Chapter 17 Spine Fusion
Anatomy and Biomechanics of Bone-Bone Interface
In the late 19th century, Sir William Macewen firmly established bone grafting as a treatment option for replacing missing bone and enhancing bone formation. His interest in bone grafting led him to perform allografts and autografts in his patients.1 In the United States, spine fusion was first reported in the early 1900s by Albee2 for the treatment of Pott disease and by Hibbs,3 who used fusion surgery to halt the progression of scoliotic deformity. Since that time the indications for and number of spine fusions have increased. In fact, the numbers doubled between 1980 to 1990,4 with an increase of 77% between 1996 and 2001.5 Spine arthrodesis is now one of the most common surgical procedures performed in the United States.
Unfortunately, a number of complications have been associated with spine fusion. Pseudarthrosis can occur in as many as 35% to 40% of multilevel lumbar fusions.6 Donor site morbidity can also be considerable.7 To achieve successful bony fusion, minimize complications, and achieve a good functional outcome, it is important to understand the various structural, biologic, and biomechanical aspects of bone fusion.
Bone grafts involve transplanting bone tissue from one site to another in order to obtain bone fusion. The terms used for describing them are usually derived from the bone’s origin, anatomic placement, or composition. Autograft is a transplanted tissue within the same individual; allografts are tissues coming from a genetically different individual of the same species; xenografts are tissues transplanted from one species to a member of a different species; isograft is tissue obtained from a monozygotic twin. A graft transplanted to an anatomically appropriate site is defined as orthotopic, whereas if it is transplanted to an anatomically dissimilar site, it is termed heterotopic. Grafts are also categorized by composition as cortical, cancellous, corticocancellous, or osteochondral.8
Anatomy of the Bone-Bone Interface
Histologic Components
Cancellous bone is porous and appears as a lattice of rods, plates, and arches individually known as trabeculae. It has a greater surface area and can be readily influenced by adjacent bone marrow cells. Because of this structural difference, cancellous bone has a higher metabolic activity and responds more readily to changes in mechanical loads.9
Cortical and cancellous bone may consist of woven (primary) or lamellar (secondary) bone. Woven bone forms the embryonic skeleton and is then resorbed and replaced by mature bone as the skeleton develops.10 In the adult, woven bone is found only in pathologic conditions, such as fracture healing and in tumors. Woven and lamellar bones differ in formation, composition, organization, and mechanical properties. Woven bone has an irregular pattern of collagen fibers, contains approximately four times as many osteocytes per unit volume, and has a rapid rate of deposition and turnover. The osteocytes of woven bone vary in orientation, and the mineralization of woven bone follows an irregular pattern in which mineral deposits vary in size and in their relationship to collagen fibrils. In contrast, the osteocytes of lamellar bone are relatively uniform, with their principle axis oriented parallel to that of other cells and to the collagen fibrils of the matrix. The collagen fibrils of lamellar bone lie in tightly organized, parallel sheets, with uniform distribution of mineral within the matrix.11,12
The irregular structure of woven bone makes it more flexible, more easily deformed, and weaker than lamellar bone.9 For these reasons the restoration of normal mechanical properties to bone tissue at the site of a healing fracture requires eventual replacement of the woven bone of the fracture callus with mature lamellar bone.11
Biomechanical Properties of Graft Material
In vivo, the mechanical performance of a bone graft is a function of the intrinsic property of the graft and the properties of the graft-host interface.13 Intrinsic properties of a graft are related to its geometry and composition and include its fracture toughness, yield strength, and elastic modulus.8
In a clinical setting, where the graft has geometric and mechanical properties similar to the host bone, it may function almost immediately.14 Nevertheless, in the case of inferior bone graft mechanical properties, the construct should be designed with additional graft material or incorporate internal fixation until remodeling occurs and the graft can provide adequate load-bearing function.14 A graft’s load-bearing capacity is achieved after complete biologic incorporation by the host, which is related to the mechanical and biologic properties of the graft-host interface.
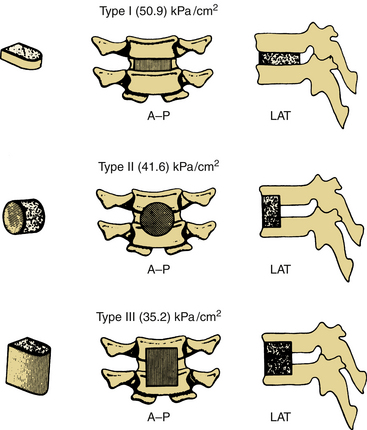
Iliac crest wedges are the most commonly used graft material. The percentages of cortical and cancellous bone remain constant at 41% and 59%, respectively, regardless of the total cross-sectional area of the wedge. Donor age also does not affect this physical parameter.15
To reduce the immune response and also as methods of preservation and sterilization, allografts undergo certain modifications. These modifications have a profound effect on the biomechanical properties of the graft. Freezing has minimal effects compared with freeze-drying, which significantly reduces both the yield strength and stiffness of the bone graft.16 Autoclaving produces a dose-dependent decrease in strength and stiffness.17 The relationship between gamma radiation and mechanical properties has yet to be established at doses between 0 and 25 kGy (standard dose). But it becomes dose-dependent at 25 kGy for cortical bone or 60 kGy for cancellous bone.18 Complete demineralization of the bone graft results in loss of almost all of its mechanical properties. Comparison testing of various graft materials shows allograft or fresh-frozen cancellous bone to be the weakest, failing at 863 N of compression. Air-dried, ethylene oxide-sterilized, tricortical bone failed at an average load of 2308 N, and fresh-frozen, tricortical allograft bone failed at an average load of 2257 N. Rehydrated iliac crest wedges are more deformable than freeze-dried wedges.19 During loading, freeze-dried wedges fail dramatically, fracturing into many small pieces; this occurrence is secondary to its brittle nature. Rehydrated wedges fail with a circumferential fracture along the side of the wedge where the cortical bone is thinnest. It has been recommended that freeze-dried wedges be rehydrated in a vacuum before clinical use.19 When water or saline is added to the vacuum-sealed container holding the wedge, the wedges gain 100% of their wet weight within 5 minutes of addition of the fluid. Graft collapse occurred more frequently with freeze-dried allografts (30%) than with autografts in anterior cervical fusions.
The loads at the lumbar spine have been well documented in various positions and levels of activity.20 Either autograft or allograft iliac crest wedges are biomechanically sound in an interbody fusion of the lumbar spine, since such fusions would provide load-bearing capacities approximately fourfold greater than would be applied in vivo. Specimens from the anterosuperior iliac spine could bear substantially greater axial loads (average 3230 N) compared with specimens from the posterosuperior iliac spine (average 1458 N).21 Fibular strut grafts are the strongest and have been shown to have a compressive strength of 5070 N.22 However, their cross-sectional area, which is important in preventing telescoping of the graft, is much smaller. In interbody fusion, the cross-sectional area of the graft should be substantially greater than 30% of the end plate to provide a margin of safety.23
Incorporation of Bone Graft
Bone graft incorporation is a prolonged process with a sequence of complicated steps involving the interrelationship of the graft and host. This ultimately leads to the envelopment of a complex of necrotic old bone with viable new bone.24 The complex develops through resorption of the necrotic old bone with viable new bone being laid down. The incorporation of the bone graft is a dynamic process involving the following processes: osteoinduction, osteoconduction along with the availability of osteogenic cells, and the structural integrity, which provides mechanical support.14,19,25,26 This ultimately leads to the replacement of the graft by host bone in a predictable pattern under the influences of load bearing.14,27
At the beginning, the inflammatory response at the host-graft interface results in migration of inflammatory cells and fibroblasts into the bone graft. In addition, the developing hematoma enhances the release of both cytokines and growth factors. Osteoinduction is the process whereby a tissue is influenced to form osteogenic elements through chemotaxis, mitosis, and differentiation of the host osteoprogenitor cells. Induction requires an inducing stimulus, such as a piece of bone or an osteogenic cell, and an environment favorable for osteogenesis. Osteoconduction is the process by which capillaries, perivascular tissue, and osteoprogenitor cells from the recipient bed grow into the graft. It can occur within a framework of nonbiologic materials or nonviable biologic materials. In viable bone grafts, osteoconduction is facilitated by osteoinductive processes and therefore occurs more rapidly than in nonviable or nonbiologic materials.28 Ultimately, this process results in the resorption of the original graft tissue and replacement with new host bone. Remodeling is a response to weight bearing.
Differences in Cancellous and Cortical Bone Graft Incorporation
Cancellous grafts are revascularized more rapidly and completely than cortical grafts. The open trabecular pattern of cancellous bone facilitates vessel ingrowth. Revascularization has been reported to begin within a few hours after grafting29 and may be complete by 2 weeks. In contrast, the dense structure of cortical bone prevents neovascular penetration during the first several weeks after grafting, and hence revascularization of cortical bone may take several months. Because of the dense architectural structure of cortical bone, new vessel incorporation follows preexisting haversian and Volkmann canals.30
Several differences exist between the cellular process of repair in cancellous and cortical grafts. With cancellous grafts, primitive mesenchymal cells that originate in the trabeculae may differentiate directly into osteoblasts, thereby resulting in relatively early new bone formation. The new bone forms on the dead trabeculae of the graft. This is followed by a resorptive phase. Cancellous bone initially undergoes an appositional new bone formation phase called creeping substitution, which is the process of new tissue invading along channels made by invasive blood vessels or along preexisting channels in the transplanted bone.31 The necrotic areas within the cancellous bone graft eventually are entirely resorbed by osteoblastic activity and totally replaced with new viable bone. As the revascularization of cancellous bone graft proceeds, primitive mesenchymal cells differentiate into osteogenic cells. These osteogenic cells form osteoblasts that line the edges of dead trabeculae and deposit a seam of osteoid that is annealed to, and eventually surrounds, a central core of dead bone. This process of alignment of osteoblasts on existing bone surfaces, with the synthesis of osteoid in successive layers to form lamellae, is termed appositional bone formation. Thus initially, there is an increase in the size of the graft. Cancellous grafts tend to repair completely with time. The areas of entrapped necrotic bone are resorbed by osteoclasts. In time the cancellous bone graft is completely replaced by viable new bone.
Cortical grafts must undergo osteoclastic resorption before osteoblastic new bone formation occurs. In cortical grafts the repair process is initiated by osteoclasts with preferential early resorption of the external cortical surface. Osteoblasts appear only after bone resorption has begun, and the initial deposition of osteoid usually occurs in resorbed areas. Cortical grafts remain as admixtures of necrotic and viable bone. In cortical grafts, revascularization is primarily the result of vascular infiltration through Volkmann and haversian canals.19 Osteoclasts initiate resorption of bone approximately 2 weeks after vascularization. Resorption is maximal at 6 weeks, and then gradually the graft recovers normal strength by 1 year. New bone is formed and seals off the remaining necrotic bone from further encroachment beginning at around 12 weeks. Thus if a biopsy specimen is obtained from a cortical graft years after placement, it demonstrates an admixture of necrotic and viable bone.
Biomechanics of Graft Incorporation
Porosity is a dominant factor in determining the material properties of bone. It is directly related to the stiffness of the tissue and yield of strength.13,14,32 Therefore, any change in porosity result in important effects on the bone graft material properties. Cortical bone grafts initially may have as little as 5% to 10% porosity, whereas cancellous grafts may be as high as 70% to 80%. This explains the material strength of cancellous graft, which is roughly equivalent to 4% of that of cortical bone.13
Cancellous grafts are incorporated by an early appositional phase. New bone formation onto the necrotic trabeculae of the graft tissue leads to an early increase in graft strength. It has been shown that necrotic bone maintains its mechanical strength.30 Cancellous grafts therefore initially strengthen with the addition of new bone. As the necrotic cores are resorbed, the mechanical strength of the graft area normalizes.
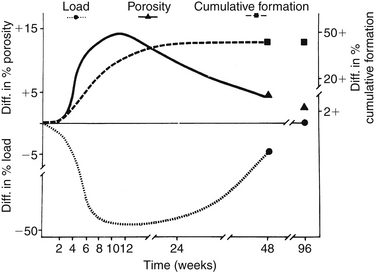
Cortical bone grafts first undergo osteoclastic bone resorption, which significantly increases graft porosity and thus decreases the graft strength. In the canine model of autogenous cortical transplant, the greatest compromise in mechanical strength occurs at 12 weeks30 (Fig. 17-1). The strength returns to normal between 1 and 2 years after transplantation. Human data suggest that cortical grafts lose approximately half their biomechanical strength during the first 6 months—a decline that persists for another 6 months.33 This process is related to osteoclastic graft resorption and is slowly reversed during the second year after implantation. These observations correlate with the highest incidence of mechanical graft failure between 6 and 8 months after transplantation. If the graft is allogenic, this process is further prolonged. Hence it is important to protect segmental grafts during the critical phase when the resorptive phase outstrips the appositional phase. This is usually accomplished by load sharing with spinal instrumentation or a spinal orthosis.
Temporal Profile of Graft Incorporation
During the first week after grafting, both cancellous and cortical grafts have similar histologic features. Both are surrounded by coagulated blood, and the graft is the focus of a tissue response characterized by vascular buds infiltrating the grafted bed. By the second week, fibrous granulation tissue becomes increasingly dominant in the graft bed, the number of inflammatory cells decrease, and osteoclastic activity increases. Within the confines of the graft, osteocytic autolysis proceeds, resulting from anoxia and injury by surgery, with necrosis delineated by vacant lacunae. Some cells, however, survive by diffusion of nutrients from surrounding host tissues. Creeping substitution of cortical bone grafts progresses transversely and parallel to the long axis of the transplanted segment. Thus the repair is found to be greater at the graft-host junctions.34
A study done in rabbits35 showed the sequence of events during the process of dorsolateral intertransverse fusion. Three phases were identified. Phase 1 represents the early reparative phase (1–3 weeks). It consists of hematoma formation and granulation tissue. There is minimal ossification. Phase 2 represents the middle reparative phase (4–5 weeks), when the fusion solidifies. Finally, phase 3 represents the late remodeling phase (6–10 weeks).35
Host Response and Incorporation of Autograft and Allograft
Autograft remains the gold standard in most fusion applications. In certain situations in which available autologous bone is insufficient or when large structural grafts are needed, allograft fusion rates can approach or equal those of autograft rates, without donor site morbidity. A successful spine fusion requires a sufficient area of decorticated host bone, ample graft material, minimal motion at the fusion site, and a rich vascular supply.36
Histocompatibility matching has an important influence on the process of incorporation. Allograft that is mismatched for major histocompatibility complex antigens functions poorly compared with autogenous grafts.37,38 Bone cells display class I and class II histocompatibility antigens, and there are both cellular and humoral responses to bone allografts.39 Syngeneic grafts are the most successful. Grafts with major histocompatibility mismatch have delayed and incomplete revascularization, compared with syngeneic grafts. In addition, marked resorption of bone often occurs, resulting in almost complete loss of graft.38 Freezing the graft, followed by thawing, disrupts and kills the cells. It mutes the antigenicity in major mismatches and thus enhances incorporation of such grafts. However, the killing of cells also diminishes the biologic activity of the graft. It is the osteoinductive component that is mainly affected. The function of an allograft as an osteoconductive system seems virtually unimpaired.
Modeling and Remodeling Associated with Spine Fusion
The bone modeling associated with spine fusion is extremely complex. Variables that may affect bone remodeling after graft insertion include (1) the design of implant, materials used, and methods of fixation; (2) the local bone, including its density and shape; and (3) the patient characteristics, including age, gender, hormonal balance, and activity.40 Osteoblasts and osteoclasts are influenced by the magnitude and state of strain imposed on them by load applied to the bone. Stresses or strains within a given range seem to be required to maintain a steady state remodeling of bone in which the rate of bone formation equals the rate of resorption. Stresses below the optimum are often associated with stress shielding, leading to bone resorption. Stresses and strains exceeding upper limits can also produce resorption of bone as a result of pressure necrosis. Cyclic stresses are required to maintain osseous homeostasis. Constant loads, even when within the desired range, can result in insufficient stimulus to maintain bone mass. Observations of strain-related electric potentials in bone, biopotentials, and electrical stimulation of osteogenesis suggest that bioelectric phenomena function as the regulators of adaptive remodeling of bone.
Growth Factors and Cytokines in Regulating Bone Remodeling
Bone formation in spine fusion is a complex and regulated process. The cellular events involved in bone formation include chemotaxis of osteoblast precursors, proliferation of committed osteoblast precursors, and differentiation and expression of regulatory factors and structural proteins of bone and mineralization.41 These processes require tight regulatory control. They may be modulated by systemic hormones, such as parathyroid hormone, but predominant control is by local factors or cytokines. Cytokines are small proteins that serve as signaling agents for cells. Cytokines are classified based on their cellular origin and principal biologic activities.42 The main families include interleukins, tumor necrosis factors, growth factors, colony-stimulating factors, interferons, and chemokines.
Bone morphogenetic protein (BMP) is a member of the transforming growth factor beta (TGF-β) superfamily. The BMP constitutes a growth family of more than 12 proteins, 9 of which have been shown individually to induce ectopic bone formation.43 They are water soluble, noncollagenous substances found in the bone matrix with osteoinductive activity. BMPs 2, 4, and 7 are specially increased in the primitive mesenchymal and osteoprogenitor cells, fibroblasts, and proliferating chondrocytes present at the fracture site.18,44,45 During the phases of healing, the expression of BMPs 2, 4, and 7 is strongly present in undifferentiated mesenchymal cells during the inflammatory phase. During intramembranous ossification, these BMPs are strongly present in the proliferating osteoblasts. During chondrogenesis and endochondral ossification, BMPs 2 and 4 are found in proliferating chondrocytes and strongly in osteoblasts near the endochondral ossification front. BMP 7 is found in later stages of healing in proliferating chondrocytes and weakly in mature chondrocytes.18,45 BMPs also affect the expression of other growth factors that may function to mediate the effects of BMPs on bone formation.
They are the most widely investigated osteoinductive growth factor in spine fusion.43,46 Several animal studies have shown that recombinant human (rh) BMPs 2, 4, and 7 induce bone formation at an orthotopic site at which the integration with the preexisting bone is structurally sound. It has also been shown that BMP plus marrow yields the highest union rates (100%) and is three times better than autogenous cancellous graft.46–54
Biomechanics of Fusion
There is a wealth of literature on the biomechanics of fusion, but the vast majority involves in vitro models. In vivo, a variety of biologic factors influence the mechanical properties of fusion mass. The type of surgical construct and choice of bone graft should be individualized, based on the biologic and mechanical considerations. The main indications for a spine fusion are listed in Box 17-1.
BOX 17-1 Primary Indications for Spine Fusion
Deformity: To correct and prevent progression of deformity
Instability: To restore the structural integrity
Painful motion segment: Includes low back pain caused by segmental instability
Positioning of Bone Graft
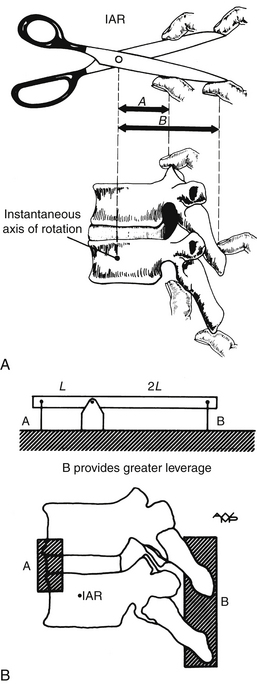
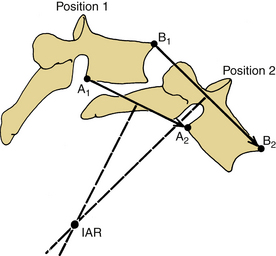
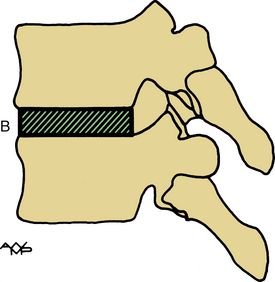
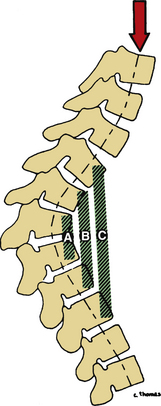
As White and Panjabi20 describe, “The placement of a fusion mass at the maximum distance from the instantaneous axes of rotation will be more effective in preventing the movement around those axes” (Fig. 17-2). The instantaneous axis of rotation (IAR) is defined as the point in the body, or some hypothetical extension of it, that does not move when a rigid body moves in a plane. An axis perpendicular to the plane of motion and passing through the point is the IAR for that motion at that instant (Fig. 17-3). It can be defined more simply as the axis around which the vertebral body rotates. It is like a fulcrum. Usually, but not always, the IAR passes through the confines of the vertebral body. With isolated destruction of columns of the spine, the IAR migrates to the remaining intact structures, as shown in Figure 17-4.
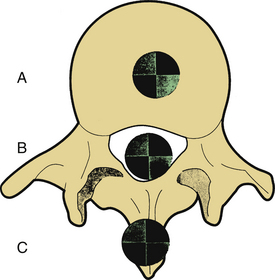
The concept of rigidity is also important. A fusion mass that involves all the dorsal elements and transverse processes provides more rigidity than a fusion that only involves the spinous process. In some situations it can be disadvantageous to place the graft at a distance from the IAR. For example, after a dorsal fusion to treat discogenic pain, motion may still occur at the disc interspace, even when all dorsal elements except the pedicle are fixed.55,56 In such situations an interbody fusion may be considered (Fig. 17-5).57
The production of biomechanical changes as a function of different types of lumbar fusion has been studied.58,59 The three types of fusion evaluated included dorsal, bilateral lateral, and ventral. All types of fusion increased bending and axial stiffness. There is increased stress on the adjoining segments that were not fused, especially the facet joints. Overall, bilateral intertransverse fusion is a superior method because it provides good stabilization to the fused segments and has less effect on adjacent, unfused segments, especially the facet joints. Dorsal (intraosseous) fusion is the least beneficial, producing the highest amount of stress in adjoining segments and allowing superficial motion in the disc space.
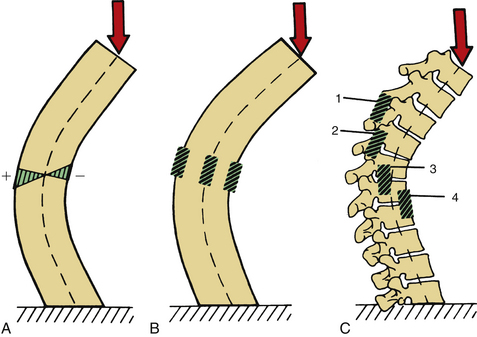
The spine experiences compressive forces on the concave side and tensile forces on the convex side of a curve. In the lumbar spine, if the graft material is placed in the intervertebral disc space, it is subjected to compressive loading. It is believed that compressive forces acting on the graft will promote fusion by stimulating the osteoconductive healing process. In contrast, a graft placed in a dorsal location experiences only tensile forces and will not be stimulated in a similar manner (Fig. 17-6).
Kyphotic Deformity and Bone Graft Positioning
At the IAR, there is neither compression nor tension. The farther instrumentation or bone graft is placed from the IAR, the greater the stress. For instance, in a kyphotic deformity, dorsal instrumentation is subjected to severe tensile stress. To reduce stress on a dorsal implant, some structural graft should be placed as ventrally as possible, away from the IAR. This counteracts the tensile stress dorsally. At times, with severe kyphotic deformity, multiple ventral grafts may be required (Fig. 17-7). It has been demonstrated that ventral and dorsal fusions are associated with a better correction and maintenance of correction than the dorsal group, but only with congenital kyphosis.60
Load Sharing
Denis61 introduced the three-column theory of the spine to classify and assist with the management of thoracolumbar spine injuries. Of these three columns, the anterior and posterior columns are the principle support structures.62 The anterior column resists compression and axial loading, and the posterior column maintains the tension. To maintain an erect posture, all forces and movements must be balanced about the IAR. The IAR is located dorsal to the anulus fibrosus in the intact spine.63
Deficiencies in the anterior or posterior column in the thoracolumbar spine usually lead to kyphosis.64 Kyphosis is corrected by lengthening the anterior column or shortening the posterior column. If the anterior or middle column is destroyed, alignment can be restored by a ventral structural graft and the resulting fusion. In this situation the axial load is shared by both anterior and posterior columns. When deciding on whether to perform a ventral or dorsal fusion, or a combination of both, the principles of load sharing should be considered. If both the ventral and dorsal elements are involved, both columns usually must be instrumented and fused. For example, a burst fracture will be compromised if the dorsal elements frequently require both ventral and dorsal spine reconstruction. With persistent posttraumatic kyphosis after a dorsal instrumentation procedure to treat a cervical or thoracolumbar fracture, anterior column load sharing is eliminated. Instrumentation such as a pedicle screw implant is exposed to high cantilever bending loads and may therefore fail.65 With correction of a kyphotic deformity, ventral surgery may not be necessary if the weight-bearing line is shifted behind the axis of rotation.66 By shifting the center of gravity dorsally, the anterior column does not have to support as much axial load. The prerequisites for such a strategy include (1) correction or overcorrection, if surgically feasible; (2) intact dorsal elements; and (3) good osteogenic potential. If sagittal correction is not accomplished, the load on the anterior column is high, and anterior column reconstruction is needed to prevent dorsal instrumentation failure.
Ventral instrumentation, without structural bone grafting, usually fails. A strong structural graft is required to resist axial loading and flexion.67 Tricortical ilium, fibula, humerus, or titanium cages packed with autogenous graft provide excellent anterior column support. However, single-rib grafts do not provide adequate structural support. Load sharing, in this case, implies a balance between ventral structural bone grafts and ventral or dorsal instrumentation. As the fixation length of ventral and dorsal constructs is reduced, load sharing with the anterior column has become increasingly important in reducing the incidence of failure of the shorter devices. The conditions frequently requiring both ventral and dorsal reconstruction include tumors involving both anterior and posterior columns, fractures involving all three columns, and postlaminectomy kyphosis.64
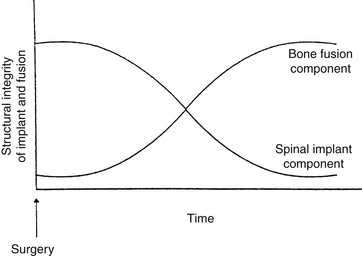
In dorsolateral spine fusion, instrumentation adds to the stability of the fusion by significant load sharing. In a human spine model where bilateral facetectomies were performed and transpedicular screws were used to restore stability, the spinal instrumentation provided 68% of the load sharing, along with the anterior and middle columns.68 As the fusion mass develops in vivo the load-sharing component of the instrumentation decreases. If an adequate fusion mass does not develop, the cyclical stresses placed on the instrumentation will lead to hardware failure (Fig. 17-8).
Stress Shielding
In a canine model, dorsolateral fusion without instrumentation resulted in fusion in only 57%, compared with a 100% fusion rate with pedicle screw fixation and a 71% fusion rate with Luque rods. Histologic evaluation of the vertebral body at the level of the fusion demonstrated osteoporosis in animals that had received instrumentation. This has been corroborated in humans.69 Patients who had undergone instrumented dorsolateral lumbar fusion were found to have decreased vertebral body mineral density at the level of fusion, compared with matched controls. This phenomenon has been termed stress shielding. However, in animal models the spine fusions that had been instrumented demonstrated increased areas of bone incorporation and biomechanical stability,70–72 and for any preexisting osteoporosis, compensation was more than adequate. In general, rigid fixation results in better union. Ventral interbody fusions are more prone than dorsal-only fusions to the negative effects of stress shielding.
Biomechanical Consideration at Specific Sites
Ventral cervical spine fusions are commonly performed using the Smith-Robinson technique. It achieves a wide decompression and provides an optimal load-bearing capacity (Fig. 17-9). The end plates are left intact. The cancellous portion of the graft is in contact with the vertebral end plates and readily permits revascularization. It is important to remember that transplanted bone weakens as resorption proceeds and, consequently, the graft is weaker at 6 months than at the time of implantation.
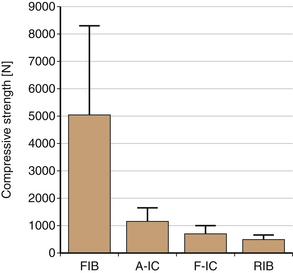
In the thoracic spine, segments of ribs may be used to provide structural support. However, they have a low compressive strength, which is related to their unfavorable length-to-width ratio, curvature, and small area of contact with the end plate. Fibular strut grafts or iliac crest grafts can be used, if structural support is important. Figure 17-10 demonstrates the relative strengths of various grafts used in ventral thoracic/lumbar fusion.
In the lumbar spine, despite the potential for surgical complications, interbody fusions are being increasingly performed. The lumbar spine experiences static loads in the range of 759 to 1600 pounds and up to 2000 pounds for high loading. The compressive strength of iliac allografts ranges from 396 to 1475 pounds, whereas femoral cortical rings have a strength in excess of 15,000 pounds. Some surgeons thus prefer femoral cortical allografts.73 Interbody cages are another option because they eliminate the associated iliac crest harvest complications.
The dorsolateral intertransverse fusion is the most commonly performed fusion procedure. It involves the facet joints, the pedicles, the transverse process, and the gutter between them. This fusion provides greater stability with axial rotation and lateral bending. Motion can persist, even after solid fusion, and can cause discogenic pain, particularly when the facet joints are not included in the arthrodesis. This motion occurs through the pedicles and can be minimized by augmenting the fusion with spinal instrumentation.74,75
Boden S.D., Schimandle J.H., Hutton W.C., et al. The use of an osteoinductive growth factor for lumbar spinal fusion. Part 1: The biology of spinal fusion. Spine (Phila Pa 1976). 1995;20:2626-2632.
Bostrom M.P., Lane J.M., Berberian W.S., et al. Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J Orthop Res. 1995;13(3):357-367.
Davy D. Biomechanical issues in bone transplantation. Orthop Clin North Am. 1999;30(4):553-563.
Lieberman J.R., Friedlaender G.E. Bone regeneration and repair: biology and clinical applications. Totowa, NJ: Humana Press; 2005. pp 57–65
McCormack T., Karikovic E., Gaines R.W. The load sharing classification of spine fractures. Spine (Phila Pa 1976). 1994;19:1741-1744.
Pelker R., Friedlaender G., Markham T. Biomechanical properties of bone allograft. Clin Orthop Relat Res. 1983;174:54.
Urist M. Bone transplants and implants. In: Urist M., editor. Fundamental and clinical bone physiology. Philadelphia: Lippincott; 1980:331-368.
1. Macewen W. The growth of bone. Glasgow: James Maclehose and Sons; 1912.
2. Albee F.H. Transplantation of a portion of the tibia into the spine for Pott’s disease. JAMA. 1911;57:885-886.
3. Hibbs R.A. An operation for progressive spinal deformities. A preliminary of three cases from the service of the Orthopedic Hospital. NY State J Med. 1911;93:1013-1016.
4. Davis H. Increasing rates of cervical and lumbar spine surgery in the United States, 1979–1990. Spine (Phila Pa 1976). 1994;19:1117-1124.
5. Deyo R.A., Mirza S.K. The case for restraint in spinal surgery: does quality management have a role to play? Eur Spine J. 2009;18(Suppl 3):331-337. 2009
6. Cotler J.M., Star A.M. Complications of spinal fusion. In: Cotler J.M., Cotier H.M., editors. Spinal fusion: science and technique. New York: Springer-Verlag; 1990:361-387.
7. Banwart J.C., Asher M.A., Hassanein R.S. Iliac crest bone graft harvest donor site morbidity. A statistical evaluation. Spine (Phila Pa 1976). 1995;20(9):1055-1060.
8. Lieberman J.R., Friedlaender G.E. Bone regeneration and repair: biology and clinical applications. Totowa, NJ: Humana Press; 2005. pp 57–65
9. Torzilli O.A., Burstein A.H., Takebe K., et al. The material and structural properties of maturing bone. In: Cowin S.C., editor. Mechanical properties of bone. New York: American Society of Mechanical Engineers; 1981:145-161.
10. Sevitt S., editor. Bone repair and fracture healing in man. Edinburgh, Scotland: Churchill Livingstone. 1981:1-24.
11. Buckwalter J.A., Glimcher M.J., Cooper R.R., et al. Bone Biology 1: structure, blood supply, cells matrix and mineralization. Instr Course Lect. 1996;41:371-386.
12. Currey J.D. Function and form of bone. Mow V.C., Ratcliffe A., Woo S.L.-Y., editors. Biomechanics of diarthrodial joints. New York: Springer-Verlag. 1990;vol 2:3-30.
13. Davy D. Biomechanical issues in bone transplantation. Orthop Clin North Am. 1999;30(4):553-563.
14. Goldberg V. Selection of bone grafts for revision total hip arthroplasty. Clin Orthop Relat Res. 2000;381:68-76.
15. Wolfinbarger L.Jr., Zhang Y., Adam B.T., et al. A comprehensive study of physical parameters, biomechanical properties and statistical correlations of iliac crest bone wedges used for spinal fusion surgery. I. Physical parameters and their correlations. Spine (Phila Pa 1976). 1994;19:277-283.
16. Pelker R., Friedlaender G., Markham T. Biomechanical properties of bone allograft. Clin Orthop Relat Res. 1983;174:54.
17. Taguchi R., Pereira B.P., Kour A.K., et al. Autoclaved autograft bone combined with vascularized bone and bone marrow. Clin Orthop Relat Res. 1995;320:220-230.
18. Nguyen H., Morgan D.A., Forwood M.R. Sterilization of allograft bone: effects of gamma irradiation on allograft biology and biomechanics. Cell Tissue Bank. 2007;8(2):93-105. Epub 2006 Oct 25
19. Prolo D.J., Rodrigo J.J. Contemporary bone graft physiology and surgery. Clin Orthop Relat Res. 1991;200:322-342.
20. White A.A., Panjabi M.M. Clinical biomechanics of the spine. Philadelphia: JB Lippincott; 1990.
21. Smith M.D., Cody D.D. Load bearing capacity of corticocancellous bone grafts in the spine. J Bone Joint Surg [Am]. 1993;75:1206-1213.
22. Wittenberg R.H., Moeller J., Shea M., et al. Compressive strength of autogenous bone grafts for thoracolumbar and cervical spine fusion. Spine (Phila Pa 1976). 1990;15:1073-1078.
23. Closkey R.F., Parsons R., Lee C.K., et al. Mechanics of interbody spinal fusion: analysis of cortical bone graft area. Spine (Phila Pa 1976). 1993;18:1011-1015.
24. Burchardt H. The biology of bone graft repair. Clin Orthop Relat Res. 1983;174:28-42.
25. Lane K., Sandhu H.S. Current approaches to experimental bone grafting. Orthop Clin North Am. 1987;18:213-225.
26. Zipfel G.J., Guiot B.H., Fessler R.G. Bone grafting. Neurosurg Focus. 2003;14(2):e8.
27. Urist M. Bone transplants and implants. In: Urist M., editor. Fundamental and clinical bone physiology. Philadelphia: Lippincott; 1980:331-368.
28. Urist M.R. Osteoinduction in undemineralized bone implants modified by chemical inhibitors of endogenous matrix enzymes. Clin Orthop Relat Res. 1972;87:132-137.
29. Deleu J., Trueta J. Vascularization of bone grafts in the anterior chamber of the eye. J Bone Joint Surg [Am]. 1965;47:319-329.
30. Enneking W.F., Burchardt H., Puhl J.J., et al. Physical and biologic aspects of repair in dog cortical bone transplants. J Bone Joint Surg [Am]. 1975;57:232-252.
31. Ray R.D. Bone grafts and bone implant. Otolaryngol Clin North Am. 1972;5:389-398.
32. McCalden R.W., McGeough J.A., Barker M.B., et al. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg [Am]. 1993;75:1193-1205.
33. Enneking W.F., Morris J.L. Human autologous cortical bone transplants. Clin Orthop Relat Res. 1972;87:28-35.
34. Stevenson J.S., Bright R.W., Dunson G.L., et al. Technetium-99m phosphate bone imaging: a method for assessing bone graft healing. Radiology. 1973;110:391-394.
35. Boden S.D., Schimandle J.H., Hutton W.C., et al. The use of an osteoinductive growth factor for lumbar spinal fusion. Part 1: The biology of spinal fusion. Spine (Phila Pa 1976). 1995;20:2626-2632.
36. Steiranan J.C., Herkowitz H.N. Pseudarthrosis of the spine. Clin Orthop Relat Res. 1992;284:80-90.
37. Burchardt H., Enneking W.F. Transplantation of bone. Surg Clin North Am. 1978;58:403-427.
38. Stevenson S., Li X.Q., Davy D.T., et al. Critical biological determinants of incorporation of nonvascularized cortical bone grafts. J Bone Joint Surg [Am]. 1997;79:1-16.
39. Stevenson S. The immune response to osteochondral allografts in dogs. J Bone Joint Surg [Am]. 1987;69:573-582.
40. Buckwalter J.A., Glimcher M.J., Cooper R.R., et al. Bone Biology 11: formation, form, modeling, remodeling and regulation of cell function. Instr Course Lect. 1996;42:387-399.
41. Mundy G.R. Regulation of bone formation by bone morphogenetic proteins and other growth factors. Clin Orthop Relat Res. 1996;323:24-28.
42. Goldring S.R., Goldring M.B. Cytokines and skeletal physiology. Clin Orthop Relat Res. 1996;324:13-23.
43. Riley E.H., Lane J.M., Urist M.R., et al. Bone morphogenetic protein-2. Biology and applications. Clin Orthop Relat Res. 1996;324:39-46.
44. Bostrom M.P., Lane J.M., Berberian W.S., et al. Immunolocalization and expression of bone morphogenetic proteins 2 and 4 in fracture healing. J Orthop Res. 1995;13(3):357-367.
45. Nakase T., Nomura S., Yoshikawa H., et al. Transient and localized expression of bone morphogenetic protein 4 messenger RNA during fracture healing. J Bone Miner Res. 1994;9(5):651-659.
46. Sandhu H.S., Linda E.A., Kabo J.M., et al. Effective doses of recombinant human morphogenetic protein-2 in experimental spinal fusion. Spine (Phila Pa 1976). 1996;21:2115-2122.
47. Boden S.D., Schimandle J.H., Hutton W.C. The use of an osteoinductive growth factor for lumbar spinal fusion. Part 11. Study of dose, carrier, and species. Spine (Phila Pa 1976). 1995;20:2633-2644.
48. Boden S.D., Moskovitz P.A., Morone M.A., et al. Video-assisted lateral intertransverse process arthrodesis. Validation of a new minimally invasive lumbar spinal fusion technique in the rabbit and nonhuman primate (rhesus) models. Spine (Phila Pa 1976). 1996;21:2689-2697.
49. Helm G.A., Sheehan J.M., Sheehan J.P., et al. Utilization of type I collagen gel, demineralized bone matrix, and bone morphogenetic protein-2 to enhance autologous bone lumbar spinal fusion. J Neurosurg. 1997;86:93-100.
50. Muschler G.F., Hyodo A., Manning T., et al. Evaluation of human bone morphogenetic protein in a canine spinal fusion model. Clin Orthop Relat Res. 1994;308:229-240.
51. Sandhu H.S., Kanim L.E.A., Kabo J.M., et al. Evaluation of rhBMP-2 with an OPLA carrier in a canine posterolateral (transverse process) spinal fusion model. Spine (Phila Pa 1976). 1995;20:2669-2682.
52. Schimandle J.H., Boden S.D., Hutton W.C. Experimental spinal fusion with recombinant human bone morphogenetic protein-2. Spine (Phila Pa 1976). 1995;20:1326-1337.
53. Sheehan J.P., Kallmes D.F., Sheehan J.M., et al. Molecular methods of enhancing lumbar spine fusion. Neurosurgery. 1996;39:548-554.
54. Yasko A.W., Cole B.J., Lane J.M., et al. Comparison of recombinant human BMP-2 versus cancellous bone to heal segmental bone defects. Trans Orthop Res Soc. 1993;17:331-368.
55. Goel V.K., Kim Y.E., Lim T.H., et al. An analytical investigation of spinal information. Spine (Phila Pa 1976). 1988;13:1-11.
56. Rolander S.D. Motion of the lumbar spine with special reference to the stabilizing effect of posterior fusion. Acta Orthop Scand. 1966;90(Suppl):1-144.
57. Fraser R.D. Interbody, posterior and combined lumbar fusions. Spine (Phila Pa 1976). 1995;20:S167-S177.
58. Esses S.I., Doherty B.J., Crawford M.J., et al. Kinematic evaluation of lumbar fusion techniques. Spine (Phila Pa 1976). 1996;21(6):676-684.
59. Lee K.C., Langrana N.A. Lumbosacral spinal fusion. A biomechanical study. Spine. 1984;9:574-581.
60. Winter R.B., Moe J.H., Lonstein J.E. The surgical treatment of congenital kyphosis. A review of 94 patients age 5 years or older with 2 years or more follow-up in 77 patients. Spine (Phila Pa 1976). 1984;10:224-231.
61. Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine (Phila Pa 1976). 1983;8:817-831.
62. James K.S., Wenger K.H., Schlegel J.D., et al. Biomechanical evaluation of the stability of thoracolumbar burst fractures. Spine (Phila Pa 1976). 1994;19:1731-1740.
63. Haher T.R., O’Brien M., Felmly W.T., et al. Instantaneous axis of rotation as a function of the three columns of the spine. Spine (Phila Pa 1976). 1992;17(Suppl 6):S149-S154.
64. Bridwell K.H. Load sharing principles. The role and use of anterior structural support in adult deformity. Instr Course Lect. 1996;45:109-115.
65. McCormack T., Karikovic E., Gaines R.W. The load sharing classification of spine fractures. Spine (Phila Pa 1976). 1994;19:1741-1744.
66. Edwards C.C., Osbome V.A. Correction of chronic post traumatic kyphosis with the kyphoreduction construct and stress relaxation (abstract). Proceedings of the American Academy of Orthopaedic Surgeons 61st Annual Meeting. New Orleans, Rosemont, IL: American Academy of Orthopedic Surgeons; 1994. p 160
67. Gurr K.R., McAfee P.C., Shih C.M. Biomechanical analysis of anterior and posterior instrumentation systems after corpectomy: a calf-spine model. J Bone Joint Surg [Am]. 1988;70:1182-1191.
68. Kotani Y., Cunningham B.W., Cappuceino A., et al. The role of spinal instrumentation in augmenting lumbar posterolateral fusion. Spine (Phila Pa 1976). 1996;21:278-287.
69. Myers M.A., Casciani T., Whitbeck M.G.Jr., et al. Vertebral body osteopenia associated with posterolateral spine fusion in humans. Spine (Phila Pa 1976). 1996;21:2368-2371.
70. Feighan J.E., Stevenson S., Emery S.E. Biologic and biomechanic evaluation of posterior lumbar fusion in the rabbit. The effect of fixation rigidity. Spine (Phila Pa 1976). 1995;20:1561-1567.
71. Johnston C.E., Welch R.D., Baker K.J., et al. Effect of spinal construct stiffness on short segment fusion mass incorporation. Spine (Phila Pa 1976). 1995;20:2400-2407.
72. McAfee P.C., Farey I.D., Sutterlin C.E., et al. Device related osteoporosis with spinal instrumentation. Spine (Phila Pa 1976). 1989;14:919-926.
73. Kozak J.A., Heilman A.E., O’Brien J.P. Anterior lumbar fusion options. Clin Orthop Relat Res. 1994;300:45-51.
74. Jacobs R.R., Montesano P.X., Jackson R.P. Enhancement of lumbar spinal fusion by use of translaminar facet joint screws. Spine (Phila Pa 1976). 1989;14:12-15.
75. Zdeblick T.A. A prospective, randomized study of lumbar fusion: preliminary results. Spine (Phila Pa 1976). 1993;18:983-991.