CHAPTER 35 Spondyloarthropathies
INTRODUCTION
These disorders frequently manifest initially with back complaints. For example, back complaints are the first symptoms in 75% of patients with ankylosing spondylitis and may be present in 89% of patients with undifferentiated spondyloarthropathy.1,2
DEFINITION AND CLASSIFICATION
The spondyloarthropathies are a cluster of overlapping and interrelated chronic inflammatory rheumatic disorders which include ankylosing spondylitis (often considered the prototype), reactive arthritis, arthritis associated with psoriasis, arthritis associated with Crohn’s disease and ulcerative colitis, Reiter’s syndrome, and undifferentiated spondyloarthropathy.3
These disorders are often referred to as the seronegative spondyloarthropathies, which are considered together since they share clinical, epidemiologic, and imaging features. The spondyloarthropathies usually have a negative rheumatoid factor (seronegativity), association with HLA-B27, familial clustering, predominant axial and peripheral joint involvement, and extra-articular manifestations.4
EPIDEMIOLOGY AND GENETICS
Spondyloarthropathies are a group of diseases heavily influenced by genetic factors, particularly HLA. There is a clinical spectrum of these disorders. Undifferentiated spondyloarthropathy is the most common disorder; with ankylosing spondylitis (AS) being second most common. The estimated incidence is likely more common than previously realized as newer classification systems have been developed. Prevalence of SpA is correlated with the presence of HLA-B27 in a particular population.5,6 Both men and women are affected. In ankylosing spondylitis, males are disproportionally affected in a 3:1 ratio.7 Psoriatic arthritis affects men and women equally.8 Postvenereal Reiter’s syndrome is more common in men, whereas postdysenteric Reiter’s syndrome equally affects men and women.9,10
GENERAL CLINICAL FEATURES
In general, spondyloarthropathies demonstrate the following clinical features:
PATHOLOGY
Enthesopathy
The ‘enthesis’ is the region of insertion of a tendon, ligament, capsule, or fascia into bone. The enthesis is now understood to be a complex structure that extends into the bone and marrow cavity.13 Recent work suggests that the entheseal fibrocartilage is the major target of the immune response and the primary site of the immunopathology.14 The bone marrow demonstrates edema and contains cellular infiltrates. T lymphocytes are abundant in these areas with a preponderance of CD8+ cells.15 Pathologic studies have demonstrated inflammatory infiltration and destruction which affect the whole anulus fibrosus, not just the enthesis of the intervertebral disc.16
Synovitis
Patients with spondyloarthropathy may have peripheral arthritis, typically mono- or oligoarticular, and often affecting one or both knees. Microscopic analysis reveals fibrin, synovial cell proliferation, lymphocytes, and plasma cells in the synovium.17 A more recent hypothesis suggests that bacterial antigens and microorganisms in a susceptible HLA-B27-postitive patient may interact to produce inflammation and arthritis in ankylosing spondylitis.18 It is well established in reactive arthritis that synovial fluid demonstrates bacteria-specific T-cell responses to the bacterium that causes the arthritis.19,20
Sacroiliitis
Studies of the sacroiliac joint reveal evidence of synovitis, osteitis, and enthesitis. Biopsy and autopsy specimens demonstrate pannus formation, myxoid marrow, superficial cartilage destruction, intra-articular fibrous strands, new bone formation, and bony ankylosis. Biopsy samples demonstrate cellular infiltrates of T lymphocytes, with both CD4+ and CD8+ cells.21,22 Contrast-enhanced magnetic resonance imaging (MRI) studies of the sacroiliac joints in inflammatory back pain can demonstrate the following: sacroiliitis is more often bilateral in AS (84%) than in undifferentiated SpA (48%); the dorsocaudal parts of the synovial joint and the bone marrow are the most frequently inflamed structures early in the disease; in contrast, the entheses and ligaments are more commonly involved in later stages.23
DIFFERENTIAL DIAGNOSIS
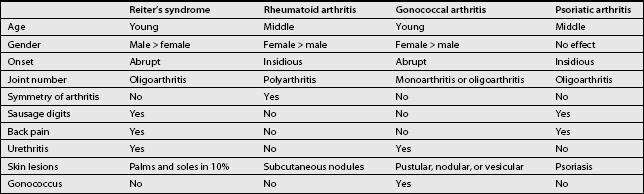
The differential diagnosis of sacroiliitis is narrow and is summarized in Table 35.1.7
| SPONDYLOARTHROPATHIES | |
Spinal pain and restriction may also be caused by diffuse idiopathic skeletal hyperostosis (DISH). In contrast to SpA, DISH usually presents with later age of onset, normal sedimentation rate, larger and more flowing ligamentous ossifications (syndesmophytes), and the absence of sacroiliitis.24
DIAGNOSIS
Ankylosing spondylitis
The classification criteria for AS were reassessed in 1984 and are referred to as the ‘modified New York criteria for ankylosing spondylitis.’ The criteria include both clinical and radiographic categories.25,26 The three clinical criteria include:
The two radiologic criteria include:
Clinical features
Clinical features of AS are heralded by chronic low back pain and stiffness as the initial symptoms in 75% of patients.27 Often, the symptoms develop spontaneously and progress insidiously. Buttock pain that radiates into the thigh may be erroneously blamed on sciatica. This pain may reflect involvement of the sacroiliac joints.28,29 A history of nocturnal back pain, diurnal variation with prolonged morning stiffness, and improvement with exercise should raise the suspicion of an inflammatory etiology to chronic back pain. A good response to nonsteroidal antiinflammatory drug (NSAID) therapy and an age younger than 40 also increase the likelihood of inflammatory back pain.30 Another, less common presentation of AS may be enthesitis or peripheral arthritis, mono- or oligoarticular.31 The enthesitis may involve the Achilles or plantar tendon insertions. The knee is often involved in the arthritis. These findings are not unique to AS. The differential diagnosis may include Reiter’s syndrome or reactive arthritis.
Physical examination
The earliest physical examination finding is often tenderness in the region of the sacroiliac joints or pain on provocative test maneuvers such as hip hyperextension and sacral compression tests. The two most sensitive maneuvers are pressure over the anterior-superior iliac spines and pressure over the lower half of the sacrum.32 As the disease progresses, physical examination findings will reflect restricted ranges of motion. As an example, reduced chest expansion is measured from maximal exhalation to maximal inhalation at the level of the fourth intercostal space. An expansion of less than 2.5 cm is considered abnormal.27 The restricted motion reflects fusion of the costovertebral joints.
Schober’s test and finger-to-floor test will also become abnormal. The Schober’s test is performed by marking the fifth lumbar vertebra and a point in the midline 10 cm above. The patient is then asked to flex forward maximally while maintaining the knees straight. The distance between the two points exceeds 15 cm in normal individuals.1
Laboratory studies
Laboratory studies in AS are often non-specific. Acute-phase reactants such as erythrocyte sedimentation rate and C-reactive protein are often elevated, but are not specific for AS and do not necessarily reflect disease activity.33,34 A mild normochromic normocytic anemia may be present. Serologic tests for lupus and rheumatoid arthritis should be negative.
HLA-B27 is present in approximately 90% of Caucasian patients and 50–60% of African-American patients with AS.3 It is present in only 6% of the general population.
Undifferentiated spondyloarthropathy, Reiter’s syndrome, and reactive arthritis
The classification criteria for SpA is based on clinical features, as there are no specific confirmatory blood tests. There are two sets of clinical criteria that have been developed and validated in Europe and are used widely. These are the European Spondyloarthropathy Study Group (ESSG) and the multiple-entry criteria by Bernard Amor.1
The European Spondyloarthropathy Study Group criteria
Indications for the designation of spinal pain as ‘inflammatory’ are:
Indications for the designation of synovitis are:
The ESSG criteria have been evaluated in many studies, including those in Europe, Brazil, and Alaska.35–38
The Amor criteria
The Amor criteria are a series of items which are weighted with a point scoring system.1,39,40
In order to qualify for a diagnosis of spondyloarthropathy, a patient must score a total of at least six from among the list of features detailed in Table 35.2.
Table 35.2 Clinical features scored in the Amor classification
| Feature | Score | |
|---|---|---|
| CLINICAL | ||
| Night pain or morning stiffness of the thoracic or lumbar spine | 1 | |
| Asymmetrical oligoarthritis | 2 | |
| Buttock pain (uni- or bilateral) | 1 or 2 | |
| Sausage-like toe or digit | 2 | |
| Heel pain | 2 | |
| Iritis | 2 | |
| Nongonococcal urethritis or cervicitis within 1 month prior to arthritis | 1 | |
| Acute diarrhea within 1 month prior to arthritis | 1 | |
| Presence or h/o psoriasis, balanitis, inflammatory bowel disease | 2 | |
| RADIOLOGIC | ||
| Sacroiliitis (grade >2 if bilateral; grade >3 if unilateral) | 2 or 3 | |
| GENETIC | ||
| HLA-B27 present and/or family h/o spondyloarthropathy | 2 | |
| RESPONSE TO TREATMENT | ||
| Clear-cut response to NSAIDs | 2 |
Specific diagnoses
Reactive arthritis
Inflammatory arthritides developing after a distant infection are labeled reactive.41 Inciting organisms may be: Chlamydia, Yersinia, Salmonella, Shigella, Campylobacter, Clostridium difficile, Brucella, and Giardia.42 The infection should have occurred within 6 weeks of clinical presentation of the arthritis. The presence of HLA-B27 renders the host susceptible; however, there is an interplay between HLA-B27 and environmental/infectious triggers in the development of reactive arthritis.43
Reiter’s syndrome
Reiter’s syndrome represents one example of reactive arthritis. The classic triad of uveitis, urethritis, and arthritis defines Reiter’s syndrome. The pathogenesis is similar to reactive arthritis since both are triggered by an infectious agent and are more common in those patients with the HLA-B27 gene.44
Not all patients present with all three features of the triad. The American College of Rheumatology requires peripheral arthritis (longer than 1 month’s duration) in association with urethritis or cervicitis.45
Undifferentiated spondyloarthropathy
Among patients who meet ESSG or Amor criteria for spondyloarthropathy, there is a large group that does not fit into the above discrete categories. These patients are labeled as undifferentiated spondyloarthropathy.1 In a recent study from Spain,46 68 patients with the diagnosis of undifferentiated spondyloarthropathy (uSpA) were followed for 2 years. At the end of this period, 75% retained the diagnosis of uSpA; disease remission occurred in 13%; ankylosing spondylitis 10%; and psoriatic arthritis 2%. In addition, a subset of patients with uSpA may be found to have reactive arthritis.47
Arthritis associated with psoriasis
Psoriasis is a chronic autoimmune disorder affecting the skin and can be associated with inflammatory arthritis. Ten to forty percent of patients with psoriasis develop a chronic inflammatory arthritis. Psoriatic arthritis (PSA) occurs as a result of interplay of genetic, immunologic, and environmental factors.48,49 Clinically, PSA may resemble RA, except that PSA patients are seronegative and express cytokines preferentially at the enthesis in addition to the synovium. The most common presentation is either oligoarthritis or symmetric polyarthritis. There are several proposed subtypes: monoarthritis and oligoarthritis, polyarthritis, arthritis of distal interphalangeal joints with nail changes, arthritis mutilans, and spondylitis.6,50 This is often associated with flexor tenosynovitis. Axial spinal involvement of sacroiliitis and spondylitis does occur in PSA but usually occurs after years of illness, and is not a common presenting complaint.8
Enteropathic arthritis
Enteropathic arthritis refers to inflammatory arthritis in association with inflammatory bowel disease, ulcerative colitis, or Crohn’s disease.51 Conversely, two-thirds of patients with spondyloarthropathy show subclinical histologic signs of gut inflammation and approximately 6% will go on to develop inflammatory bowel disease.52 In a study by de Vlam et al.,53 39% of 103 consecutive patients followed in a gastroenterology clinic for ulcerative colitis or Crohn’s disease had enteropathic arthritis. Ninety percent met criteria for spondyloarthropathy, while 10% fulfilled criteria for ankylosing spondylitis. An additional 18% had asymptomatic sacroliliitis.6 Approximately 25% of patients with enteropathic arthritis have axial disease. Peripheral joint arthritis occurs more frequently in patients with enteropathic colitis compared with AS.
Please refer to Table 35.3 which represents a summary of some of the key clinical aspects of the differential diagnosis of systemic causes of arthritis. This may clarify the recognition of systemic arthritis for the practicing spine specialist.1
Radiographic imaging in spondyloarthropathies
The main musculoskeletal features include: sacroiliitis, spondylitis, and peripheral joint lesions. Sacroiliitis is the hallmark feature which unifies the group.54 The distribution of joint involvement may give a clue to diagnosis. For example, ankylosing spondylitis primarily involves the axial joints and enthesis, with less consistent findings in the appendicular skeleton,55 and psoriatic arthritis distinctively may involve the interphalageal joints.56 Multiple imaging modalities are available to assess seronegative spondyloarthropathies, provide early diagnosis, and possibly follow disease activity.
Radiographic assessment
Standard radiographs are still the appropriate first images to obtain in practice. Radiographic features common to all spondyloarthropathies include: erosion, periostitis, bone proliferation at the entheses, and normal bone mineralization.54 Radiographic analysis of early sacro-iliitis may demonstrate erosions on the iliac side of the joint. Late-stage radiographic appearance is one of SI fusion and ankylosis.57 The shortcomings of plain radiography for the diagnosis of sacroiliitis include the large variability in interpretation among radiologists, and the relative insensitivity in early sacroiliitis.58
Scintigraphy (bone scan)
Bone scanning is well documented as a modality to identify hyperemia and joint inflammation that may not be apparent radiographically. Quantitative bone scanning has approximately 80% predictability for detection of active sacroiliitis. This compares to 100% for MRI.59 Periarticular radionuclide uptake around peripheral joints and at the entheses are demonstrated with bone scan.60 The problem with scintigraphy is that it is non-specific and must be correlated with other clinical and radiologic investigations. Single photon emission computed tomography (SPECT) has improved localization of areas of increased uptake and may be a useful supplement.54
Computed tomography
Computed tomography (CT) scanning is superior to plain radiography for visualization of early sacroiliac erosions and sclerosis.61 The true synovial sacroiliac joint is the inferior two-thirds, with the superior one-third being ligamentous. Comparison of CT with MRI scanning suggests that CT is superior for evaluation of chronic bone changes in the ligamentous portion of the joint; however, it is insensitive for detection of inflammatory changes in the subchondral bone.62 In addition, CT should be considered if further information about spinal fracture or bony canal stenosis is needed. A recent study demonstrates efficacy of CT-guided sacroiliac injections for treatment of sacroiliitis.63
Magnetic resonance imaging
Magnetic resonance imaging (MRI) has emerged as a sensitive and detailed modality for imaging of spondyloarthropathy. MRI is excellent at depicting the normal sacroiliac joint and clearly separates the synovial and ligamentous compartments. The tissue resolution permits visualization and evaluation of bone marrow, synovium, articular cartilage, ligaments, tendons, muscles, entheses, and various stages of inflammation. MRI can identify joint effusion, synovitis, bone marrow edema, and bone erosions.54 In patients with a high clinical likelihood of spondyloarthropathy and negative standard radiography, MRI (especially with gadolinium enhancement) provides excellent radiation-free evidence of sacroiliitis and enthesitis.64
DIAGNOSIS OF SPONDYLOARTHROPATHIES
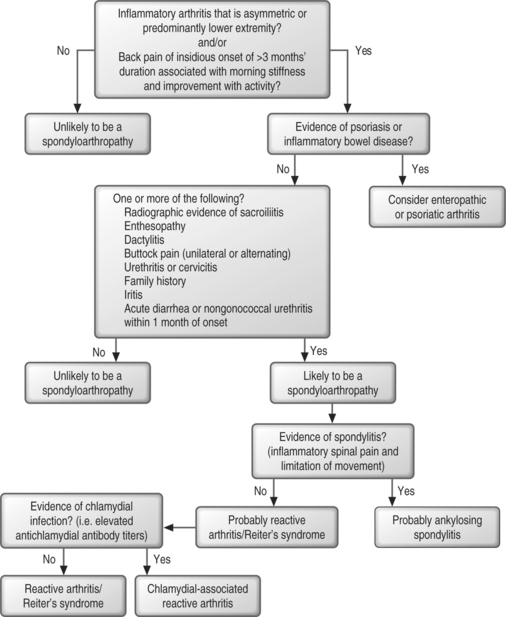
As an overview, there are four basic steps to follow if a clinician suspects the diagnosis of spondyloarthropathy (Fig. 35.1):
Systemic features of spondyloarthropathies
Ocular
Eye involvement can occur in all of the spondylotic variants. The most common finding is uveitis, and this can be seen in 25–40% of patients who have ankylosing spondylitis.65 Symptoms include eye pain, blurred vision, and photosensitivity. The eye can appear red and injected. In cases in which uveitis is suspected, patients should immediately be referred to an ophthalmologist, as the diagnosis can only be made by slit-lamp examination. The treatment generally includes topical nonsteroidal eye drops or steroid drops. In severe or refractory cases, systemic immunosuppression and aggressive treatment with disease-modifying agents is necessary. While most patients will recover, severe cases can result in visual loss. A less common ocular manifestations of the spondylotic variants includes Sjögren’s syndrome and optic neuritis.66
Gastrointestinal
Gastrointestinal involvement, in particular bowel inflammation and ulceration, can be seen in all of the spondylotic variants. Up to 44% of patients with ankylosing spondylitis have gastrointestinal involvement.67 Gut inflammation in patients with ankylosing spondylitis is histologically similar to the lesions found in Crohn’s disease.68 Moreover, in one series subclinical sacroiliitis was found in 24% of patients with inflammatory bowel disease.69 Therefore, patients with spondylitis should be monitored for symptoms suggestive of occult inflammatory bowel disease and, conversely, patients with documented inflammatory bowel disease should be monitored for spondylitis.
Cardiac
Aortitis and aortic root disease that can sometimes lead to valvular dysfunction is the most common cardiac lesion found in patients with ankylosing spondylitis. In one case series, 82% of patients with ankylosing spondylitis had evidence of either aortic root disease or valvular disease. Valve regurgitation was found in close to half of the patients and many of these patients went into heart failure or required valve replacement therapy.70 Conduction abnormalities affecting the atrioventricular node and myocardial involvement are found less commonly.71
Skin
There have been reports of an association between vitiligo and the spondyloarthropathies. In men, circinate balanitis can also occur.72,73
Pulmonary
In patients who have thoracic spine and costovertebral joint involvement, decreased chest wall expansion during inspiration can lead to decreased lung capacity and dyspnea. This can lead to recurrent infections as well.74 In addition, patients can develop fibrobullous disease of the upper lobes.75
Patient management
Functional assessment
This functional evaluation should include a functional assessment and an evaluation of how much fixed damage has been done to the spine and the joints. Patients with spondylitis can develop functional impairment leading to long-term disability. Various assessment tools can be used but the most common are the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), the Bath Ankylosing Spondylitis Functional Index (BASFI) and the Dougados Functional Index (DFI).77 More recently, the World Health Organization Disability Assessment Schedule II (WHODAS II) was shown to be useful for evaluating functional capacity in patients with ankylosing spondylitis.78 The BASDAI focuses on pain, joint involvement, and swelling while the BASFI, DFI, and the WHODAS II assess functional impairment.
Prognostic indicators
In addition to functional assessment, various disease characteristics portend prognostic outcome. In particular, worse outcome is found in persons who have disease onset prior to age 16, hip joint involvement, peripheral joint involvement (in particular a sausage digit), decreased range of motion of the lumbar spine, high ESR, and poor response to nonsteroidal antiinflammatory drugs.79
Physical therapy
One of the mainstays of therapy for spondylitis is exercise and physical therapy. The theory is that even in cases of spinal fusion and severe restriction in range of motion of the spine, physical therapy and exercise can maximize function and maintain as much mobility as possible. In Europe, spa therapy is included as part of the treatment regimen.80
Unfortunately, there are few well-designed studies that clearly demonstrate long-term efficacy of physical therapy. In one meta-analysis, group physical therapy was better than home exercise in decreasing pain and stiffness.81 In another study, recreational exercise for longer than 200 minutes a week was shown to decrease pain and stiffness, but not to improve HAQ scores in patients with disease for less than 15 years. In patients with longer-standing disease, 5–7 days a week of back exercises decreased pain and had a modest improvement of HAQ scores.82
Pharmacologic management
Nonsteroidal antiinflammatory drugs and COX-2 inhibitors
Historically, the most commonly used NSAID has been indometacin.83 This was based upon the sense that this medication was more effective than other antiinflammatory agents although controlled studies have failed to substantiate this finding.84 In general, any of the nonsteroidals may be used to treat the pain and inflammation of the spondylotic variants. Phenylbutazone was once used with high frequency in patients with ankylosing spondylitis but is no longer used secondary to its high toxicity. In those patients who are intolerant of NSAIDs or who have had gastrointestinal toxicity from NSAIDs, the COX-2 inhibitors can be used. Both the NSAIDs and the COX-2 inhibitors can unmask occult colitis in these patients, so the treating healthcare provider should be aware of the potential for gastrointestinal toxicity. Moreover, the COX-2 inhibitors have been associated with increased risk of cardiovascular disease and should be used judiciously.
Sulfasalazine
Sulfasalazine has been used for many years in the treatment of inflammatory bowel disease and for rheumatoid arthritis. The data on its utility in spondylitis are murkier. While inflammatory markers such as the C-reactive protein clearly improve with sulfasalazine it is unclear whether the spondylitis and those symptoms benefit. It is particularly beneficial for patients who have extraspinal manifestations of spondylitis such as psoriatic arthritis, peripheral arthritis, and inflammatory bowel disease. Dosing is in the range of 2000–3000 mg a day. The major toxicity is bone marrow or liver toxicity.85
Methotrexate
Methotrexate was approved in the early 1980s for use in rheumatoid arthritis. It has also been used for the treatment of inflammatory bowel disease and psoriatic arthritis. The efficacy of this medication in spondylitis is less clear. In one double-blind, placebo-controlled study of ankylosing spondylitis, there was no benefit of methotrexate treatment compared with placebo.86
Tumor necrosis factor-alpha antagonists
The new biologic agents, especially those directed against tumor necrosis factor-alpha, have only been used recently for the treatment of spondylotic variants but represent a large advance in the pharmacologic therapy of ankylosing spondylitis. Those that are approved include etanercept, infliximab, and adalimumab. These agents have been shown to be effective in the treatment of patients with ankylosing spondylitis. Both infliximab and etanercept have been shown to cause a rapid and significant improvement in BASDAI scores and improvement in morning stiffness, spinal pain, and inflammatory markers such as the ESR and CRP.87,88 As these agents are used with greater frequency and earlier in the course of the disease, it will be interesting to see whether they can impact disease outcome.
Corticosteroids
Systemic corticosteroids are of limited use in patients with ankylosing spondylitis and are generally not used.85
Experimental therapy
Thalidomide has been used for refractory ankylosing spondylitis. In one open label study minimal improvement in joint symptoms and function was observed. Further studies need to be performed before this medication is used on a regular basis.91
Pamidronate has been studied in a few open label trials. Long-lasting improvement in pain, stiffness, and function were found although several patients developed arthralgias and myalgias after the infusions. Further studies need to be done before accepting the utility of this medication in treating spondylitis.92
Complementary therapy
There are limited data on the use of complementary therapy in the treatment of spondylitis. In one case study, chiropractic manipulation was helpful in a patient with advanced ankylosing spondylitis. However, given the degree of fusion found in patients’ spines, caution should be used.93
CASE STUDIES
1 Yu DT, Wiesenhutter CW. Clinical manifestations and diagnosis of ankylosing spondylitis. UpToDate. on line 12.1:1–21.
2 Yu DT, Wiesenhutter CW. Definition and diagnosis of undifferentiated spondyloarthropathy, Reiter’s syndrome, and reactive arthritis. UpToDate. online 12.1:1–18.
3 Espinoza L. Spondyloarthropathies. Lippincotts Prim Care Pract. 1998;2:81-86.
4 Grigoryan M, Roemer FW, Mohr A, et al. Imaging in spondyloarthropathies. Curr Rheumatol Rep. 2004;6:102-109.
5 Reveille JD. The genetic basis of spondyloarthritis. Curr Rheumatol Rep. 2004;6:117-125.
6 Khan MA. Update on spondyloarthropathies. Ann Intern Med. 2002;136:896-907.
7 Arnett FC. Ankylosing spondylitis. In: Koopman WJ, Moreland LW, editors. Arthritis and allied conditions – a textbook of rheumatology. Philadelphia: Lippincott, Williams & Wilkins, 2001. Chap. 66
8 Bennett RB. Psoriatic arthritis. In: Koopman WJ, Moreland LW, editors. Arthritis and allied conditions-a textbook of rheumatology. Philadelphia: Lippincott, Williams & Wilkins, 2001. Chap. 68
9 Kvien TK, Glennaos A, Melby K, et al. Reactive arthritis: incidence, triggering agents and clinical presentation. J Rheumatol. 1994;21:115-122.
10 Michet CJ, Machado EB, Ballard DJ, et al. Epidemiology of Reiter’s syndrome in Rochester, Minnesota: 1950–1980. Arthritis Rheum. 1996;39:1172-1177.
11 Miceli-Richard C, et al. Spondyloarthropathy for practicing rheumatologists: diagnosis, indication for disease-controlling antirheumatic therapy, and evaluation of the response. Rheum Dis Clin North Am. 2003;29:449-462.
12 Dougados M, van der Heijde D. Ankylosing spondylitis: how should the disease be assessed? Best Pract Res Clin Rheumatol. 2002;16:605-618.
13 Granfors K, Marker-Hermann E, de Keyser F, et al. The cutting edge of spondyloarthropathy research in the millennium. Arthritis Rheum. 2002;46:606.
14 Benjamin M, McGonagle D. The anatomical basis for disease localization in seronegative spondyloarthropathy at spondylotic and related sites. J Anat. 2001;199:503-526.
15 Laloux L, Voisin MC, et al. Immunohistological study of entheses in spondyloarthropathies: comparison of rheumatoid arthritis and osteoarthritis. Ann Rheum Dis. 2001;60:316-321.
16 Bywaters EGL. Pathology of the spondyloarthropathies. In: Calin A, editor. Spondyloarthropathies. Orlando: Grune & Stratton; 1984:43-68.
17 Chang CP, Schumacher HR. Light and electron microscopic observations on the synovitis of ankylosing spondylitis. Semin Arthritis Rheum. 1992;22:54.
18 Granfors K. Do bacterial antigens cause reactive arthritis? Rheum Dis Clin North Am. 1992;18:37-48.
19 Hermann E. T cells in reactive arthritis. APMIS. 1993;101:177-186.
20 Urgrinovic S, Mertz A, et al. A single monamer from the Yersinia 60-kD heat shock protein is the target of HLA-B27 restricted CTL response in Yersinia-induced reactive arthritis. J Immunol. 1997;159:5715-5723.
21 Braun J, Bollow M, Neure L, et al. Use of immunohistologic and in situ hybridization techniques in the examination of sacroiliac joint biopsy specimens from patients with ankylosing spondylitis. Arthritis Rheum. 1995;38:499.
22 Bollow M, Fischer T, Reisshauer H, et al. Quantitative analyses of sacroiliac biopsies in spondyloarthropathies: T cells and macrophages predominate in early and active sacroiliitis – cellularity correlates with the degree of enhancement detected by magnetic resonance imaging. Ann Rheum Dis. 2000;59:135.
23 Muche B, Bollow M, Francois RJ, et al. Anatomic structures involved in early- and late-stage sacroiliitis in spondylarthritis: a detailed analysis by contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 2003;48:1374-1384.
24 Mader R. Diffuse idiopathic skeletal hyperostosis: a distinct clinical entity. Isr Med Assoc J. 2003;5:506-508.
25 van der Linden S, Valkenburg HA, Cats A. Evaluation of diagnostic criteria for ankylosing spondylitis. Arthritis Rheum. 1984;27:361.
26 Gran JT, Husby G. The epidemiology of ankylosing spondylitis. Semin Arthritis Rheum. 1993;22:319.
27 Gran JT. An epidemiologic survey of the signs and symptoms of ankylosing spondylitis. Clin Rheum Dis. 1985;4:161-169.
28 Calin A, Porta J, Fries JF, et al. Clinical history as a screening test for ankylosing spondylitis. JAMA. 1977;237:2613-2614.
29 Blackburn WDJr, Alarcon GS, Ball GV. Evaluation of patients with back pain of suspected inflammatory nature. Am J Med. 1988;85:766-770.
30 Maksymowych WP. Ankylosing spondylitis. Not just another pain in the back. Can Fam Physician. 2004;50:205-207. 213–215
31 Olivieri I, Barozzi L, Padula A. Enthesopathy: clinical manifestations, imaging and treatment. Baillieres Clin Rheumatol. 1998;12:665-681.
32 Bower PW, Griffin AJ. Clinical sacroiliac tests in ankylosing spondylitis and other causes of low back pain – 2 studies. Ann Rheum Dis. 1984;43:192-195.
33 Ruof J, Stucki G. Validity aspects of erythrocyte sedimentation rate and C-reactive protein in ankylosing spondylitis: a literature review. J Rheumatol. 1999;26:966-970.
34 Spoorenberg A, van der Heijde D, et al. Radiological scoring methods in ankylosing spondylitis: reliability and sensitivity to change over one year. J Rheumatol. 1999;26:997.
35 Cury SE, Vilar MJ, Ciconelli RM, et al. Evaluation of the European spondyloarthropathy study group preliminary classification criteria in Brazilian patients. Clin Exp Rheumatol. 1997;15:79-82.
36 Gomariz EM, Guijo VP, et al. The potential of ESSG spondyloarthropathy classification criteria as a diagnostic aid in rheumatologic practice. J Rheumatol. 2002;29:326-330.
37 Collantes-Estevez E, Cisnal del Mazo A, Munoz-Gomariz. Assessment of 2 systems of spondyloarthropathy diagnostic and classification criteria (Amor and ESSG): a Spanish multicenter study. European Spondyloarthropathy Study Group. J Rheumatol. 1995;22:246-251.
38 Boyer GS, Templin DW, Goring WP. Evaluation of the European spondyloarthropathy group preliminary classification criteria in Alaskan Eskimo populations. Arthritis Rheum. 1993;36:534-538.
39 Amor B, Dougados M, Listrat V, et al. Are classification criteria for spondyloarthropathy useful as diagnostic criteria? Rev Rhum Engl Ed. 1995;62:10-15.
40 Amor B, Dougados M, Mijiyawa M. Criteria of the classification of spondyloarthropathies. Rev Rhum Mal Osteoartic. 1990;57:85.
41 Toivanen P, Toivanen A. Two forms of reactive arthritis? Ann Rheum Dis. 1999;58:737-741.
42 Hill Gaston JS, Lillicrap MS. Arthritis associated with enteric infection. Best Pract Res Clin Rheumatol. 2003;17:219-239.
43 Cush JJ, Lipsky PE. Reiter’s syndrome and reactive arthritis. In: Koopman WJ, Moreland LW, editors. Arthritis and allied conditions – a textbook of rheumatology. Philadelphia: Lippincott, Williams & Wilkins; 2001:1-37.
44 Parker CT, Thomas D. Reiter’s syndrome and reactive arthritis. J Am Osteopath Assoc. 2000;100:101-104.
45 Willkens RF, Arnett FC, Bitter T, et al. Reiter’s syndrome: evaluation of preliminary criteria for definite disease. Arthritis Rheum. 1981;24:844-849.
46 Sampaio-Barros PD, Bertolo MB, et al. Undifferentiated spondyloarthropathies: a 2-year follow-up study. Clin Rheumatol. 2001;20:201-206.
47 Aggarwal A, Misra R, Chandrasekhar S, et al. Is undifferentiated seronegative spondyloarthropathy a forme fruste of reactive arthritis? Br J Rheumatol. 1997;36:1001-1004.
48 Scarpa R, Cosentini E, Manguso F, et al. Clinical and genetic aspects of psoriatic arthritis ‘sine psoriasis.’. J Rheumatol. 2003;30(12):2638-2640.
49 Gladman DD. Psoriatic arthritis: recent advances in pathogenesis and treatment. Rheum Dis Clin North Am. 1992;18:247-256.
50 Hohler T, Marker-Hermann E. Psoriatic arthritis: clinical aspects, genetics, and the role of T cells. Curr Opin Rheumatol. 2001;28:3-5.
51 Holden W, Orchard T, Wordsworth P. Enteropathic arthritis. Rheum Dis Clin North Am. 2003;29:513-530.
52 De Keyser F, Baeten D, et al. Gut inflammation and spondyloarthropathies. Curr Rheumatol Rep. 2002;4:525-532.
53 de Vlam K, Mielants H, et al. Spondyloarthropathy is underestimated in inflammatory bowel disease; prevalence and HLA association. J Rheumatol. 2000;27:2860-2865.
54 Grigoryan M, Roemer FW, Mohr A, et al. Imaging in spondyloarthropathies. Curr Rheumatol Rep. 2004;6:102-109.
55 Bennett DL, Ohashi K, El-Khoury GY. Spondyloarthropathies: ankylosing spondylitis and psoriatic arthritis. Radiol Clin North Am. 2004;42:121-134.
56 Taylor WJ, Porter GG, Helliwell PS. Operational definitions and observer reliability of the plain radiographic features of psoriatic arthritis. J Rheumatol. 2003;30:2645-2658.
57 Muche B, Bollow M, et al. Anatomic structures involved in early- and late-stage sacroiliitis in spondylarthritis: a detailed analysis by contrast-enhanced magnetic resonance imaging. Arthritis Rheum. 2003;48:1374-1384.
58 van Tubergen A, Heuft-Dorenbosch L, Schulpen G, et al. Radiographic assessment of sacroiliitis by radiologists and rheumatologists: does training improve quality? Ann Rheum Dis. 2003;62:519-525.
59 Battafarano DF, West SG, et al. Comparison of bone scan, computed tomography and magnetic resonance imaging in the diagnosis of active sacroiliitis. Semin Arthritis Rheum. 1993;23:161-176.
60 Baumgarten DA, Taylor ATJr. Enthesopathy associated with seronegative spondyloarthropathy: 99mTc-methylene diphosphonate scintigraphic findings. Am J Roentgenol. 1993;160:1249-1250.
61 Yu W, Feng F, Dion E, et al. Comparison of radiography, computed tomography and magnetic resonance imaging in the detection of sacroiliitis accompanying ankylosing spondylitis. Skeletal Radiol. 1998;27:311-320.
62 Puhakka KB, Jurik AG, Egund N, et al. Imaging of sacroiliitis in early seronegative spondyloarthropathy: assessment of abnormalities by MR in comparison with radiography and CT. Acta Radiol. 2003;44:218-229.
63 Pulisetti D, Ebraheim NA. CT-guided sacroiliac joint injections. J Spinal Disord. 1999;12:310-312.
64 Braun J, Bollow M, Sieper J. Radiologic diagnosis and pathology for the spondyloarthropathies. Rheum Dis Clin North Am. 1998;24:697-735.
65 Maksymowych WP, Chou CT, Russell AS. Matching prevalence of peripheral arthritis and anterior uveitis in individuals with ankylosing spondylitis. Ann Rheum Dis. 1995;54:28.
66 Brandt J, Rudwaleit M, Eggens U, et al. Increased frequency of Sjögren’s syndrome in patients with spondyloarthropathy. J Rheum. 1998;25:718.
67 Leirisalo-Repo M, Turenen U, Stenman S, et al. High frequency of silent inflammatory bowel disease in spondyloarthropathy. Arthritis Rheum. 1994;37:23.
68 Baeten D, De Keyser F, Mielants H, et al. Ankylosing spondylitis and bowel disease. Best Pract Res Clin Rheumatol. 2002;16:537-549.
69 Queiro R, Maiz O, Intxausti J, et al. Subclinical sacroiliitis in inflammatory bowel disease: a clinical and follow-up study. Clin Rheumatol. 2000;19:445-449.
70 Roladan CA, Chavez J, Wiest PW, et al. Aortic root disease and valve disease associated with ankylosing spondylitis. J Am Coll Cardiol. 1998;2:1397-1404.
71 Lautermann D, Braun J. Ankylosing spondylitis – cardiac manifestations. Clin Exp Rheumatol. 2002;20:S11-S15.
72 Padula A, Ciancio G, La Civita L, et al. Association between vitiligo and spondyloarthritis. J Rheumatol. 2001;28:313-314.
73 Angualo J, Espinoza LR. The spectrum of skin, mucosa and extra-articular manifestations. Baillieres Clin Rheumatol. 1998;12:649-666.
74 Hunningnake GW, Fauci AS. Pulmonary involvement in the collagen vascular diseases. Ann Rev Respir Dis. 1979;119:471-503.
75 Rumanak WM, Firooznia H, Davis MJ, et al. Fibrobullous disease of the upper lobes: an extraskeletal manifestations of ankylosing spondylitis. J Comput Tomogr. 1984;8:225-229.
76 Lange U, Teichmann J. Ankylosing spondylitis and genitourinary infection. Eur J Med Res. 1999;4:1-7.
77 Ruof J, Sangha O, Stucki G. Comparative responsiveness of 3 functional indices in ankylosing spondylitis. J Rheumatol. 1999;26:1959.
78 Van Tubergen A, Landewe R, Heuft-Dorenbosch L, et al. Assessment of disability with the World Health Organization Disability Assessment Schedule II in patients with ankylosing spondylitis. Ann Rheum Dis. 2003;62:140-145.
79 Amor B, Santos RS, Nahal R. Predictive factors for the long-term outcome of the spondyloarthropathies. J Rheumatol. 1994;21:1883.
80 Van Tubergen A, Hidding A. Spa and exercise treatment in ankylosing spondylitis: Fact or fancy? Best Pract Res Clin Rheumatol. 2002;16:653-666.
81 Dagfinrud H, Kvien TK, Hagen K. Physiotherapy interventions for ankylosing spondylitis. Cohrane Database Syst Rev. (4):2004. CD002822
82 Uhrin A, Kuzis S, Ward M. Exercise and changes in health status in patients with ankylosing spondylitis. Arch Intern Med. 2000;160:2969-2975.
83 Harrison TR, Wilson JD. Ankylosing spondylitis and reactive arthritis. In: Jeffers HD, Boynton SD, editors. Principles of internal medicine. 12th ed. New York: McGraw-Hill; 1991:1453.
84 Barlle-Gualda E, Figueroa M, Ivorra J, et al. The efficacy and tolerability of aceclofenac in the treatment of patients with ankylosing spondylitis: a multicenter controlled clinical trial. Aceclofenac Indomethacin Study Group. J Rheum. 1996;23:1200-1206.
85 Dougados M, Dijkmans B, Khan M, et al. Conventional treatments for ankylosing spondylitis. Ann Rheum Dis. 2002;61:11140-11150.
86 Roychowdhury B, Bintly-Bagot S, Bulgen DY, et al. Is methotrexate effective in ankylosing spondylitis? Rheumatology. 2002;41:1330-1332.
87 Braun J, Brandt J, Listing J, et al. Long-term efficacy and safety of infliximab in the treatment of ankylosing spondylitis: an open, observational, extension study of a three-month, randomized, placebo-controlled trial. Arthritis Rheum. 2003;42:2224-2233.
88 Gorman JD, Sack KE, Davis JC. Treatment of ankylosing spondylitis by inhibition of tumor necrosis factor alpha. N Engl J Med. 2002;246:1349-1356.
89 Karabackakoglu A, Karakose S, Ozerbilo M, et al. Fluoroscopy-guided intra-articular corticosteroid injections into the sacroiliac joints in patients with ankylosing spondylitis. Acta Radiol. 2002;43:425-427.
90 Bollow M, Braun J, Taupitz M, et al. CT-guided intra-articular corticosteroid injection into the sacroiliac joints in patients with spondyloarthropathy: indication and follow-up with contrast-enhanced MRI. J Comput Assist Tomogr. 1996;20:512-521.
91 Wei JC, Chan TW, Lin HS. Thalidomide for severe refractory ankylosing spondylitis: a 6-month open-label trial. J Rheumatol. 2003;30:2627-2632.
92 Maksymowych WP, Jhangri GS, Leclercq S, et al. An open study of pamidronate in the treatment of refractory ankylosing spondylitis. J Rheumatol. 1998;25:714-717.
93 Rose KA, Kim WS. The effect of chiropractic care for a 30-year-old male with advanced ankylosing spondylitis: a time series case report. J Manip Phys Ther. 2003;26:E1-E9.