75 Spine Trauma and Spinal Cord Injury
• Patients with spinal pain and spine fractures should receive a thorough neurologic examination to look for spinal cord injury.
• Spine fractures are associated with a high incidence of concurrent noncontiguous spine fractures and spinal cord injuries.
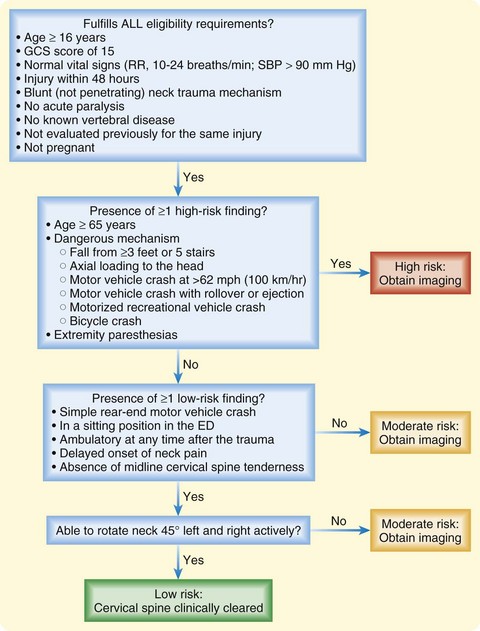
• The National Emergency X-radiography Utilization Study criteria or the Canadian Cervical-Spine Rule criteria can be used to identify low-risk patients who do not need cervical spine imaging.
• Imaging with plain films versus computed tomography of the cervical spine should be based on the pretest probability of a significant injury and the irradiation risk with computed tomography.
• Spinal shock, or transient physiologic transection of the spinal cord as a result of trauma, is different from neurogenic shock, which is physiologic sympathectomy of the upper spinal cord leading to peripheral vasodilation.
• Patients with a spinal cord injury caused by blunt trauma are often given high-dose corticosteroids within 8 hours of injury, although such therapy is controversial.
Epidemiology
The estimated annual cost of spine injuries, including inability to work and health care costs, exceeds $5 billion in the United States.1
In the emergency department (ED), all trauma victims are screened for vertebral fractures, ligamentous disruptions, and spinal cord injuries because of the potentially devastating neurologic consequences of overlooking these injuries. Patients with a delayed diagnosis of spinal fracture are 7.5 times more likely to sustain secondary neurologic deficits.2 Neurologic deficits from spinal cord injury may be subtle and can easily be missed if not specifically evaluated. Adding to these difficulties, plain film radiographs of the spine, though an adequate screening tool for other fractures, can miss 23% to 42% of cervical spinal fractures3,4 and 13% to 50% of lumbar fractures.5,6
Pathophysiology
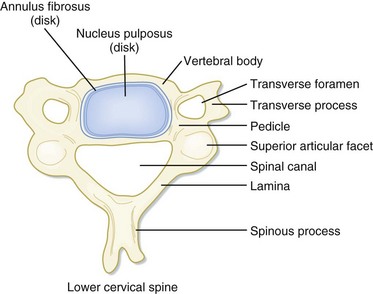
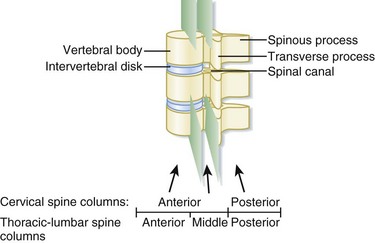
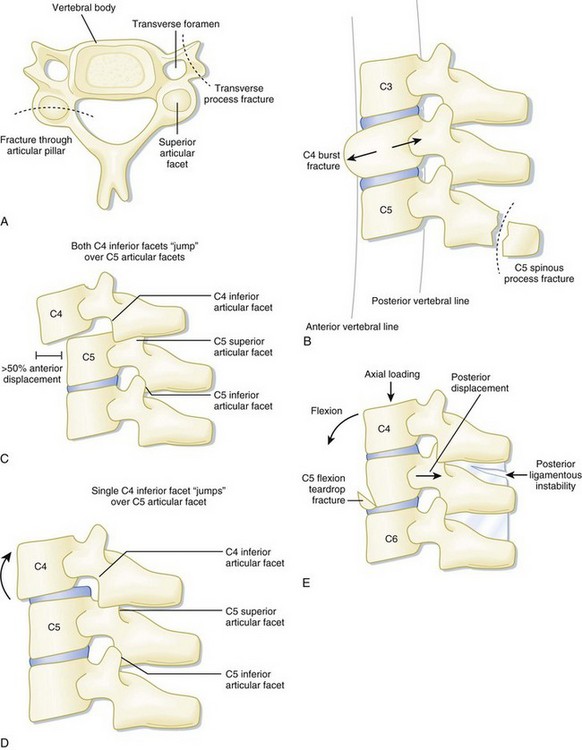
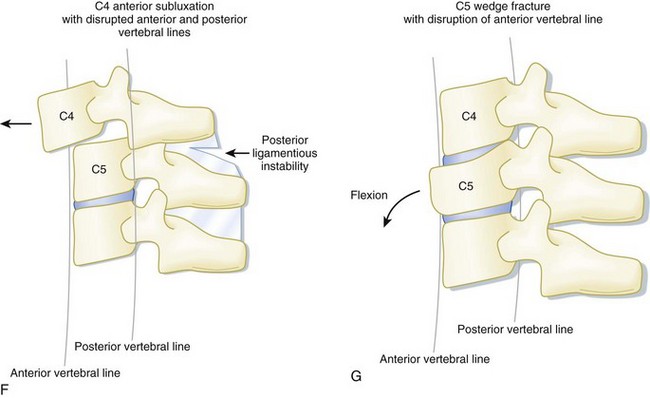
In the setting of spinal trauma, the bone, ligaments, spinal cord, and vascular structures may be injured. Anatomically, the vertebral bony spine can be divided into structural columns. The cervical spine is traditionally divided into two columns—anterior and posterior. The anterior column consists of the load-bearing vertebral bodies, intervertebral disks, anterior longitudinal ligament, and posterior longitudinal ligament (Fig. 75.1). The posterior column consists of the more posterior structures, including the pedicles, laminae, and transverse and spinous processes (Fig. 75.2).
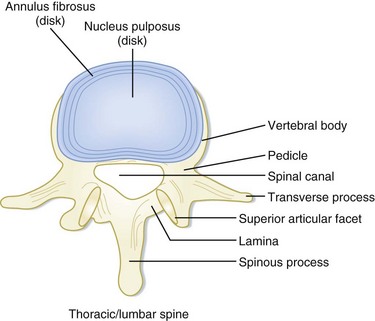
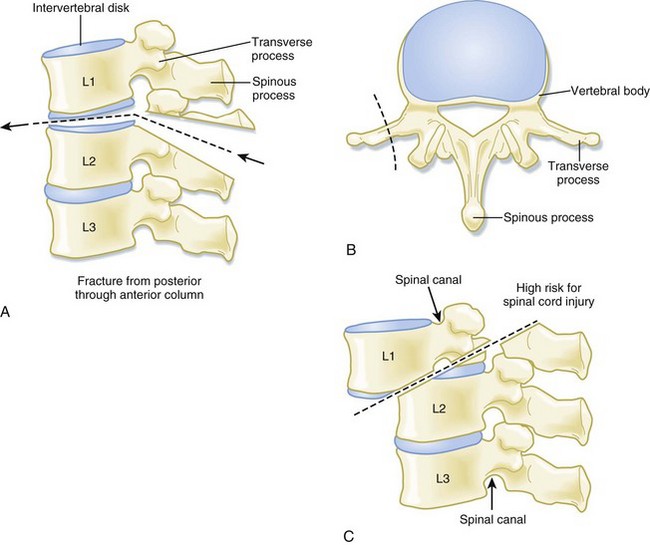
In contrast, the thoracic and lumbar vertebral spines are divided into three columns based on the modified Denis model—anterior, middle, and posterior (Fig. 75.3). The anterior column consists of the anterior longitudinal ligament, the anterior two thirds of the vertebral body, and the intervertebral disk. The middle column consists of the posterior longitudinal ligament, the posterior third of the vertebral body, and the intervertebral disk. Any disruption of the middle column predisposes a patient to significant spinal cord injury because the middle column abuts the spinal canal. The posterior column consists of the remaining posterior structures.
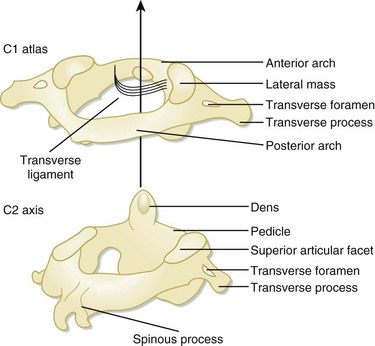
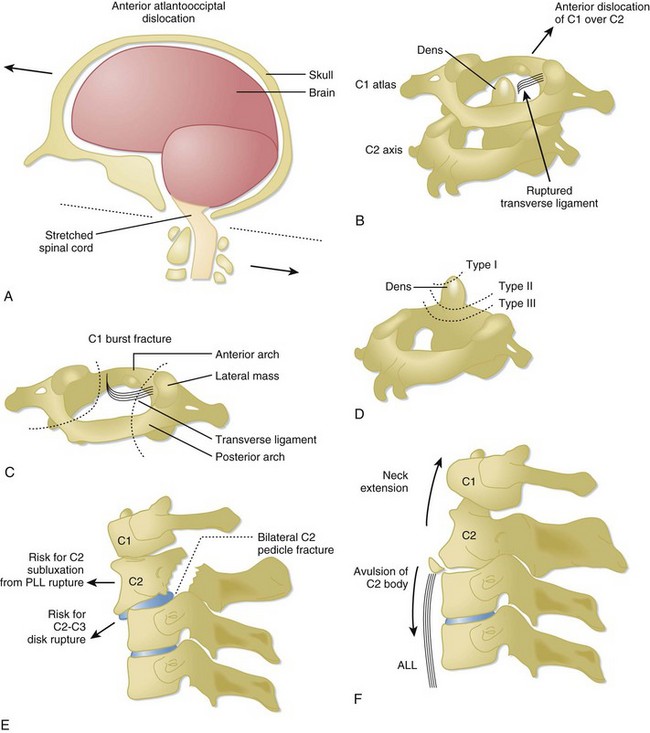
The C1 and C2 vertebrae are anatomically unique (Fig. 75.4). C1 (atlas) is a ring-link structure without a vertebral body. It articulates superiorly with the occipital condyles. This articulation allows 50% of normal neck flexion and extension. C2 (axis) projects the dens superiorly to articulate with C1. The transverse ligament tethers the dens to the anterior arch of C1. This atlantoaxial articulation allows 50% of normal neck rotation left and right.
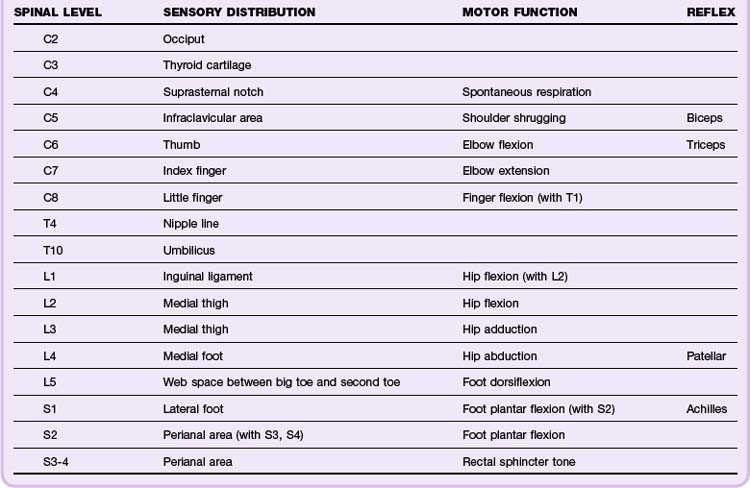
The spinal cord spans from the foramen magnum to the L1 level, whereupon the spinal cord tapers into the conus medullaris and cauda equina, a collection of peripheral lower lumbar and sacral nerve roots. Because the spinal cord is thickest in the cervical spine, there is relatively less spinal canal space in the cervical levels than in the thoracic or lumbar spine. Thus spinal cord injuries occur more frequently with cervical spine trauma than with thoracic or lumbar spine trauma. The neurologic dermatomes can help localize the injury (Table 75.1).
Presenting Signs and Symptoms
Patients with spinal cord injuries may have a spectrum of findings ranging from subtle neurologic deficits to grossly obvious paralysis. Spinal cord injuries should be suspected in any trauma victim who complains of neck or back pain, especially pain exacerbated by movement. Neurologic symptoms suggesting spinal cord injury include numbness, tingling, paresthesias, focal weakness, and paralysis. Other worrisome symptoms include urinary or fecal incontinence and urinary retention. Unconscious patients and those with impaired consciousness secondary to intoxication may harbor occult spinal cord injuries. Physical examination should focus on the spine and areas where associated injuries may occur (Tables 75.2 and 75.4).
Table 75.2 Physical Examination Findings Associated with Vertebral Fractures and Spinal Cord Injuries
| INJURY | PHYSICAL EXAMINATION AREA | ASSOCIATED FINDINGS |
|---|---|---|
| Vertebral fracture | Spine | Tenderness of the neck and/or back. Examine the entire spine because vertebral fractures may occur in multiples. |
| Neurologic | See spinal cord injury below. | |
| Chest | Thoracic spine fractures: Check for chest tenderness, unequal breath sounds, and arrhythmia, which are suggestive of an associated intrathoracic injury or myocardial contusion. | |
| Abdomen/pelvis | Thoracolumbar and lumbar spine fractures: Check for abdominal or pelvic tenderness. For instance, up to 50% of patients with a transverse process fracture7 and 33% of patients with a Chance fracture8 have concurrent intraabdominal pathology. A transverse area of ecchymosis on the lower abdominal wall (seat belt sign) increases the chance of an abdominopelvic injury. | |
| Extremity | Thoracolumbar and lumbar spine fractures: Check for calcaneal tenderness because 10% of calcaneal fractures are associated with a low thoracic or lumbar fracture. Mechanistically, these areas are fractured as a result of axial loading. | |
| Spinal cord injury | Neurologic, motor (anterior column) | Assess motor function on a scale of 0 to 5 (see Table 75.3). motor level is defined as the most caudal segment with at least 3/5 strength. Injuries to the first eight cervical segments result in tetraplegia (previously known as quadriplegia); lesions below the T1 level result in paraplegia. |
| Neurologic, sensory (spinothalamic tract) | Assess sensory function via pinprick and light touch on the following scale: 0 = absent; 1 = impaired; 2 = normal. The sensory level is defined as the most caudal segment of the spinal cord with normal sensory function. The highest intact sensory level should be marked on the patient’s spine to monitor for progression. | |
| Neurologic, sensory (dorsal column) | Assess vibratory sensory function on a scale of 0 to 2 by using a tuning fork over bony prominences. Assess position sense (proprioception) by flexing and extending the great toe. | |
| Neurology, deep tendon reflex | On a scale of 0 to 4, assess the deep tendon reflexes in the upper (biceps, triceps) and lower (patellar, Achilles) extremities (see Table 75.4). | |
| Anogenital | Assess rectal tone, sacral sensation, signs of urinary or fecal retention or incontinence, and priapism. Also check the anogenital reflexes: an anal wink (S2-S4) is present if the anal sphincter contracts in response to stroking the perianal skin area. The bulbocavernosus reflex (S3-S4) is elicited by squeezing the glans penis or clitoris (or pulling on an inserted Foley catheter), which results in reflexive contraction of the anal sphincter. | |
| Head-to-toe examination | A spinal cord injury may mask a patient’s ability to perceive and localize pain. Imaging of high-risk areas, such as the abdomen, and areas of bruising or swelling may be required to exclude occult injuries. |
Table 75.3 Graded Assessment of Motor Function
| GRADE | ASSESSMENT ON PHYSICAL EXAMINATION |
|---|---|
| 0 | No active contraction |
| 1 | Trace visible or palpable contraction |
| 2 | Movement with gravity eliminated |
| 3 | Movement against gravity |
| 4 | Movement against gravity and resistance |
| 5 | Normal power |
Table 75.4 Graded Assessment of Deep Tendon Reflexes
| GRADE | ASSESSMENT ON PHYSICAL EXAMINATION |
|---|---|
| 0 | Reflexes absent |
| 1 | Reflexes diminished but present |
| 2 | Normal reflexes |
| 3 | Reflexes increased |
| 4 | Clonus present |
Differential Diagnosis and Medical Decision Making
Indications for Cervical Spine Imaging
In the year 2000, in the hope of reducing the number of low-risk patients undergoing cervical spine plain film radiography, a multicenter study by the National Emergency X-radiography Utilization Study (NEXUS) group validated a set of five low-risk criteria for determining which patients do not require radiographic imaging if all the criteria are met (Box 75.1). This clinical decision tool demonstrated a sensitivity of 99.6% and a specificity of 12.9% for detecting clinically significant cervical spine fractures. It was thus extrapolated that 4309 (12.6%) of the 34,069 patients enrolled could have avoided plain film radiography.9
Box 75.1
NEXUS Low-Risk Criteria for a Cervical Spine Injury
1. No posterior midline neck pain or tenderness
2. No focal neurologic deficit
NEXUS, National Emergency X-radiography Utilization Study.
From Hoffman JR, Mower WR, Wolfson AB, et al. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. N Engl J Med 2000;343:94-9.
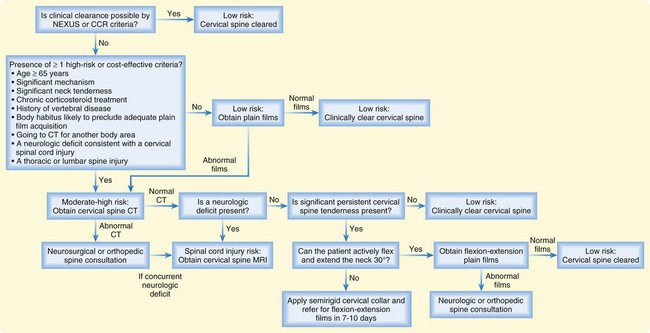
Following development of the NEXUS criteria, the Canadian Cervical-Spine Rule (CCR) was developed (Fig. 75.5). The validated sensitivity and specificity for this decision rule were 99.4% and 45.1%, respectively.10
Choosing the Imaging Modality to Evaluate the Cervical Spine (Fig. 75.6)
When patients have at least one high-risk criterion for a spinal fracture, imaging begins with either plain films or computed tomography (CT) scans. The pros and cons of both imaging approaches are listed in Table 75.5.
Table 75.5 Advantages and Disadvantages of Plain Film Imaging and Computed Tomography of the Cervical Spine
| PLAIN FILM RADIOGRAPHY | COMPUTED TOMOGRAPHY | |
|---|---|---|
| Advantages | Less irradiation of the thyroid, breast, and lens Can be performed at the bedside |
98% sensitivity in detecting fractures More cost-effective than plain films Less delay in patient management, especially if the patient is already going to CT scanner for imaging of another body part |
| Disadvantages | Only 53% sensitivity in detecting fractures Three-view films are inadequate >50% of the time, especially films of the cervicocranial and cervicothoracic junction Inefficient use of radiology personnel, who are often repeating films because of image inadequacy A suspicious fracture or one detected on plain films requires additional evaluation by CT for confirmation and further delineation |
More irradiation of the thyroid, breast, and lens Requires the patient to be hemodynamically stable because of being transported out of the emergency department to the CT scanner |
Computed Tomography
With increasing evidence in the literature showing that CT is much more sensitive (98%) than plain film radiography (53%) in detecting cervical spine fractures, future recommendations will probably recommend cervical spine CT as the first-line diagnostic approach for most patients because of the neurologic significance of a missed cervical spine injury.11 Conventional radiography is especially difficult to interpret in the high cervical spine (occiput, C1, C2) and cervicothoracic junction (C6, C7, T1), where coincidentally most cervical spine fractures occur.12 It is important to obtain sagittal CT reconstructions, in addition to the traditional axial views, to adequately assess spinal alignment.
Cost analyses have shown that cervical spine CT scans are actually less expensive than conventional radiography in high-risk patients. These studies factored personnel time, delays in patient management while obtaining films, and the neurologic sequelae of initially missing a cervical spine injury. Cost savings are especially evident if the patient is already undergoing CT imaging of other body parts, such as head scanning for a closed head injury. With multidetector scanners being more readily available, an additional cervical spine scan would add less than 5 minutes of scan time at a relatively small cost.13
The risk for cancer from irradiation serves as the major deterrent against universally performing CT in all patients with neck trauma. It is estimated that up to 2% of cancers in the United States are attributable to CT studies.14 The thyroid gland, breast tissue, and lens are exposed to especially high levels of radiation in cervical spine CT, thus placing the patient at high risk for the development of thyroid cancer, breast cancer, and cataracts. Patients receive an effective dose of 0.2 millisievert (mSv) and 6 mSv for cervical spine plain films and CT, respectively. In contrast, the effective dose of a posteroanterior and lateral chest radiograph is just 0.1 mSv.15 The overall lifetime carcinogenic risk from CT imaging, however, varies depending on the patient’s age at the time of irradiation. Younger patients have greater risk, partly because they have more years of life left for the development of cancer. Furthermore, children are more radiosensitive. If irradiated after 40 years of age, the risk reaches its nadir, with an estimated lifetime attributable risk for death from cancer of less than 0.2%.14
Older and Osteopenic Patients
Patients older than 65 years old and those taking corticosteroids on a long-term basis are probably osteopenic. They can sustain spinal fractures with mild trauma, such as a fall from a standing position, and often exhibit minimal associated pain. Specifically, patients older than 65 years have an increased risk for cervical spine fracture (relative risk of 2.09).16 In addition, acute back pain in chronic corticosteroid users is correlated with 99% specificity for a spinal compression fracture.17 Thus, imaging should be performed in these potentially osteopenic patients in the setting of neck or back pain.
Clinical Clearance of the Cervical Spine
![]() Facts and Formulas
Facts and Formulas
Ten percent of spinal fractures have a second noncontiguous fracture along the vertebral spine.
Ten percent of patients with a calcaneal fracture have an associated thoracic or lumbar fracture.
The most commonly fractured cervical spine level is C2, especially in the elderly.
Approximately 20% of computed tomography–confirmed burst fractures in the thoracic and lumbar spine appear as wedge fractures on plain film radiography.18
High-dose methylprednisolone is administered as a 30-mg/kg bolus and then as a 5.4-mg/kg/hr infusion for 24 hours (if started within 3 hours of injury) or for 48 hours (if started within 8 hours of injury).
Consider early endotracheal intubation in spinal cord injury patients with a negative inspiratory force of less than −25 cm H2O or a vital capacity of less than 15 mL/kg.
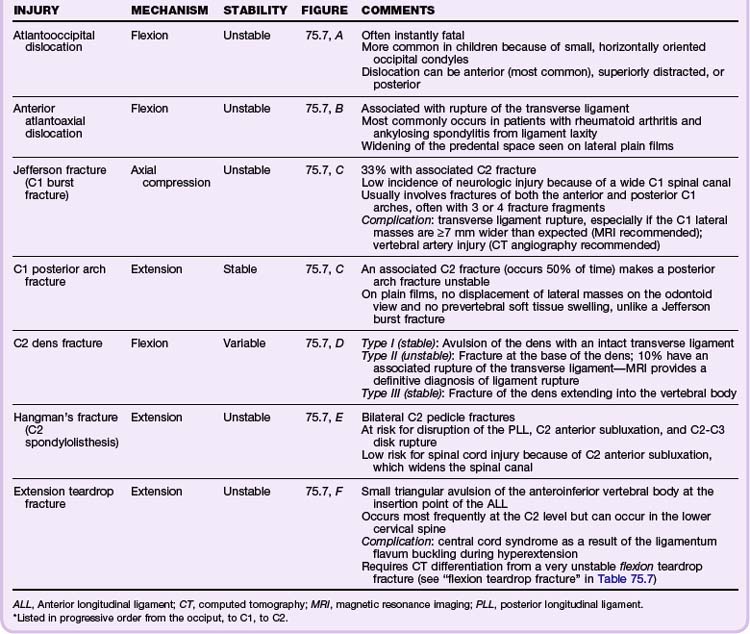
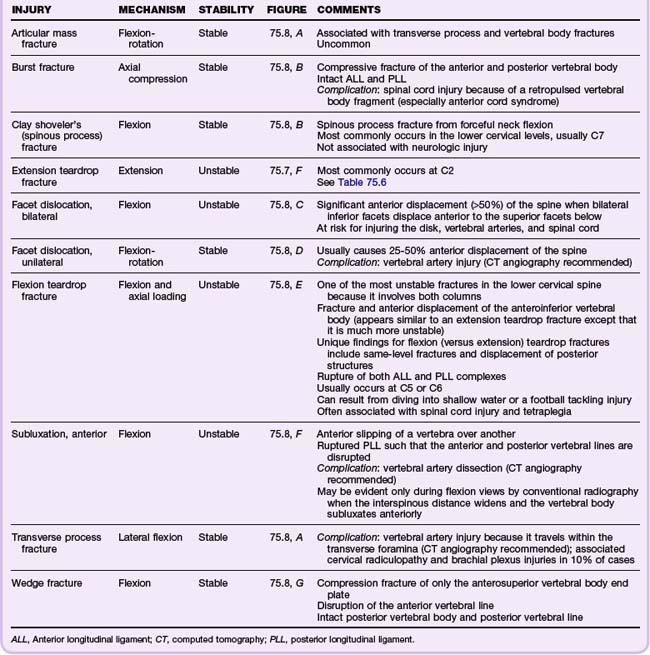
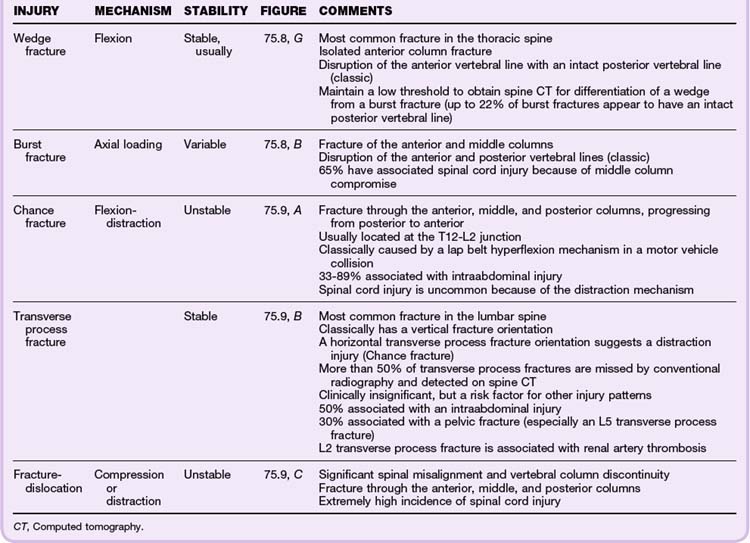
Classic Fracture Patterns (Tables 75.6 to 75.8; Figs. 75.7 to 75.9)
Cervical Spine Injuries
Based on the NEXUS study of 818 patients with cervical spine injury, fractures occurred most commonly at the level of C2 (24% of all fractures), C6 (20%), and C7 (19%). Anatomically, the most commonly fractured part of the cervical spine was the vertebral body, which accounted for 30% of fractures at the C3 to C7 levels. It was more common than fractures of the spinous process (21%), lamina (16%), and articular process (15%). Subluxations occurred most commonly at the C5-C6 (25%) and C6-C7 (23%) levels.19
Classification of Spinal Cord Injuries
Treatment
In-Line Immobilization of the Cervical Spine
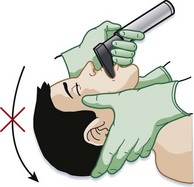
During the initial resuscitation phase of trauma victims, patients with a potential cervical spine injury may require endotracheal intubation before a definitive diagnosis can be made. By preventing neck hyperextension during direct laryngoscopy, in-line cervical spine immobilization during intubation maintains cervical spine neutrality (Fig. 75.10).
Corticosteroid Therapy for Spinal Cord Injury
Though controversial, treatment of blunt spinal cord injury with high-dose methylprednisolone is common. This therapeutic recommendation is based on the findings of the National Acute Spinal Cord Injury Study (NASCIS), which demonstrated improved neurologic function in patients receiving high-dose corticosteroids within 8 hours of injury. Improved neurologic function, however, was defined as a modest gain in motor scores but not functional improvement. In NASCIS, a loading dose of 30 mg/kg of methylprednisolone administered over a 15-minute period was followed by an infusion of 5.4 mg/kg/hr and continued for 24 hours (in patients treated within 3 hours of injury) or 48 hours (in patients treated 3 to 8 hours after injury).20,21 No benefit was found when steroids were administered more than 8 hours after injury.
Finally, systemic corticosteroid therapy is not benign. Complications of steroid therapy include gastrointestinal hemorrhage and wound infection in patients treated with corticosteroid infusions for 24 hours and higher rates of severe sepsis and severe pneumonia in those treated for 48 hours. The use of steroids for blunt traumatic spinal cord injury is far from the standard of care.22 More research is needed to verify or refute this controversial therapy.
Surgical Management of Spinal Cord Injury
Timely reduction of the displaced spinal column plus decompression of the spinal cord has been associated with recovery from otherwise devastating spinal cord injuries.23 The optimal timing of surgery following a spinal injury remains controversial. Some argue for immediate surgery, whereas others advocate delayed surgery because of the initial posttraumatic swelling. The sole absolute indication for immediate surgery is progressively worsening neurologic status in patients with spinal fracture-dislocations who initially have incomplete or absent neurologic deficits.24
In a series of patients with traumatic central cord syndrome, those who underwent early surgery (<24 hours after injury) and had an underlying disk herniation or fracture-dislocation exhibited significantly greater overall motor improvement than did those who underwent late surgery (>24 hours after injury).25 Unfortunately, early decompressive surgery does not uniformly improve outcome following spinal cord injury.
![]() Priority Actions
Priority Actions
Maintain full spinal precautions until the spine can be cleared radiographically or clinically.
If intubating a trauma patient, an assistant should provide in-line cervical spine immobilization until the cervical spine can be assessed more definitively at a later time.
Perform a careful initial neurologic examination, especially in patients who are about to undergo sedation or neuromuscular blockade.
If a spinal fracture is suspected or detected, evaluate for associated injuries:
Obtain urgent spine imaging if a fracture or spinal cord injury is suspected.
Obtain emergency magnetic resonance imaging of the spine if a spinal cord injury is suspected.
Consider administering corticosteroids if an adult patient has sustained blunt spinal trauma and exhibits neurologic deficits within 8 hours of injury.
Tips and Tricks
Prolonged immobilization on a rigid backboard is uncomfortable for the patient and places the patient at risk for aspiration and early pressure sores. Aim to remove the backboard as soon as possible and ideally within 2 hours of patient arrival. A standard hospital gurney provides adequate thoracic and lumbar stability.
Perform serial neurologic examinations on patients with suspected or known spinal injuries to document neurologic improvement or deterioration. Neurologic deterioration involving the cervical and upper thoracic levels may require empiric endotracheal intubation for impending respiratory failure.
Once a spinal injury is detected, carefully reexamine the entire cervical, thoracic, and lumbar spine. Obtain plain films or computed tomography scans of any levels with pain or tenderness because of the high risk for a second spinal injury.
When performing “clinical clearance” of a patient’s cervical spine or obtaining flexion-extension cervical spine plain films, do not passively range the neck for the patient. This may cause an iatrogenic spinal injury. Pain with active movement will prevent the patient from overranging the neck.
![]() Red Flags (Pitfalls)
Red Flags (Pitfalls)
Failure to identify occult injuries in hypoesthetic areas. For example, in a patient with a midthoracic sensory level deficit, occult intraabdominal injuries may be hidden because the abdomen may be insensate.
Failure to consider a spinal cord injury in a patient with normal radiographic and computed tomographic (CT) findings.
Failure to repeat plain films or obtain CT imaging when plain film radiographs of the cervical, thoracic, or lumbar spine are inadequate.
Failure to exclude other causes of hypotension in a trauma patient before assuming that it is neurogenic shock. A search for occult blood loss should first be done.
Failure to consider a distracting injury, particularly fractures, as a reason for a patient’s ability to localize neck and back pain.
Follow-up, Next Steps in Care, and Patient Education
![]() Documentation
Documentation
Document neck and back tenderness, along with the neurologic examination, in all trauma patients.
In spinal cord injury patients, mark the initial level of sensory deficit to monitor progression of the patient’s neurologic status.
For patients with neurologic deficits, perform and document the bulbocavernosus reflex and sacral-sparing examination to assess for spinal shock.
1 Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604.
2 Hoffman JR, Mower WR, Wolfson AB, et al. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. N Engl J Med. 2000;343:94–99.
3 Stiell IG, Clement CM, McKnight RD, et al. The Canadian C-Spine Rule versus the NEXUS low-risk criteria in patients with trauma. N Engl J Med. 2003;349:2510–2518.
1 Berkowitz M. Assessing the socioeconomic impact of improved treatment of head and spinal cord injuries. J Emerg Med. 1993;11:63–67.
2 Reid DC, Henderson R, Saboe L, et al. Etiology and clinical course of missed spine fractures. J Trauma. 1987;27:980–986.
3 Nunez DB, Jr., Zuluaga A, Fuentes-Bernardo DA, et al. Cervical spine trauma: how much more do we learn by routinely using helical CT? Radiographics. 1996;16:1307–1318.
4 Woodring JH, Lee C. Limitations of cervical radiography in the evaluation of acute cervical trauma. J Trauma. 1993;34:32–39.
5 Hauser CJ, Visvikis G, Hinrichs C, et al. Prospective validation of computed tomographic screening of the thoracolumbar spine in trauma. J Trauma. 2003;55:228–234.
6 Lucey BC, Stuhlfaut JW, Hochberg AR, et al. Evaluation of blunt abdominal trauma using PACS-based 2D and 3D MDCT reformations of the lumbar spine and pelvis. AJR Am J Roentgenol. 2005;185:1435–1440.
7 Patten RM, Gunberg SR, Brandenburger DK. Frequency and importance of transverse process fractures in the lumbar vertebrae at helical abdominal CT in patients with trauma. Radiology. 2000;215:831–834.
8 Tyroch AH, McGuire EL, McLean SF, et al. The association between Chance fractures and intra-abdominal injuries revisited: a multicenter review. Am Surg. 2005;71:434–438.
9 Hoffman JR, Mower WR, Wolfson AB, et al. Validity of a set of clinical criteria to rule out injury to the cervical spine in patients with blunt trauma. N Engl J Med. 2000;343:94–99.
10 Stiell IG, Clement CM, McKnight RD, et al. The Canadian C-Spine Rule versus the NEXUS low-risk criteria in patients with trauma. N Engl J Med. 2003;349:2510–2518.
11 Mahadevan SV, Navarro M. The evaluation and clearance of the cervical spine in adult trauma patients: clinical concepts, controversies, and advances. Trauma Rep. 2004;5:1–12.
12 Velmahos GC, Theodorou D, Tatevossian R, et al. Radiographic cervical spine evaluation in the alert asymptomatic blunt trauma victim: much ado about nothing? J Trauma Injury. 1994;40:768–774.
13 Blackmore CC, Ramsey SD, Mann FA, et al. Cervical spine screening with CT in trauma patients: a cost-effective analysis. Radiology. 1999;212:117–125.
14 Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. N Engl J Med. 2007;357:2277–2284.
15 Mettler FA, Jr., Huda W, Yoshizumi TT, et al. Effective doses in radiology and diagnostic nuclear medicine: a catalog. Radiology. 2008;248:254–263.
16 Lowery DW, Wald MM, Browne BJ, et al. Epidemiology of cervical spine injury victims. Ann Emerg Med. 2001;38:12.
17 Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268:760–765.
18 Ballock RT, Mackersie R, Abitbol JJ, et al. Can burst fractures be predicted from plain radiographs? J Bone Joint Surg Br. 1992;74:147–150.
19 Goldberg W, Mueller C, Panacek E, et al. Distribution and patterns of blunt traumatic cervical spine injury. Ann Emerg Med. 2001;38:17–21.
20 Bracken MB, Holford TR. Effects of timing of methylprednisolone or naloxone administration on recovery of segmental and long-tract neurological function in NASCIS 2. J Neurosurg. 1993;79:500–507.
21 Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604.
22 Spencer MT, Bazarian JJ. Evidence-based emergency medicine/systematic review abstract. Are corticosteroids effective in traumatic spinal cord injury? Ann Emerg Med. 2003;41:410–413.
23 Brunette DD, Rockswold GL. Neurologic recovery following rapid spinal realignment for complete cervical spinal cord injury. J Trauma. 1987;27:445–447.
24 Lindsey RW, Pneumaticos SG, Gugala ZG. Management techniques in spinal injuries. In: Browner BD, Jupiter JB, Levine AM, et al. Skeletal trauma: basic science, management, and reconstruction. 3rd ed. Philadelphia: Saunders; 2003:746–747.
25 Guest J, Eleraky MA, Apostolides PJ, et al. Traumatic central cord syndrome: results of surgical management. J Neurosurg. 2002;97:25–32.