CHAPTER 104 SPINAL TRAUMA
Spinal cord injury (SCI) is a devastating event for patients and their families, with many severe medical, social, and economic sequelae. Patients may be permanently disabled and may ultimately have a lifelong dependence on support services. The neurological dysfunction after traumatic SCI results from a “primary” mechanical insult, followed by a downstream cascade of “secondary” processes that disrupt normal cord anatomy and function. The primary insult is determined by the mechanism of injury, energy applied to the cord, level of SCI, and patient factors such as medical comorbid conditions and the preinjury space available to the cord. Secondary injury mechanisms include, but are not limited to, disruption of the microcirculation, loss of autoregulation, edema, ischemia, calcium toxicity, glutamate excitotoxicity, lipid peroxidation, and free radical activation.1
Greater understanding of the pathophysiology of the secondary cascade and effective early resuscitation measures have improved the outcomes for these patients. Treatment of SCI is aimed at preserving residual neurological function, avoiding secondary injury to the cord, and restoring spinal alignment and stability. Currently, there is also burgeoning activity in basic research aimed at repair and regeneration of the injured spinal cord. This may facilitate higher levels of independence and productivity and may markedly improve the quality of life for patients with SCI.2,3
DEFINITIONS
SCI can be categorized as incomplete paraplegia, complete paraplegia, incomplete tetraplegia, and complete tetraplegia. According to the classification of the American Spinal Injury Association,4 tetraplegia is the “impairment or loss of motor and/or sensory function in the cervical segments of the spinal cord.” Paraplegia refers to “impairment or loss of motor and/or sensory function in the thoracic, lumbar, or sacral segments of the spinal cord.”
The neurological injury level is determined primarily by clinical examination and is defined as the most caudal spinal cord segment with normal sensory and motor function on both sides of the body. The sensory level refers to the most caudal spinal cord segment with normal sensory function. The motor level is defined similarly with regard to motor function as the lowest key muscle that has a grade of at least 3/5 (Table 104-1).
| Score | Clinical Finding |
|---|---|
| 0 | Total paralysis |
| 1 | Palpable or visible contraction |
| 2 | Full range of motion with gravity eliminated |
| 3 | Full range of motion against gravity |
| 4 | Full range of motion but less than normal strength |
| 5 | Normal strength |
| NT | Not testable |
ANATOMY OF SPINAL CORD INJURY
Cervical Injury
Atlanto-Occipital Dislocation
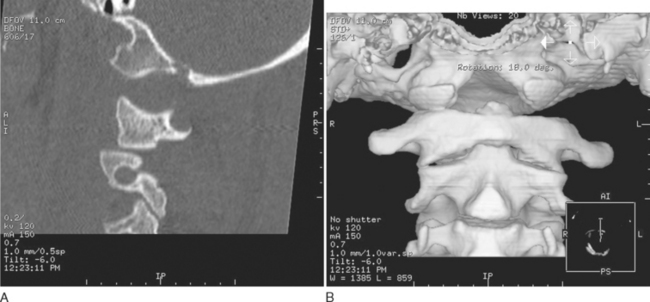
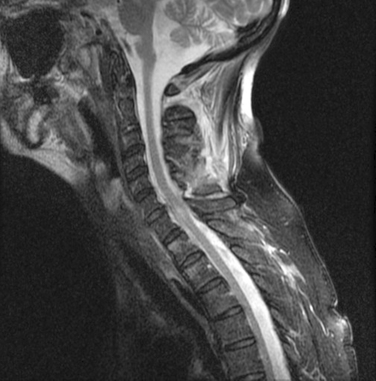
This is caused by traumatic hyperflexion and extension, in which the ligamentous connections between the skull and C1 and C2 are disrupted (Fig. 104-1). Because of the highly unstable nature of this injury, these patients often either die of brainstem destruction and apnea or are profoundly neurologically impaired (ventilator dependent and tetraplegic). On occasion, a patient may survive if prompt resuscitation is available at the injury scene. This injury may be identified in up to 20% of patients with fatal cervical spine injuries and is a common cause of death in cases of shaken baby syndrome in which the infant died immediately after being shaken.
Atlas Fracture (C1)
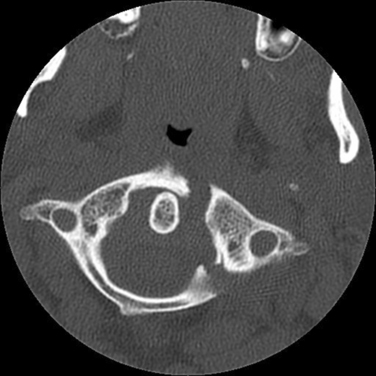
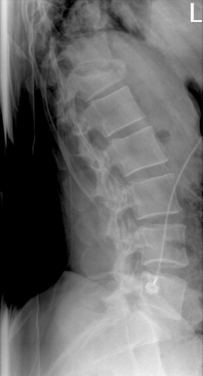
This injur represents 10% of all cervical fractures and usually results from axial loading, such as when a large load falls vertically on the head or in a fall in which the patient lands on the head in a relatively neutral position. The most common C1 fracture is a burst fracture (Jefferson’s fracture), which involves disruption of both the anterior and posterior rings of C1 with lateral displacement of the lateral masses (Fig. 104-2). In patients who survive, these fractures are usually not associated with SCIs but may be accompanied with significant retropharyngeal swelling. The trauma team should be alert to this possibility and should consider prophylactic intubation. Approximately 40% of atlas fractures occur in combination with fractures of the axis (C2).
Axis (C2) Fractures
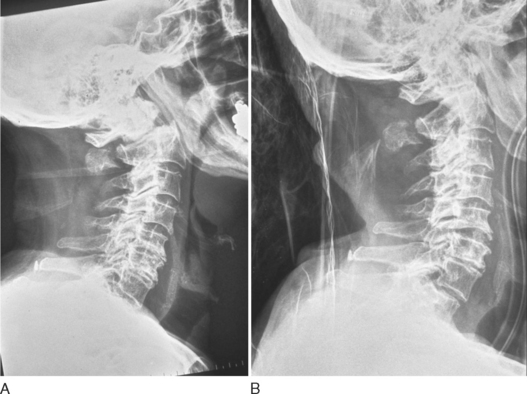
Approximately 60% of C2 fractures involve the odontoid process. These are classified according to the scale of Anderson and D’Alonzo. Type 1 odontoid fractures involve the tip of the odontoid and are relatively uncommon. Type II odontoid fractures occur at the waist of the odontoid and C2 body and are the most common type (Fig. 104-3). Type III odontoid fractures occur at the base of the dens and extend obliquely into the body of C2.
Fractures and Dislocations (C3 to C7)
Bony injury to the lower cervical area occurs in the form of compression fracture, burst fracture, or teardrop fracture. Compression fractures arise from a flexion injury, with no greater than 25% compression of the middle column and no injury to the posterior longitudinal ligament. Burst fractures are the result of compression and flexion. Teardrop fractures are caused by flexion with rotation and compression and are notably unstable injuries (Fig. 104-4).
The Thoracic Spine
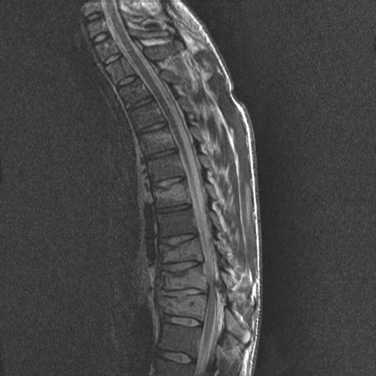
The mobility of the thoracic spine is much more restricted than that of the cervical spine, because it has additional support from the rib cage. This region requires greater force to disrupt its integrity and thus has a much lower incidence of fractures (Fig. 104-5). However, because the thoracic canal is relatively narrow, a fracture dislocation here frequently results in a severe neurological deficit. Because thoracic spine fractures result from violent forces, they are associated with a high incidence of concomitant injuries, such as rib fractures, pneumothorax, hemothorax, pulmonary contusion, cardiac contusion, and sometimes aortic shearing injury.
The Thoracolumbar Junction
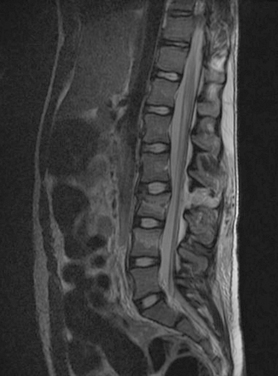
The thoracic spine has a natural kyphosis (concave forward), whereas the lumbar spine has a lordosis (convex forward). Because of the change of curvature in the transition zone, the thoracolumbar junction acts like a fulcrum between the inflexible thoracic region and the stronger lumbar levels, which predisposes it to injury.5 Consequently, approximately 15% of spinal injuries are found in this region (Fig. 104-6).
Lumbar Spine
Compression fractures usually result from failure of the anterior column with intact middle and posterior columns, frequently from an anterior flexion force accompanied by a posterior tensile force. These injuries are generally not associated with neurological deficit. With lumbar burst fractures, loss of height of the anterior and middle columns is characteristically shown on radiographs, with retropulsion of bone into the canal and widening of the interpedicle distance. These fractures are inherently unstable. Flexion and distraction injuries, frequently described as Chance fractures (Fig. 104-7), represent a failure of the middle and posterior columns in tension, with the anterior column acting like a hinge. Fracture dislocations are associated with failure of all three columns with a combination of forces, including flexion rotation, flexion distraction, or shearing. Because of their inherent instability these injuries are probably associated with a high incidence of severe SCI.
HISTOLOGY OF SPINAL CORD INJURY
According to Belanger and Levi (2000) and Park and associates (2004), the histological changes in SCI can be categorized as immediate, acute, intermediate, and late phases.6,7
Acute Phase (Hours to 1 to 2 Days)
The early inflammatory response is a complex process involving vascular changes, including the disruption of the blood-brain barrier and the upregulation of endothelial cell adhesion molecules that allow the activation and extravasation of cells from the bloodstream (neutrophils, macrophages, lymphocytes) into the injured tissue. These cells also release a large number of soluble immune mediators (cytokines, chemokines) that consequently activate resident glial cells.1,3 A mild influx of neutrophils has been described as beginning within 1 day after SCI, with a peak at 2 days and a decrease by 3 days. This is in sharp contrast to other tissues, in which the neutrophilic influx is often marked. The neutrophilic response in the central nervous system (CNS) is likely to be neurotoxic in nature, because once these cells are activated and accumulated at the lesion site, they release potent free radicals that attack the integrity of the lipid bilayer of the cellular membrane. The extent of neutrophil infiltration has been associated with blood-brain barrier dysfunction and tissue damage. The neuronal and axonal changes in the acute phase include marked axonal swelling and ultimately fiber disconnection. Microscopically, this is marked by the formation of typical retraction balls. Myelin breakdown is another feature of the early period after SCI. This is initially characterized by swelling of the myelin sheaths, and ultimately by fragmentation of myelin and its phagocytosis by macrophages. Myelin loss generally occurs in association with axonal pathology. Oligodendrocytes are also exquisitely sensitive to SCI. Much of the injury to these cells appears to be necrotic, but oligodendrocyte apoptosis has also been documented both in experimental animals and in humans.
Excitotoxicity
In SCI, excitotoxicity has been demonstrated to be predominantly caused by glutamate, the most prevalent excitatory neurotransmitter in the CNS.6 Release and accumulation of glutamate occur rapidly after SCI, reaching toxic levels as early as 15 minutes after experimental injury. Glutamate activates N-methyl-D-aspartate (NMDA) receptors and thereby allows extracellular calcium (and sodium) to move down a massive concentration gradient into the cell. NMDA receptor activation may also trigger the release of calcium into the cytoplasmic compartment from intracellular stores. Elevated cytosolic and mitochondrial calcium concentrations can trigger a multitude of calcium-dependent processes that reduce energy available to the cell, potentially leading to apoptosis.
Late Phase (Weeks to Months/Years)
Syrinx
This is a cavity with a denser gliotic wall that is under pressure. This is problematic, for as the pressure increases, the cavity enlarges, compressing the adjacent cord parenchyma and aggravating the neurological deficits. The mechanism of syrinx formation is not understood but may often be associated with cord tethering. Surgical drainage is the only available treatment.
SPINAL CORD SYNDROMES
Incomplete lesions may be classified into a number of neurologic syndromes that reflect the anatomical level of cord injury. Recognition of the type of lesion enables the clinician to gather information about the mechanism of injury and guides selection of appropriate treatment. The various syndromes also have different prognoses for recovery.8
Central Cord Syndrome
Because the motor fibers to the cervical segments are topographically arranged toward the center of the cord, damage to the central cord affects the arms and hands more severely than the lower extremities. The degree of sensory loss is variable. This syndrome is usually seen after a hyperextension injury in a patient with preexisting cervical canal stenosis (often resulting from degenerative osteoarthritic changes). The history is commonly that of a forward fall that resulted in a facial impact. The syndrome may occur with or without cervical spine fracture or dislocation. Central cord syndrome in young patients has a relatively good prognosis. Recovery usually follows a characteristic pattern. The lower extremities recover strength first, bladder function next, and the proximal upper extremities and hands last (Fig. 104-8).
Penetrating Injuries
Penetrating SCIs are most commonly caused by gunshots or stabbings. A complete neurological deficit may result if the path of injury passes directly through the vertebral canal. Complete deficits can also be produced by energy transfer from a high-velocity missile (e.g., a rifle bullet) passing close to the spinal cord rather than through it. Spinal instability from penetrating injury is exceedingly rare. Associated injuries, however, should be highly suspected. Penetrating cervical injuries are combined with a high incidence of vascular lesions, whereas penetrating thoracic trauma is associated with pulmonary and cardiac injuries. Knife or gunshot trauma to the lumbar spine may be accompanied by damage to the abdominal viscera, genitourinary system, or major vascular structures, and severe infection may follow bullet injuries that traverse the colon. Penetrating injuries can be complicated by the formation of cerebrospinal fluid fistulae. All patients with penetrating spinal injuries should therefore receive treatment with tetanus prophylaxis and broad-spectrum antibiotics.
Spinal Cord Injury without Radiographic Abnormality (SCIWORA)
In a child with SCI without abnormal findings on plain radiographs, a hyperextension cervical SCIWORA should be considered. A high index of suspicion is required. Because the flexibility of the spine is reduced with increasing age, SCIWORA is rarer in the adult trauma population, but it should be suspected in all patients with a neurological deficit and apparently normal radiographs.9,10
MANAGEMENT OF SPINAL TRAUMA
Victims of high-speed motor vehicle accidents, persons ejected from a vehicle, and patients with falls from a height are at particularly high risk of SCI. However, because severe SCI can also result from a seemingly minor insult, all trauma patients should be presumed to have a SCI until proved otherwise.7,11–13
Prehospital Management
 If the airway is compromised, a nasotracheal or orotracheal intubation should be considered with simultaneous in-line stabilization, whereby a second person manually maintains the patient’s head and the neck in neutral alignment with the torso. Cricothyroidotomy may be required if these techniques fail to secure the airway.
If the airway is compromised, a nasotracheal or orotracheal intubation should be considered with simultaneous in-line stabilization, whereby a second person manually maintains the patient’s head and the neck in neutral alignment with the torso. Cricothyroidotomy may be required if these techniques fail to secure the airway.Surgical Treatment
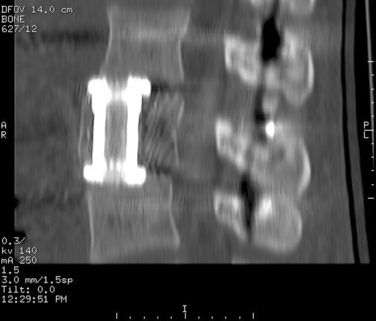
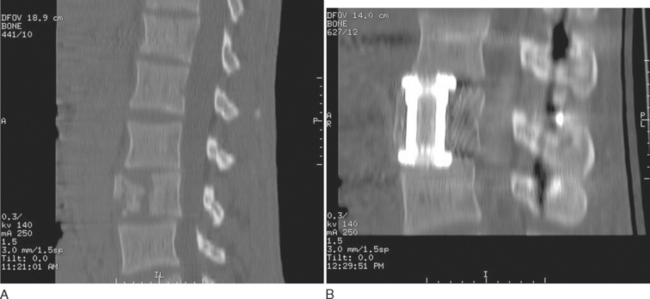
Kossmann and colleagues (2004) recommended two-staged “damage control” approach for spine trauma.14 Relatively safe procedures, such as posterior internal stabilization of thoracic or lumbar fractures or temporary external fixation of cervical fractures in a halo thoracic brace, are performed early. Once the patient is medically stable, a second operation for reconstruction of the anterior column may be performed. This delay allows for general recovery of the patient and facilitates safer anesthesia. Eventually, the definitive surgery can be electively scheduled at a time when an experienced spine surgeon is available. This may enable use of new minimally invasive techniques that are now available for the reconstruction of the anterior column of the thoracic and lumbar spine (Fig. 104-9).15
Pharmacological Therapy
Most traumatic injuries to the spinal cord do not involve actual transection of the cord but rather entail damage of the fibers from contusive, compressive, or stretch injury. Often, portions of the ascending and descending tracts remain intact. Although it is not currently known how much of the spinal cord needs to remain intact to mediate meaningful neurological function, the observation of anatomical continuity of the spinal cord has led to the notion that pharmacological treatments, if applied early, may be able to interrupt the secondary cascade and thereby improve the survival of damaged tissue.1
Methylprednisolone
In experimental studies, the glucocorticoid methylprednisolone has been shown to reduce lipid peroxidation, lessen edema and inflammation, lower excitatory amino acid release, and inhibit tumor necrosis factor α expression and nuclear factor κB activation. The nonglucocorticoid 21-aminosteroid tirilazad has also been shown to have these antioxidant neuroprotective properties. Treatment with methylprednisolone quickly became the standard of care for acute SCI; clinical studies indicated that initiation of treatment within the first 3 hours is optimal. However, use of high-dose methylprednisolone is controversial. Some researchers argue that the risks associated with methylprednisolone, such as increased wound infection rates, pneumonia, and severe sepsis, outweigh what they believe are usually modest neurological benefits. Other researchers have criticized the interpretations and conclusions drawn from the National Acute Spinal Cord Injury Studies that supported administration of methylprednisolone. Currently, many centers administer methylprednisolone if it can be given within 8 hours of the injury. In addition to methylprednisolone and other steroids, there has also been interest in the tetracycline antibiotic minocycline and the immunosuppressants FK-506 and cyclosporine. In animal models of contusive SCI, preliminary results with minocycline suggest a promising reduction in apoptotic death at the injury site and improved locomotor function. Cyclosporine acts at the mitochondrial membrane to impede apoptosis and has been shown to promote tissue sparing and inhibit lipid peroxidation in models of brain injury and SCI. In experimental models, FK-506 has been shown to promote axonal regeneration within the CNS.
New Pharmacological Approaches
Transplantation Strategies
Transplantation of embryonic stem cells and more mature cells by use of fragments of peripheral nerve, fetal tissue, or Schwann cell bridges is often assessed in conjunction with the administration of neurotrophins, such as brain-derived neurotrophic factor or neurotrophin 3, which have been shown to promote the growth of regenerating axons from transplanted tissue into the injured spinal cord. However, to restore neurological function, replenishment of cells at the injury site with stem cells needs to be followed by successful differentiation and targeted formation of operational connections by the new fibers. The complexity of these multifactorial events makes successful transplantation a challenging task. Research in this area is being pursued.
CONCLUSION
Anderson LD, D’Alonzo RT. Fractures of the odontoid process of the axis. J Bone Joint Surg Am. 1974;56:1663-1674.
Belanger E, Levi AD. The acute and chronic management of spinal cord injury. J Am Coll Surgeons. 2000;190:603-618.
Denis F. The three column spine and its significance in the classification of acute thoracolumbar spinal injuries. Spine. 1983;8:817-831.
Kossmann T, Trease L, Freedman I, et al. Damage control surgery for spine trauma. Injury. 2004;35:661-670.
Kwon BK, Tetzlaff W, Grauer JN, et al. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451-464.
Maynard FM, Bracken MB, Creasey G. International standard for neurological and functional classification of spinal cord injury. Spinal Cord. 1997;35:266-274.
Park E, Velumian AA, Fehlings MG. The role of excitoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754-774.
1 Kwon BK, Tetzlaff W, Grauer JN, et al. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004;4:451-464.
2 Mohta M, Sethi AK, Tyagi A, et al. Psychological care in trauma patients. Injury. 2003;34:17-25.
3 Lee TT, Green BA. Advances in the management of acute spinal cord injury. Orthop Clin North Am. 2002;33:311-315.
4 Maynard FM, Bracken MB, Creasey G. International standard for neurological and functional classification of spinal cord injury. Spinal Cord. 1997;35:266-274.
5 Hsu JM, Joseph T, Ellis AM. Thoracolumbar fracture in blunt trauma patients: guidelines for diagnosis and imaging. Injury. 2003;34:426-433.
6 Park E, Velumian AA, Fehlings MG. The role of excitoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754-774.
7 Belanger E, Levi AD. The acute and chronic management of spinal cord injury. J Am Coll Surgeons. 2000;190:603-618.
8 Vale F, Burns J, Jackson AB, et al. Combined medical and surgical treatment after spinal cord injury: result of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87:129-146.
9 Kothari P, Freeman B, Grevitt M, et al. Injury to the spinal cord without radiological abnormality (SCIWORA) in adults. J Bone Joint Surg Br. 2000;82:1034-1037.
10 Pang D, Pollack I. Spinal cord injury without radiographic abnormality in children: the SCIWORA syndrome. J Trauma. 1989;29:654-664.
11 Guidelines for initial management and assessment of spinal injury. British Trauma Society. Injury. 2003;34:405-425.
12 Gilbert J. Critical care management of the patient with acute spinal cord injury. Crit Care Clin. 1987;3:549-567.
13 Patel RV, Delong WJr, Vresilovic EJ. Evaluation and treatment of spinal injuries in the patient with polytrauma. Clin Orthop Relat Res. 2004;422:43-54.
14 Kossmann T, Trease L, Freedman I, et al. Damage control surgery for spine trauma. Injury. 2004;35:661-670.
15 Kossmann T, Jacobi D, Trentz O. The use of a retractor system (SynFrame) for open, minimal invasive reconstruction of the anterior column of the thoracic and lumbar spine. Eur Spine J. 2001;10:396-402.