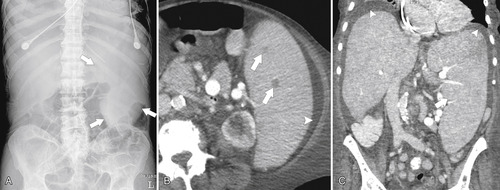
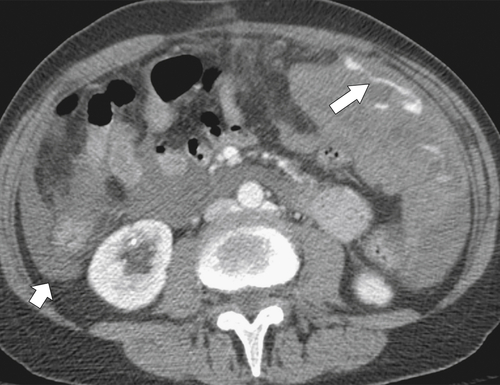
). Splenic tissue can also be imaged with technetium 99m (99mTc) labeled sulfur colloid, which is rapidly sequestered by the mononuclear phagocytic system (including in the liver and bone marrow). The vascularity of the red pulp creates a variable enhancement pattern, particularly in the arterial phase, and should not be mistaken for splenic disease. Any difficulty is usually resolved by imaging during the portal venous phase (PVP) when the spleen is homogeneously enhanced (Fig. 7-2).
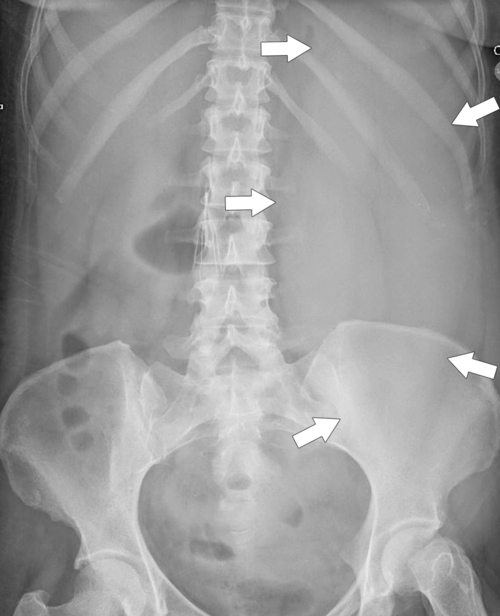
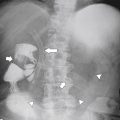
Figure 7-1 Plain abdominal radiograph in a 37-year-old woman with leukemic splenic infilration and splenomegaly (arrows).
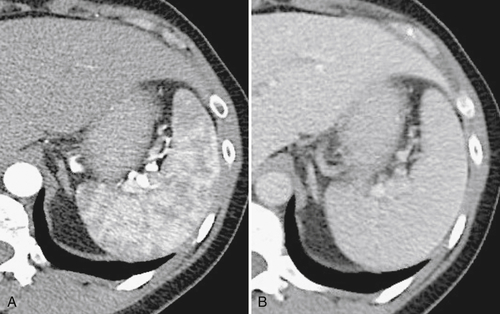
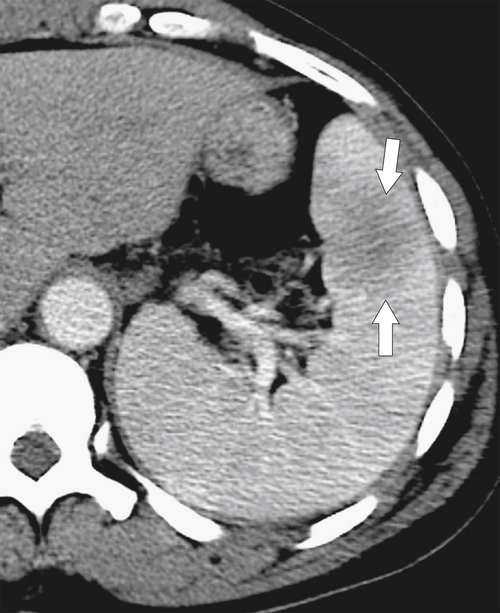
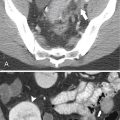
Figure 7-2 Axial arterial and PVP CT in a 36-year-old woman demonstrating marked irregular arterial enhancment (A) becoming uniform during the PVP (B).
Being highly vascular, the spleen is susceptible to many blood-borne pathogens, particularly metastatic and infectious disease, either as discrete lesions or as diffuse infiltration of the whole organ with or without splenomegaly. Differentiation between benign and malignant splenic lesions, either single or multiple, may ultimately require positron emission tomography (PET) or preferably PET/CT or even percutaneous biopsy, a relatively safe procedure when performed by experienced interventionalists.
Splenic Disorders
Congenital Disorders
Accessory Spleen
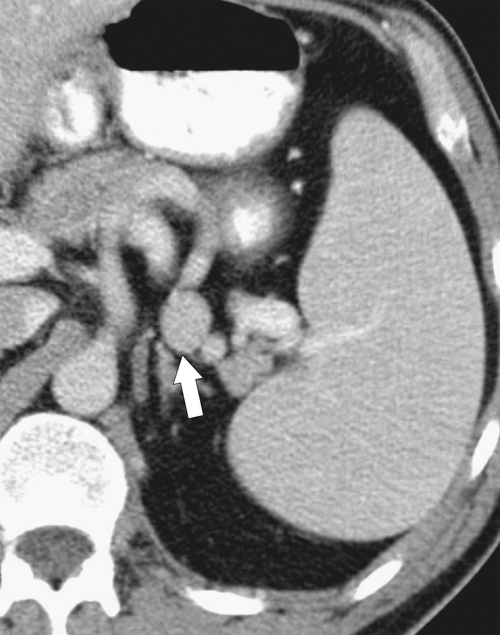
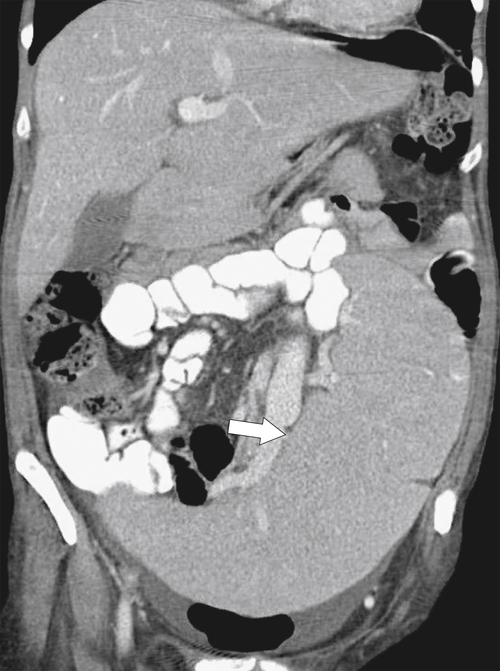
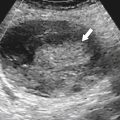
Typically the spleen is a single organ, but it is not uncommon for smaller amounts of splenic tissue (splenule or accessory spleen) to surround the main body, particularly close to the pancreatic tail. Usually these are single, but sometimes a few are present. Splenules are characteristically rounded, smooth-walled masses, most often 1 to 2 cm (but can be larger), that are located in the proximity of the spleen (usually the splenic hilum). They typically demonstrate a similar density to the spleen, whether on contrast-enhanced or noncontrast imaging (
Fig. 7-3), which usually differentiates them from lymphadenopathy or peritoneal and omental masses.
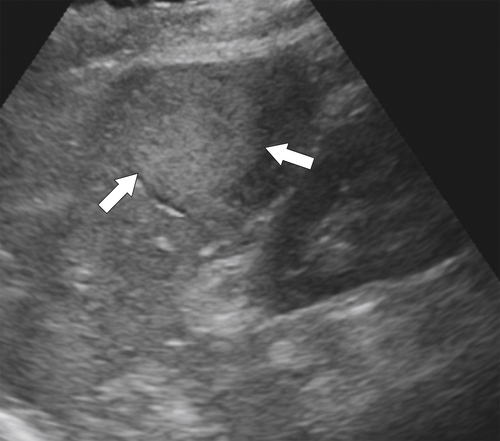
Figure 7-3 Axial contrast-enhanced CT in a 45-year-old woman with a splenule (accessory splenic tissue) (arrow).
Asplenia and Polysplenia Syndromes
Asplenia and polysplenia syndromes belong to a spectrum of heterotaxic syndromes referring to abnormal positioning of the internal organs. Situs solitus refers to the normal position, and situs inversus to the mirror image. When the position of the organs is between the two (or ambiguous), it is referred to as situs ambiguus. There are two primary classifications of situs ambiguus, which depend on the cardiac atrial morphology. If both atria have right-sided morphologies, it is known as right isomerism or asplenic syndrome (the spleen is absent). Conversely, if both atria have left atrial morphologies, this is known as left isomerism or polysplenism (multiple small splenic masses). However, the features and positioning of the abdominal organs in situs ambiguus are inconsistent, and precise definition of the type is often difficult.
Right isomerism (asplenia) usually presents in infancy with numerous other congenital anomalies, such as imperforate anus, Hirschsprung
∗ disease, and annular pancreas. It is often fatal. Left isomerism (polysplenia) can be fatal, but not usually as early as right isomerism. Patients with left isomerism are prone to biliary obstruction, esophageal and duodenal atresia, and biliary atresia, as well as other anomalies.
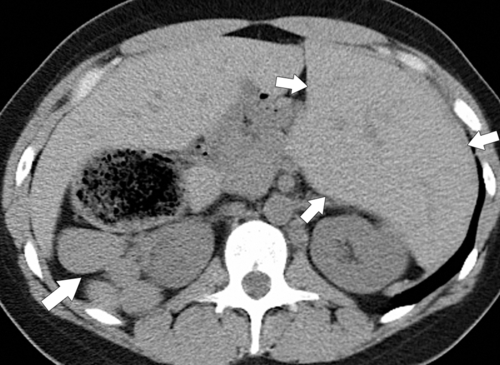
On CT, right isomerism is identified with asplenia, a centrally located liver, the aorta and inferior vena cava on the same side (usually the right), both lungs trilobed, and bilateral morphological right atria. Left isomerism is characterized by multiple splenic
nodules, intrahepatic inferior vena cava interruption with azygous or hemiazygous continuation of the inferior vena cava, bilobed lungs, and bilateral left-sided atria (Fig. 7-4).
Figure 7-4 Axial noncontrast CT in a 26-year-old man with polysplenia (large arrow). The liver is predominantly on the left (small arrows) with splenic tissue on the right.
Wandering Spleen
The so-called wandering spleen is caused by an abnormal congenital development of the lienorenal ligament, resulting in a long splenic vascular pedicle that allows the spleen the “freedom to wander” within the peritoneum. Given that it is on a long pedicle, its position within the abdomen or pelvis can vary from one scan to another (
Fig. 7-5). For similar reasons, it is susceptible to torsion.
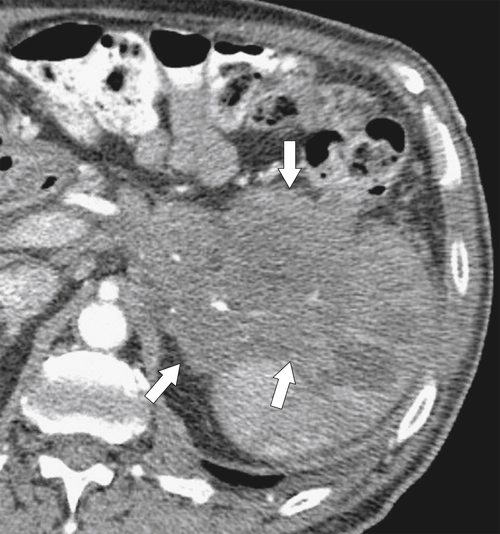
Figure 7-5 Coronal contrast–enhanced CT in a 57-year-old woman with splenomegaly caused by polycythemia rubra vera and a “wandering” spleen in the left lower quadrant (arrow).
Splenosis and Residual Splenic Tissue
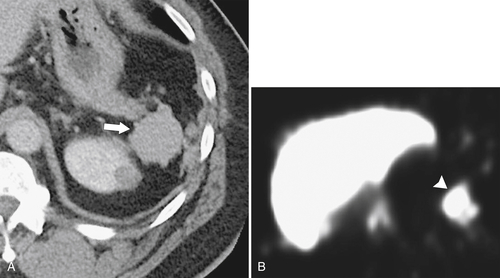
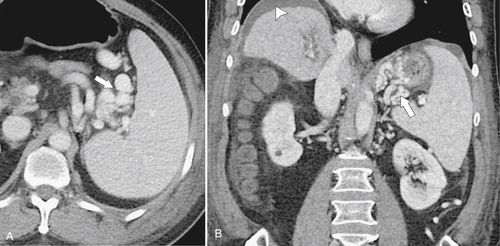
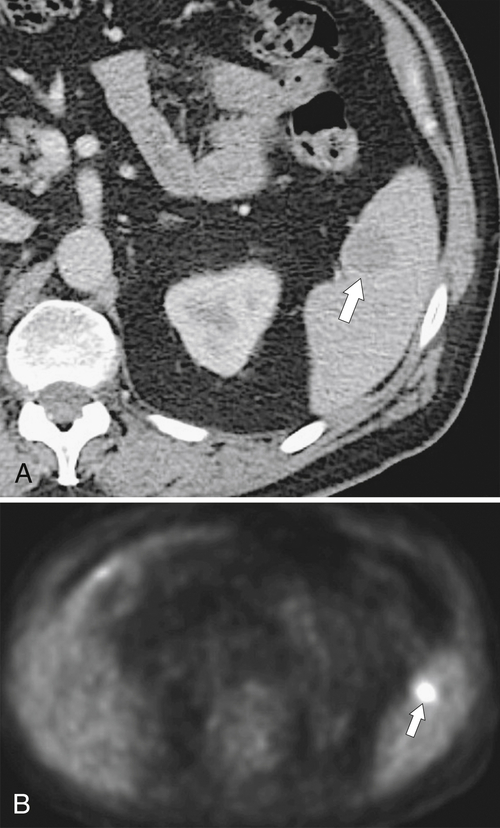
Splenosis is usually secondary to blunt splenic trauma, in which either the whole or parts of the shattered spleen distribute splenic tissue throughout the abdomen and pelvis. The chest may be involved if the diaphragm is breached, and sometimes other organs are affected if they also underwent traumatic laceration. Similarly, splenic remnants can remain after splenectomy (
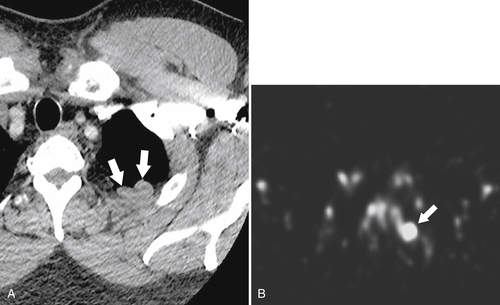
Fig. 7-6). These nodules, which are often multiple, must be differentiated from other abdominal masses, although their smooth-walled, homogeneous, and rounded appearance and their enhancement characteristics, similar to those of the spleen, should be a clue to the diagnosis. The diagnosis may ultimately require 99mTc-labeled red blood cells or sulfur colloid tests to confirm the splenic nature of the mass, whether in the abdomen (Fig. 7-6) or in the mediastinum (Fig. 7-7).
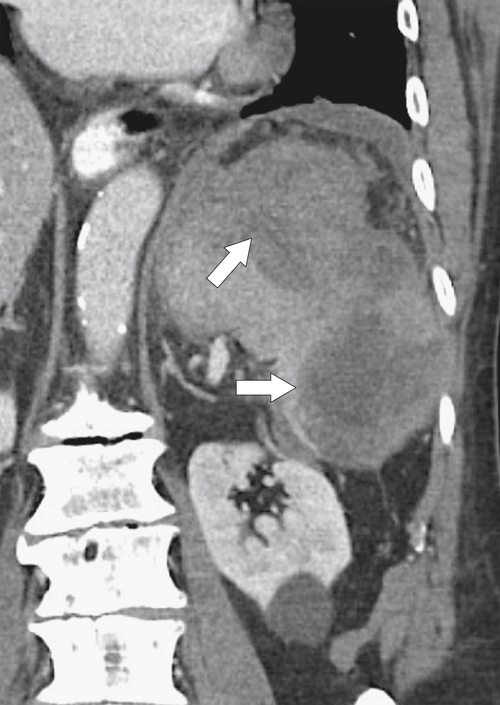
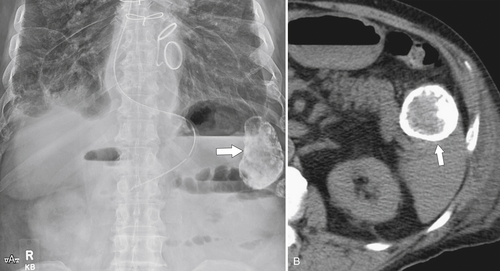
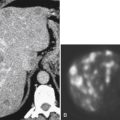
Figure 7-6 Axial contrast–enhanced CT (A) in a 47-year-old woman with prior splenectomy and now a 3-cm left upper quadrant mass (arrow), proved to be splenic tissue on 99mTc sulfur colloid scan (B; arrowhead). There is also normal hepatic uptake.
Figure 7-7 Axial contrast–enhanced CT (A) in the same patient as in Figure 7-6 with a 2-cm pleural-based left upper lobe mass (arrows) demonstrating splenic activity at 99mTc sulfur colloid imaging (B).
Splenomegaly
Splenomegaly is defined as splenic enlargement and should not be confused with hypersplenism (see later in the chapter), which is caused by splenic functional abnormalities in distinction to simple splenic enlargement. There are numerous disparate causes of an enlarged spleen (
Box 7-1), usually defined on US as >13 cm in long axis or >500 cm3 volume, although these measurements are relative given the size and morphology of the individual.
Box 7-1 Causes of Splenomegaly
Vascular Congestion
Portal hypertension
Sickle cell disease
Hereditary spherocytosis
Thalassemia
Right heart failure
Malignancy
Lymphoma
Leukemia
Metastases
Myelofibrosis
Multiple myeloma
Hematological
Polycythemia rubra vera
Trauma (hemorrhage)
Infectious
Infectious mononucleosis
Leptospirosis
Brucellosis
Malaria
Leishmaniasis
Acute tuberculosis and histoplasmosis
Typhoid
Fungal infection
Schistosomiasis
HIV-related
Multiple splenic infected emboli (endocarditis)
Storage
Gaucher disease
Niemann-Pick disease
Mucopolysaccharidosis
Langerhans cell histiocytoses
Amyloidosis
Autoimmune
Rheumatoid arthritis (Felty syndrome)
Sjögren syndrome
Systemic lupus erythematosus
Idiopathic thrombocytopenia purpura
Autoimmune hemolytic anemia
Congenital Splenomegaly
Hematological Abnormalities
Hematological genetic abnormalities usually result in splenomegaly because the continuously produced defective red blood cells are sequestered in a steadily increasing spleen. These include thalassemia, early sickle cell disease (in the later stages, the spleen infarcts and becomes small), hereditary spherocystosis, and elliptocytosis.
Depositional Metabolic Disease
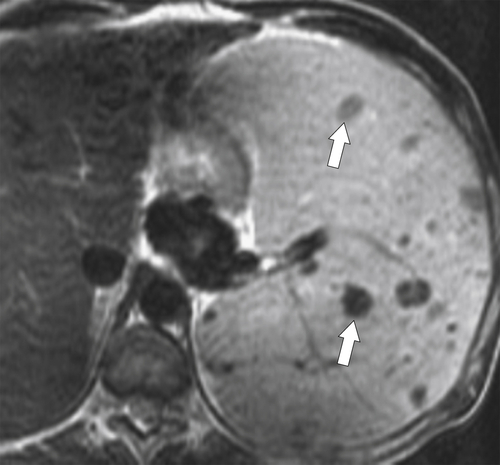
Gaucher
∗ disease is the most common lysosomal storage disease caused by a genetic defect (autosomal recessive) in the enzyme glucosylceramidase, resulting in glucosylceramide accumulation, predominantly in tissues abundant in mononuclear leukocytes (macrophages), namely the spleen, lungs, kidneys, liver, brain, and bone marrow. There are types I, II, and III, and patients with types I and III usually survive into adulthood. These may present with hepatosplenomegaly (
Fig. 7-8) as the unmetabolized lipids steadily accumulate within the spleen, or they may be deposited as complex focal masses known as gaucheromas.
Figure 7-8 Axial T2-weighted MRI in a 46-year-old man with Gaucher disease and multiple splenic gaucheromas (arrows).
Niemann-Pick
∗ disease is a lysosomal storage disorder in which sphingomyelin accumulates (owing to lack of sphingomyelinase) in the liver, spleen, brain, lungs, and bone marrow.
Mucopolysaccharidoses are a group of lysosomal storage disorders caused by deficiency of lysosomal enzyme activity (the subclassification is dependent on the missing enzyme) with accumulation of glycosaminoglycans, which are deposited in the peripheral nervous cysts, eyes, liver, and spleen.
Langerhans
† cell histiocytoses (LCH) are a group of disorders with excessive deposition of Langerhans histiocytic cells. The systemic manifestations involve multiple organs and include splenomegaly. Subtypes (now all referred to as LCH) were known as histiocytosis X, eosinophilic granuloma, Hand-Schüller-Christian
∗ disease, and Letterer-Siwe
† disease.
Vascular Congestion
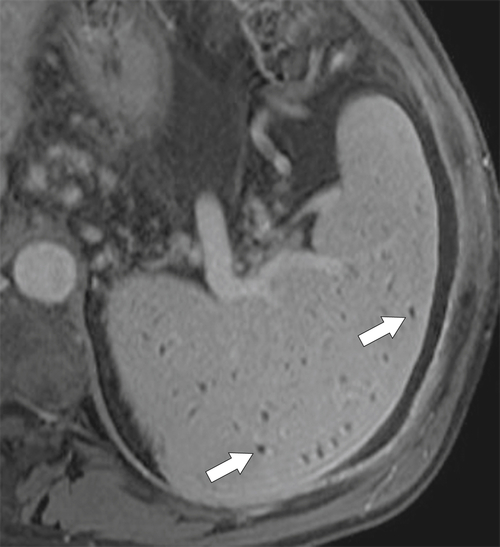
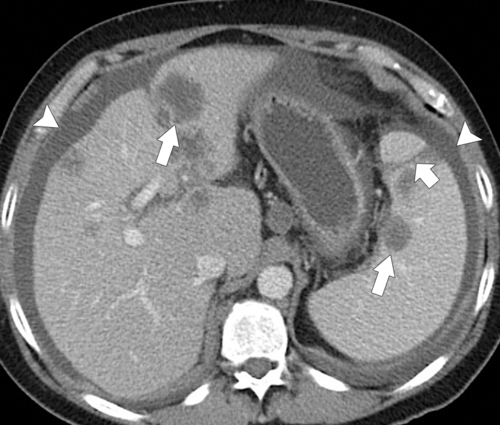
The most common cause of splenomegaly is portal hypertension as a result of liver cirrhosis, although any cause of portal hypertension can lead to splenomegaly, including right-sided heart failure, hepatic fibrosis, Budd-Chiari syndrome, portal or splenic vascular thrombosis, and occlusion. The findings are readily appreciated with CT, particularly when signs of liver cirrhosis are obvious. Splenic venous enlargement and tortuosity are caused by the chronically raised venous pressure, and collaterals commonly form, particularly around the splenic hilum (
Fig. 7-9). At MRI, multiple small T1 and T2 hypointense lesions, known at Gamna-Gandy∗ bodies, can be observed, which represent areas of hemosiderosis, probably from prior microhemorrhages induced by the vascular congestive process (Fig. 7-10).
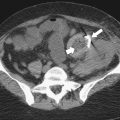
Figure 7-9 Axial (A) and coronal (B) contrast–enhanced CT in a 57-year-old man with cirrhosis, ascites (arrowhead), splenic varices (arrows), and splenomegaly.
Figure 7-10 Axial T1-weighted fat-saturated postcontrast MRI in a 44-year-old man with cirrhosis and multiple hypointense foci in the spleen (arrows) caused by Gamna-Gandy bodies.
Lymphoma
Lymphoma is the most common malignant tumor of the spleen and frequently a site of both Hodgkin
† (30%) and non-Hodgkin disease (30%). Secondary or metastatic involvement from lymphoma elsewhere is more common than isolated primary splenic lymphoma. For staging purposes, splenomegaly is considered nodal in Hodgkin disease and extranodal in non-Hodgkin disease (see
“Lymphoma Staging,”Chapter 4).
Figure 7-11 Axial contrast-enhanced CT in a 66-year-old woman with almost complete splenic replacement by a primary lymphomatous mass (arrows). There is an incidental hemangioma in the liver (small arrow).
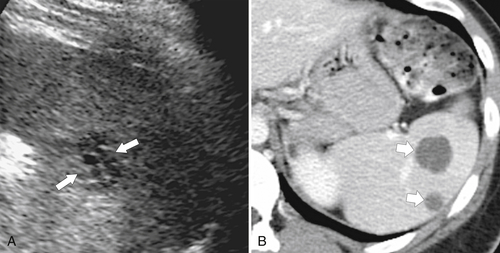
Figure 7-12 Axial contrast–enhanced CT in a 75-year-old woman with multiple tiny splenic lesions (arrows) caused by small lymphocytic lymphoma.
Figure 7-13 Axial contrast–enhanced CT in a 51-year-old woman with multiple hypodense splenic lesions (arrows) caused by B-cell lymphoma.
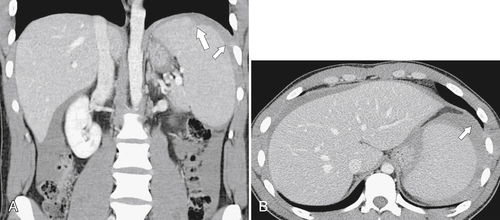
Figure 7-14 Axial noncontrast CT in a 37-year-old man with splenomegaly (arrows) caused by diffuse infiltration with Epstein-Barr virus舐induced lymphoma. There is associated ascites.
Figure 7-15 Axial (A) and coronal (B) contrast-enhanced CT in a 66-year-old woman demonstrating homogeneous splenomegaly (short arrows) caused by non-Hodgkin lymphoma. There is diffuse intraabdominal adenopathy (arrows).
Leukemia
Most leukemias involve the spleen, although this is less common with acute lymphoblastic leukemia. The disease typically affects
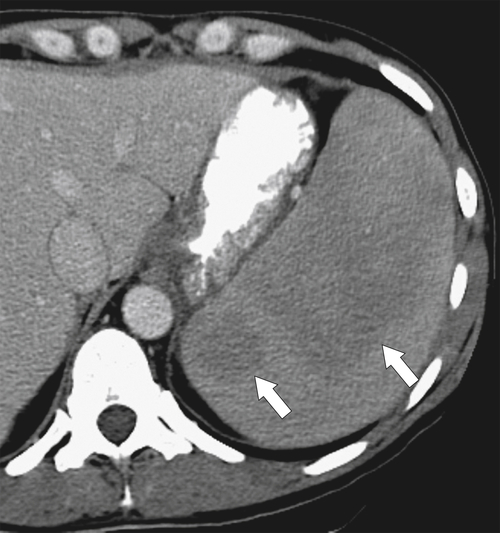
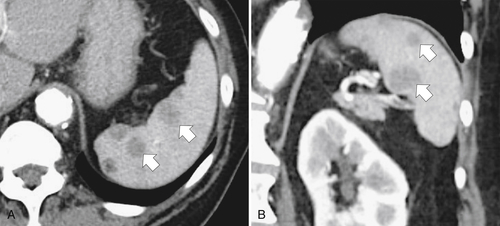
the spleen uniformly as an infiltrative process of malignant leukemic cells. Splenomegaly can reach massive sizes, particularly with chronic leukemias (especially chronic myelocytic leukemia) (Fig. 7-16). At imaging, the spleen appears uniformly and homogeneously enlarged. With massive splenomegaly the spleen may demonstrate some heterogeneous regions because of either tumor deposits or areas of splenic infarction (wedge shaped or rounded), since some anatomy of the speen is arterially compromised owing to its massive size (Fig. 7-16).
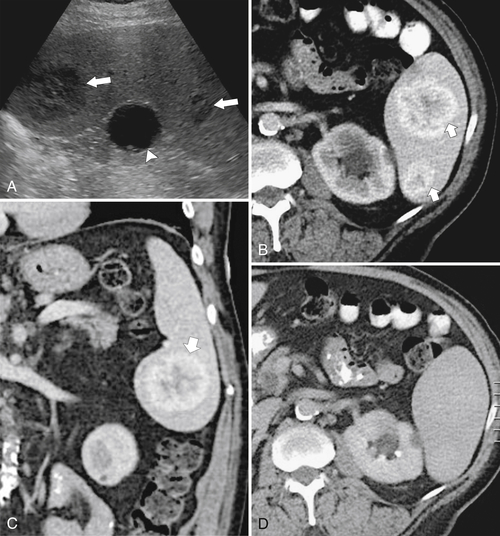
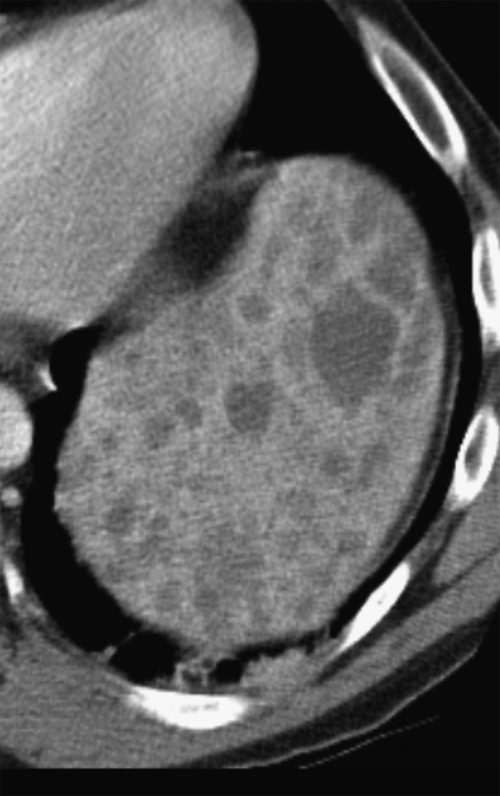
Figure 7-16 Sagittal US (A), axial (B), and coronal (C) contrast-enhanced CT in a 76-year-old woman with splenomegaly resulting from chronic lymphocytic leukemia. On US the spleen measures 21 cm in length. CT demonstrates small associated peripheral splenic infarctions (arrows).
Myeloproliferative Disease
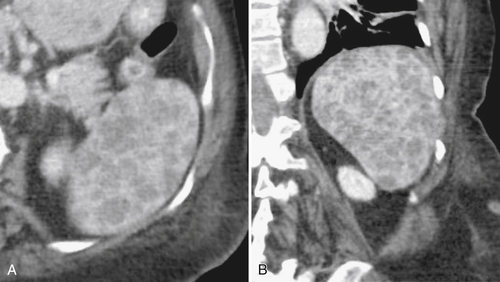
Figure 7-17 Plain frontal abdominal radiograph (A) in a 77-year-old woman with myelofibrosis and massive splenomegaly (arrows). Axial (B) and coronal (C) contrast-enhanced CT demonstrates splenomegaly and several small hypodense splenic tumors (small arrow). There is associated ascites.
Figure 7-18 Axial contrast-enhanced CT in a 58-year-old man with polycythemia rubra vera with splenomegaly and ill-defined splenic hypodensities (arrows) caused by extramedullary hematopoiesis.
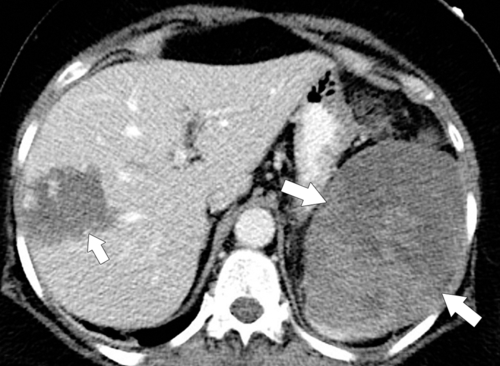
Splenic Metastases
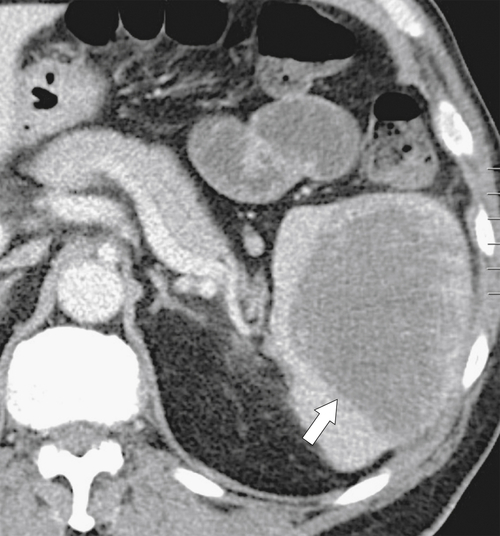
Hematogenous metastases are common because of the rich splenic vascular supply and are observed in numerous malignancies, but particularly melanoma, breast cancer, and lung cancer. There is often a history of malignant disease, which helps to differentiate single or multiple splenic lesions from other neoplastic, infectious, or infiltrative causes. Should metastatic disease cause splenomegaly, it is usually because of mass effect from single or, more often, multiple lesions rather than homogeneous enlargement (
Fig. 7-19).
Figure 7-19 Axial (A) and coronal (B) contrast-enhanced CT in a 74-year-old man with lung cancer demonstrating splenomegaly and numerous hypodense splenic metastases.
Infectious Splenomegaly
Viral Splenic Infection
Similar to other infective agents, viral splenic infection is usually associated with hepatomegaly (see
Chapter 6). It can be part of a generalized acute viral hepatitis (hepatitis A through E, human immunodeficiency virus [HIV]) or infectious mononucleosis, but can also be seen in cytomegalovirus and rubella infection. Infectious mononucleosis is secondary to infection with the Epstein-Barr
∗ virus (a herpes virus), and there is characteristic preferential splenic enlargement, which is at particular risk of spontaneous rupture, especially following contact sport injuries (
Fig. 7-21).
Figure 7-21 Coronal (A) and axial (B) contrast-enhanced CT in a 24-year-old woman with infectious mononucleosis and splenomegaly, splenic rupture (large arrow), and intraabdominal hemorrhage (small arrows).
Bacterial Splenic Infection
A number of bacterial infections can cause diffuse splenomegaly, particularly brucellosis, leptospirosis, and typhoid fever.
Mycobacterium tuberculosis and histoplasmosis often cause diffuse hepatosplenomegaly in the acute phase and are usually associated with concomitant multiple, hypodense lesions (
Fig. 7-22). Repeated embolic assault, particularly from bacterial endocarditis, can cause splenomegaly, including splenic abscess (Fig. 7-23).
Figure 7-22 Axial (A) and coronal (B) contrast-enhanced CT in a 53-year-old man with tuberculosis and mild splenomegaly and multiple hypodense tuberculous splenic lesions.
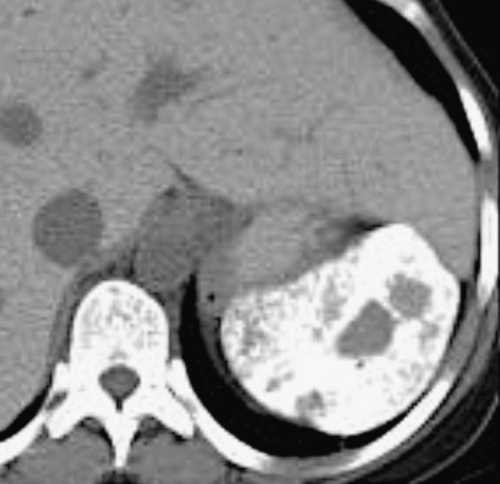
Figure 7-23 Axial contrast-enhanced CT in a 66-year-old man with splenomegaly and a 10-cm splenic abscess with air/fluid level (arrow) caused by gas-forming organisms.
Depositional Disease
Splenomegaly is usually secondary to primary systemic amyloidosis (AL-type) with accumulation of monoclonal immunoglobulin light chains within the spleen and multiple other organs.
Hypersplenism
Hypersplenism is a pancytopenia (erythrocytes, platelets, and granulocytes) to a variable degree caused by an enlarged spleen that is responsible for their premature destruction. Hypersplenism results from splenomegaly from almost any cause (
Box 7-2), but not all cases of splenomegaly cause hypersplenism. Hypersplenism may or may not be associated with increased bone marrow activity as a counter to the pancytopenia.
Autoimmune Disease
Many autoimmune conditions can cause splenomegaly (
Box 7-1).
Autoimmune Hemolytic Anemia
As its name suggests, autoimmune hemolytic anemia is an autoimmune reaction to red blood cells. The primary disease is idiopathic, whereas secondary disease can be caused by a number of lymphoproliferative or autoimmune disorders (systemic lupus erythematosus, ulcerative colitis, rheumatoid arthritis, and scleroderma). The damaged red blood cells are sequestered in the spleen, leading to splenomegaly.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is a systemic autoimmune disease with repeated episodes of vasculitis and inflammatory disease. In the acute phase, this can result in hepatosplenomegaly, although it more commonly causes glomerulonephritis, dermatological rashes, arthritis, and neuropsychiatric disorders.
Granulomatosis with Polyangiitis (Wegener Granulomatosis)
A multisystemic vasculitis, usually requiring lifelong immunosuppressive therapy, Wegener
∗ granulomatosis (granulomatosis with polyangiitis) can cause repeated splenic infarctions leading ultimately to a “shrunken” spleen (
Fig. 7-24).
Figure 7-24 Axial contrast-enhanced CT in a 41-year-old man with Wegener granulomatosis and a shrunken heterogeneous spleen (arrow) as a result of multiple infarctions.
Felty Syndrome
Splenomegaly is part of Felty
∗ syndrome with associated neutropenia (secondary to splenic sequestration from granulocytic abnormalities) and rheumatoid arthritis. The spleen can be markedly enlarged. Anemia and thrombocytopenia often accompany this syndrome.
Sjögren Syndrome
Sjögren
† syndrome is a systemic autoimmune disease that predominantly affects the exocrine glands (parotid and salivary glands). However, it is often associated with other autoimmune connective tissue disorders, and splenomegaly may result (
Fig. 7-25).
Figure 7-25 Axial (A) and coronal (B) contrast-enhanced CT in a 28-year-old woman with Sjögren syndrome and spelnomegaly (arrows).
Small or Shrunken Spleen (Box 7-2)
Congenital (Fanconi Syndrome)
A small spleen as a congenital condition is very uncommon but has been described in Fanconi‡ syndrome, a disease of the proximal renal tubules with loss of bicarbonate renal tubular acidosis and phosphate and rickets formation. Causes of Fanconi syndrome include cystinosis and Wilson∗ disease, among others.
Box 7-2 Causes of Small Spleen
Congenital (Fanconi syndrome)
Sickle cell anemia
Essential thrombocytopenia
Irradiation
Thorotrast
Wegener granulomatosis
Sickle Cell Disease
Although the spleen is commonly enlarged in the more acute phases of sickle cell disease, the repetitive splenic infarctions ultimately lead to a small, often fibrotic spleen that is effectively nonfunctioning, such that it is frequently termed an autosplenectomy (
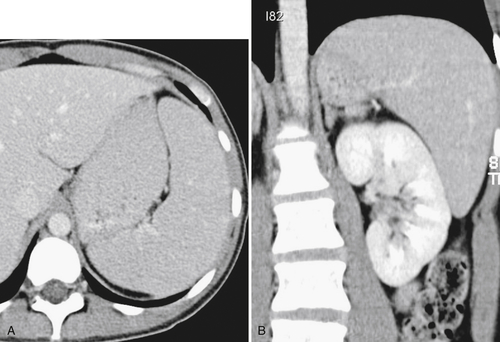
Fig. 7-26). The small residual mass may calcify (Fig. 7-27).
Figure 7-26 Axial contrast-enhanced CT in a 39-year-old woman with sickle cell disease and a small spleen (arrow) resulting from repeated thrombotic episodes.
Figure 7-27 Axial noncontrast CT in a 44-year-old woman with sickle cell disease and a small calcified “shrunken” spleen (arrow). The stomach is filled with oral contrast material (arrowheads).
Essential Thrombocythemia (Thrombocytosis)
Essential thrombocythemia, or thrombocytosis, is a myeloproliferative disease with overproduction of platelets by megakaryocytes in the bone marrow. Initially splenomegaly occurs, but ultimately numerous splenic infarcts caused by platelet aggregates can lead to a small spleen, as in sickle cell disease.
Splenic Irradiation
Radiation change that produces endarteritis obliterans and chronic multiple small splenic infarctions results in hyposplenism.
Thorotrast
The presence of Thorotrast is now rarely identified because its use as an intravascular angiographic contrast agent was terminated in the 1950s following the discovery that its alpha-emitting particles could lead to malignancies (mainly splenic angiosarcoma). It was readily sequestered by the mononuclear phagocytic system, mainly the liver and spleen, resulting in hyperdense organs (particularly the spleen). Thorotrast in the spleen sets up a chronic reactive process, ultimately leading to fibrosis and splenic contraction (
Fig. 7-28).
Figure 7-28 Axial contrast-enhanced CT in a 79-year-old man with previous Thorotrast contrast agent administration and a hyperdense spleen (arrow), representing sequestered Thorotrast.
Splenic Mass Lesions
Splenic mass lesions can be classified as either single or multiple, benign (
Table 7-1) or malignant (Table 7-2) lesions. Benign and malignant primary splenic lesions tend to be single, whereas metastatic and infectious lesions are multiple.
Table 7-1
Benign Splenic Lesions
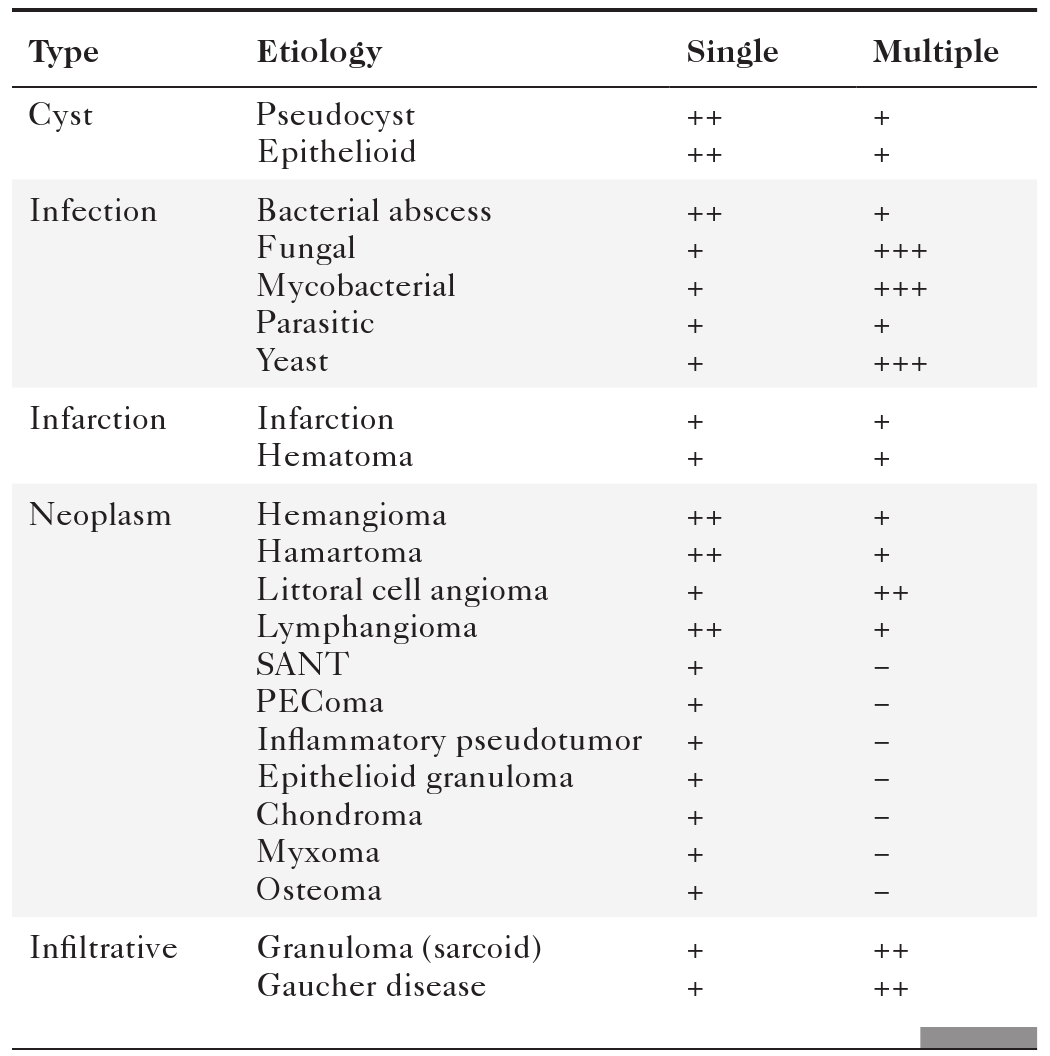
PEComa, Perivascular epitheliod cell tumor; SANT, sclerosing angiomatoid nodular transformation.
Table 7-2
Malignant Splenic Lesions
| Etiology |
Single |
Multiple |
| Lymphoma |
+ |
+ |
| Metastasis |
+ |
+ |
| Angiosarcoma |
++ |
+ |
| Hemangiosarcoma |
++ |
+ |
| Hemangiopericytoma |
++ |
− |
| Kaposi sarcoma |
+ |
++ |
| Fibrosarcoma |
+ |
− |
| Leiomyosarcoma |
+ |
− |
Benign Splenic Lesions
Gaucher Disease
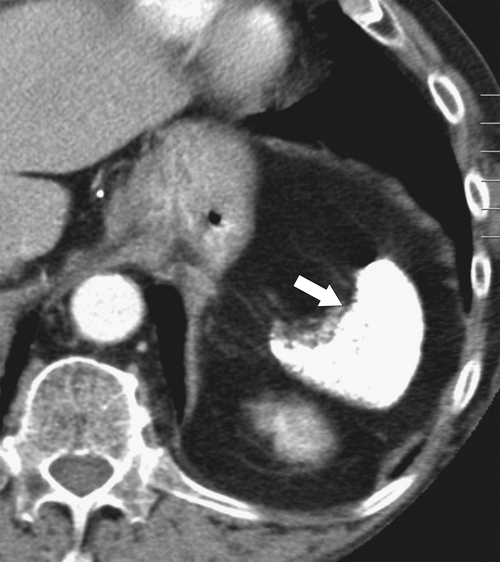
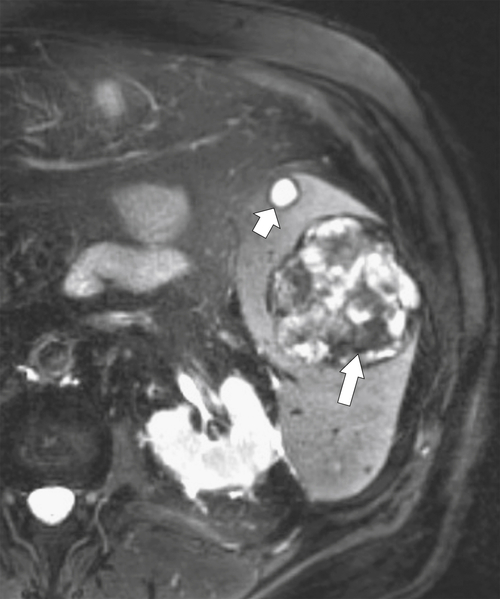
Figure 7-29 Axial T2 fat-saturated MRI in a 77-year-old with Gaucher disease demonstrating a complex splenic mass consistent with “gaucheroma” (large arrow). There is an incidental simple splenic cyst in the anterior spleen (small arrow).
Splenic Cysts
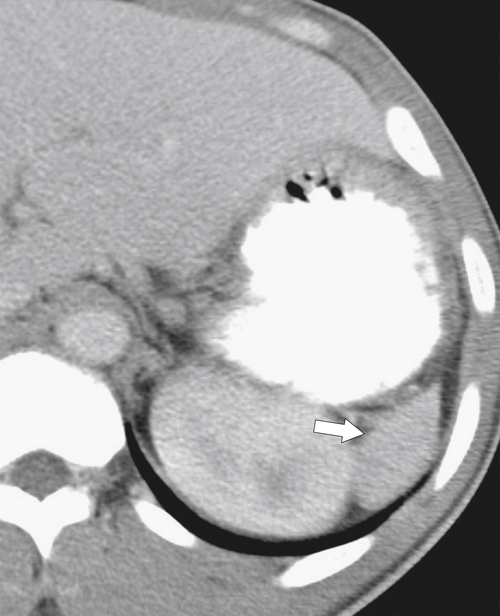
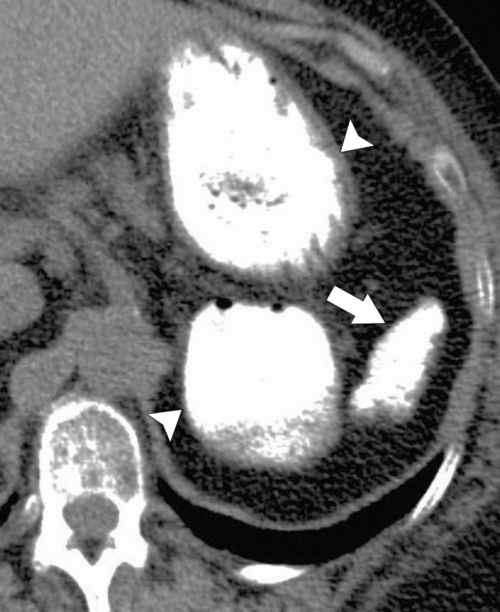
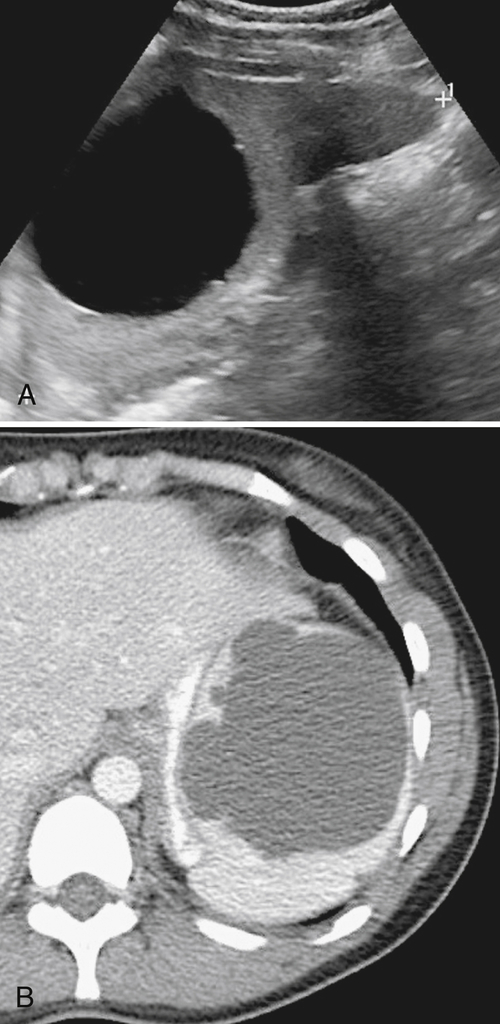
The majority of splenic cysts are thought to be pseudocysts and probably posttraumatic because they have no epithelial lining (
Fig. 7-30). True epidermoid cysts are less common, lined by epithelial tissue, and most likely congenital (Fig. 7-31). The walls of both types can calcify. They are not usually associated with cystic disease elsewhere (kidney, liver, pancreas). On imaging they are generally simple and are uniformly hypodense on CT or uniformly sonolucent on US (Fig. 7-31). On MRI they demonstrate typical cystic features of low T1-weighted and high T2-weighted signal. Occasionally they are complicated by secondary infection or hemorrhage.
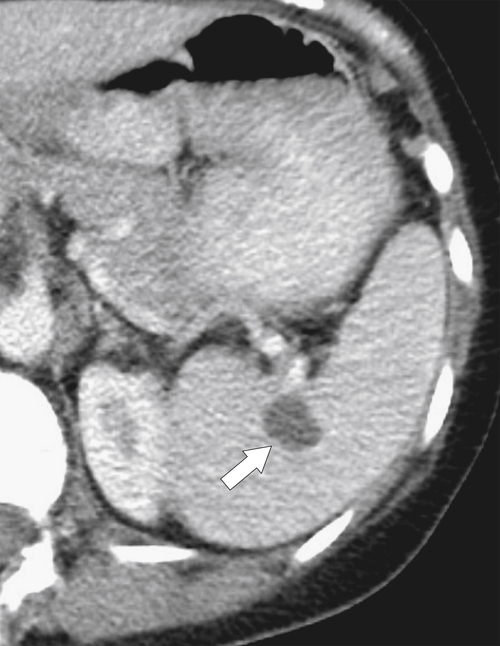
Figure 7-30 Axial contrast-enhanced CT in a 66-year-old woman with a 1.8-cm simple splenic cyst (arrow).
Figure 7-31 Sagittal US (A) and axial contrast-enhanced CT (B) in a 28-year-old woman with a sonolucent 7-cm lesion that is uniformly hypodense at CT and represents a splenic epidermoid cyst.
Splenic Infections
Most infective splenic lesions are multiple rather than solitary, although isolated splenic lesions are sometimes found.
Bacterial Abscess
Pyogenic abscesses may be single (
Fig. 7-22) but are more often multiple (
Fig. 7-33). They can be unilocular or multilocular and present with the expected symptoms of fever, pain, and elevated white blood cell count. They are often caused by septic emboli, particularly endocarditis. On CT they are nonenhancing, complex, low-density masses (Figs. 7-22 and 7-32).
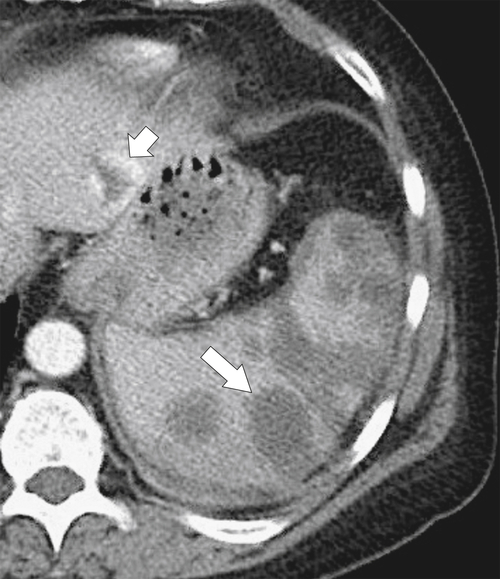
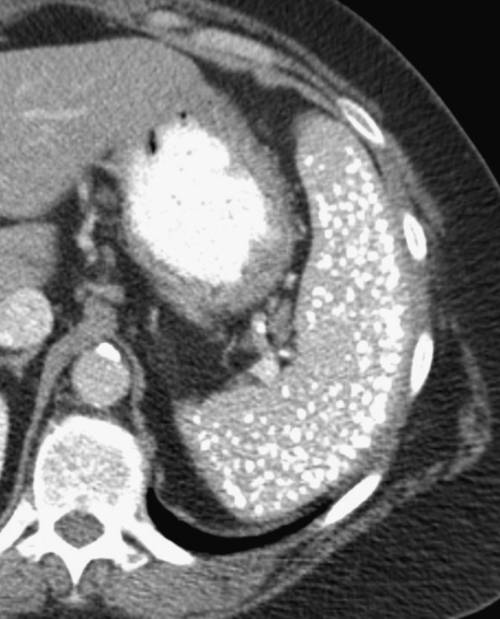
Figure 7-33 Axial contrast-enhanced CT demonstrating multiple hypodense fungal splenic masses (long arrow) in a 28-year-old woman taking high-dose steroids. There is an incidental liver hemangioma (short arrow).
Figure 7-32 Axial contrast-enhanced CT in a 17-year-old man with Bartonella sp. infection, or “cat-scratch fever,” demonstrating multiple splenic and hepatic hypodense bacterial lesions (arrows). There are also subtle hepatic lesions (small arrows).
Fungal Abscess
Fungal (
Candida, Cryptococcus, and
Aspergillus spp.) splenic abscesses may be single but are usually multiple (
Fig. 7-33) and often smaller than pyogenic abscesses. A history of immunosuppression strongly suggests the diagnosis. Histoplasmosis often heals by calcification (
Fig. 7-34).
Figure 7-34 Axial noncontrast CT in a 55-year-old man with previous histoplasmosis and multiple calcified splenic granulomata (arrow). An associated calcified granuloma is in the right lower lobe (short arrow).
Mycobacterial Infection
Tuberculous infection is rarely limited to the spleen. Patients are often immunocompromised. The splenic findings are nonspecific, with single or multiple low-density lesions that can be large (
Fig. 7-21) or small (
Fig. 7-35) on CT imaging. Similar to tuberculosis elsewhere, the infection often heals by calcification. Mycobacterium avium-intracellulare infection gives a similar appearance but is usually confined to patients with AIDS.
Figure 7-35 Axial contrast-enhanced CT in a 49-year-old man with numerous small hepatic and splenic hypodense lesions caused by tuberculosis. There is associated intraabdominal adenopathy (arrow).
Parasitic Disease
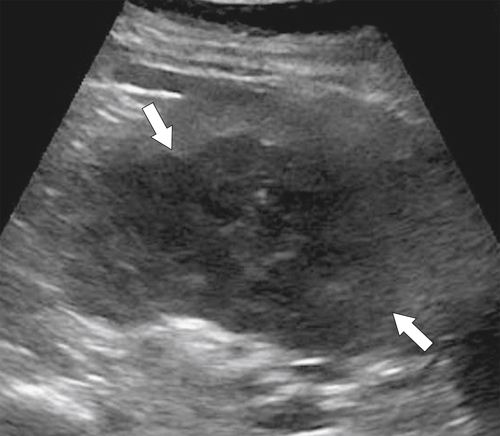
Hydatid (
Echinococcus granulosus) disease of the spleen is rare and usually occurs in association with hepatic disease. The splenic disease, like that of the liver, is much more likely to originate in countries where animal husbandry is common. The CT features are similar to those in the liver and include single or multiple irregular low-density cysts, some with daughter cysts, and a thick wall. The “drooping lily” sign of a collapsing cyst wall from cyst death is also recognized (
Fig. 7-36).
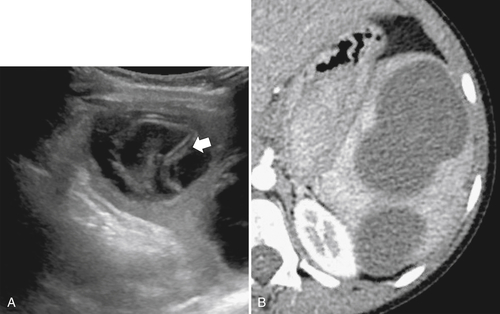
Figure 7-36 Sagittal US (A) and axial contrast-enhanced CT (B) in a 10-year-old girl with complex splenic cysts from hydatid disease. The “drooping lily” sign is better identified with US (arrow).
Yeast Infections
Infection with
Pneumocystis jirovecii (previously known as
Pneumocystis carinii and misclassified as a protozoan) usually occurs in patients with AIDS and may infiltrate both the liver and spleen and is commonly associated with pneumocystis pneumonia. CT imaging features demonstrate multiple low-density lesions, but these are nonspecific findings. Yeast infection differs from fungal disease as it may demonstrate calcification in some lesions. Lesions also tend to be larger than those seen in other splenic infective diseases (
Fig. 7-37).
Figure 7-37 Axial noncontrast CT in a 38-year-old man with AIDS and multiple low-density splenic lesions caused by Pneumocystis jirovecii infection.
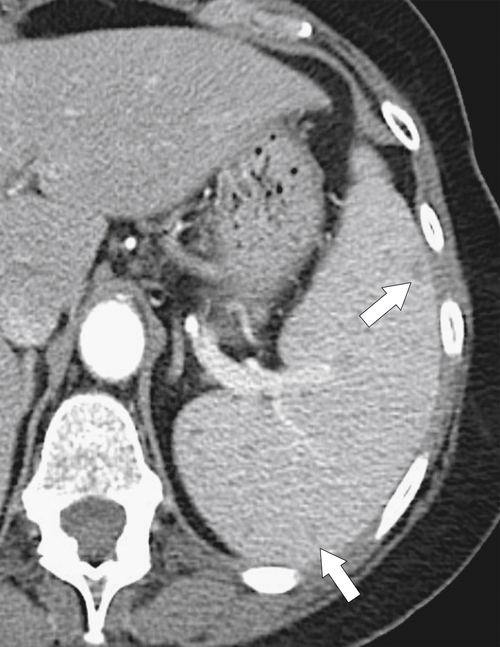
Splenic Infarct
Splenic infarcts are the most common cause of solitary or multiple splenic defects and should therefore always be considered in the differential dignosis for any discrete splenic lesion (
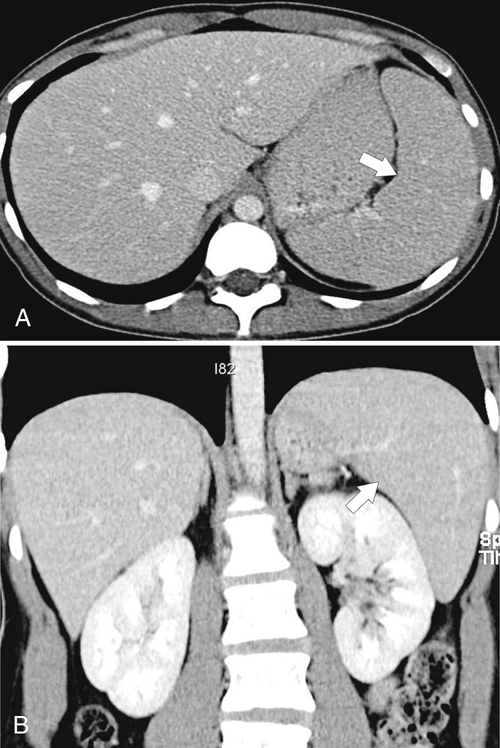
Box 7-3). Most demonstrate peripheral wedge-shaped hypoattenuating defects at CT (Fig. 7-38), best visualized after the administration of intravenous contrast material. Many splenic infarcts, however, do not demonstrate the classic peripheral wedge-shaped defect and are more irregular or even rounded in shape, both at the periphery and within the spleen (Figs. 7-13 and 7-39).
Box 7-3 Causes of Splenic Infarct
Embolic
Infectious endocarditis
Atheroma
Valve vegetations, atrial fibrillation
Mitral stenosis
Tumor
Lymphoma (hematogenous)
Leukemia
Pancreatic (local invasion)
Inflammatory
Pancreatitis
Connective tissue diseases
Thrombotic
Sickle cell disease
Polycythemia rubra vera
Hypercoagulable states (diffuse intravascular dissemination, malignancy)
Mechanical
Splenic torsion (i.e., wandering spleen)
Splenic trauma
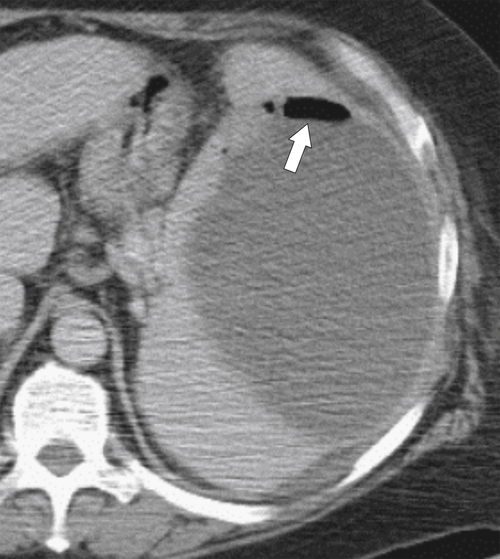
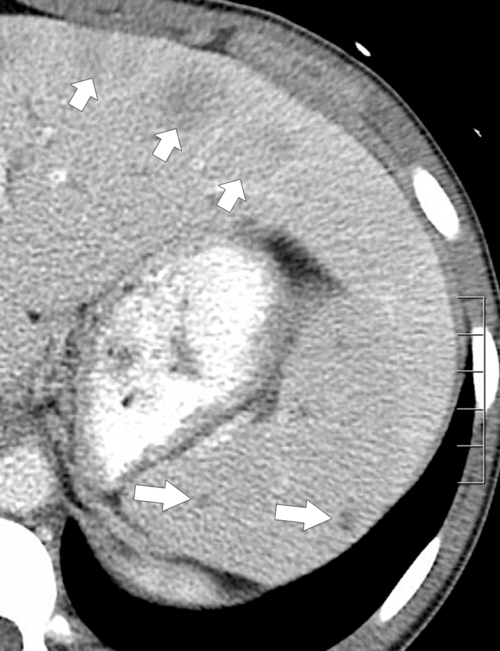
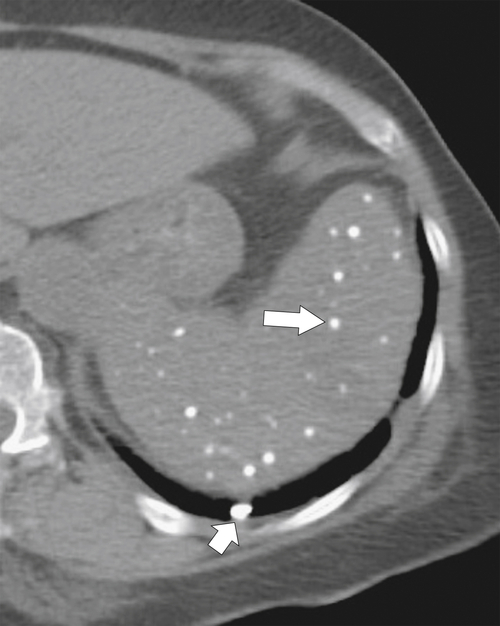
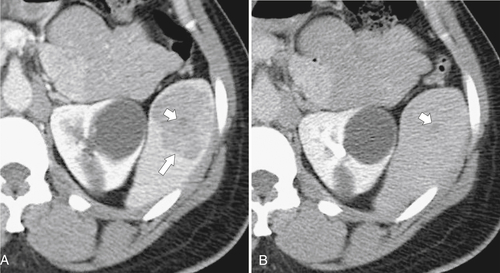
Figure 7-38 Axial contrast-enhanced CT in an 83-year-old woman with peripheral hypoattenuating nonenhancing splenic defects (arrows) caused by infarcts.
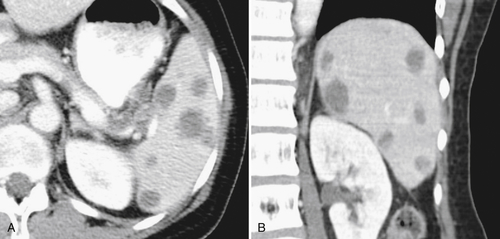
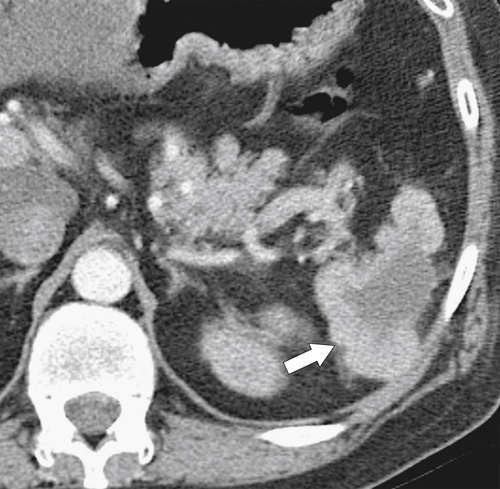
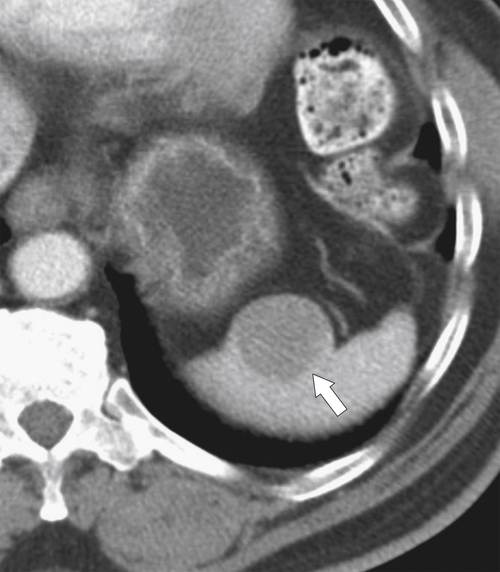
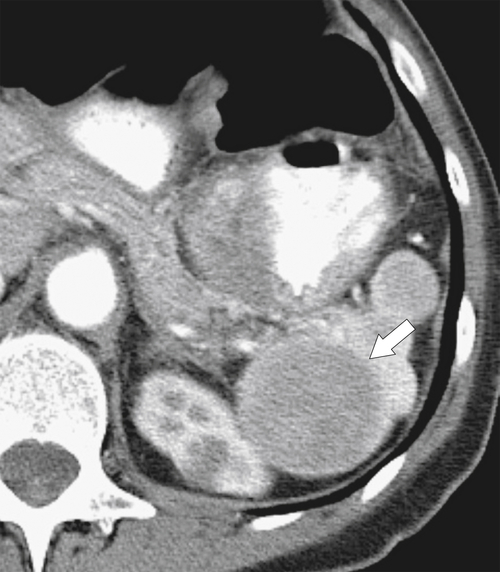
Figure 7-39 Coronal contrast-enhanced CT in a 43-year-old woman with a partially rounded hypodense splenic lesion (arrow) caused by an infarct. There is also splenomegaly.
Sarcoidosis
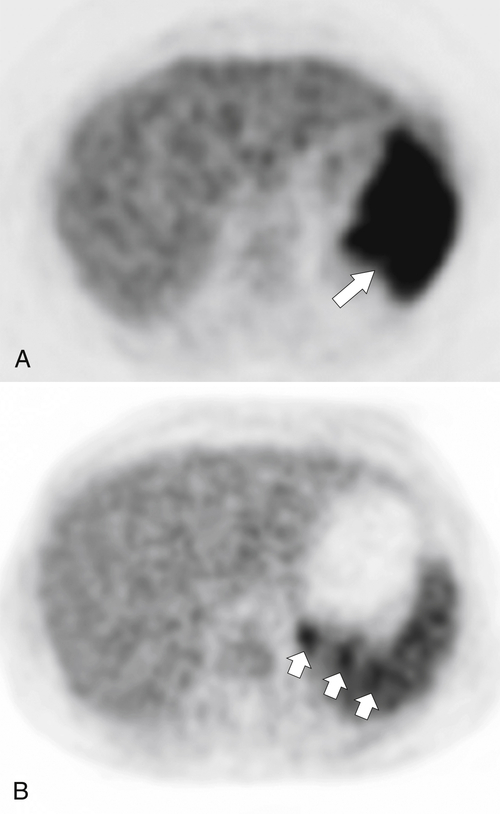
Granulomatous splenic infiltration can occur with sarcoidosis, recognized as numerous, smaller, hypodense lesions (
Fig. 7-40). PET imaging can determine the degree of splenic sarcoid as either diffuse or isolated (Fig. 7-41).
Figure 7-40 Axial contrast-enhanced CT in a 34-year-old woman with multiple splenic granulomas (arrows) from sarcoidosis.
Figure 7-41 PET imaging in two patients with splenic sarcoid, one with diffuse and marked FDG uptake (A,arrow), the other with discrete subtle areas of uptake (B,small arrows).
Epithelioid Granuloma
Epithelioid granuloma is a very rare, sometimes large, granulomatous mass with or without necrosis. It is caused by various systemic intraabdominal diseases involving multiple organs (
Fig. 7-42).
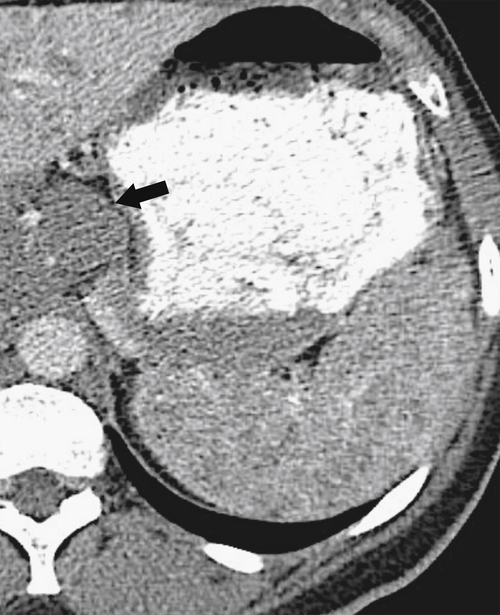
Figure 7-42 Axial contrast-enhanced CT in a 39-year-old man with an ill-defined, 3.5-cm splenic mass (arrows) due to an epithelioid granuloma.
Splenic Hemangioma
Although uncommonly seen by imaging, hemangioma is the most common benign splenic tumor and has been noted in up to 14% of autopsy studies. The sonographic appearances are variable, sometimes hyperechoic (
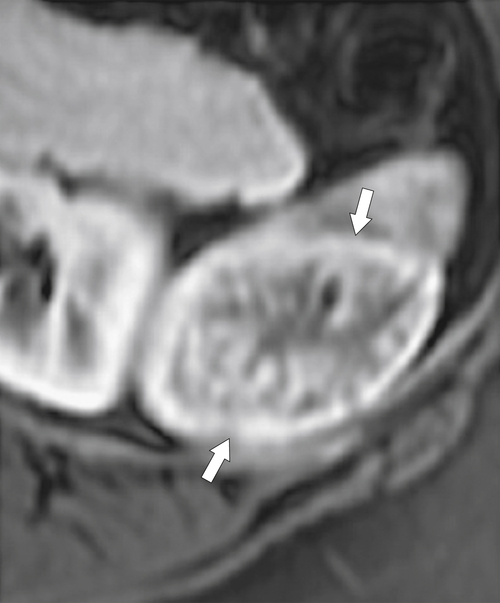
Fig. 7-43), like hepatic hemangiomas but in other cases complex and of mixed echogenicity (Fig. 7-44). The tumors can be solid or cystic, heterogeneous or homogeneous. They can uncommonly calcify. On contrast-enhanced cross-sectional imaging they demonstrate less reliable appearances, and so, unlike hemangiomas in the liver, many are difficult to characterize. For instance, they may demonstrate peripheral enhancement after the administration of IV contrast medium that progressively fills in toward the center (Fig. 7-44), but these appearances are unpredictable. At MRI, they may or may not show hyperintense T2 signal and their enhancement is similar to that observed with CT. The major differential diagnosis is with splenic hamartoma, whose enhancement characteristics can be identical.
Figure 7-43 Sagittal US in a 36-year-old woman with a 3.5-cm hyperechoic splenic mass (arrows) representing a hemangioma.
Figure 7-44 Sagittal US (A), axial (B, D), and coronal (C) contrast-enhanced CT in a 70-year-old man with splenic hemangiomas. US demonstrates hypoechoic splenic lesions (arrows), and CT demonstrates heterogeneous but predominantly peripherally enhancing lesions (small arrows). The lesions show complete contrast “fill-in” on delayed images such that they are barely perceptible against the normal parenchyma. There is an incidental renal cyst (arrowhead).
Splenic Hamartoma
Hamartomas are uncommon vascular tumors of the spleen that are usually an incidental finding. They are generally hyperechoic on US and can be difficult to visualize. On CT they appear similar to hemangiomas, although the enhancement tends to be more heterogeneous with variable filling-in toward the center on delayed images (
Fig. 7-45). They can therefore be difficult or impossible to differentiate from hemangiomas by imaging. On MRI they are often isointense on T1-weighted imaging and slightly hyperintense on T2-weighted images (Fig. 7-45). As with CT, they generally show peripheral nodular enhancement and variable centripetal filling-in of contrast material. Similar to hemangiomas, they can also show areas of calcification.
Figure 7-45 Axial (A) and coronal (B) contrast-enhanced CT and coronal T2-weighted (C) and postcontrast fat-saturated T1-weighted (D) MRI in a 45-year-old woman with a slightly T2 hyperintense and heterogeneously enhancing 5-cm splenic mass (arrows). The appearance was similar to a hemangioma but proved to be a hamartoma.
Splenic Lymphangioma
Splenic lymphangiomas are rare and classically of low density at CT, sometimes cyst like, with thin walls and sharp margins, often in a subcapsular location. The walls may demonstrate curvilinear calcifications. Fine internal septa are frequently seen (
Fig. 7-46) and are better identified after the administration of IV contrast agent or with MRI.
Figure 7-46 Sagittal US (A) and axial (B) contrast-enhanced CT in a 38-year-old woman with splenic lymphangioma. US demonstrates an ill-defined, small, complex, cyst-like structure (arrows) with internal septa. CT demonstrates low-density complex cysts with the septa barely visible (small arrows).
Littoral Cell Angioma
Littoral cell angiomas are rare benign vascular splenic tumors arising from littoral cells in the red pulp sinuses. They can be as large as 6 to 8 cm and are often multiple. They appear hypodense on noncontrast CT imaging and demonstrate irregular enhancement after administration of IV contrast material (
Fig. 7-47). They may become relatively isodense with the spleen on delayed imaging, which makes differentiation from splenic hamartomas and hemangiomas difficult because they can show similar features. Usually the diagnosis can be made only by percutaneous biopsy or splenectomy.
Figure 7-47 Axial (A) and coronal (B) contrast-enhanced CT in a 66-year-old woman with multiple hypodense splenic lesions (arrows) that proved to be littoral cell angiomas.
Sclerosing Angiomatoid Nodular Transformation
Sclerosing angiomatoid nodular transformation (SANT) is a rare benign vascular tumor composed of multiple red pulp nodules made from endothelial cells interspersed with fibrous bands, sometimes with a central scar. At imaging, unless the scar is identified, there are no particular identifying features. The tumors are typically hypodense but may demonstrate some peripheral enhancement and, like hemangiomas and hamartomas, can become isodense with the spleen on delayed imaging (
Fig. 7-48).
Figure 7-48 Axial portal venous phase (A) and delayed (B) contrast-enhanced CT in a 48-year-old woman with a 4-cm heterogeneously enhancing mass (arrows) representing a sclerosing angiomatoid nodular transformation (SANT) that is almost isodense with the spleen on delayed imaging. There is a small central nonenhancing scar (small arrows).
Perivascular Epithelioid Cell Tumor
The perivascular epithelioid cell tumor (PEComa; clear cell “sugar” tumor) is a very rare benign lesion, far more common in women, that more commonly occurs in the lung. It has been described anywhere in the abdomen. It is a mesenchymal neoplasm related to angiomyolipoma and lymphangiomyomatosis, both of which are more common in tuberous sclerosis. Contrast-enhanced CT demonstrates diffuse heterogeneity with some areas of intense enhancement and other areas with little or no enhancement (
Fig. 7-49).
Figure 7-49 Axial contrast-enhanced CT in a 70-year-old woman with a complex heterogeneous mass (arrows) in the spleen that is a PEComa (“sugar” tumor).
Inflammatory Pseudotumor
Inflammatory pseudotumors, also known as inflammatory myofibroblastic tumors, are rare benign tumors that contain inflammatory cells (lymphocytes, plasma cells, and eosinophils) and can occur within any organ in the body. The pathogenesis is uncertain. At imaging they typically demonstrate enhancement (
Fig. 7-50), but it is not possible to differentiate them from other benign or malignant splenic lesions. Biopsy or surgical removal is required for diagnosis.
Figure 7-50 Axial T1-weighted fat-saturated contrast-enhanced MRI in a 44-year-old man with a rounded heterogeneously enhancing splenic mass (arrows) that proved to be an inflammatory pseudotumor.
Malignant Splenic Lesions
Lymphoma (see also Chapter 4)
Lymphomatous splenic involvement is the most common malignancy to affect the spleen. In addition to diffuse splenomegaly described earlier in this chapter, lymphoma can present with focal or multiple discrete masses (
Figs. 7-10 and
7-11), often indistinguishable from other splenic diseases. The lymphoma is usually secondary to non-Hodgkin or Hodgkin lymphoma elsewhere, but when primary, it is commonly associated with AIDS.
Metastases
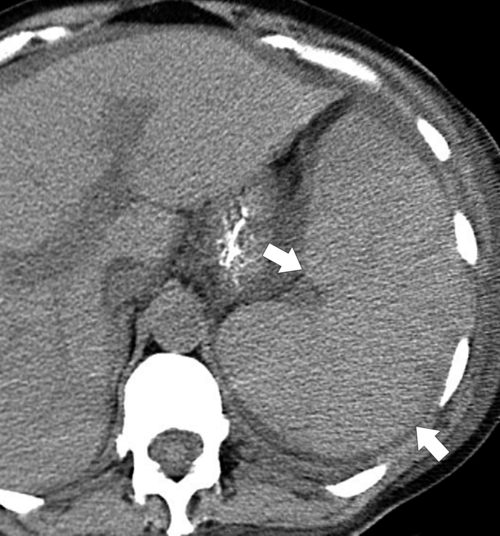
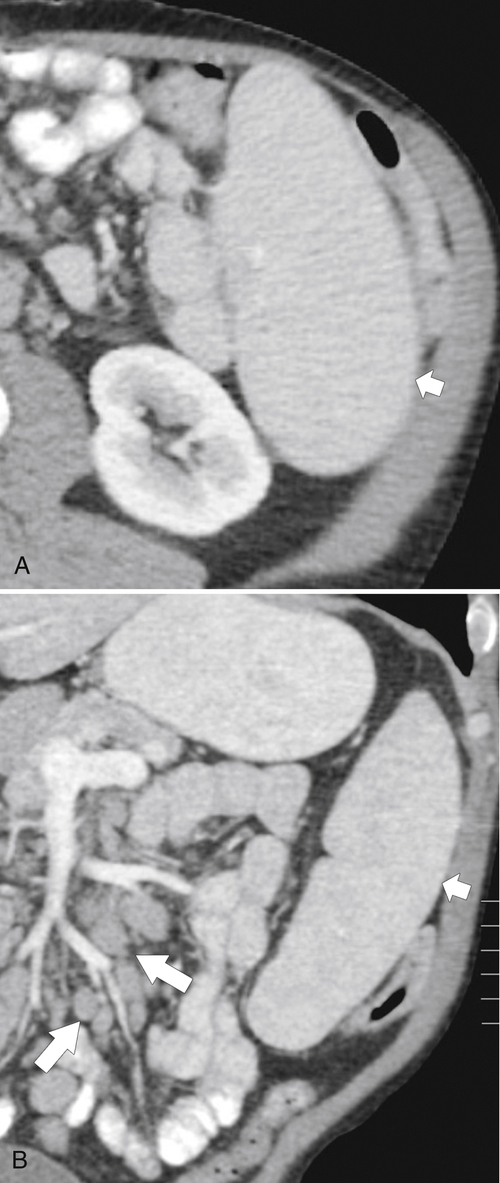
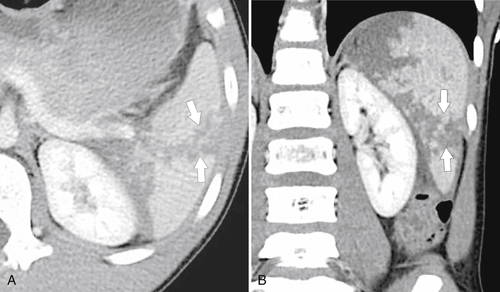
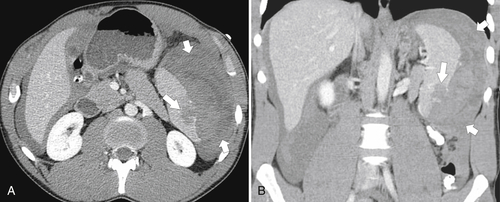
Splenic metastases are less common than hepatic metastases, but given the vascularity of the spleen, it is a relatively common site for hematogenous spread of metastatic disease. The most common splenic metastases are from breast, lung, stomach, and ovarian cancer and from melanoma. They may be single (
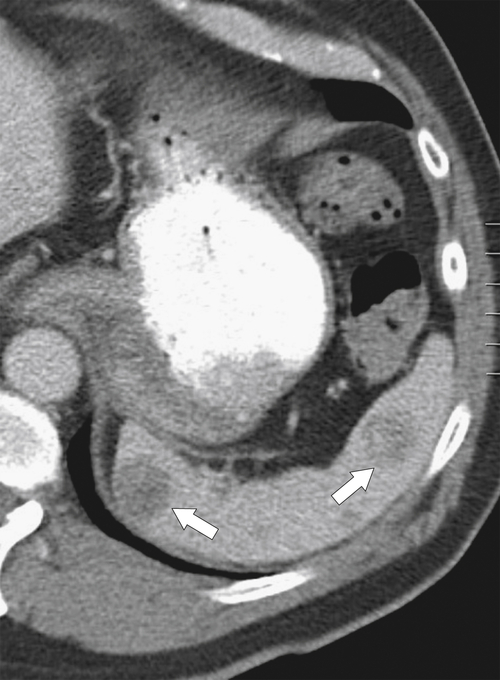
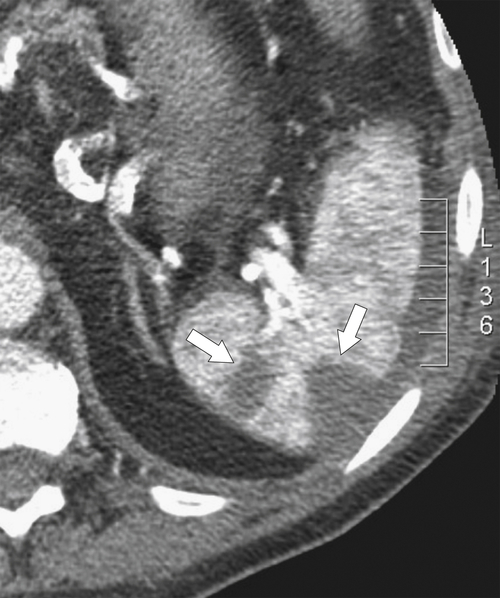
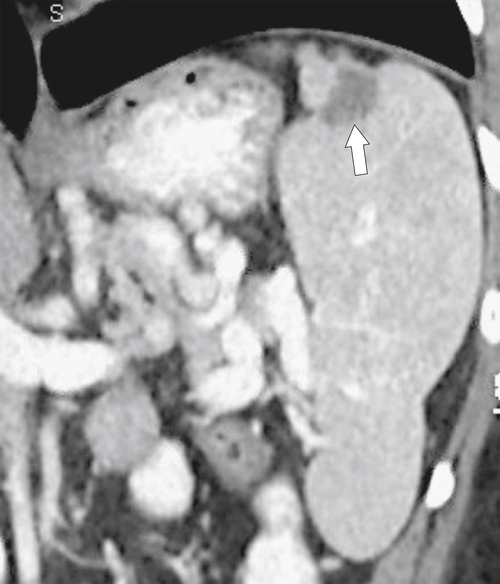
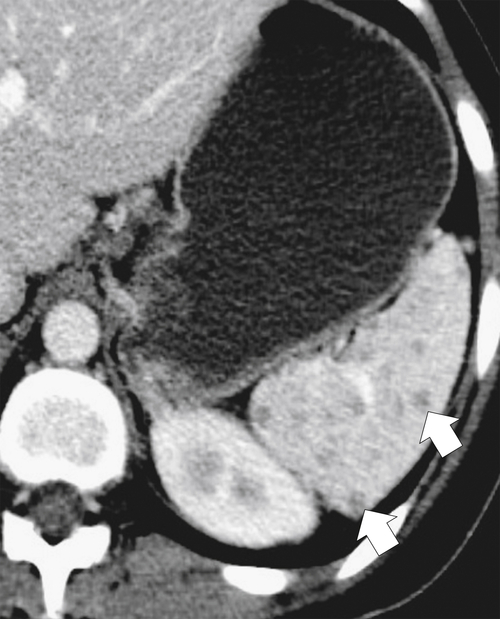
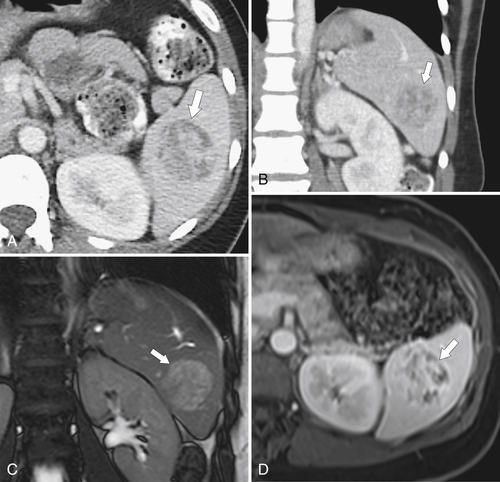
Fig. 7-51), or multifocal, solid or cystic (Fig. 7-52), They are typically hypoechoic on US (Fig. 7-53) and hypodense on CT and are difficult to differentiate from other solitary or multiple splenic lesions. If disease has spread to the spleen by the peritoneal route (e.g., gastric and ovarian cancer), the lesions may be capsular (Fig. 7-54). Other tumors (e.g., pancreas, colon, stomach) may invade the spleen by direct invasion (Fig. 7-55).
Figure 7-51 A and B, Axial contrast-enhanced PET/CT in a 48-year-old man with splenic metastases from melanoma (arrows). This solitary lesion cannot be differentiated from many other benign splenic lesions by CT alone.
Figure 7-52 Axial contrast-enhanced CT in a 60-year-old man with lung cancer and multiple cystic-appearing splenic and hepatic metastases (arrows). Abdominal ascites (arrowheads) and a splenic infarct (small arrow) are also present.
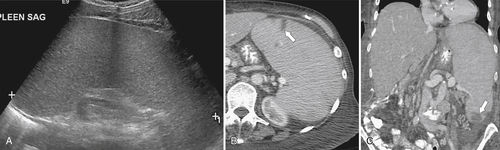
Figure 7-53 Sagittal US in a 71-year-old woman with a 7-cm hypoechoic splenic metastasis (arrows) from breast carcinoma.
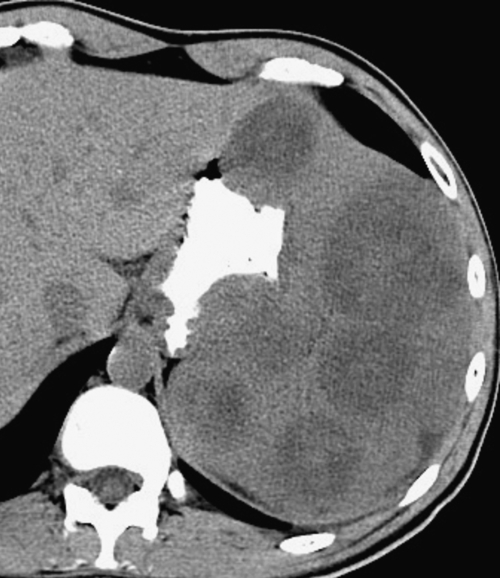
Figure 7-54 Coronal contrast-enhanced CT in a 49-year-old woman with splenic capsular invasion (arrows) from ovarian cancer.
Figure 7-55 Axial contrast-enhanced CT in a 68-year-old man demonstrating a large pancreatic tail adenocarcinoma with direct invasion into the spleen (arrows).
Angiosarcoma
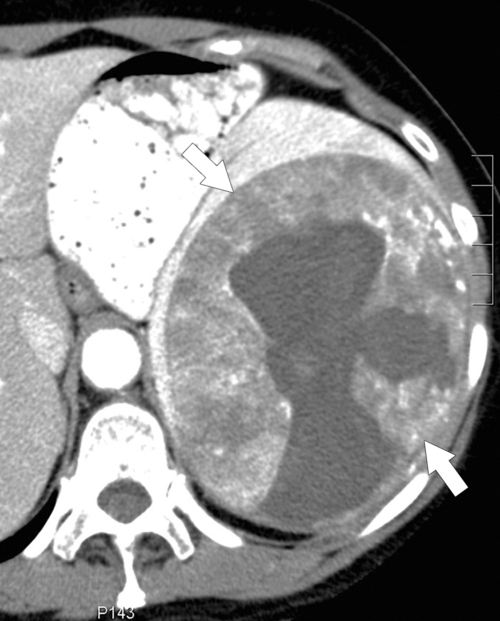
Angiosarcoma is the second most common primary splenic malignant tumor after lymphoma. A rare, aggressive tumor associated with a poor prognosis, angiosarcoma is sometimes associated with prior Thorotrast use because of its alpha-emitting properties. As its name suggests, angiosarcoma is highly vascular in appearance and metastasizes early. Contrast-enhanced CT imaging often demonstrates splenomegaly with a heterogeneous hypervascular mass, which can be either focal or diffuse. Not all tumors avidly enhance, however (
Fig. 7-56). The tumor may demonstrate increased density before contrast enhancement because of its highly vascular nature. There is often tumoral calcification.
Figure 7-56 Axial contrast-enhanced CT in a 55-year-old man with a 3-cm splenic mass (arrow) that has relatively poor enhancement and proved to be angiosarcoma at surgery.
Kaposi Sarcoma
Induced virally (human herpes virus 8), Kaposi sarcoma is often associated with AIDS and typically presents with cutaneous lesions, but it can be present anywhere in the mediastinum and
abdomen. Kaposi sarcoma is rare in the spleen but can present there as either a single mass or multiple masses (Fig. 7-57).
Figure 7-57 Axial contrast-enhanced CT in a 33-year-old man with HIV infection and multiple splenic masses caused by Kaposi sarcoma.
Very Rare Splenic Neoplasms
Very rare splenic neoplasms include epithelioid tumors, hemangioendothelioma, hemangiopericytoma (
Fig. 7-58), malignant fibrous histiocytoma, follicular dendritic cell tumor (Fig. 7-59), fibrosarcoma, and leiomyosarcoma. They are usually focal, heterogeneous, hypervascular, splenic masses on contrast-enhanced CT, and tissue diagnosis is usually made by splenectomy or percutaneous biopsy.
Figure 7-58 Axial contrast-enhanced CT in a 55-year-old man with a solid poorly enhancing splenic mass (arrow) representing hemangiopericytoma.
Figure 7-59 Axial contrast-enhanced CT in a 75-year-old man with follicular dendritic cell tumor (arrow). The mass is heterogeneous at CT and cannot be differentiated from other malignant splenic lesions by CT alone.
Splenic Trauma
Usually occurring as blunt trauma (e.g., motor vehicle crash injuries) rather than penetrating injuries (e.g., gunshot or knife injuries), splenic trauma may also be caused by spontaneous rupture from marked splenomegaly (e.g., in infectious mononucleosis) (
Fig. 7-20). The diagnosis is usually based on the appropriate history, signs of left upper quadrant pain, and contrast-enhanced
CT, which demonstrates a low-attenuation splenic laceration that fails to “fill in” with IV contrast medium on delayed imaging. The laceration may be severe enough to cause splenic fracture (Fig. 7-60) or a contained laceration (Fig. 7-61), both of which can cause severe hemorrhage. There may be active extravasation as evidenced by a high-density focus (similar in attenuation to the aorta), with surrounding, slightly lower density hemorrhage (but still hyperdense relative to the spleen and surrounding tissues) (Fig. 7-62). Hemorrhage may be within the spleen, in a subcapsular region, or freely into the peritoneum. With severe trauma there may be splenic avulsion, with little or no enhancement within the spleen but diffuse hemorrhage from the ruptured splenic artery.
Figure 7-60 Axial (A) and coronal (B) contrast-enhanced CT in a 24-year-man who has a motor vehicle injury with splenic fracture (arrows) and perisplenic hemorrhage.
Figure 7-61 Axial (A) and coronal (B) contrast-enhanced CT in a 23-year-old man with splenic trauma incurred during a hockey game. An inferior splenic laceration (arrows) and extensive perisplenic hyperdense blood and peritoneal hemorrhage (small arrows) can be seen.
Figure 7-62 Axial contrast-enhanced CT in a 56-year-old woman after a motor vehicle injury with splenic trauma and active arterial extravasation (large arrow) and abdominal hemorrhage (small arrow).
Splenic Calcification
Splenic calcification most commonly results from prior infection, tuberculosis, histoplasmosis (
Fig. 7-34), or hydatid disease (
Fig. 7-63 and Box 7-4). Repeated splenic infarctions from sickle cell disease often create either a completely (Fig. 7-26) or partially calcified spleen (Fig. 7-64). Some vasculitic diseases may heal with diffuse splenic calcification (Fig. 7-65).
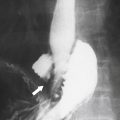
Figure 7-63 Plain upper abdominal radiograph (A) and axial noncontrast CT (B) in a 64-year-old man with a calcified splenic mass (arrows) resulting from prior treated splenic echinococcal infection.
Box 7-4 Splenic Calcification
Vascular calcification
Tuberculosis
Histoplasmosis
Hydatid disease
Sickle cell disease
Vasculitis (SLE)
Prior hemorrhage
Figure 7-64 Axial noncontrast CT in a 38-year-old woman with irregular splenic calcification caused by sickle cell disease.
Figure 7-65 Axial contrast-enhanced CT in a 56-year-old woman with systemic lupus erythematosus and multiple splenic calcifications.
Suggested Readings
Andrews M.W. Ultrasound of the spleen. World J Surg 2000;24(2):183–187.
Anis M. et al. Imaging of abdominal lymphoma. Radiol Clin North Am 2008;46(2):265–285: viii-ix.
Bensinger T.A. et al. Thorotrast-induced reticuloendothelial blockage in man. Am J Med 1971;51:663–668.
Bessoud B. et al. Nonoperative management of traumatic splenic injuries: is there a role for proximal splenic artery embolization? AJR 2006;186:779–785.
Brancatelli G. et al. Case 80: splenosis. Radiology 2005;234(3):728–732.
Brancatelli G. et al. Fibropolycystic liver disease: CT and MR imaging findings. Radiographics 2005;25(3):659–670.
Chua S.C. et al. Iimaging features of primary extranodal lymphomas. Clin Radiol 2009;64(6):574–588.
Dachman A.H. et al. Nonparasitic splenic cysts: a report of 52 cases with radiologic-pathologic correlation. AJR 1986;147:537–542.
de Jong P.A. et al. CT and 18F-FDG PET for noninvasive detection of splenic involvement in patients with malignant lymphoma. AJR 2009;192:745–753.
Doody O. et al. Blunt trauma to the spleen: ultrasonographic findings. Clin Radiol 2005;60(9):968–976.
Elsayes K.M. et al. MR imaging of the spleen: spectrum of abnormalities. Radiographics 2005;25(4):967–982.
Freeman J.L. et al. CT of congenital and acquired abnormalities of the spleen. Radiographics 1993;13(3):597–610.
Fulcher A.S. et al. Abdominal manifestations of situs anomalies in adults. Radiographics 2002;22(6):1439–1456.
Harris G.N. et al. Accessory spleen causing a mass in the tail of the pancreas: MR imaging findings. AJR 1994;163(5):1120–1121.
Lee W.K. et al. Abdominal manifestations of extranodal lymphoma: spectrum of imaging findings. AJR 2008;191(1):198–206.
Leite N.P. et al. Cross-sectional imaging of extranodal involvement in abdominopelvic lymphoproliferative malignancies. Radiographics 2007;27(6):1613–1634.
Levy A.D. et al. Littoral cell angioma of the spleen: CT features with clinicopathologic comparison. Radiology 2004;230(2):485–490.
Luna A. et al. MRI of focal splenic lesions without and with dynamic gadolinium enhancement. AJR 2006;186:1533–1547.
Marmery H. et al. Optimization of selection for nonoperative management of blunt splenic injury: comparison of MDCT grading systems. AJR 2007;189:1421–1427.
Mortele K.J. et al. CT features of the accessory spleen. AJR 2004;183(6):1653–1657.
Nunweiler C.G. et al. The imaging features of nontuberculous mycobacterial immune reconstitution syndrome. J Comput Assist Tomogr 2009;33(2):242–246.
Paterson A. et al. A pattern-oriented approach to splenic imaging in infants and children. Radiographics 1999;19(6):1465–1485.
Radin D.R. et al. Visceral and nodal calcification in patients with AIDS-related Pneumocystis carinii infection. AJR 1990;154:27–31.
Rezai P. et al. Splenic volume model constructed from standardized one-dimensional MDCT measurements. AJR 2011;196:367–372.
Singh A.K. et al. Image-guided percutaneous splenic interventions. Radiographics 2012;32(2):523–534.
Thanos L. et al. Percutaneous CT-guided drainage of splenic abscess. AJR 2002;179(3):629–632.
Thompson W.M. et al. Angiosarcoma of the spleen: imaging characteristics in 12 patients. Radiology 2005;235(1):106–115.
Thorelius L. Emergency real-time contrast-enhanced ultrasonography for detection of solid organ injuries. Eur Radiol 2007;17(Suppl 6):F107–F111.
Urrutia M. et al. Cystic masses of the spleen: radiologic-pathologic correlation. Radiographics 1996;16(1):107–129.
∗ Harald Hirschsprung (1830-1916), Danish physician.
∗ Phillippe Gaucher (1854-1918), French dermatologist.
∗ Albert Niemann (1880-1921), German pediatrician; Ludwig Pick (1868-1935), German physician.
† Paul Langerhans (1847-1888), German pathologist.
∗ Alfred Hand (1868-1949), US pediatrician; Artur Schüller (1822-1884), Austrian physician; Henry A. Christian (1876-1951), American internist.
† Eric Letterer (1895-1982), German pathologist; Sture A. Siwe (1897-1966), Swedish pediatrician.
∗ Carlos Gamna (1866-1950), Italian physician; Charles Gandy (1872-1943), French physician.
† Alan Lloyd Hodgkin (1914-1998), British physiologist.
∗ Michael A. Epstein (1921- ), British pathologist and virologist; Yvonne Barr (1932- ), British virologist.
∗ Friedrich Wegener (1907-1990), German pathologist
∗ Augustus R. Felty (1895-1964), U.S. internal medicine physician.
† Henrick Sjögren (1899-1986), Swedish ophthalmologist.
‡ Guido Fanconi (1892-1979), Swiss pediatrician.
∗ Samuel A.K. Wilson (1878-1937), British neurologist.