaminal epidural steroid injections.63 Seventy-five percent of patients had successful long-term outcome, reporting at least a >50% reduction between preinjection and postinjection pain scores. Numerous other medical rehabilitation and interventional spine outcome studies for lumbar spinal stenosis have been published.2, 16, 18, 21, 31 In 1992, Johnsson et al.75 reported on 32 patients followed up for an average of 49 months (range10–103 months). Fifteen percent of the patients were improved, 70% were the same, and 15% were worse. In that study, the authors did not specify if patients received epidural steroid injection.
In 1996, Atlas and colleagues,64 at 12 months, assessed the outcomes of 81 patients who were treated surgically and 67 patients who were conservatively treated. Although the conditions of those who underwent surgery were clinically and radiographically more severe than in the conservative care group, their outcomes were superior. Only 28% of patients who were treated conservatively reported that their pretreatment symptoms of pain were better or totally abated and for 15% the pain was much worse. An important point to make is that only 18% of patients in the conservative care group were treated with epidural steroid injections. Perhaps a careful strategy of oral medications, specific physical therapy, and injection techniques that are predicated on the pathology, history and exam would provide better outcomes. Certainly, that is the authors’ experience, but it remains an unproven perspective until further scientific inquiry is undertaken.
In 1996, Swezey68 reported on the outcomes of 47 patients who had been evaluated 5 years earlier for lumbar spinal stenosis. Patients had symptoms of neurogenic claudication, and CT or MRI findings of moderate to severe stenosis (43 patients) or severe spondylosis by plain radiographs (4 patients). Treatments included education regarding ergonomics and flexion exercise, analgesic medications, intermittent pelvic traction (11 patients), and epidural steroids (13 patients). Eleven patients required laminectomy. Of the patients who were treated conservatively, 43% were improved, while the symptoms of neurogenic claudication were unchanged in 30%.
The current authors studied outcomes of 49 patients with stenosis with an average 33 month follow-up (range 16–55) treated with aggressive medical rehabilitation and interventional spine care.26 Eighty-eight percent received an epidural steroid injection. At a mean follow-up time of 33 months, 9 required surgery, 5 were worse, 12 unchanged, 11 mild, and 12 sustained improvement. Thirty percent of patients felt none or mild overall pain. Twenty-two of the 49 studied patients with stenosis also had DSSS. These 22 patients tended to have worse outcomes, but without reaching statistical significance (e.g. 6 of the 22 with DSSS required surgery).
Sequence and timing considerations
Epidural steroid injection is generally indicated when other conservative therapies have failed to satisfactorily control pain, or if the intensity is such that engaging in a therapy program is not feasible. While some clinicians have advocated an administration of three epidural injections, usually spaced 1–2 weeks apart, there are no current data to support that this strategy is more effective than a wait and see approach after one initial injection. Additionally, most clinicians perform spinal injections with radiographic control.76,77 The use of fluoroscopy confirms the delivery of medication to the targeted spinal level. Such precision is a special concern for patients with spondylolisthesis. Foraminal stenosis and facet joint arthopathy, common features of the disease, are probably best treated by transforaminal and intra-articular injections which can only be performed correctly with the assistance of radiographic guidance. Most patients with severe symptoms will require more than one injection intervention. A second injection can be repeated within 1–4 weeks. A sufficient interval should transpire to allow the clinician to judge the patient response. In the event that there is no benefit realized following the first injection, a different injection should be considered. For partial responses, a second injection performed within a month of the first may help to solidify gains. Most patients are unlikely to benefit from three or more fluoroscopically confirmed epidural injections during the course of 6–12 months.
Integrating injections with overall management
Integrating different levels and stages of treatment into an overall plan is critical to the success of medial rehabilitation and interventional spine treatment for symptomatic spondylolisthesis. Usually, patients present with a mixture of back, thigh, and leg complaints. As stated throughout this chapter, a thorough study of the history, examination, and radiologic findings is essential to achieving success. Unfortunately, even though that process is undertaken, the determination of which injection to perform, where in the spine, and when to do it represents an art form. Although one conceptualizes the process in a methodical way, there are no studies that provide the necessary guidance. As a typical example, a patient suffering with degenerative spondylolisthesis may have excellent relief of leg pain by the administration of a well-placed epidural steroid injection or selective nerve root block. Residual back pain, presumably related to facet joint arthropathy and subluxation may require augmenting therapy later on with fluoroscopically guided facet injections or flexion-biased trunk stabilizing exercise programs and hip flexor range of motion. Some patients with residual back and thigh pain and additional scoliosis deformity may require a period of spinal bracing. In others, the pain may be a consequence of disc pathology, and an interlaminar injection or transforaminal epidural may be needed.
INTERVENTIONAL PROCEDURES
Trigger point injections
Trigger point injections have been widely used by clinicians over the years for problems of lower back pain and sciatica alike. Their widespread use suggests there is some basis for their efficacy but there are no scientific data to support this belief.42 Patients with spondylolisthesis may present with clinical examination findings or exaggerated tenderness in paraspinal, gluteal, or piriformis muscles. Injections to these tender areas may reach the muscles or to some underlying structures such as facet joint arthropathy, trochanteric or gluteal bursitis, and sciatic nerve. Relief may come from resolution of muscle tenderness and spasm, or (when corticosteroid is included in the injection) treatment of the underlying structure, or both. These typical areas of tenderness may be seen in some patients with spondylolisthesis and may be considered as part of the clinical presentation. It is unclear whether these symptoms will resolve with specific spinal injections or whether they require additional therapy. In the authors’ view, it is reasonable to use trigger point therapy (usually with corticosteroid and local anesthetic) on one or two occasions in such patients before proceeding to the more invasive spinal injection procedures.
Epidural steroid injections
Rationale
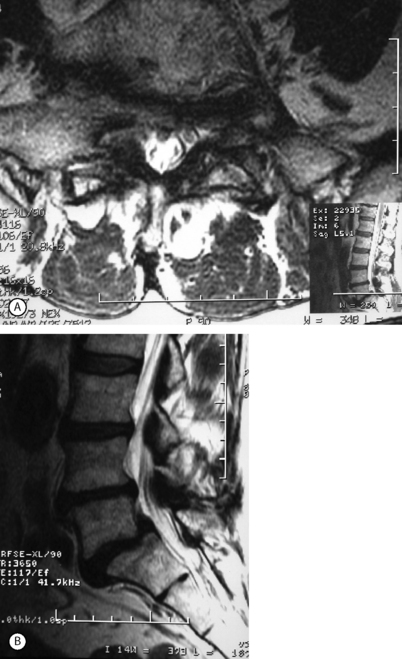
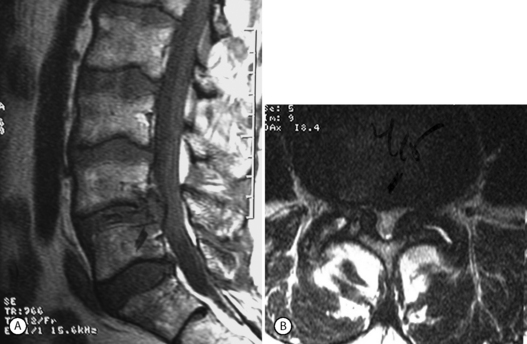
Epidural steroids in spondylolisthesis are indicated for recalcitrant leg pain that can be attributed to radiculopathy. They may also be used in patients with recalcitrant back and/or thigh pain. Many such patients will respond favorably to epidural injection, presumably because these symptoms are caused by a mild form of radiculopathy. Many, but not all, patients with degenerative spondylolisthesis will develop central spinal stenosis, especially in later stages of the disease.16, 18, 19 Usually, the most severe stenosis will occur at the listhesis level. Normally such patients will respond more favorably to injection targeting the most severely compressed nerve roots, and the rationale for the therapy is the same as has been noted for lumbar stenosis. Central stenosis seems to carry a worse outcome than isolated foraminal stenosis.78 The transiting root will usually be more diseased than the exiting (foraminal root) (Fig. 99.8). The initial injection should target this level.
Transforaminal injections
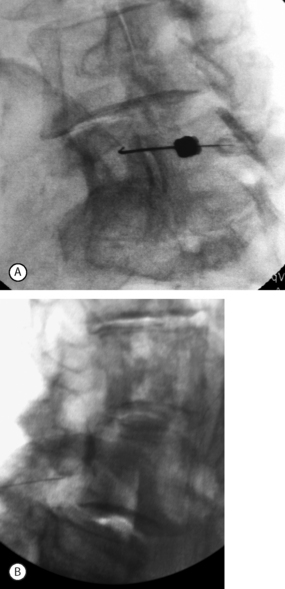
In the case of degenerative L4–5 spondylolisthesis, spinal stenosis is often present more dramatically at the level of listhesis than other spinal levels. As a result, the central canal tapers at this level. Interlaminar injection at L4–5 may be difficult to perform if there is severe segmental stenosis. Injections placed above and below this tend to diffuse away from the level of stenosis. The transforaminal epidural offers the most direct method of injection. The traversing L5 root is best approached by performing an L5–S1 transforaminal epidural (on the most severely affected side). Needle placement just under the L5 pedicle will allow medication flow first to the L5–S1 foramen and L4–5 lateral recess before proceeding into the remainder of the L4–5 epidural space (Fig. 99.9). Patients with bilateral leg pain may be treated with bilateral transforaminal injections. Alternatively, the physician may treat the more severely affected side and hope that some contralateral spread will occur, as it often does.
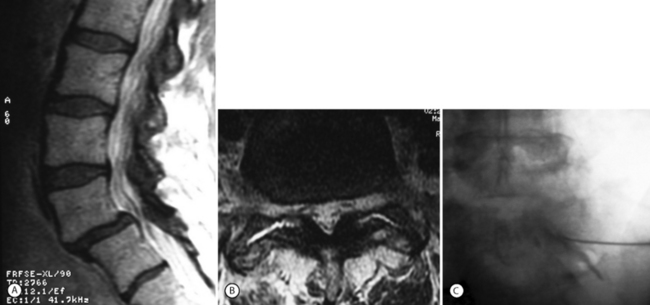
L4 radiculopathies can occur in L4–5 spondylolisthesis. Although less common, foraminal radiculopathies in spondylolisthesis often occur when there is superimposed scoliosis, severe facet arthropathy, or foraminal disc herniation (Figs 99.10, 99.11).
Some physicians shy away from performing an epidural injection at the most severely affected level and shift the injection down one level hoping to ‘float’ the medication upwards in a more gentle fashion. This may be a reasonable approach. In such a circumstance, an S1 transforaminal epidural would be used to treat an L4–5 DSSS (Fig. 99.12). Concerns with performing the L5–S1 transforaminal epidural include creating too much procedural pain, further sensitizing a severely compressed and inflamed nerve root, and the risk of causing further compression injury incurred by injecting a severely compressed level. These concerns are reasonable, but there are no available reports to support their validity. The degree of L4–5 stenosis and radiculopathy can be severe enough to effect both L5 and S1 roots, such that injections from the S1 transforaminal approach may be equally or more effective than the L5–S1 transforaminal (Fig. 99.13).
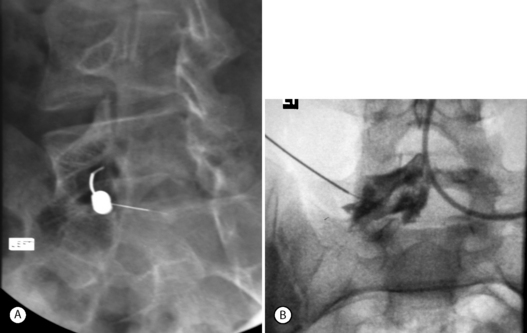
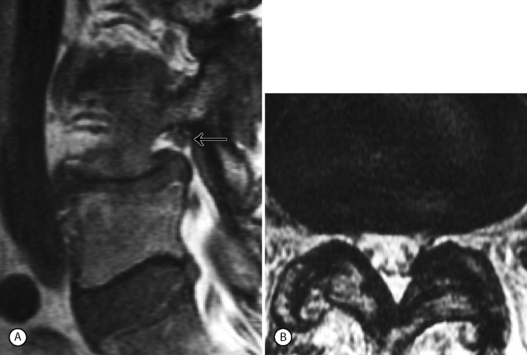
Foraminal stenosis such as that noted with lytic spondylolisthesis (IS) is also best treated by transforaminal epidural steroid injection. As in the case of L5–S1 IS, the exiting (L5) nerve root becomes entrapped in the intervertebral foramen by the pars lysis, and the onslaught of vertical (up–down) narrowing in the foramen. Performing a L5–S1 transforaminal epidural, particularly in cases of grade 2 and above spondylolisthesis, can be technically demanding. The sacral lordosis is usually significant and the sacrum has a high horizontal inclination. The L5 vertebra pitches forward and horizontal, deeper within the pelvis. The oblique needle approach is often blocked by the narrow walls of the iliac crest and posterior spine elements which are bunched up by the lordosis (Fig. 99.14). One possible solution is to use a coaxial double-needle technique with double bending. In this case the first spinal needle (usually 20-gauge, 3.5 in) is bent at its tip. The second needle (25-gauge, 6 in) is curved more dramatically than the first, such as to afford the most acute 90° turn of the needle upon passing deep to the obstructing posterior elements. This allows a passage to the deeper-seated L5–S1 foramen (Fig. 99.15).
Interlaminar and caudal epidural injections
Some clinicians will routinely use caudal or interlaminar injection as the initial procedure. The caudal approach is safe, usually easy to perform, and small needle manipulations will insure a bilateral spread of injection. Observation of contrast penetration suggests most of the injection will spread onto the L5 and sacral roots, but doubtfully above this level. In spite of this limitation, many clinicians will employ the caudal technique for stenosis problems up to the L3–4 level.
Outcome studies
There are limited scientific data available regarding the efficacy of medical rehabilitation and interventional spine treatment for lumbar spinal stenosis.26,63,64,66,79,80 The extent to which such information can be applied to patients with spondylolisthesis is unclear. Patients with spondylolisthesis and stenosis may have better or worse outcomes than patients with stenosis alone. None of the available studies regarding spinal stenosis explicitly identify the occurrence of spondylolisthesis, nor do they contain significant numbers of cases to study outcome efficacy.
Facet joint injection
Rational
The rationale for using facet injections in the treatment of patients with spondylolisthesis emerges from several observations. The degenerative deterioration of the facet joint that occurs with the progression of degenerative spondylolisthesis suggests it has a central role in pain and inflammation, prima facie. Facet joints are a primary restraint for anterior listhesis so they are presumable receiving accentuated stress and shear forces. Patients with degenerative spondylolisthesis often present in the earlier phases of disease with symptoms of back and thigh pain, which is a proven pain referral pattern of the lumbar facet joints.16, 18, 19
Radiographically guided intra-articular facet injection may be the injection treatment of choice in patients with lumbar degenerative spondylolisthesis presenting with back, buttock, and thigh pain or combinations thereof. Facet injections for these patients, especially those presenting without significant concomitant stenosis, may be more effective than epidural steroid injection. Nonetheless, may clinicians may opt to use the epidural steroid as the initial procedure. A reason underpinning this decision is the overall greater scientific evidence for their efficacy than for facet injection in patients with back pain and sciatica.69, 71, 74 A second reason is that the facet joint injection into a degenerated structure is more tedious and technically demanding to perform.
Choosing levels
Scientific data supporting the use of facet injections for spondylolisthesis are surmised from clinical outcome studies regarding the efficacy of facet injections for non-specific low back pain.69, 71, 74 There is also speculation as to whether radiographic findings such as facet joint arthropathy can be used to identify ‘facet joint pain.’73,81,82 The authors are not aware of any study with sufficient numbers of patients to specifically identify efficacy of this intervention for spondylolisthesis. At best they can postulate that the pathomechanics of DS and the increased stress to posterior elements of the spine suggest that facet injections may be effective.
The efficacy of facet injections in patients with IS is somewhat more dubious. In such cases, transmission of load forces across the neural arch is interrupted by the pars lysis. Usually, the facet joint below the lysis will appear on radiographs not to have undergone any degenerative change. Some clinicians have suggested injection of the pars defect as a diagnostic or therapeutic tool for patients with chronic low back pain. The rationale is unclear since chronic low-grade lytic spondylolisthesis does not always result in chronic low back pain and in those cases where it is imputed as the cause, it is less than clear that the pars region is a focal pain generator. It has been substantiated that the fibrous union of a pars interarticlaris fracture contains neural elements.83 Some spine interventionists attempted to perform diagnostic pars interarticualris fracture injections. In one unreported series of 50 cases, not a single diagnostic injection, using 0.5 cc of 2% xylocaine, was reported as positive (Slipman C. Unpublished).
SUMMARY
Patients presenting with back pain with or without leg pain and radiographic evidence of spondylolisthesis represent a spectrum of different clinical entities with potentially different associated outcomes. The radiographic assessment of spondylolisthesis does not automatically determine outcome or treatment. Radiographic assessment is more involved than simply identifying the presence or severity of spondylolisthesis. Specifically, DS, DSSS, and IS appear to have a different pathoanatomy and clinical course. Other radiographic variables that must be considered include sagittal and coronal plane deformity, and the extent of spondylotic changes. Most importantly, the proper diagnosis and characterization of the patient’s condition involves a synthesis of clinical and radiographic information. A clinical evaluation of the patient history of pain and disability, history of medical comorbities, and psychologic and social factors are extremely important. Additionally, physical examination findings of neuromuscular status, flexibility, soft tissue sensitivity, and general fitness also should be conducted. The implementation of medical rehabilitation and interventional spine therapeutic strategies requires the symptoms to be conceptually organized into categories of back, buttock, thigh, leg, or pain. The combination of symptom location and provocative influences aid in determining the relative contribution of facet, disc, root or myofascial pain. Using this paradigm enables the spine clinician to devise an individualized conservative care program. Similarly, such a process prevents the repeated institution of failed conservative measures when surgical treatment is required.
1 Karjalainen K, Malmivaara A, van Tulder M, et al. Multidisciplinary biopsychosocial rehabilitation for subacute low back pain among working age adults. Cochrane Database Syst Rev. 2000. CD002193
2 Schultz IZ, Crook JM, Berkowitz J, et al. Biopsychosocial multivariate predictive model of occupational low back disability. Spine. 2002;27(23):2720-2725.
3 Wand BM, Bird C, McAuley JH, et al. Early intervention for the management of acute low back pain: a single-blind randomized controlled trial of biopsychosocial education, manual therapy, and exercise. Spine. 2004;29(21):2350-2356.
4 Seers K. Review: intensive multidisciplinary biopsychosocial rehabilitation reduces pain and improves function in chronic low back pain. Evid Based Nurs. 2002;5(4):116.
5 Nielson WR, Weir R. Biopsychosocial approaches to the treatment of chronic pain. Clin J Pain. 2001;17(4 Suppl):S114-S127.
6 Truchon M. Determinants of chronic disability related to low back pain: towards an integrative biopsychosocial model. Disabil Rehabil. 2001;23(17):758-767.
7 Karjalainen K, Malmivaara A, van Tulder M, et al. Multidisciplinary biopsychosocial rehabilitation for subacute low back pain in working-age adults: a systematic review within the framework of the Cochrane Collaboration Back Review Group. Spine. 2001;26(3):262-269.
8 Waddell G. Biopsychosocial analysis of low back pain. Baillières Clin Rheumatol. 1992;6(3):523-557.
9 Wiltse LL, Newman PH, Macnab I. Classification of spondylolysis and spondylolisthesis. Clin Orthop Relat Res. 1976;117:23-29.
10 Berlemann U, Jeszenszky DJ, Buhler DW, et al. Facet joint remodeling in degenerative spondylolisthesis: an investigation of joint orientation and tropism. Eur Spine J. 1998;7(5):376-380.
11 Berlemann U, Jeszenszky DJ, Buhler, et al. The role of lumbar lordosis, vertebral end-plate inclination, disc height, and facet orientation in degenerative spondylolisthesis. J Spinal Disord. 1999;12(1):68-73.
12 Cinotti G, Postacchini F, Fassari F, et al. Predisposing factors in degenerative spondylolisthesis. A radiographic and CT study. In Orthop. 1997;21(5):337-342.
13 Farfan HF. The pathological anatomy of degenerative spondylolisthesis. A cadaver study. Spine. 1980;5(5):412-418.
14 Kirkaldy-Willis WH, Wedge JH, Yong-Hing K, et al. Pathology and pathogenesis of lumbar spondylosis and stenosis. Spine. 1978;3(4):319-328.
15 Sinaki M, Lutness MP, Ilstrup DM, et al. Lumbar spondylolisthesis: retrospective comparison and three-year follow-up of two conservative treatment programs. Arch Phys Med Rehabil. 1989;70(8):594-598.
16 Matsunaga S, Sakou T, Morizono Y, et al. Natural history of degenerative spondylolisthesis. Pathogenesis and natural course of the slippage. Spine. 1990;15(11):1204-1210.
17 Matsunaga S, Ijiri K, Hayashi K. Nonsurgically managed patients with degenerative spondylolisthesis: a 10- to 18-year follow-up study. J Neurosurg. 2000;93(2 Suppl):194-198.
18 Frymoyer JW. Degenerative spondylolisthesis: diagnosis and treatment. J Am Acad Orthop Surg. 1994;2(1):9-15.
19 Newman PH. Degenerative spondylolisthesis. J Bone Joint Surg. 1976;58(2):184-192.
20 Bassewitz H, Herkowitz H. Lumbar stenosis with spondylolisthesis: current concepts of surgical treatment. Clin Orthop. 2001;384:54-60.
21 Grobler LJ, Robertson PA, Novotny JE, et al. Decompression for degenerative spondylolisthesis and spinal stenosis at L4–5. The effects on facet joint morphology. Spine. 1993;18(11):1475-1482.
22 Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg [Am]. 1991;73(6):802-808.
23 Rosenberg NJ. Degenerative spondylolisthesis: surgical treatment. Clin Orthop. 1976;117:112-120.
24 Wiltse LL, Kirkaldy-Willis WH, McIvor GW. The treatment of spinal stenosis. Clin Orthop. 1976;115:83-91.
25 Du Priest CM. Nonoperative management of lumbar spinal stenosis. J Manip Phys Ther. 1993:411-414.
26 Simotas AC, Dorey FJ, Hansraj KK, et al. Nonoperative treatment for lumbar spinal stenosis. Clinical and outcome results and a 3-year survivorship analysis. Spine. 2000;25(2):197-203.
27 Johnson AC, Power TC. Combined neurosurgical–orthopedic approach to the correction of radiculopathy and instability in spondylolysis and spondylolisthesis. Int Surg. 1984;69(4):345-350.
28 Rosomoff HL. Lumbar spondylolisthesis: etiology of radiculopathy and role of the neurosurgeon. Clin Neurosurg. 1980;27:577-590.
29 Stears JC. Radiculopathy in spondylosis/spondylolisthesis. The laminar/ligamentum flavum process. Acta Radiol Suppl. 1986;369:723-726.
30 Saifuddin A, Burnett SJ. The value of lumbar spine MRI in the assessment of the pars interarticularis. Clin Radiol. 1997;52(9):666-671.
31 Virta L, Osterman K. Radiographic correlations in adult symptomatic spondylolisthesis: a long-term follow-up study. J Spinal Disord. 1994;7(1):41-48.
32 Lonstein JE. Spondylolisthesis in children. Cause, natural history, and management. Spine. 1999;24(24):2640-2648.
33 Danielson BI, Frennered AK, Irstam LK. Radiologic progression of isthmic lumbar spondylolisthesis in young patients. Spine. 1991;16(4):422-425.
34 Saraste H, Brostrom LA, Aparisi T. Prognostic radiographic aspects of spondylolisthesis. Acta Radiol Diagn (Stockh). 1984;25(5):427-432.
35 Saraste H. Spondylolysis and spondylolisthesis. Acta Orthop Scand Suppl. 1993:84-86.
36 Seitsalo S, Osterman K, Poussa M, et al. Spondylolisthesis in children under 12 years of age: long-term results of 56 patients treated conservatively or operatively. J Pediatr Orthop. 1988;8(5):516-521.
37 Seitsalo S. Operative and conservative treatment of moderate spondylolisthesis in young patients. J Bone Joint Surg [Br]. 1990:908-913.
38 Seitsalo S, Osterman K, Hyvarinen H, et al. Progression of spondylolisthesis in children and adolescents. A long-term follow-up of 272 patients. Spine. 1991;16(4):417-421.
39 Andersson GB. What are the age-related changes in the spine? Baillières Clin Rheumatol. 1998;12(1):161-173.
40 Ventura JM, Justice BD. Need for multiple diagnosis in the presence of spondylolisthesis. J Manipulative Physiol Ther. 1988;11(1):41-42.
41 Spivak JM. Current concepts review: degenerative lumbar spinal stenosis. J Bone Joint Surg [Am]. 1998;80(7):1053-1066.
42 Deyo RA. Drug therapy for back pain. Which drugs help which patients? Spine. 1996;21(24):2840-2849.
43 Bartleson JD. Evidence for and against the use of opioid analgesics for chronic nonmalignant low back pain: a review. Pain Med. 2002;3(3):260-271.
44 Breckenridge J, Clark JD. Patient characteristics associated with opioid versus nonsteroidal antiinflammatory drug management of chronic low back pain. J Pain. 2003;4(6):344-350.
45 Mullican WS, Lacy JR. Tramadol/acetaminophen combination tablets and codeine/acetaminophen combination capsules for the management of chronic pain: a comparative trial. Clin Ther. 2001;23(9):1429-1445.
46 van Tulder MW, Koes BW, Bouter LM, et al. Management of chronic nonspecific low back pain in primary care: a descriptive study. Spine. 1997;22(1):76-82.
47 O’Sullivan PB, Phyty GD, Twomey LT, et al. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine. 1997:2959-2967.
48 McNair PJ, Heine PJ. Trunk proprioception: enhancement through lumbar bracing. Arch Phys Med Rehabil. 1999;80(1):96-99.
49 Prateepavanich P, Thanapipatsiri S, Santisatisakul P, et al. The effectiveness of lumbosacral corset in symptomatic degenerative lumbar spinal stenosis. J Med Assoc Thai. 2001;84(4):572-576.
50 Kirkaldy-Willis WH, Burton CV. Managing low back pain. New York: Churchill Livingstone, 1992;254-256.
51 Burke JG, Conhyea D, McCormack D, et al. Human nucleus pulposus can respond to a pro-inflammatory stimulus. Spine. 2003;28(24):2685-2693.
52 Habtemariam A, Virri J, Gronblad M, et al. The role of mast cells in disc herniation inflammation. Spine. 1999;24(15):1516-1520.
53 Kang JD, Georgescu HI, McIntyre-Larkin L, et al. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21(3):271-277.
54 Miyamoto H, Saura R, Harada T, et al. The role of cyclooxygenase-2 and inflammatory cytokines in pain induction of herniated lumbar intervertebral disc. Kobe J Med Sci. 2000;46(1–2):13-28.
55 Claman HN. Corticosteroids and lymphoid cells. N Engl J Med. 1972;287(8):388-397.
56 Rinehart JJ, Sagone AL, Balcerzak SP, et al. Effects of corticosteroid therapy on human monocyte function. N Engl J Med. 1975;292(5):236-241.
57 Weinstein SM, Herring SA, Derby R. Contemporary concepts in spine care. Epidural steroid injections. Spine. 1995;20(16):1842-1846.
58 Igarashi A, Kikuchi S, Konno S, et al. Inflammatory cytokines released from the facet joint tissue in degenerative lumbar spinal disorders. Spine. 2004;29(19):2091-2095.
59 Willburger RE, Wittenberg RH. Prostaglandin release from lumbar disc and facet joint tissue. Spine. 1994;19(18):2068-2070.
60 Boden S, Davis D, Dina T, et al. Abnormal magnetic resonance scans of the lumbar spine in asymptomatic subjects. J Bone Joint Surg. 1990;72(3):403-408.
61 Herno A, Airaksinen O, Saari T. Computed tomography after laminectomy for lumbar spinal stenosis. Patients’ pain patterns, walking capacity, and subjective disability had no correlation with computed tomography findings. Spine. 1994;19(17):1975-1978.
62 Herno A, Airaksinen O, Saari T, et al. The predictive value of preoperative myelography in lumbar spinal stenosis. Spine. 1994;19(12):1335-1338.
63 Botwin KP, Gruber RD, Bouchlas CG, et al. Fluoroscopically guided lumbar transformational epidural steroid injections in degenerative lumbar stenosis: an outcome study. Am J Phys Med Rehabil. 2002;81(12):898-905.
64 Atlas SJ, Deyo RA, Keller RB, et al. The Maine Lumbar Spine Study, part iii. 1-year outcomes of surgical and nonsurgical management of lumbar spinal stenosis. Spine. 1996;21(15):1787-1794.
65 Hoogmartens M, Morelle P. Epidural injection in the treatment of spinal stenosis. Acta Orthop Belg. 1987;53(2):409-411.
66 Onel D, Sari H, Donmez C. Lumbar spinal stenosis: clinical/radiologic therapeutic evaluation in 145 patients. Conservative treatment or surgical intervention? Spine. 1993;18(2):291-298.
67 Rydevik BL, Cohen DB, Kostuik JP. Spine epidural steroids for patients with lumbar spinal stenosis. Spine. 1997;22(19):2313-2317.
68 Swezey RL. Outcomes for lumbar stenosis: A five-year follow-up study. J Clin Rheumatol. 1996:129-134.
69 Carrera GF. Lumbar facet joint injection in low back pain and sciatica: preliminary results. Radiology. 1980;137(3):665-667.
70 Carrera GF, Williams AL. Current concepts in evaluation of the lumbar facet joints. Crit Rev Diagn Imaging. 1984;21(2):85-104.
71 Dreyfuss PH, Dreyer SJ, Herring SA. Lumbar zygapophyseal (facet) joint injections. Spine. 1995;20(18):2040-2047.
72 Jackson RP, Jacobs RR, Montesano PX. 1988 Volvo Award in Clinical Sciences. Facet joint injection in low back pain. A prospective statistical study. Spine. 1988;13(9):966-971.
73 Lau LS, Littlejohn GO, Miller MH. Clinical evaluation of intra-articular injections for lumbar facet joint pain. Med J Aust. 1985;143(12–13):563-565.
74 Lilius G, Laasonen EM, Myllynen P, et al. Lumbar facet joint syndrome. A randomised clinical trial. J Bone Joint Surg [Br]. 1989;71(4):681-684.
75 Johnsson KE, Rosen I, Uden A. The natural course of lumbar spinal stenosis. Clin Orthop. 1992;279:82-86.
76 Mehta M, Salmon N. Extradural block. Confirmation of the injection site by X-ray monitoring. Anaesthesia. 1985;40(10):1009-1012.
77 White AH. Injection techniques for the diagnosis and treatment of low back pain. Orthop Clin North Am. 1983;14(3):553-567.
78 Porter RW, Hibbert C, Evans C. The natural history of root entrapment syndrome. Spine. 1984;9(4):418-421.
79 Amundsen T, Weber H, Nordal H, et al. Lumbar spinal stenosis: conservative or surgical management?: a prospective 10-year study. Spine. 2000;25(11):1424-1436.
80 Dilke TF, Burry HC, Grahame R. Extradural corticosteroid injection in management of lumbar nerve root compression. Br Med J. 1973;2(5867):635-637.
81 Revel ME, Listrat VM, Chevalier XJ, et al. Facet joint block for low back pain: identifying predictors of a good response. Arch Phys Med Rehabil. 1992;73(9):824-828.
82 Schwarzer AC, Wang SC, O’Driscoll D, et al. The ability of computed tomography to identify a painful zygapophyseal joint in patients with chronic low back pain. Spine. 1995:907-912.
83 Eisenstein SM, Ashton IK, Roberts S, et al. Innervation of the spondylolysis ‘ligament.’. Spine. 1994;19(8):912-916.