CHAPTER 222 Spine Tumors in Children
Pediatric tumors affecting the spine arrive via multiple pathologic pathways, including intrinsic tumors of the osseous elements, local invasion from adjacent disease, and metastatic disease from distant neoplastic processes. As a result, the differential diagnosis can be broad in any child with a spinal column lesion.1–8
The overwhelming majority of spinal tumors cause pain.7,9,10 Typically, this pain is localized to the spine in the general location of the lesion. Most patients report worsening of that pain at night, although many complain of pain during the day. Other initial complaints depend largely on the cranial-caudal location, the anterior-posterior position, the extent of neural compression, the presence of associated instability and spinal deformity, and the tumor’s histology. The duration of symptoms is a function of both the location and pathology. Benign lesions that are accompanied by neurological findings often have a long-standing history of preceding pain and decreased range of motion; malignant tumors typically have a shorter duration of symptoms. The deformity seen in relation to spinal tumors is thought to be caused by either destruction or deformation of normal anatomy in the growing spine or the body’s response to pain.11–16
Data describing the epidemiology of spine tumors are not as well studied as those for bone tumors in general. We obtain an understanding of the incidence when we examine bone malignancies. Cancer related to bone tumors has an incidence rate of about 0.9 per 100,000 children aged 0 to 19 years in the United States.17 Spine tumors are a subset of this population. This chapter focuses on primary pediatric tumors of the spine.
Benign Tumors
Osteoid Osteoma
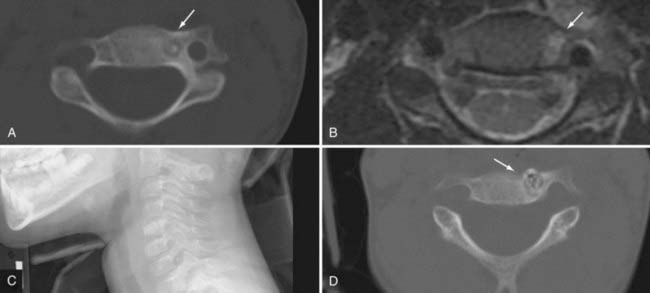
Osteoid osteomas are benign tumors of bone characterized by a small nidus of calcified osteoid tissue with a vascular connective tissue matrix.18–24 The osteoid tissue and trabeculae stimulate bone formation, which results in the surrounding dense sclerotic margin. Because the lesion is typically more vascular than the adjacent normal bone, one can often see an increased reddish blue color on gross examination. Over time, the lesions undergo calcification. The upper limit of size is 1.5 cm, although most lesions are generally smaller than 1.0 cm.
Osteoid osteoma and osteoblastoma together account for approximately 7% to 12% of all benign tumors of bone.25–30 There is a gender difference that varies from a male-to-female ratio of 2 : 1 to 6 : 1. The most common age at initial evaluation is in the second decade of life. These lesions are commonly found in the posterior elements of the spine with a predilection for the lumbar spine, followed by the cervical, thoracic, and sacral segments. The imaging findings reveal a lytic defect with a sclerotic margin. Computed tomography (CT) demonstrates the lesion better than plain films do, especially with smaller tumors. Magnetic resonance imaging (MRI) can make the lesion appear larger with increased surrounding edema. The lesion frequently has a hypointense central area surrounded by isointense to hyperintense edema on T2-weighted imaging (Fig. 222-1). Other imaging modalities such as bone scans may help confirm the diagnosis but are not necessary with adequate CT and MRI evaluation. More likely, bone scans may be useful to help localize the area of interest if the history and examination do not provide adequate clues to guide imaging.
Osteoblastoma
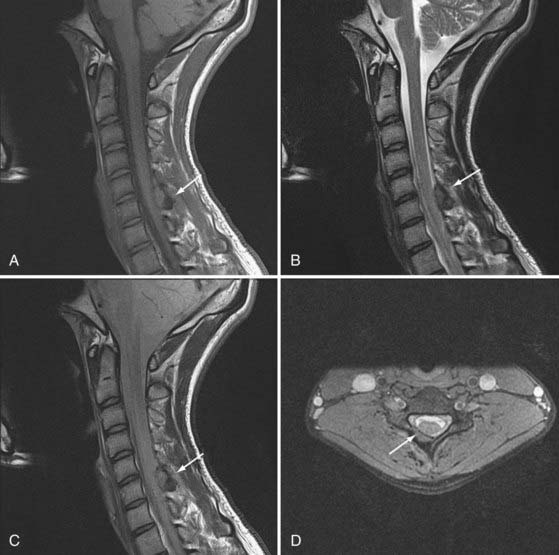
Osteoblastomas are histologically similar to osteoid osteomas except that these lesions may have a thin or absent sclerotic margin and are usually larger than 1.5 cm.12,21,23,27,30–41 These lesions consist of osteoblasts, osteoid tissue, and trabecular bone surrounded by a highly vascular matrix. Instead of a thick sclerotic margin, these lesions are more expansile with growth into the epidural space. Forty percent of osteoblastomas have been found in the spine, primarily in the posterior elements and in the first 2 decades of life. Again, there is a male predominance with a male-to-female ratio of 2 : 1.10,12,34,39
The initial symptom is pain, but it is often less severe and less localized and has less diurnal variation than seen with osteoid osteoma. Because of the indolent course and larger size, osteoblastomas can be accompanied by neurological signs of radiculopathy or myelopathy. Plain films are more likely to demonstrate this tumor as a lucent lesion with a thin sclerotic rim. These lesions are best visualized with CT or MRI. Depending on the degree of vascularity, there can be brisk enhancement (Fig. 222-2).
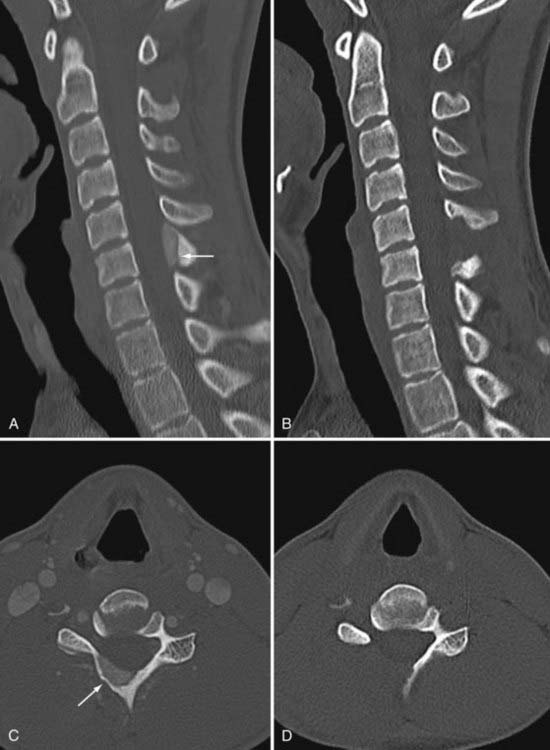
Although osteoblastomas are benign lesions, they can be locally aggressive with a higher recurrence rate. As a result, careful attention to surgical margins is important. The expansile lesion destroys adjacent bone and can create spinal instability either through destruction of normal elements or as a result of complete resection (Fig. 222-3). Malignant transformation of a benign osteoblastoma is rare, although cases have been reported. Treatment with radiation has been reported for lesions that are either surgically inaccessible or incompletely resected.34 There are reports of radiation promoting malignant transformation.42 Accordingly, radiation therapy should be given only when surgical resection is not possible for an osteoblastoma that is clinically aggressive.
Osteochondroma
Osteochondroma, also known as chondroma or osteocartilaginous exostosis, is a benign bone hamartoma. These cartilage-capped bony lesions contain normal-appearing bone. The pathology is derived from normal bone in an abnormal location, most commonly in the cervical and thoracic spine.43–49 There is a male preponderance of these lesions, and they occur in the second to fourth decades of life. Osteochondromas are thought to develop at secondary sites of ossification and are found in the spinous process, lamina, and transverse process. In the rare instances in which they are located in the pedicle or vertebral body, it is important to watch for neural compression and spinal stenosis.50 Plain film imaging reveals a pedunculated lesion coming out of normal cortex. CT shows stippled calcification. The cartilage portion is hyperintense, whereas the bony portion is isointense on MRI.
Multiple lesions are seen in patients with hereditary multiple exostoses.44,46,47,49,51 This genetic disorder has an even greater male preponderance. Although solitary lesions rarely degenerate to the malignant lesions osteosarcoma or chondrosarcoma, these tumors can develop in 5% to 10% of patients with multiple cartilaginous exostoses. When malignant transformation has taken place, imaging changes from a well-defined lesion with normal adjacent soft tissue to an indistinct, rapidly growing lesion with associated calcifying soft tissue. The cartilaginous cap is typically less than 1 cm for chondroma; chondrosarcoma develops a thicker cap, often greater than 2 to 3 cm.
Hemangioma
Hemangiomas are found incidentally in 10% to 12% of the population. These benign, osteolytic lesions contain multiple thin-walled vessels. They are found primarily in the vertebral body.21,52–54 Histologically, there are two types of hemangiomas based on the size of the vascular channels: capillary and cavernous. Cavernous hemangiomas are more common and have larger venous channels. They are most often found in the thoracic spine.
Imaging often includes plain films, CT, MRI, and angiography. CT shows a “honeycomb” appearance because of the resorption of horizontal trabeculae with thickened vertical trabeculae. MRI is useful to determine the extent of neurological compression. Angiography demonstrates an extensive vascular blush; this radiographic study is useful for identification of the vascular feeder and the venous drainage, as well as for embolization with particles or ethanol.54,55
The vast majority of hemangiomas are asymptomatic and found incidentally. Because they are more common in adults, the infrequent pediatric patient may benefit from short-term follow-up. Once it is determined that this is a static lesion, no further follow-up is recommended. In the rare patient with significant pain, neurological compression, or spinal instability, treatment is indicated.56–61 Because these lesions are highly vascular, preoperative embolization is important. With the advent of less invasive techniques, percutaneous vertebroplasty may provide definitive treatment. Other less involved approaches include posterior decompression with or without fusion. If the lesion is large or involves multiple areas of the spinal column, 360-degree resection and reconstruction will be necessary for definitive treatment. There are reports of stereotactic radiation therapy and ethanol embolization as primary treatment, but this is less described in the pediatric population and the immature spine.
Giant Cell Tumor
Giant cell tumors are lytic, expansile lesions containing multinucleated giant cells.36,62–67 The presence of increased osteoclastic giant cells explains why these tumors are also known as osteoclastomas. Giant cell tumors tend to be very vascular and often exhibit hemorrhages of varying age. The most common location for these lesions is in the long bones, but they can rarely be found in the spine. Those in the spine can be located in both the vertebral body and the posterior elements. The prognosis is thought to be better for spinal lesions than for those found in the long bones.
Pain is common; radiculopathy and weakness occur as the tumor enlarges and begins to compress the neural elements. Imaging reveals a large destructive and expansile lesion with a thin rim of bone.28 MRI and CT are important to delineate the extent of the tumor and guide surgical planning. Pathologically, homogeneous mononuclear stromal cells are admixed with giant cells.
Treatment is determined by accessibility and size, and resection and subsequent spinal stabilization are often required.36,64–66,68–72 Because these lesions are vascular and may be similar to aneurysmal bone cysts, preoperative embolization may be useful to limit bleeding. Giant cell tumors can be locally aggressive, which has prompted the use of adjuvant radiation therapy to control the disease. Malignant transformation with metastases to the lung is possible.73
Aneurysmal Bone Cyst
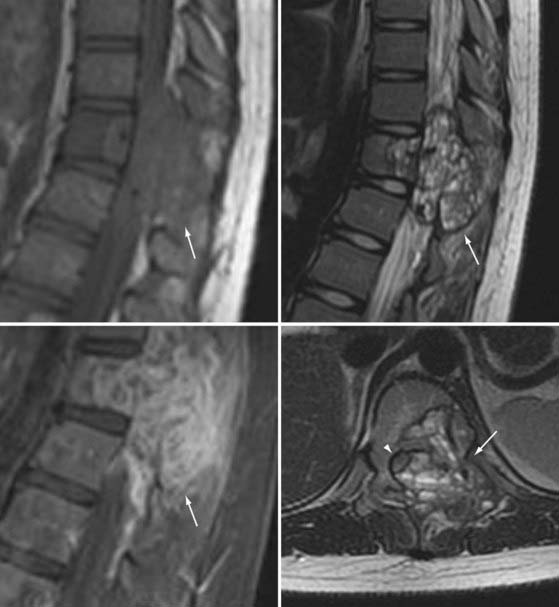
Aneurysmal bone cysts are benign, lytic, vascular lesions found in any bone but are typically seen in the long bones, spine, and ribs.74–78 The majority of aneurysmal bone cysts are found in the spine, with up to 30% to 50% being associated with other lesions such as eosinophilic granuloma, angioma, osteoblastoma, and bone cysts. Between 60% and 70% are found in the posterior elements, with the remaining being localized to the vertebral body. These lesions arise during the first 2 decades of life, with a slight female predilection.
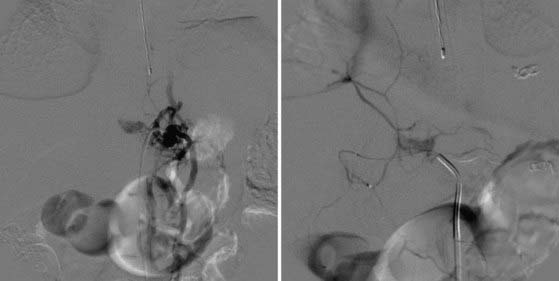
Imaging shows an irregular, osteolytic, expansile lesion with destruction of the normal anatomy.74,75,77,78 On CT and MRI, there is an associated complex heterogeneous cyst with multiple fluid-fluid levels and blood products of varying age (Fig. 222-4). Angiography can show brisk flow with large feeding and draining vessels. Histologic analysis reveals multiple blood-filled cysts separated by fibrous bands and thin trabeculae contained by a thin subperiosteal shell. Blood products of different age are seen to be undergoing organization to varying degrees.
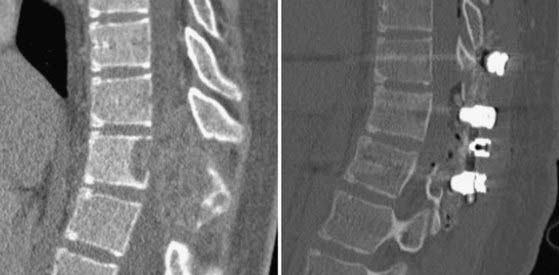
Treatment centers on resection of the lesion and stabilization of the spine.74,76,79–86 Aneurysmal bone cysts can be quite vascular; as a result, preoperative embolization is useful for limiting blood loss (Fig. 222-5). Because these lesions are often destructive of multiple columns of spinal support, stabilization with instrumentation and bone grafting is likely to be necessary (Fig. 222-6).36,74,87–91 The recurrence rate can be as high as 10% to 20%; clinical examination and imaging over long-term follow-up are required. It can be difficult to distinguish maturation of the bone graft and bone healing from recurrent tumor. Adjuvant therapy with radiation and liquid nitrogen have been used in an attempt to reduce the recurrence rate.
Langerhans Cell Histiocytosis
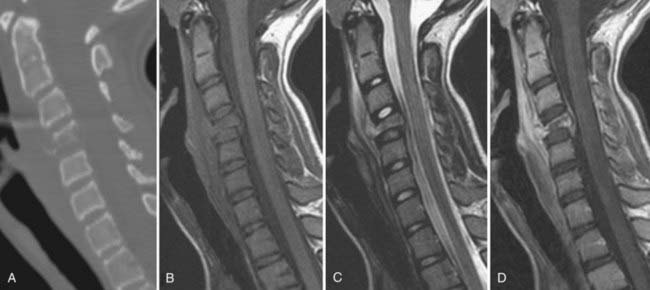
This lytic lesion is either known as or part of many named disease processes, including eosinophilic granuloma, histiocytosis X, Langerhans cell histiocytosis, Hand-Schüller-Christian disease, Letterer-Siwe disease, and Calvé’s disease.92–95 Langerhans cell histiocytosis is commonly seen in the first 2 decades of life with a male-to-female ratio of 3 : 2. Symptoms from this lesion typically begin as localized pain but can include tenderness and fever.21,40,53,63,96–99 Vertebra plana is often used to describe this lesion because of the flattened vertebral body. Progression of symptoms results from the instability created by bone destruction or from epidural compression of nerve roots and the spinal cord (Fig. 222-7).100–103
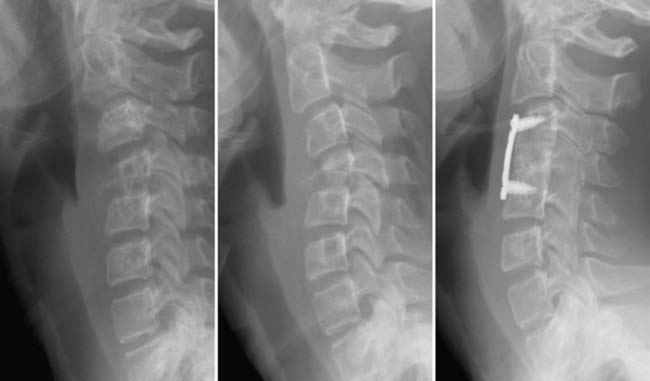
Multiorgan involvement can occur and affect the liver, lung, lymph nodes, skin, mucosal membranes, bones, and pituitary gland. As a result, the work-up should include a skeletal survey, chest radiograph, and liver function tests; a clinical history and examination focusing on endocrine dysfunction, prominent lymph nodes, and skin manifestations are also important. The most common bones affected include the skull, long bones, ribs, spine, and facial bones. It is important to determine whether the disease is solitary or multifocal. Solitary lesions can be treated locally by either intralesional steroid injection or surgical resection. Multifocal disease should be treated systemically with chemotherapy because of the high rate of recurrent disease and central nervous system lesions. Biopsy, guided by either CT or fluoroscopy, can help differentiate Langerhans cell histiocytosis from other lesions such as infections, lymphoma, neoplasia, or metastatic disease.104 In patients with high suspicion for eosinophilic granuloma, simultaneous injection of methylprednisolone may improve the pain and promote healing (Fig. 222-8).
Fibrous Dysplasia
Fibrous dysplasia is a disease that affects bone marrow, with replacement of the marrow by fibro-osseous expansion.105–109 Any bone may be affected, although the long bones, ribs, facial bones, and skull are primarily involved. It is classified as monostotic when involving one bone or as polyostotic when affecting multiple bones. The presence of polyostotic disease, endocrine dysfunction such as precocious puberty or hyperthyroidism, and skin findings of café au lait spots makes the diagnosis of McCune-Albright syndrome.105,110 In general, the signs and symptoms depend on the location and extent of the disease. If fibrous dysplasia does involve the spine, it typically affects the lumbar spine. Certainly, it can cause deformity through asymmetric vertebral growth or collapse.40,111 In patients with polyostotic disease, the spinal deformity may be compensatory to disease affecting the legs, ribs, or pelvis. Symptoms caused by compression of the exiting nerve root or canal stenosis have been described.
On pathologic examination, collagen fibers are noted to be deposited around bone trabeculae and osteoid tissue. As the fibrous mass grows, focal degeneration and cystic development occur.105,110
In a large part, both the extent and activity of the disease determine treatment.105,107,112 Treatment is targeted toward stabilization and correction of deformity, as well as decompression of the nervous system. Patients without neurological symptoms may benefit from stabilization of the spine without resection.
Malignant Tumors
Chordomas
Chordoma is a malignant tumor derived from the remnants of the notochord.4,8,41,113–115 In the pediatric age group, chordomas are equally distributed in the sacrum, the skull base, and the mobile spine. In the distribution of the mobile spine, the cervical spine is the most common location. Rostral tumors are seen in younger patients; caudal lesions are seen in older patients.
Although these lesions rarely metastasize,116,117 they are locally invasive and destructive.41,68,113,118–125 As a result, the symptoms at initial evaluation depend entirely on the location. Clival lesions can be associated with endocrinopathy, headache, and cranial nerve palsy; sacrococcygeal lesions will be accompanied by pain, weakness, and bowel and bladder incontinence. In addition to neurological compromise from compression, adjacent structures and organs are also affected. Chronic sinus disease from skull base lesions, airway obstruction from cervical tumors, and bowel and bladder compromise from sacral outgrowth all result from direct mass effect of the tumor.
Ideally, treatment involves radical, en bloc resection.* Because of the aggressive nature of these tumors, complete resection is sometimes not possible without sacrifice of neural tissue or adjacent organs and structures. These tumors can be unresponsive to both chemotherapy and radiation therapy. High-dose stereotactic photon radiation therapy and lower dose proton radiation therapy have shown some benefit against this relentless lesion.113,123,133–136
Teratoma
Sacrococcygeal teratoma is the most common pediatric spinal teratoma and the most common neonatal tumor.137–141 Teratomas typically, but not always contain all three germ cell layers—ectoderm, mesoderm, and endoderm. Teratomas are described as mature, immature, and malignant. Mature and immature teratomas may be considered benign, especially if treated early. Mature teratomas contain components that are fully differentiated. Immature lesions typically contain fetal tissue. Malignant teratomas are named for the presence of the malignant germ cell layer component. It is important to study the specimen completely because benign and malignant elements can be present simultaneously. Although the sacrococcygeal location is the most common site, teratomas found elsewhere are often associated with other spinal abnormalities, such as neural tube defect.
Treatment centers on complete resection.114,138,140,142 As part of treatment, the entire coccyx must be resected, even if the lesion involves only the distal portion. Lesions not completely resected can undergo malignant transformation.141 As a result, patients should undergo follow-up with rectal examination and serum α-fetoprotein and β-human chorionic gonadotropin assay.
Sarcoma
Osteosarcoma is a malignant tumor of bone.8,42,129,143–146 The sarcomatous mesenchyme forms osteoid and osseous tissue. Although it is the most common tumor of bone in the pediatric population, with an incidence rate of 4.8 per 1,000,000 children younger than 19 years, its appearance in the spine is more likely to be metastatic or secondary rather than a primary lesion. Pathologically, osteoid and osseous tissue are seen in the sarcomatous mesenchyme. Additional subclassification based on the predominance of formed tissue yields osteoblastic, chondroblastic, and fibroblastic types. Surgery followed by chemotherapy and radiation therapy has been used for the treatment of these tumors.
Chondrosarcoma is an extremely rare malignant sarcomatous tumor that forms cartilage and has an incidence rate of 0.2 per 1,000,000 children.42,147–151 These lesions are found primarily in adults but have rarely been reported in adolescents. They are encountered in the pediatric population when a chondroma undergoes malignant transformation. This is most commonly seen in patients with multiple hereditary exostoses.
Ewing’s sarcoma is an aggressive, malignant tumor derived from primitive noncommitted mesenchymal cells.10,152–156 It has an incidence rate of 2.8 cases per 1,000,000 children younger than 15 years. It is the second most common bone malignancy in children and has a male preponderance. Although pain is the common initial manifestation, the aggressive nature of these tumors makes neurological compromise prevalent, with weakness, sensory changes, radiculopathy, or neurogenic bladder occurring. The location in the axial spine is most commonly the sacrum and then the lumbar spine. It can be confused with osteomyelitis, but the vertebral disk is often maintained.157,158 Up to a third of patients will have metastasis at the time of diagnosis. Radiographic examination reveals destruction of the vertebral body with ill-defined margins and a soft tissue mass. Pathologic examination shows sheets or nests of small round cells.
Management starts first by making a diagnosis via either percutaneous or open biopsy.36,40,152,156,159,160 Because these lesions are sensitive to chemotherapy and radiation therapy, presurgical treatment with these modalities may improve the chance of complete resection. Problems arise when neurological compression compromises the situation. Decompression and stabilization may be required before the initiation of chemotherapy.
, Barrenechea IJ, Perin NI, Triana A, et al. Surgical management of chordomas of the cervical spine. J Neurosurg Spine. 2007;6:398.
, Blaskiewicz DJ, Sure DR, Hedequist DJ, et al. Osteoid osteomas: intraoperative bone scan–assisted resection. Clinical article. J Neurosurg Pediatr. 2009;4:237.
, Boriani S, De Iure F, Campanacci L, et al. Aneurysmal bone cyst of the mobile spine: report on 41 cases. Spine. 2001;26:27.
, Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine. 2006;31:493.
, Boriani S, De Iure F, Bandiera S, et al. Chondrosarcoma of the mobile spine: report on 22 cases. Spine. 2000;25:804.
, Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine. 1997;22:1036.
, Burn SC, Ansorge O, Zeller R, et al. Management of osteoblastoma and osteoid osteoma of the spine in childhood. J Neurosurg Pediatr. 2009;4:434.
, Denaro L, Longo UG, Papalia R, et al. Eosinophilic granuloma of the pediatric cervical spine. Spine. 2008;33:E936.
, Dreghorn CR, Newman RJ, Hardy GJ, et al. Primary tumors of the axial skeleton. Experience of the Leeds Regional Bone Tumor Registry. Spine. 1990;15:137.
, Fenoy AJ, Greenlee JD, Menezes AH, et al. Primary bone tumors of the spine in children. J Neurosurg. 2006;105:252.
, Grubb MR, Currier BL, Pritchard DJ, et al. Primary Ewing’s sarcoma of the spine. Spine. 1994;19:309.
, Hoffman HJ. Childhood and adolescent lumbar pain: differential diagnosis and management. Clin Neurosurg. 1980;27:553.
, Ippolito E, Bray EW, Corsi A, et al. Natural history and treatment of fibrous dysplasia of bone: a multicenter clinicopathologic study promoted by the European Pediatric Orthopaedic Society. J Pediatr Orthop B. 2003;12:155.
, Lee CK, Rosa R, Fernand R. Surgical treatment of tumors of the spine. Spine. 1986;11:201.
, Liu JK, Brockmeyer DL, Dailey AT, et al. Surgical management of aneurysmal bone cysts of the spine. Neurosurg Focus. 2003;15(5):E4.
, Loh JK, Lin CK, Hwang YF, et al. Primary spinal tumors in children. J Clin Neurosci. 2005;12:246.
, Lucas DR, Unni KK, McLeod RA, et al. Osteoblastoma: clinicopathologic study of 306 cases. Hum Pathol. 1994;25:117.
, Mankin HJ, Hornicek FJ, Ortiz-Cruz E, et al. Aneurysmal bone cyst: a review of 150 patients. J Clin Oncol. 2005;23:6756.
, Menezes AH, Sato Y. Primary tumors of the spine in children—natural history and management. 1990. Pediatr Neurosurg. 1995;23:101.
, Myles ST, MacRae ME. Benign osteoblastoma of the spine in childhood. J Neurosurg. 1988;68:884.
, O’Donnell J, Brown L, Herkowitz H. Vertebra plana–like lesions in children: case report with special emphasis on the differential diagnosis and indications for biopsy. J Spinal Disord. 1991;4:480.
, Ozaki T, Flege S, Liljenqvist U, et al. Osteosarcoma of the spine: experience of the Cooperative Osteosarcoma Study Group. Cancer. 2002;94:1069.
, Srikantha U, Bhagavatula ID, Satyanarayana S, et al. Spinal osteochondroma: spectrum of a rare disease. J Neurosurg Spine. 2008;8:561.
, Weinstein JN, McLain RF. Primary tumors of the spine. Spine. 1987;12:843.
1 Hoffman HJ. Childhood and adolescent lumbar pain: differential diagnosis and management. Clin Neurosurg. 1980;27:553.
2 Lee CK, Rosa R, Fernand R. Surgical treatment of tumors of the spine. Spine. 1986;11:201.
3 Weinstein JN, McLain RF. Primary tumors of the spine. Spine. 1987;12:843.
4 Dreghorn CR, Newman RJ, Hardy GJ, et al. Primary tumors of the axial skeleton. Experience of the Leeds Regional Bone Tumor Registry. Spine. 1990;15:137.
5 Menezes AH, Sato Y. Primary tumors of the spine in children—natural history and management. 1990. Pediatr Neurosurg. 1995;23:101.
6 Piper JG, Menezes AH. Management strategies for tumors of the axis vertebra. J Neurosurg. 1996;84:543.
7 Beer SJ, Menezes AH. Primary tumors of the spine in children. Natural history, management, and long-term follow-up. Spine. 1997;22:649.
8 Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine. 1997;22:1036.
9 Conrad EU3rd, Olszewski AD, Berger M, et al. Pediatric spine tumors with spinal cord compromise. J Pediatr Orthop. 1992;12:454.
10 Loh JK, Lin CK, Hwang YF, et al. Primary spinal tumors in children. J Clin Neurosci. 2005;12:246.
11 Boriani S, Capanna R, Donati D, et al. Osteoblastoma of the spine. Clin Orthop Relat Res. 1992;278:37.
12 Saifuddin A, Sherazi Z, Shaikh MI, et al. Spinal osteoblastoma: relationship between paravertebral muscle abnormalities and scoliosis. Skeletal Radiol. 1996;25:531.
13 Sinha AK, Seki JT, Moreau G, et al. The management of spinal metastasis in children. Can J Surg. 1997;40:218.
14 Kim FM, Poussaint TY, Barnes PD. Neuroimaging of scoliosis in childhood. Neuroimaging Clin N Am. 1999;9:195.
15 Jose Alcaraz Mexia M, Izquierdo Nunez E, Santonja Garriga C, et al. Osteochondroma of the thoracic spine and scoliosis. Spine. 2001;26:1082.
16 Shindle MK, Khanna AJ, McCarthy EF, et al. Desmoid tumor of the spinal canal causing scoliosis and paralysis. Spine. 2002;27:E304.
17 U.S. Cancer Statistics Working Group, United States Cancer Statistics: 1999-2005 Incidence and Mortality Web-based Report. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Available at. www.cdc.gov/uscs, 2009.
18 Bucci MN, Feldenzer JA, Phillips WA, et al. Atlanto-axial rotational limitation secondary to osteoid osteoma of the axis. Case report. J Neurosurg. 1989;70:129.
19 D’Angelo G, Donzelli O. Osteoid osteoma of the vertebral body: a clinical case. Chir Organi Mov. 1998;83:303.
20 Saifuddin A, White J, Sherazi Z, et al. Osteoid osteoma and osteoblastoma of the spine. Factors associated with the presence of scoliosis. Spine. 1998;23:47.
21 Kostas JP, Dailiana Z, Xenakis T, et al. Back pain caused by benign tumors and tumor-like lesions of the thoracolumbar spine. Am J Orthop. 2001;30:50.
22 Guzey FK, Seyithanoglu MH, Sencer A, et al. Vertebral osteoid osteoma associated with paravertebral soft-tissue changes on magnetic resonance imaging. Report of two cases. J Neurosurg. 2004;100:532.
23 Bruneau M, Cornelius JF, George B. Osteoid osteomas and osteoblastomas of the occipitocervical junction. Spine. 2005;30:E567.
24 Lin HH, Yu CT, Chang IL, et al. Painful scoliosis secondary to osteoid osteoma of the lumbar spine in adolescents. Int Surg. 2008;93:32.
25 Fielding JW, Keim HA, Hawkins RJ, et al. Osteoid osteoma of the cervical spine. Clin Orthop Relat Res. 1977;128:163.
26 Maiuri F, Signorelli C, Lavano A, et al. Osteoid osteomas of the spine. Surg Neurol. 1986;25:375.
27 Myles ST, MacRae ME. Benign osteoblastoma of the spine in childhood. J Neurosurg. 1988;68:884.
28 Kroon HM, Schurmans J. Osteoblastoma: clinical and radiologic findings in 98 new cases. Radiology. 1990;175:783.
29 Blaskiewicz DJ, Sure DR, Hedequist DJ, et al. Osteoid osteomas: intraoperative bone scan–assisted resection. Clinical article. J Neurosurg Pediatr. 2009;4:237.
30 Burn SC, Ansorge O, Zeller R, et al. Management of osteoblastoma and osteoid osteoma of the spine in childhood. J Neurosurg Pediatr. 2009;4:434.
31 Rothschild EJ, Savitz MH, Chang T, et al. Primary vertebral tumor in an adolescent girl. Spine. 1984;9:695.
32 Afshani E, Kuhn JP. Common causes of low back pain in children. Radiographics. 1991;11:269.
33 Hladky JP, Lejeune JP, Singer B, et al. Osteoblastoma of the odontoid process. Pediatr Neurosurg. 1994;21:260.
34 Lucas DR, Unni KK, McLeod RA, et al. Osteoblastoma: clinicopathologic study of 306 cases. Hum Pathol. 1994;25:117.
35 Berberoglu S, Oguz A, Aribal E, et al. Osteoblastoma response to radiotherapy and chemotherapy. Med Pediatr Oncol. 1997;28:305.
36 Sar C, Eralp L. Surgical treatment of primary tumors of the sacrum. Arch Orthop Trauma Surg. 2002;122:148.
37 Denaro V, Denaro L, Papalia R, et al. Surgical management of cervical spine osteoblastomas. Clin Orthop Relat Res. 2007;455:190.
38 Saglik Y, Atalar H, Yildiz Y, et al. Surgical treatment of osteoblastoma: a report of 20 cases. Acta Orthop Belg. 2007;73:747.
39 Berry M, Mankin H, Gebhardt M, et al. Osteoblastoma: a 30-year study of 99 cases. J Surg Oncol. 2008;98:179.
40 Menezes AH. Craniovertebral junction neoplasms in the pediatric population. Childs Nerv Syst. 2008;24:1173.
41 Puri A, Agarwal MG, Shah M, et al. Decision making in primary sacral tumors. Spine J. 2009;9:396.
42 Weatherby RP, Dahlin DC, Ivins JC. Postradiation sarcoma of bone: review of 78 Mayo Clinic cases. Mayo Clin Proc. 1981;56:294.
43 Albrecht S, Crutchfield JS, SeGall GK. On spinal osteochondromas. J Neurosurg. 1992;77:247.
44 Robbins SE, Laitt RD, Lewis T. Hereditary spinal osteochondromas in diaphyseal aclasia. Neuroradiology. 1996;38:59.
45 Silber JS, Mathur S, Ecker M. A solitary osteochondroma of the pediatric thoracic spine: a case report and review of the literature. Am J Orthop. 2000;29:711.
46 Bergh P, Gunterberg B, Meis-Kindblom JM, et al. Prognostic factors and outcome of pelvic, sacral, and spinal chondrosarcomas: a center-based study of 69 cases. Cancer. 2001;91:1201.
47 Gille O, Pointillart V, Vital JM. Course of spinal solitary osteochondromas. Spine. 2005;30:E13.
48 Arkader A, Dormans JP, Gaugler R, et al. Spontaneous regression of solitary osteochondroma: reconsidering our approach. Clin Orthop Relat Res. 2007;460:253.
49 Srikantha U, Bhagavatula ID, Satyanarayana S, et al. Spinal osteochondroma: spectrum of a rare disease. J Neurosurg Spine. 2008;8:561.
50 Loftus CM, Rozario RA, Prager R, et al. Solitary osteochondroma of T4 with thoracic cord compression. Surg Neurol. 1980;13:355.
51 Moon KS, Lee JK, Kim YS, et al. Osteochondroma of the cervical spine extending multiple segments with cord compression. Pediatr Neurosurg. 2006;42:304.
52 Bohlman HH, Sachs BL, Carter JR, et al. Primary neoplasms of the cervical spine. Diagnosis and treatment of twenty-three patients. J Bone Joint Surg Am. 1986;68:483.
53 Graham GN, Browne H. Primary bony tumors of the pediatric spine. Yale J Biol Med. 2001;74:1.
54 Jenny B, Radovanovic I, Haenggeli CA, et al. Association of multiple vertebral hemangiomas and severe paraparesis in a patient with a PTEN hamartoma tumor syndrome. Case report. J Neurosurg. 2007;107:307.
55 Esparza J, Castro S, Portillo JM, et al. Vertebral hemangiomas: spinal angiography and preoperative embolization. Surg Neurol. 1978;10:171.
56 Bandiera S, Gasbarrini A, De Iure F, et al. Symptomatic vertebral hemangioma: the treatment of 23 cases and a review of the literature. Chir Organi Mov. 2002;87:1.
57 Bas T, Aparisi F, Bas JL. Efficacy and safety of ethanol injections in 18 cases of vertebral hemangioma: a mean follow-up of 2 years. Spine. 2001;26:1577.
58 Gailloud P, Beauchamp NJ, Martin JB, et al. Percutaneous pediculoplasty: polymethylmethacrylate injection into lytic vertebral pedicle lesions. J Vasc Interv Radiol. 2002;13:517.
59 Hadjipavlou A, Tosounidis T, Gaitanis I, et al. Balloon kyphoplasty as a single or as an adjunct procedure for the management of symptomatic vertebral haemangiomas. J Bone Joint Surg Br. 2007;89:495.
60 Ryoppy S, Poussa M, Heiskanen O, et al. Resection of a thoracic vertebra for hemangioma—operation under deep hypothermia and circulatory arrest. A case report. J Bone Joint Surg Am. 1990;72:1245.
61 Smith TP, Koci T, Mehringer CM, et al. Transarterial embolization of vertebral hemangioma. J Vasc Interv Radiol. 1993;4:681.
62 Boriani S, Biagini R, Laus M, et al. Giant cell tumor of the vertebral column. Chir Organi Mov. 1996;81:233.
63 Fenoy AJ, Greenlee JD, Menezes AH, et al. Primary bone tumors of the spine in children. J Neurosurg. 2006;105:252.
64 Goldenberg RR, Campbell CJ, Bonfiglio M. Giant-cell tumor of bone. An analysis of two hundred and eighteen cases. J Bone Joint Surg Am. 1970;52:619.
65 Junming M, Cheng Y, Dong C, et al. Giant cell tumor of the cervical spine: a series of 22 cases and outcomes. Spine. 2008;33:280.
66 Leggon RE, Zlotecki R, Reith J, et al. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin Orthop Relat Res. 2004;423:196.
67 Ozaki T, Liljenqvist U, Halm H, et al. Giant cell tumor of the spine. Clin Orthop Relat Res. 2002;401:194.
68 Boriani S, Biagini R, De Iure F, et al. Resection surgery in the treatment of vertebral tumors. Chir Organi Mov. 1998;83:53.
69 Boriani S, Biagini R, De Iure F, et al. Lumbar vertebrectomy for the treatment of bone tumors: surgical technique. Chir Organi Mov. 1994;79:163.
70 Flatley TJ, Anderson MH, Anast GT. Spinal instability due to malignant disease. Treatment by segmental spinal stabilization. J Bone Joint Surg Am. 1984;66:47.
71 Hart RA, Boriani S, Biagini R, et al. A system for surgical staging and management of spine tumors. A clinical outcome study of giant cell tumors of the spine. Spine. 1997;22:1773.
72 Kawahara N, Tomita K, Matsumoto T, et al. Total en bloc spondylectomy for primary malignant vertebral tumors. Chir Organi Mov. 1998;83:73.
73 Turcotte RE, Sim FH, Unni KK. Giant cell tumor of the sacrum. Clin Orthop Relat Res. 1993;291:215.
74 Boriani S, De Iure F, Campanacci L, et al. Aneurysmal bone cyst of the mobile spine: report on 41 cases. Spine. 2001;26:27.
75 DiCaprio MR, Murphy MJ, Camp RL. Aneurysmal bone cyst of the spine with familial incidence. Spine. 2000;25:1589.
76 de Kleuver M, van der Heul RO, Veraart BE. Aneurysmal bone cyst of the spine: 31 cases and the importance of the surgical approach. J Pediatr Orthop B. 1998;7:286.
77 Vergel De Dios AM, Bond JR, Shives TC, et al. Aneurysmal bone cyst. A clinicopathologic study of 238 cases. Cancer. 1992;69:2921.
78 Mankin HJ, Hornicek FJ, Ortiz-Cruz E, et al. Aneurysmal bone cyst: a review of 150 patients. J Clin Oncol. 2005;23:6756.
79 DeRosa GP, Graziano GP, Scott J. Arterial embolization of aneurysmal bone cyst of the lumbar spine. A report of two cases. J Bone Joint Surg Am. 1990;72:777.
80 Dysart SH, Swengel RM, van Dam BE. Aneurysmal bone cyst of a thoracic vertebra. Treatment by selective arterial embolization and excision. Spine. 1992;17:846.
81 Koci TM, Mehringer CM, Yamagata N, et al. Aneurysmal bone cyst of the thoracic spine: evolution after particulate embolization. AJNR Am J Neuroradiol. 1995;16:857.
82 Konya A, Szendroi M. Aneurysmal bone cysts treated by superselective embolization. Skeletal Radiol. 1992;21:167.
83 Mohit AA, Eskridge J, Ellenbogen R, et al. Aneurysmal bone cyst of the atlas: successful treatment through selective arterial embolization: case report. Neurosurgery. 2004;55:982.
84 Ohashi M, Ito T, Hirano T, et al. Percutaneous intralesional injection of calcitonin and methylprednisolone for treatment of an aneurysmal bone cyst at C-2. J Neurosurg Pediatr. 2008;2:365.
85 Papagelopoulos PJ, Choudhury SN, Frassica FJ, et al. Treatment of aneurysmal bone cysts of the pelvis and sacrum. J Bone Joint Surg Am. 2001;83:1674.
86 Stella G, De Sanctis N, Boero S, et al. Benign tumors of the pediatric spine: statistical notes. Chir Organi Mov. 1998;83:15.
87 Beiner JM, Sastry A, Berchuck M, et al. An aneurysmal bone cyst in the cervical spine of a 10-year-old girl: a case report. Spine. 2006;31:E475.
88 Ameli NO, Abbassioun K, Saleh H, et al. Aneurysmal bone cysts of the spine. Report of 17 cases. J Neurosurg. 1985;63:685.
89 Parrish FF, Pevey JK. Surgical management of aneurysmal bone cyst of the vertebral column. J Bone Joint Surg Am. 1967;49:1597.
90 Liu JK, Brockmeyer DL, Dailey AT, et al. Surgical management of aneurysmal bone cysts of the spine. Neurosurg Focus. 2003;15(5):E4.
91 Dormans JP, Hanna BG, Johnston DR, et al. Surgical treatment and recurrence rate of aneurysmal bone cysts in children. Clin Orthop Relat Res. 2004;421:205.
92 Cheyne C. Histiocytosis X. J Bone Joint Surg Br. 1971;53:366.
93 Hosalkar HS, Greenberg JS, Wells L, et al. Isolated Langerhans cell histiocytosis of the T12 vertebra in an adolescent. Am J Orthop. 2007;36:E21.
94 Glotzbecker MP, Carpentieri DF, Dormans JP. Langerhans cell histiocytosis: a primary viral infection of bone? Human herpes virus 6 latent protein detected in lymphocytes from tissue of children. J Pediatr Orthop. 2004;24:123.
95 Weber K, Damron TA, Frassica FJ, et al. Malignant bone tumors. Instr Course Lect. 2008;57:673.
96 Sohn MJ, Park HC, Park HS, et al. Anterior cervical corpectomy and fusion using miniplate and screws in a 7-year-old child with eosinophilic granuloma of the cervical spine. Spine. 2001;26:1193.
97 Denaro L, Longo UG, Papalia R, et al. Eosinophilic granuloma of the pediatric cervical spine. Spine. 2008;33:E936.
98 Silberstein MJ, Sundaram M, Akbarnia B, et al. Eosinophilic granuloma of the spine. Orthopedics. 1985;8:264.
99 Kamimura M, Kinoshita T, Itoh H, et al. Eosinophilic granuloma of the spine: early spontaneous disappearance of tumor detected on magnetic resonance imaging. Case report. J Neurosurg. 2000;93:312.
100 van Giffen NH, van Rhijn LW, van Ooij A, et al. Benign fibrous histiocytoma of the posterior arch of C1 in a 6-year-old boy: a case report. Spine. 2003;28:E359.
101 Maggi G, de Sanctis N, Aliberti F, et al. Eosinophilic granuloma of C4 causing spinal cord compression. Childs Nerv Syst. 1996;12:630.
102 Ngu BB, Khanna AJ, Pak SS, et al. Eosinophilic granuloma of the atlas presenting as torticollis in a child. Spine. 2004;29:E98.
103 Buonocore S, Valente AL, Nightingale D, et al. Histiocytic sarcoma in a 3-year-old male: a case report. Pediatrics. 2005;116:e322.
104 O’Donnell J, Brown L, Herkowitz H. Vertebra plana–like lesions in children: case report with special emphasis on the differential diagnosis and indications for biopsy. J Spinal Disord. 1991;4:480.
105 Ippolito E, Bray EW, Corsi A, et al. Natural history and treatment of fibrous dysplasia of bone: a multicenter clinicopathologic study promoted by the European Pediatric Orthopaedic Society. J Pediatr Orthop B. 2003;12:155.
106 Leet AI, Magur E, Lee JS, et al. Fibrous dysplasia in the spine: prevalence of lesions and association with scoliosis. J Bone Joint Surg Am. 2004;86:531.
107 Mancini F, Corsi A, De Maio F, et al. Scoliosis and spine involvement in fibrous dysplasia of bone. Eur Spine J. 2009;18:196.
108 Przybylski GJ, Pollack IF, Ward WT. Monostotic fibrous dysplasia of the thoracic spine. A case report. Spine. 1996;21:860.
109 Troop JK, Herring JA. Monostotic fibrous dysplasia of the lumbar spine: case report and review of the literature. J Pediatr Orthop. 1988;8:599.
110 Isaia GC, Lala R, Defilippi C, et al. Bone turnover in children and adolescents with McCune-Albright syndrome treated with pamidronate for bone fibrous dysplasia. Calcif Tissue Int. 2002;71:121.
111 Bahk WJ, Mirra JM. Pseudoanaplastic tumors of bone. Skeletal Radiol. 2004;33:641.
112 Guille JT, Bowen JR. Scoliosis and fibrous dysplasia of the spine. Spine. 1995;20:248.
113 Boriani S, Bandiera S, Biagini R, et al. Chordoma of the mobile spine: fifty years of experience. Spine. 2006;31:493.
114 Deutsch H, Mummaneni PV, Haid RW, et al. Benign sacral tumors. Neurosurg Focus. 2003;15(2):E14.
115 Noel G, Feuvret L, Calugaru V, et al. Chordomas of the base of the skull and upper cervical spine. One hundred patients irradiated by a 3D conformal technique combining photon and proton beams. Acta Oncol. 2005;44:700.
116 Arnautovic KI, Al-Mefty O. Surgical seeding of chordomas. J Neurosurg. 2001;95:798.
117 Shinmura Y, Miura K, Yajima S, et al. Sacrococcygeal chordoma in infancy showing an aggressive clinical course: an autopsy case report. Pathol Int. 2003;53:473.
118 Boriani S, Chevalley F, Weinstein JN, et al. Chordoma of the spine above the sacrum. Treatment and outcome in 21 cases. Spine. 1996;21:1569.
119 Fuchs B, Dickey ID, Yaszemski MJ, et al. Operative management of sacral chordoma. J Bone Joint Surg Am. 2005;87:2211.
120 Kaiser TE, Pritchard DJ, Unni KK. Clinicopathologic study of sacrococcygeal chordoma. Cancer. 1984;53:2574.
121 Munting E, Faundez A, Manche E. Vertebral reconstruction with cortical allograft: long-term evaluation. Eur Spine J. 2001;10(suppl 2):S153.
122 Ramamurthy R, Bose JC, Muthusamy V, et al. Staged sacrectomy—an adaptive approach. J Neurosurg Spine. 2009;11:285.
123 Samson IR, Springfield DS, Suit HD, et al. Operative treatment of sacrococcygeal chordoma. A review of twenty-one cases. J Bone Joint Surg Am. 1993;75:1476.
124 Sundaresan N, Rosen G, Boriani S. Primary malignant tumors of the spine. Orthop Clin North Am. 2009;40:21.
125 Wuisman P, Lieshout O, Sugihara S, et al. Total sacrectomy and reconstruction: oncologic and functional outcome. Clin Orthop Relat Res. 2000;381:192.
126 Ahmed R, Traynelis VC, Menezes AH. Fusions at the craniovertebral junction. Childs Nerv Syst. 2008;24:1209.
127 Barrenechea IJ, Perin NI, Triana A, et al. Surgical management of chordomas of the cervical spine. J Neurosurg Spine. 2007;6:398.
128 Chang DW, Friel MT, Youssef AA. Reconstructive strategies in soft tissue reconstruction after resection of spinal neoplasms. Spine. 2007;32:1101.
129 Kelley SP, Ashford RU, Rao AS, et al. Primary bone tumours of the spine: a 42-year survey from the Leeds Regional Bone Tumour Registry. Eur Spine J. 2007;16:405.
130 Randall RL, Bruckner J, Lloyd C, et al. Sacral resection and reconstruction for tumors and tumor-like conditions. Orthopedics. 2005;28:307.
131 Richards AT, Stricke L, Spitz L. Sacrococcygeal chordomas in children. J Pediatr Surg. 1973;8:911.
132 Sung HW, Shu WP, Wang HM, et al. Surgical treatment of primary tumors of the sacrum. Clin Orthop Relat Res. 1987;215:91.
133 Amendola BE, Amendola MA, Oliver E, et al. Chordoma: role of radiation therapy. Radiology. 1986;158:839.
134 Fagundes MA, Hug EB, Liebsch NJ, et al. Radiation therapy for chordomas of the base of skull and cervical spine: patterns of failure and outcome after relapse. Int J Radiat Oncol Biol Phys. 1995;33:579.
135 Ryu SI, Chang SD, Kim DH, et al. Image-guided hypo-fractionated stereotactic radiosurgery to spinal lesions. Neurosurgery. 2001;49:838.
136 Sundaresan N, Boriani S, Rothman A, et al. Tumors of the osseous spine. J Neurooncol. 2004;69:273.
137 Avni FE, Guibaud L, Robert Y, et al. MR imaging of fetal sacrococcygeal teratoma: diagnosis and assessment. AJR Am J Roentgenol. 2002;178:179.
138 De Backer A, Erpicum P, Philippe P, et al. Sacrococcygeal teratoma: results of a retrospective multicentric study in Belgium and Luxembourg. Eur J Pediatr Surg. 2001;11:182.
139 Fadler KM, Askin DF. Sacrococcygeal teratoma in the newborn: a case study of prenatal management and clinical intervention. Neonatal Netw. 2008;27:185.
140 Perrelli L, D’Urzo C, Manzoni C, et al. Sacrococcygeal teratoma. Outcome and management. An analysis of 17 cases. J Perinat Med. 2002;30:179.
141 Rescorla FJ, Sawin RS, Coran AG, et al. Long-term outcome for infants and children with sacrococcygeal teratoma: a report from the Children’s Cancer Group. J Pediatr Surg. 1998;33:171.
142 Raffensperger JG. Massive sacrococcygeal teratoma using hypothermic perfusion with extracorporeal membrane oxygenation. J Pediatr Surg. 1996;31:1467.
143 del Prever AB, Fagioli F, Berta M, et al. Long-term survival in high-grade axial osteosarcoma with bone and lung metastases treated with chemotherapy only. J Pediatr Hematol Oncol. 2005;27:42.
144 Ozaki T, Flege S, Liljenqvist U, et al. Osteosarcoma of the spine: experience of the Cooperative Osteosarcoma Study Group. Cancer. 2002;94:1069.
145 Shives TC, Dahlin DC, Sim FH, et al. Osteosarcoma of the spine. J Bone Joint Surg Am. 1986;68:660.
146 Wang VY, Potts M, Chou D. Sarcoma and the spinal column. Neurosurg Clin N Am. 2008;19:71.
147 Belhachmi A, Akhaddar A, Gazzaz M, et al. Primary spinal intradural mesenchymal chondrosarcoma. A pediatric case report. J Neuroradiol. 2008;35:189.
148 Boriani S, De Iure F, Bandiera S, et al. Chondrosarcoma of the mobile spine: report on 22 cases. Spine. 2000;25:804.
149 Boriani S, Laus M. Chondromas and chondromatosis (a study of 265 cases, 200 with long term follow up). Ital J Orthop Traumatol. 1978;4:353.
150 Kruse R, Simon RG, Stanton R, et al. Mesenchymal chondrosarcoma of the cervical spine in a child. Am J Orthop. 1997;26:279.
151 Shives TC, McLeod RA, Unni KK, et al. Chondrosarcoma of the spine. J Bone Joint Surg Am. 1989;71:1158.
152 Bacci G, Toni A, Avella M, et al. Long-term results in 144 localized Ewing’s sarcoma patients treated with combined therapy. Cancer. 1989;63:1477.
153 Grubb MR, Currier BL, Pritchard DJ, et al. Primary Ewing’s sarcoma of the spine. Spine. 1994;19:309.
154 Ilaslan H, Sundaram M, Unni KK, et al. Primary Ewing’s sarcoma of the vertebral column. Skeletal Radiol. 2004;33:506.
155 Pierz KA, Womer RB, Dormans JP. Pediatric bone tumors: osteosarcoma Ewing’s sarcoma, and chondrosarcoma associated with multiple hereditary osteochondromatosis. J Pediatr Orthop. 2001;21:412.
156 Yang RS, Eckardt JJ, Eilber FR, et al. Surgical indications for Ewing’s sarcoma of the pelvis. Cancer. 1995;76:1388.
157 Caksen H, Odabas D, Demirtas M, et al. A case of metastatic spinal Ewing’s sarcoma misdiagnosed as brucellosis and transverse myelitis. Neurol Sci. 2004;24:414.
158 Evans RG, Nesbit ME, Gehan EA, et al. Multimodal therapy for the management of localized Ewing’s sarcoma of pelvic and sacral bones: a report from the second intergroup study. J Clin Oncol. 1991;9:1173.
159 Krepler P, Windhager R, Bretschneider W, et al. Total vertebrectomy for primary malignant tumours of the spine. J Bone Joint Surg Br. 2002;84:712.
160 Tasdemiroglu E, Bagatur E, Ayan I, et al. Primary spinal column sarcomas. Acta Neurochir (Wien). 1996;138:1261.