CHAPTER 46 Spinal Meningiomas
INTRODUCTION
According to Guidetti,1,2 in 1887, Sir William Gowers had been treating a British Army major with paraplegia. He eventually diagnosed a spinal tumor and referred the patient to Sir Victor Horsley for operation. Sir Horsley planned thoracic laminectomy, which was only successful in locating the tumor after removing additional laminae, though with some trepidation. He removed an intradural extramedullary tumor that was consequently typed as a fibromyxoma.1,3 Remarkably, with rehabilitation the major regained his gait, and this case has come to be known as the first spinal tumor in medical history to be cured surgically. In 1925, Elsberg4 compiled his experience with the diagnosis and treatment of all spinal tumors in which he described the surgical techniques of intradural tumor removal that rank him high in the history of neurosurgery. In 1938, Cushing collaborated with Eisenhardt to publish his meticulous and refined surgical techniques in a book in which they defined the successful removal of a spinal meningioma as “one of the most gratifying of all operative procedures.”5,6
In the following four decades no major advancement was achieved in terms of surgical techniques for spinal tumors, and most of the progress henceforth owes more to the advances made in related fields such as myelography, angiography, and electromyography, followed by computer-assisted tomography (CT) and magnetic resonance imaging (MRI), which have revolutionized the diagnosis of intraspinal tumors, allowing for early detection and improved anatomic location. Introduction of microneurosurgery by Yasargil in the 1970s, together with developments in neuroanesthesiology, computer-assisted planning of operations, and a better understanding of microanatomy have resulted in more aggressive surgical approaches for even complex spinal meningiomas.7
INCIDENCE
Spinal intradural tumors can be categorized as extra- or intramedullary tumors. Although intramedullary tumors have a higher incidence in the pediatric age group, extramedullary tumors have a higher incidence (70%) in adults.8,9 The annual incidence of primary intraspinal neoplasm is approximately five per million for females and three per million for males and is expected to rise as life expectancy is increasing in industrialized nations.10 Spinal meningiomas occur less frequently than intracranial ones and account for approximately 7.5% to 12.7% of all meningiomas.11 Meningiomas have a very low incidence in the first two decades of life,12,13 and in the first five decades they occur more frequently in the cervical spine (39% of spinal meningiomas).14 Meningiomas in middle-aged women between the fourth and fifth decades account for 80% of all spinal meningioma cases.15
Various reports consistently show the most common distribution of meningiomas along the spinal axis to be the thoracic segment (67%–84%), followed by the less frequent high cervical levels (14%–27%). They are extremely rare at lumbar levels (2%–14%).7,12,16–21 Although most of the spinal meningioma cases are intradural, 5% to 14% occur at extradural or extraspinal locations.7,13,16,22,23
PATHOLOGY AND ETIOLOGY
Spinal meningiomas are thought to originate from arachnoid cap cells that are most densely located within the arachnoid villi at the entry zone of the nerve roots or at the junction of dentate ligaments and dura mater, where the spinal arteries penetrate.24,25 For this reason, lateral tumors are more common than dorsal and ventral lesions.17,20,25,26 Gottfried and colleagues7 performed a meta-analysis including 566 cases from 6 published series, reaching the conclusion that most spinal meningiomas were located lateral to the spinal cord or had a component that extended laterally assuming a posterolateral or anterolateral location, of which they determined the posterolateral was the more frequent. Interestingly, Levy and colleagues18 reported that cervical meningiomas were more likely to be located ventrally.
The striking preponderance of meningiomas for postmenopausal women has led to a controversy over a causal relationship between the sex hormones and meningiomas.27 Hormonal studies that have shown the existence of various receptor types in meningioma, of which progesterone is the most notable, followed by estrogen and others, support the argument for this relationship.27–29 Preston-Martin and colleagues30 suggested that the overwhelming tendency of spinal meningiomas to occur in the thoracic segments of postmenopausal women may be due to increased trauma of the meninges related to the osteoporotic collapse of thoracic vertebrae. Ionizing radiation is another known risk factor for the development of these tumors.31,32
Multiple meningiomas occurring both at intracranial and spinal locations is a rare entity. Only 18 such cases have been reported worldwide, for which in only 10 cases were surgery and histopathologic investigations performed separately for the tumors at both locations. In five of these cases, the tumors revealed the same histopathologic classification, only one being malignant. In the other five cases, which revealed different histopathology for intracranial versus intraspinal lesions, the meningiomas had a benign character.33 These cases strongly support the idea that spinal or cranial meningiomas may be derived from the same clone of cells and provide foundation for the theory of development of multiple meningiomas from the spreading of tumor cells via cerebrospinal fluid (CSF) as a possible mechanism, which eventually may progress to assume different histologic subtypes.34
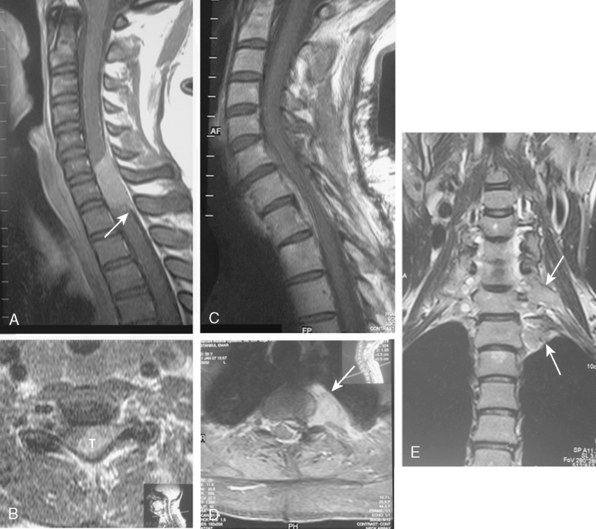
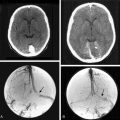
The most important histologic difference between the spinal and cranial meninges is that the former has an intermediate layer and the latter does not. Electron microscopic investigations of human spinal meninges demonstrate an intermediate leptomeningeal layer lying between the arachnoid and pia mater. This intermediate layer is attached to the inner aspect of the arachnoid mater and extends in an arborizing fashion over the dorsal surface of the spinal cord, encasing the nerve roots and blood vessels.26,35 The intermediate layer is not observed in human cerebral leptomeninges. The presence of the intermediate layer in spinal meninges might explain the hypointense rim detected via MRI around the tumor and may also be the reason why no penetration and no tumoral edema are observed in spinal meningiomas which is common with intracranial meningiomas26,36 (Fig. 46-1). Caroli and colleagues37 suggested that incomplete development of the intermediate layer may underlie the en plaque expansion of meningiomas and facilitate the involvement of the pial layer. Salpietro and colleagues26 considered the intermediate layer responsible for two types of interfaces between the tumor and pial membrane. Accordingly, what they call a “floating interface” correlates with the presence of the hypointense peritumoral rim revealed by MRI. This interface is characterized by a small preserved subarachnoid space confined between the arachnoid and the intermediate leptomeningeal layer, making the tumor easily dissectible from the spinal cord. In the deep-type interface, however, no hypointense rim is observed on MRI scans. The inner arachnoid layer is difficult to identify or absent, and the blood vessels and spinal roots are extremely adherent to the tumor, making dissection of the tumor more difficult, although generally achievable as no pial disruption is present.
Most spinal meningiomas are globoid, but a few exceptions assume a carpet-like, en plaque configuration. Reflecting the variable proportions of neoplastic cells, collagen, and psammoma bodies, meningiomas range in consistency from soft to firm to even gravel-like.18 Most meningiomas remain within the intradural space, although a few penetrate the dura or exit through root sleeves to reach the adipose tissue of the epidural compartment, rarely resulting in exclusively epidural tumors.24,38 Only rarely do these lesions continue their infiltration into the regional soft tissue and bony structures.39
Although spinal meningiomas exhibit the same histologic spectrum as the intracranial meningiomas, psammomatous lesions are overrepresented.16,40 Intradurally, meningothelial and psammomatous meningiomas are the most common histologic subtypes of spinal meningioma.23,40 On the other hand, atypical (WHO grade II) and malignant (WHO grade III) meningiomas are rare in the spinal meninges, with a combined incidence of 1.3% among all craniospinal meningiomas.7,20,40,41 An unusual variant, the clear cell meningioma (WHO grade II), is rare in the spine, prone to appear in the lumbar region, and is associated with a worse prognosis.42,43 Despite the relative frequency of psammomatous lesions, calcifications that are visible on plain radiographs are rarer than calcified intracranial meningiomas.16,44,45 They emerge from the incipient hydroxyapatite crystals precipitate and recruit collagen fibers as they coalesce into psammoma bodies, eventually resulting in calcified or ossified meningiomas.37,46 However, Kitagawa and colleagues47 did not found a correlation between the ossification process and density of psammoma bodies in meningiomas and suggested that ossification is a result of metaplasia in the arachnoid cells.45,47
The occurrence of differential patterns of genetic abnormalities and gene expression profiles in spinal meningiomas versus cranial meningiomas may provide new insights into molecular pathways involved in tumor genesis and progression of spinal meningiomas and could explain their particular clinical and histologic features.48,49 Sayagues and colleagues49 found differential expressions in 35 genes in spinal versus cranial meningiomas, of which only MN1 was encoded in chromosome 22, the usual suspect in meningioma pathogenesis.
PRESENTATION
Owing to the slow growing rate of these tumors, patients have a slow, indolent course of symptoms. In most patients, the presenting symptoms largely depend on tumor location and duration. Various authors reported that with the introduction of MRI into clinical practice, the average history until diagnosis was shortened by about 6 months, and consequently, patients suffered less severe neurologic deficits at the time of surgery.7,16,20,44
Invariably, the most common presentations are motor deficit and spinal pain.7,16,18–20,40 However, when the natural courses of symptoms are considered, two developmental stages of the tumor can be recognized. In the first stage, symptoms appear due to irritation of nerve roots. In the second stage, symptoms develop due to compression of the spinal cord.
In the first stage, the most notable symptom of nerve root involvement is pain, which predominates and may precede all other symptoms by months or years. The pain may assume a radicular, radiating character or may present as a localized back pain. The other common symptoms of this stage are partial weakness and sensory loss (hypoesthesia, paresthesia). As the tumor begins to compress on the spinal cord, symptoms of the second stage develop that may progress simultaneously with pain or in a small percentage of cases without pain.25 If the tumor is situated anterolaterally, the symptoms will progress to produce a Brown–Sequard-like syndrome that presents as motor loss and impairment of tactile and deep sensations on the same side below the level of the lesion, together with loss or diminution of pain and temperature perception on the opposite side. In posteriorly located tumors the posterior columns of the cord are the first to be compressed by the tumor, the deep sensibility is decreased, and ataxia appears.
Sphincter disturbances are generally late findings in the course of symptoms and are ominous signs for the recovery of functions that can be expected after surgery.7,20
It should be kept in mind that the clinical picture created by spinal meningiomas is not specific, and the differential diagnosis should consider spinal tumors of all types even after most advanced imaging techniques.50
DIAGNOSIS
In the era before the revolutionary introduction of MRI, during which myelography was the radiological modality of choice for diagnosis, spinal meningiomas were often confused with other medical conditions such as multiple sclerosis, syringomyelia, and herniated disc.1,7,16,19,25,44 Levy and colleagues18 reported a 33% rate of misdiagnosis in their series for cases antedating MRI, which led to delayed treatment and sometimes, to inappropriate surgeries. Today, the use of myelography is confined to cases with previous surgical implants incompatible with MRI.
In the past, plain radiography was valued for its ability to detect calcified meningiomas, although calcifications are only visible on 1% to 5% of plain x-ray films.46 Further, spinal meningiomas with calcifications that are visible on plain radiographs are rarer than calcified intracranial meningiomas. This is thought to be due to the fact that intraspinal spaces are much smaller than intracranial spaces and symptoms appear more rapidly leaving less time for visible calcifications to occur.51
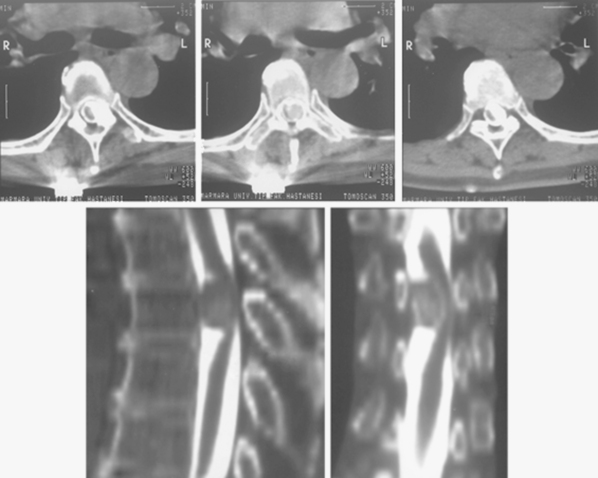
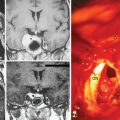
CT is more reliable than plain radiographs in demonstrating these tumoral calcifications,52 because it is highly sensitive to alterations in bone mineralization and is particularly useful in evaluating the bony matrix. Combined with MRI, it provides valuable information as to the aggressiveness of the tumor. This is because, in general, it is possible to infer the benign or malignant nature of the lesion from the pattern of bony destruction. Bony changes that occur in an expansive manner respectful of the original topography suggest a slowly expanding benign lesion. Generally, long-standing tumors demonstrate an enlarged spinal canal with scalloping of the vertebral bodies, pedicle erosion, and thinning of the lamina.52 Particularly, contrast-enhanced CT, with its ability to demonstrate tumoral lesions and their interactions with bony structures, provides more superior information than non-contrast CT. Further, myelo-CT, although an invasive procedure, can still be used today to demonstrate intradural tumors where MRI is unavailable or for patients with MRI-incompatible instruments (Fig. 46-2).
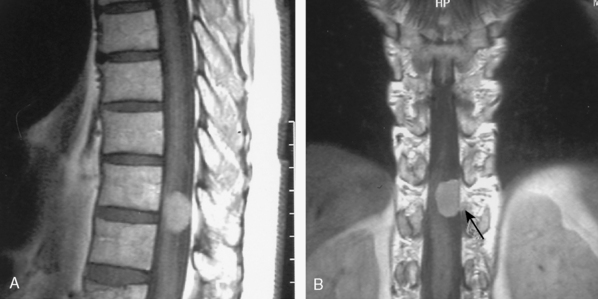
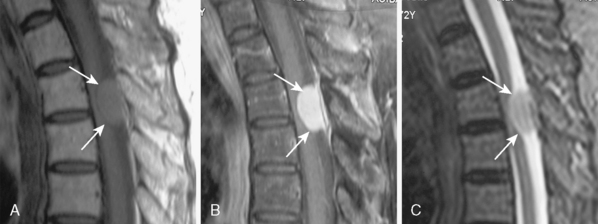
With its superior contrast sensitivity and multiplanar capabilities, MRI is the gold standard for diagnosing spinal meningiomas. It provides crucial information for surgical planning, as it clearly delineates the level of the tumor and its relationship to the cord. Contrast-enhanced MRI defines the relationship of the tumor to the spinal cord, nerve roots, and thecal sac, differentiating the tumor from peritumoral edema and cysts, determining tumor extent, and evaluating the cord’s intrinsic signal abnormalities.52 When investigating an intraspinal pathology, both T1-weighted images (T1WI) and T2-weighted images (T2WI) should be obtained in the axial and sagittal planes. T1-weighted slices should also be obtained following contrast injection. Coronal imaging is particularly useful for laterally situated tumors or for tumors extending longitudinally over multiple levels (Fig. 46-3). MRI is an excellent tool to delineate both intradural and extradural meningiomas. In principle, when an intradural, extramedullary lesion is detected on MRI, only one of a few benign lesions should be considered in differential diagnosis.50 Typically, spinal meningiomas are isointense to the normal spinal cord on T1- and T2-weighted images, and they display intense enhancement after gadolinium (Fig. 46-4).40,51,53 Coronal scanning planes are especially very helpful in detecting dural tails, which are very suggestive of meningiomas.53–55 Although the dural tail sign was initially thought to be highly specific for meningioma, it is now considered as only highly suggestive of meningioma after its description in a plethora of other extra-axial and intra-axial lesions, including schwannomas.51,56 Dural tail sign may be a reflection of a conglomeration of tumor cells invading and occluding small vessels at the point of tumor attachment to the dura, leading to congestion in the dural vicinity, which sometimes after contrast injection results in the dural tail enhancing more strongly than the tumor itself.31,56 In line with this argument, dural tail sign on MR imaging holds an essential role in surgical planning, as it may be the reason behind tumor recurrence after apparent total surgical excision of meningiomas.56 Further, the dural tail sign is one of the parameters that help in differentiation between spinal meningiomas and schwannomas, together with location of the tumor, T2 signal intensity, and enhancement pattern.54
Since its introduction for cerebral pathologies, diffusion-weighted MRI has found some application in spinal neoplasms. Various studies reported that the calculated mean apparent diffusion coefficient (ADC) values measured in meningiomas with malignant histologic findings or cellular atypical were significantly lower than the mean ADC values measured in meningiomas with benign histologic findings. It seems that the quantification of the diffusion constant may reliably predict the histopathologic aspect of meningiomas and provide guidance in surgical planning.57,58
Many spinal lesions demonstrate similar clinical symptoms. Without the use of modern imaging techniques the physician can easily arrive at a wrong diagnosis. MRI is the best noninvasive neuroimaging technique in preventing misdiagnosis. The MRI findings that make it possible to distinguish benign from malignant tumors mainly include parameters such as tumor outline, invasive behavior, and edematous reactions.40
However, extradural meningiomas can easily be confused with other neoplasms, especially metastatic tumors, as their location and growth patterns may mimic malignant behavior. Because there are essential differences between the surgical managements of metastasis and meningiomas, it is important to use a high index of suspicion when faced with these cases.59
TREATMENT
Because spinal meningiomas are mostly benign tumors, their total surgical resection provides the best treatment option for a definitive cure for these patients. Even in elderly patients with symptomatic spinal meningiomas or poor preoperative neurologic condition, whenever there is an acceptable risk from the anesthesiologic aspect, surgical excision should be performed.50
The fact that normal dural pulsations will be detected above the level of the lesion but not below the level of the lesion provides guidance to the surgeon. Typically, the laminectomy should be started on the lamina corresponding to tumor and extended rostrally until dural pulsations are seen, which signifies the rostral limit of the tumor. The tips of the adjacent spines, above and below the level of laminectomy, should also be removed, which may obscure access to the tumor. Depending on the dural attachment, a facet joint or the proximal part of a rib can be resected to gain access for tumors with anterior orientation.11 However, in principle, maximum exposure and secure access to meningioma should be gained through minimal bone removal without hindering spinal biomechanics, which may complicate recovery of the patient.
After adequate dural exposure, most meningiomas can be recognized by the contour they create on the dura. However, for small-sized tumors intraoperative ultrasonography can provide useful information about the location and size of the lesion, as well as the degree to which the spinal cord is displaced.7 Typical ultrasonographic features include echogenicity, irregular surfaces, and tight adherence to the dura.7 In addition, the echogenicity of the tumor may help differentiate between meningioma and nerve sheath tumors in cases where the MRI is equivocal. In contrast to neuromas, meningiomas appear uniformly hyperechoic, and typically they lack intraparenchymal cysts.60
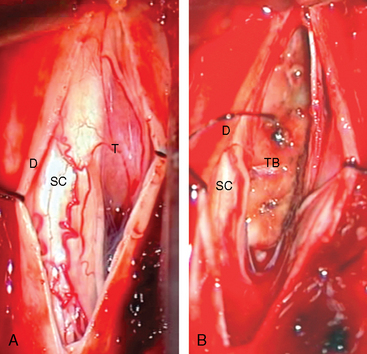
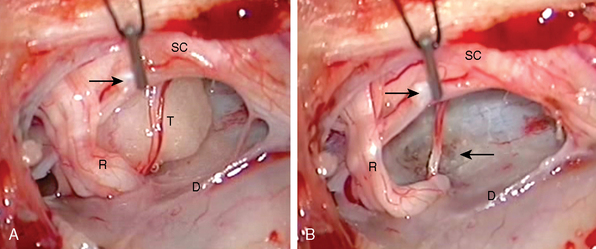
After the tumor is localized, the dura should be opened only after absolute homeostasis has been achieved and the head of the patient tilted downward. These maneuvers will prevent blood and air from entering the subarachnoid space, which may result in arachnoiditis and air embolism complicating the postoperative period.1 The dura should be opened longitudinally in the midline and retracted laterally over the tumor with dural sutures, which will facilitate access from both sides (Fig. 46-5A). After dural opening, conventionally, we use surgical microscope and microsurgical instruments for the rest of the procedure. Microscopically, it is easier to visualize and preserve the arachnoid sheath between the tumor and the spinal cord (Fig. 46-6A). Most meningiomas are located in a “floating space” created by this arachnoid sheath.26 The floating space can be recognized under the microscope as a distinct, CSF-containing tumoral interface, contained between a dense, continuous, watertight outer layer and an inner leptomeningeal layer which has strand-like attachments to the pial surface. Rarely, bridging vessels run within this space, which should be taken into consideration during dissection.
However, for tumors with a ventral location, dentate ligaments may be sectioned, so that spinal cord can be moved aside. If the operative field is still too narrow due to considerable size of the tumor, one or more nerve roots may be sectioned to prevent spinal cord trauma due to retraction.7,11,60 Alternatively, facet, rib head, and pedicle resection can be performed to this end.11 Tumor debulking can be started at this point, especially for anteriorly situated tumors, where it usually becomes necessary to remove the tumor in a piecemeal fashion in order to avoid undue traction or pressure on the spinal cord.
Bipolar diathermy, microscissors, and ultrasonic aspirators are the most widely used tools for tumor debulking.7,16,19,20 However, laser debulking is another means used by some groups.61
After the removal of the tumor, frequently it is possible to see a marked indentation on the cord. In advanced cases, these indentations may have reduced the thickness of the cord to less than half its normal size. Even for these cases, as long as the blood supply is not impeded, a complete recovery can be expected. The recovery is thought to occur due to gradual growth of the tumor producing destruction and absorption of the myelin before any injury occurs on the axonal shaft which retains a stronger capability for regeneration.25
After total tumor removal, the surgical management of spinal meningioma with extensive involvement of dura mater is still controversial. Our standard practice in treating the area of dural attachment is to perform bipolar coagulation to eradicate any residual tumor cells (Figs. 46-5B and 46-6B). Alternatively, some groups perform an extensive dural excision and dural reconstruction, as excision of dural margins has been reported to be associated with lower recurrence rate, in contrast to simply cauterizing margins (4%–8% for dural margin cauterization and 0–5.6% for dural margin excision).60,62 However, some reports suggest that dural resection has no correlation with the recurrence rate of these tumors and coagulation of dural base yields a rate of recurrence of the tumor equal to that with dural excision.16,20,23 In light of these conflicting results, it should be borne in mind that in the majority of cases total intradural tumor excision is possible with a clear dissection from dural surface, where coagulation of the dural tail or tumor origin is sufficient to prevent recurrence.
Nevertheless, meningiomas that extensively involve the dura mater are still surgical challenges. The principal difficulty in performing a total resection is the potential for complications associated with extensive dural removal. Radical dural excision followed by novel dural reconstruction involving the implantation of a fascia lata graft or a bovine pericardium allograft has been effectively used to reconstruct the dura and create an adequate barrier to CSF.62 Another option for the treatment of tumors with exceedingly large sizes can be two-staged surgery removing posterolateral and anterior portions separately.22
On occasion, spinal meningiomas grow en plaque or in a collar-like manner around the spinal cord and infiltrate the pia mater. Most en plaque meningiomas are intradural tumors, but sometimes there is a concurrent presence of an intradural and an extradural component of growth.37 These tumors have a peculiar tendency to induce arachnoiditis with intense adhesive reactions, which can be detected only intraoperatively. In these cases, symptoms may stem from a compressive arachnoiditis rather than tumor growth. This kind of arachnoiditis has been reported to occur more often with en plaque meningiomas than with encapsulated ones.44 Klekamp and Samii44 suggested that en plaque and infiltrative meningiomas should be seen as a separate entity, as they continue to be surgical challenges due to their extensive tumor matrix and infiltrative nature.
In addition to en plaque dural involvement, anterior location, ossification, and recurrence are considered to lessen the likelihood of a favorable postoperative neurologic outcome.7,44 Recurrent meningiomas rarely bear a tumor capsule and the arachnoidal sheath that creates the floating interface between the meningiomas and the pial surface is no longer present, leading to infiltration of the pia, the nerve roots, and the surrounding neighboring structures.15,44
The place of intraoperative spinal evoked potential monitoring has not been well established in spinal meningioma surgery. Generally, this monitoring is not considered to add useful information. However, two groups advocated the use of neuromonitoring for spinal meningiomas.7,63
After surgical intervention for tumors involving multiple vertebral levels, especially with anterior approaches where corpectomy and interference with facet joints may be performed, spinal instability is a serious long-term complication and source of pain for the patient, which may hinder functional recovery.60 So, surgeons must be prepared to perform fixation and fusion in such patients prophylactically or to monitor them closely by obtaining follow-up serial radiographs.62
When there is early recurrence after a total resection or residual tumor is detected, radiotherapy should be performed as an adjuvant therapy. New radiosurgery techniques, such as CyberKnife® robotic stereotactic radiosurgery, are expected to be useful in the treatment of these patients.64
RESULTS
Clearly, a favorable postoperative neurologic outcome necessitates complete resection of the spinal meningioma. Therefore, the first surgeon to operate on a spinal meningioma is in a position of great responsibility, as the subsequent operations tend to have a lower total resection and higher recurrence rates.44
Advances in imaging and surgical techniques, such as MRI, ultrasonography, neuromonitoring, the operative microscope, and ultrasonic aspirator have resulted in refinements in the treatment of spinal meningiomas.7 Because of the excellent outcome of surgery for benign spinal meningiomas and the correlation between duration of symptoms and poor functional outcome, early operation is the treatment of choice.63
Early diagnosis may prevent irreversible neurologic deficits and enhances the likelihood of full recovery of neurologic functions.65
In the meta-analysis of Gottfried and colleagues7 causes of death included pulmonary embolism, aspiration pneumonia, stroke, and myocardial infarction. The incidence of CSF leakage was between 0 and 4%.
In line with their predominantly benign nature, the recurrence of spinal meningiomas is rare and ranges from 1.3% to 10%.44,63,66 This has been partly attributed to the overwhelmingly more frequent occurrence of lower grade meningiomas which have been found to not have the genetic aberrations found in recurrent intracranial meningiomas.67 The slow growth of spinal meningiomas and their proclivity to occur at advanced ages should also contribute to the low recurrence rates of spinal meningiomas.
En plaque and recurrent meningiomas are the greatest challenges facing the surgeon operating on spinal meningiomas. Klekamp and Samii44 reported a 53% of total resection for en plaque meningiomas compared to 97% of encapsulated meningiomas. In their series, they also reported only a 45% rate of complete resections for recurrent tumors compared with 95% of the first operations. They presented arachnoid scarring as an independent factor preventing total excision (70% total resection when it exists versus 94% when it does not). Thus, arachnoid scarring is at the crux of problems faced by a neurosurgeon operating on en plaque and recurrent meningiomas. The scarring leads to disappearance of the intermediate leptomeningeal layer obscuring the dissection plane between the pial surface and the tumor. In both situations, the arachnoid scarring does not seem to have a specific histopathologic basis.37
PROGNOSIS
With life expectancy in the industrial nations increasing, the number of patients in their eighth decade of life with spinal meningiomas is expected to increase.10 Various series have reported a favorable neurologic outcome after surgical removal in the range of 60% to 98%.18,23,40,44,61 Advanced age, profound neurologic deficits, long duration of symptoms before diagnosis, along with pial invasion and subtotal removal of tumor are generally considered to be predictors of poor outcome after surgery.50,63 In addition, of all neurologic deficits, sphincter dysfunction is considered a harbinger of a worse prognosis.20
Psammomatous meningiomas of the spine, postoperatively, have been associated with less favorable neurologic outcome, comparing to spinal meningiomas of other pathological subtypes.21 Further, anterior location in the spinal canal, the second to third decades of life, highly calcified, and clearly, en plaque tumors still indicate a poorer prognosis.13,14,68 In contrast, posterior or lateral location in the spinal canal, below C4 vertebra level, age younger than 60 years, and short duration of preoperative symptoms seem to correlate with good outcome.21 In addition to total surgical resection of tumor, the presence of mechanical and ischemic trauma during surgery plays an important part in the speed and possibility of recovery in these patients.68 Nevertheless, a good outcome can be expected in the vast majority of cases, even for the patients harboring spinal meningioma with severe preoperative deficits.
Slinko and colleagues11 determined the prognostic positive factors to be operation before the appearance of severe neurologic symptoms, young age of the patient, total removal of the tumor, moderate spinal cord compression, no intraoperative spinal cord traction during surgery, and use of microsurgical techniques. They suggested that as a rule, the severity of both neurological signs and spinal cord compression correlates with degree of recovery of neurologic functions and surgical intervention always resulted in the resolution of pain or radicular signs even when neurologic signs persisted.11
Accumulated evidence conclusively suggests that recovery of neurologic deficits can almost always be expected. Our own experience with these patients indicates that a good neurologic outcome can be obtained in the better half of these patients after surgery, even with severe neurologic signs. Therefore, surgery should be followed by rehabilitation programs even for patients of advanced age.44
In patients who continue to deteriorate, the progression is generally related to recurrence, delayed postoperative arachnoid scarring, syringomyelia or instability, and myelopathy.44
AUTHOR’S EXPERIENCE
Forty-nine spinal meningioma cases were operated in our institution between 1986 and 2007. Forty (20%) cases were females and 9 (18%) were males with a female to male ratio of 4.4:1. The age of patients ranged between 10 and 75 years. The average age was 55 for males and 48 for females, in agreement with other reports. Pain was the predominant symptom leading to presentation in 44 patients (90%). The pain was a localized back pain in 31 (63%) patients and a radicular pain in 13 (27%) patients. Lower extremity weakness was seen in 21 (43%), paresthesia in 14 (29%), and numbness in 16 (33%) patients. Sphincter dysfunction was present in only 2 (4%) of the cases (Table 46-1).
| Findings | Preoperative |
|---|---|
| Back pain | 31 |
| Radicular pain | 13 |
| Lower extremity weakness | 21 |
| Paresthesia | 14 |
| Numbness | 16 |
| Babinski | 10 |
| Sphincter dysfunction | 2 |
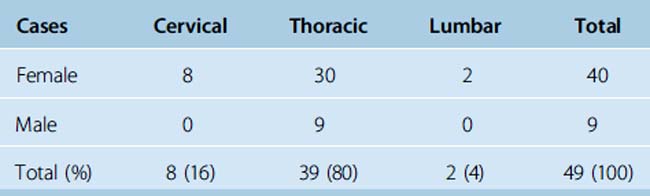
The tumor was located at the cervical levels in 8 females (16%), and at thoracic levels in 30 females and 9 males (80% of all patients). Remarkably, all male patients had thoracic meningiomas and the two lumbar meningiomas were in females (Table 46-2).
The most common histopathological type was meningotheliomatous meningioma, found in 22 (45%) of our cases, followed by, in order of frequency, psammomatous in 11 cases (22%), transitional in 10 cases (20%), fibroblastic in 3 cases (6%) (Table 46-3).
TABLE 46-3 Pathological diagnosis of the patients
| Pathologic type | Cases (%) |
|---|---|
| Meningothelial | 22 (46) |
| Psammomatous | 11 (22) |
| Transitional | 10 (20) |
| Fibroblastic | 3 (6) |
| Other | 3 (6) |
[1] Guidetti B. Removal of extramedullary benign spinal cord tumours. Krayenbuehl H., Brihaye J., Loew F., editors. Advances and Technical Standards in Neurosurgery, vol. 1. Wien, Springer Verlag, 1974;173-197.
[2] Powell M. Sir Victor Horsley—an inspiration. BMJ. 2006;333(7582):1317-1319.
[3] Gowers W.R., Horsley V. A case of tumour of the spinal cord. Removal; recovery. Med-Chir Trans. 1888;53(2nd):377-428.
[4] Elsberg C.A. Tumors of Spinal Cord and the Symptoms of Irritation and Compression of the Spinal Cord and Nerve Roots: Pathology, Symptomatology, Diagnosis, and Treatment. New York: Paul B. Hoeber, 1925.
[5] Cohen-Gadol A.A., Spencer D.D., Krauss W.E. The development of techniques for resection of spinal cord tumors by Harvey W. Cushing. J Neurosurg Spine. 2005;2:92-97.
[6] Cushing H., Eisenhardt L. Meningiomas: Their Classification, Regional Behavior, Life History and Surgical End Results. Springfield, IL: Charles C Thomas, 1938.
[7] Gottfried O.N., Gluf W., Quinones-Hinojosa A., et al. Spinal meningiomas: surgical management and outcome. Neurosurg Focus. 2003;15:14.
[8] DeSousa A.L., Kalsbeck J.E., Mealey Jr J., et al. Intraspinal tumors in children: a review of 81 cases. J Neurosurg. 1979;51:437-445.
[9] Epstein F., Epstein N. Surgical treatment of spinal cord astrocytomas of childhood: a series of 19 patients. J Neurosurg. 1982;57:685-689.
[10] Helseth A., Mork S.J. Primary intraspinal neoplasms in Norway, 1955 to 1986. A population-based survey of 467 patients. J Neurosurg. 1989;71:842-845.
[11] Slin’ko E.I., Al-Qashqish I.I. Intradural ventral and ventrolateral tumors of the spinal cord: surgical treatment and results. Neurosurg Focus. 15,17, 2004. ECP2 [Review]
[12] Gelabert-González M., García-Allut A., Martínez-Rumbo R. Spinal meningiomas. Neurocirugia (Astur). 2006;17:125-131.
[13] Messori A., Rychlicki F., Salvolini U. Spinal epidural en-plaque meningioma with an unusual pattern of calcification in a 14–year-old girl: case report and review of the literature. Neuroradiology. 2002;44:256-260.
[14] Cohen-Gadol A.A., Zikel O.M., Koch C.A., et al. Spinal meningiomas in patients younger than 50 years of age: a 21–year experience. J Neurosurg. 2003;98:258-263.
[15] Souweidane M.M., Benjamin V. Spinal cor meningiomas. Neurosurg Clin North Am. 1994;5:283-291.
[16] King A.T., Sharr M.M., Gullan R.W., Bartlett J.R. Spinal meningiomas: a 20–year review. Br J Neurosurg. 1998;12:521-526.
[17] Kuday C. Spinal meningiomas. Türk Nöroşirurji Dergisi. 1994;4:53-56.
[18] Levy W.J.Jr, Bay J., Dohn D. Spinal cord meningioma. J Neurosurg. 1982;57:804-812.
[19] Namer I.J., Pamir M.N., Benli K., et al. Spinal meningiomas. Neurochirurgia (Stuttg). 1987;30:11-15.
[20] Peker S., Cerçi A., Ozgen S., et al. Spinal meningiomas: evaluation of 41 patients. J Neurosurg Sci. 2005;49:7-11.
[21] Schaller B. Spinal meningioma: relationship between histological subtypes and surgical outcome? J Neurooncol. 2005;75:157-161.
[22] Buchfelder M., Nomikos P., Paulus W., Rupprecht H. Spinal-thoracic dumbbell meningioma: a case report. Spine. 2001;26:1500-1504.
[23] Solero C.L., Fornari M., Giombini S., et al. Spinal meningiomas: review of 174 operated cases. Neurosurgery. 1989;25:153-160.
[24] Hassin G. Villi (pacchionian bodies) of the spinal arachnoid. Arch Neurol Psychiatry. 1930;23:65-78.
[25] Rasmussen T., Kernohan J., Adson A. Pathologic classification with surgical consideration of intraspinal tumors. Ann Surg. 1940;111:513-530.
[26] Salpietro F.M., Alafaci C., Lucerna S., et al. Do spinal meningiomas penetrate the pial layer? Correlation between magnetic resonance imaging and microsurgical findings and intracranial tumor interfaces. Neurosurgery. 1997;41:254-258.
[27] Pravdenkova S., Al-Mefty O., Sawyer J., Husain M. Progesterone and estrogen receptors: opposing prognostic indicators in meningiomas. J Neurosurg. 2006;105:163-173.
[28] Gruber T., Dare A.O., Balos L.L., et al. Multiple meningiomas arising during long-term therapy with the progesterone agonist megestrol acetate. Case report. J Neurosurg. 2004;100:328-331.
[29] Milenković S., Berisavac J., Cvetković-Dozić D., et al. Hormone receptors in benign intracranial meningiomas. J BUON. 2004;9:295-298.
[30] Preston-Martin S., Monroe K., Lee P.J., et al. Spinal meningiomas in women in Los Angeles County: investigation of an etiological hypothesis. Cancer Epidemiol Biomarkers Prev. 1995;4:333-339.
[31] Kawahara Y., Niiro M., Yokoyama S., Kuratsu J. Dural congestion accompanying meningioma invasion into vessels: the dural tail sign. Neuroradiology. 2001;43:462-465.
[32] Preston-Martin S., Mack W., Henderson B.E. Risk factors for gliomas and meningiomas in males in Los Angeles County. Cancer Res. 1989;49:6137-6143.
[33] Bhatoe H.S. Simultaneous occurrence of multiple meningiomas in different neuraxial compartments. Neurol India. 2003;51:263-265. [Review]
[34] Durmaz R., Arslantaş A., Artan S., et al. The deletion of 22q13 region in both intracranial and spinal meningiomas in a patient (case report). Clin Neurol Neurosurg. 1998;100:219-223.
[35] Nicholas D.S., Path M.R.C., Weller R.O., Path F.R.C. The fine anatomy of the human spinal meninges: A light and scanning electron microscopy study. J Neurosurg. 1988;69:276-282.
[36] Neuta H.J.W., Dolan E., Yasargil M.G. Microsurgical anatomy of spinal subarachnoid space. Surg Neurol. 1983;19:431-437.
[37] Caroli E., Acqui M., Roperto R., et al. Spinal en plaque meningiomas: a contemporary experience. Neurosurgery. 2004;55:1275-1279. discussion 1279 [Review]
[38] Gambardella G., Toscano S., Staropoli C., et al. Epidural spinal meningioma. Role of magnetic resonance in differential diagnosis. Acta Neurochir (Wien). 1990;107:70-73.
[39] Calogero J.A., Mossy J. Extradural spinal meningiomas: report of 4 cases. J Neurosurg. 1972;37:442-447.
[40] Gezen F., Kahraman S., Canakci Z., Bedük A. Review of 36 cases of spinal cord meningioma. Spine. 2000;25:727-731.
[41] Sade B., Chahlavi A., Krishnaney A., et al. World Health Organization Grades II and III meningiomas are rare in the cranial base and spine. Neurosurgery. 2007;61:1194-1198. discussion 1198
[42] Holtzman R.N., Jonmark S.C. Nondural-based lumbar clear cell meningioma: case report. J Neurosurg. 1996;84:264-266.
[43] Zorludemir S., Scheithauer B.W., Hirose T., et al. Clear cell meningioma: clinicopathologic study of potentially aggressive variant of meningioma. Am J Surg Pathol. 1995;19:493-505.
[44] Klekamp J., Samii M. Surgical results for spinal meningiomas. Surg Neurol. 1999;52:552-562.
[45] Naderi S., Yilmaz M., Canda T., Acar U. Ossified thoracic spinal meningioma in childhood: a case report and review of the literature. Clin Neurol Neurosurg. 2001;103:247-249. [Review]
[46] Doita M., Harada T., Nishida K., et al. Recurrent calcified spinal meningioma detected by plain radiograph. Spine. 2001;26:E249-E252.
[47] Kitagawa M., Nakamura T., Aida T., et al. Clinicopathologic analysis of ossification in spinal meningioma. Brain Tumor Pathol. 1994;11:115-119.
[48] Arslantas A., Artan S., Oner U., et al. Comparative genomic hybridization analysis of genomic alterations in benign, atypical and anaplastic meningiomas. Acta Neurol Belg. 2002;102:53-62.
[49] Sayagués J.M., Tabernero M.D., Maíllo A., et al. Microarray-based analysis of spinal versus intracranial meningiomas: different clinical, biological, and genetic characteristics associated with distinct patterns of gene expression. J Neuropathol Exp Neurol. 2006;65:445-454.
[50] Morandi X., Haegelen C., Riffaud L., et al. Results in the operative treatment of elderly patients with spinal meningiomas. Spine. 2004;29:2191-2194.
[51] Alorainy I.A. Dural tail sign in spinal meningiomas. Eur J Radiol. 2006;60:387-391.
[52] Bloomer C.W., Ackerman A., Bhatia R.G. Imaging for spine tumors and new applications. Top Magn Reson Imaging. 2006;17:69-87.
[53] Schroth G., Thron A., Guhl L., et al. Magnetic resonance imaging of spinal meningiomas and neurinomas. Improvement of imaging by paramagnetic contrast enhancement. J Neurosurg. 1987;66:695-700.
[54] De Verdelhan O., Haegelen C., Carsin-Nicol B., et al. MR imaging features of spinal schwannomas and meningiomas. J Neuroradiol. 2005;32:42-49.
[55] Quekel L.G., Versteege C.W. The “dural tail sign” in MRI of spinal meningiomas. J Comput Assist Tomogr. 1995;19:890-892.
[56] Guermazi A., Lafitte F., Miaux Y., et al. The dural tail sign—beyond meningioma. Clin Radiol. 2005;60:171-188. [Review]
[57] Eastwood J.D., Turner D.A., McLendon R.E., Provenzale J.M. Diffusion-weighted imaging in a patient with vertebral and epidural abscesses. AJNR Am J Neuroradiol. 2002;23:496-498.
[58] Filippi C.G., Edgar M.A., Ulug A.M., et al. Appearance of meningiomas on diffusion-weighted images: correlating diffusion constants with histopathologic findings. AJNR. 2001;22:65-72.
[59] Zevgaridis D., Thomé C. Purely epidural spinal meningioma mimicking metastatic tumor: case report and review of the literature. Spine. 2002;27:E403-E405.
[60] Misra S.N., Morgan H.W. Avoidance of structural pitfalls in spinal meningioma resection. Neurosurg Focus. 2003;1514:e1.
[61] Roux F.X., Nataf F., Pinaudeau M., et al. Intraspinal meningiomas: review of 54 cases with discussion of poor prognosis factors and modern therapeutic management. Surg Neurol. 1996;46:458-463. discussion 463–4
[62] Horn E.M., Deshmukh V.R., Lekovic G.P., Dickman C.A. Durectomy and reconstruction for the treatment of a recurrent spinal meningioma. Case report. J Neurosurg Spine. 2006;5:76-78.
[63] Setzer M., Vatter H., Marquardt G., et al. Management of spinal meningiomas: surgical results and a review of the literature. Neurosurg Focus. 2007;23:E14.
[64] Sahgal A., Chou D., Ames C., et al. Image-guided robotic stereotactic body radiotherapy for benign spinal tumors: the University of California San Francisco preliminary experience. Technol Cancer Res Treat. 2007;6:595-604.
[65] Katz K., Reichenthal E., Israeli J. Surgical treatment of spinal meningiomas. Neurochirurgia (Stuttg). 1981;24:21-22.
[66] McCormick P.C., Post K.D., Stein B.M. Intradural extramedullary tumors in adults. Stein B.M., McCormick P.C., editors. Neurosurg Clin North Am, vol. 1, 1990;591-608.
[67] Ketter R., Rahnenführer J., Henn W., et al. Correspondence of tumor localization with tumor recurrence and cytogenetic progression in meningiomas. Neurosurgery. 2008;62:61-69. discussion 69–70
[68] Ciappetta P., Domenicucci M., Raco A. Spinal meningiomas: prognosis and recovery factors in 22 cases with severe motor deficits. Acta Neurol Scand. 1988;77:27-30.