CHAPTER 34 Cavernous Sinus Meningiomas
HISTORY
It is widely cited that Galen had performed dissections in lower vertebrates, described that the internal carotid artery formed a vascular rete in the neck, and transferred this finding to his teachings on the human body.1 As Galen’s teachings formed the backbone of medicine during this time, his description was not challenged. A clearer understanding of the anatomy of the parasellar area began to emerge after the former dogma was challenged in the second half of the 17th century. In 1658 Wepfer described that the carotid artery passed through a deep and conspicuous sinus during its course through the skull base. In 1685 Vieussens described the relation of cranial nerves to the outer wall of the sinus. These initial references were followed by Ridley’s first detailed description of the CS in 1695. Winslow, however, was the first to use the term “cavernous sinus” in 1734, as an analogy to the corpus cavernosum of the penis. Winslow clearly described the anatomy including the placement of the internal carotid artery and the IIIrd, IVth, Vth, and VIth cranial nerves within the sinus. The medical literature contains only very few reports from the period between the 18th and the second half of the 20th centuries. These sporadic reports include the first tumor removal from the CS, performed by Krogius in 1896. Browder2 performed the first successful surgery in 1937 for a caroticocavernous fistula. Although the state of the art in treatment of caroticocavernous fistulas at that time was carotid ligation, Browder had surgically explored the sinus and electrocoagulated the fistula. Despite such single achievements, the approach to the CS was outside the scope of neurosurgery. The dogma of “no man’s land” was challenged first by Parkinson. The first direct and systematic approach to the CS was performed by Parkinson in 1965.3 This operation was performed again for a high-flow caroticocavernous fistula, using hypothermia and extracorporeal circulation. The initial report drew attention to the so-called “anatomic jewel box,” and systematic studies on the microsurgical anatomy by Taptas,4 Dolenc,5 Parkinson,3 and Umansky and Nathan6 were followed by others. A better appreciation of the microsurgical anatomy led to a description of novel surgical approaches. Hakuba7 described the combined orbitozygomatic infratemporal epidural and subdural approach in 1982. This was followed by the very popular frontotemporal epidural approach of Dolenc in 1983.8 Sekhar9 reported the preauricular infratemporal approach in 1986 and Al-Mefty10 reported the cranio-orbitozygomatic and extended middle fossa zygomatic approaches in 1988, which led to the popularization of CS surgery. The start of the skull-base era marked the appearance of reports describing very high total resection rates and consistent decreases in mortality. However, the initial enthusiasm in the computed tomography (CT) era of 1980s was soon followed by the demonstration of a high incidence of tumor residuals as detected with the more sensitive magnetic resonance imaging (MRI) technology. Today we know more of the biology of CS meningiomas. Accumulating expertise has taught us that total resection of CS meningiomas is possible but that it can rarely be achieved for meningiomas that involve the CS proper. Seen from an oncologic perspective, such total resections can only be palliative, considering the high incidence of local invasion of meningiomas. In addition, we now know that CS exploration is associated with significant cranial nerve morbidity.
SURGICAL ANATOMY
The CS is a parasellar anatomic compartment that contains bilateral internal carotid arteries, the IIIrd, IVth, first two divisions of the Vth and VIth cranial nerves, the pericarotid sympathetic plexus, and a network of interconnected venous spaces.5 This anatomic compartment is located at both sides of the sellar space, at the junction of the anterior and middle cranial fossae. Anterolaterally it neighbors the sphenoid ridge and posterolaterally the petroclival ridge. The space is limited on all sides by dura mater. The inferior and medial walls are formed as a continuum of the periosteal layer of the sellar dura. The superior and lateral walls are formed by fusion of the dural sheaths of IIIrd, IVth, and first divisions of the Vth cranial nerve in the embryologic period. In the adult, the lateral wall of the CS is composed of two layers, and invasion by laterally situated meningiomas may involve the lateral or the medial cavernous component. The CS is a meeting point for the intracranial venous circulation. This pair of venous sinuses on either side of the sella is connected by intercavernous sinuses and receives blood from the superior and inferior ophthalmic veins; it connects posteriorly to the petroclival venous plexus, to the sigmoid sinus via the superior petrosal sinus, to the jugular bulb through the inferior petrosal sinus, and to the venous sinuses along the sphenoid wing and is connected with the deep facial veins through the pterygoid venous plexus. Within the CS, the internal carotid artery (ICA) is the most medial structure and sits against the carotid sulcus of the sphenoid bone. Laterally the ICA neighbors the VIth cranial nerve, which is the only cranial nerve to course within the “cavernous sinus proper.” The ICA enters the CS as it pierces through the sinus membrane, by the foramen lacerum. The foramen lacerum contains a fibrocartilaginous tissue in adult and the ICA courses tangential to this. At this point the “lacerum segment” of the ICA is covered by the “inferior sphenopetrosal ligament,” which was first described by Lang and Strobel. The authors described two parts: the pars sagittalis (which is the petrolingual ligament spanning from the petrous apex to the lingual sphenoidale) and the pars transversalis (which is formed by the endosteal dura of the superior aspect of the petrosphenoidal suture). Both of these structures blend into a fibrous ring circumferentially surrounding the ICA. Sekhar has stated that this ring is formed by dural fibers from the carotid canal. Dolenc has called this structure “the lateral ring.” In this region the ICA is neighbored laterally by the greater superficial petrosal nerve, which exits the foramen lacerum and enters the pterygoid canal. The artery forms a siphon within the CS by assuming an anterosuperior bend posteriorly and a superomedial bend anteriorly before it leaves the CS. At its exit from the cavernus sinus, the ICA pierces through the carotico-oculomotor membrane and travels for a short segment, extradurally under the anterior clinoid process, before it becomes intradural again. The two dural rings are called the proximal and distal dural rings and are a continuum of the roof of the CS, which in turn is formed as a continuum of the tentorial dura. The superior wall sends two reflections to the anterior clinoid process forming the two membranes. The extradural segment of the ICA behind the ACP was named the “clinoid segment” by Bouthilier and colleagues, “the siphon segment” by Fukushima and Day, or is simply the Dolenc’s space.11 The proximal dural ring is formed by the medial and inferior periosteoum of the ACP and only partially surrounds the ICA. Although the clinoid segment is extracevernous, venous plexi may continue into this incomplete dural ring. The distal dural ring, which was first described by Perneczky in 1985, surrounds the ICA circumferentially and it blends laterally into the adventitia of the ICA, the roof of the CS, and the dural fold known as the falciform ligament.12 The tough fibrous coverings at the entry and exit of the ICA to the CS are called dural rings and retain the bends of the ICA siphon under high pulsatile pressures. Within the CS, the ICA gives off three main branches. The posterior or the meningohypohyseal truncus leaves the posterior genu at its superior surface. The distal branches of this truncus are the tentorial artery of Bernasconi and Cassinari, the dorsal meningeal artery, and the inferior hypophyseal arteries. At the midpoint of the horizontal segment the ICA gives off the lateral main stem or the inferior CS artery at its lateral side. This stem forms an important source of anastomosis with the external carotid artery (ECA) via the arteria meningea media at the foramen spinosum, the maxillary artery at the foramen rotundum, and at the foramen ovale. The third and last branch of the cavernous ICA is McConnell’s capsullary artery, which supplies the capsule of the pituitary gland.
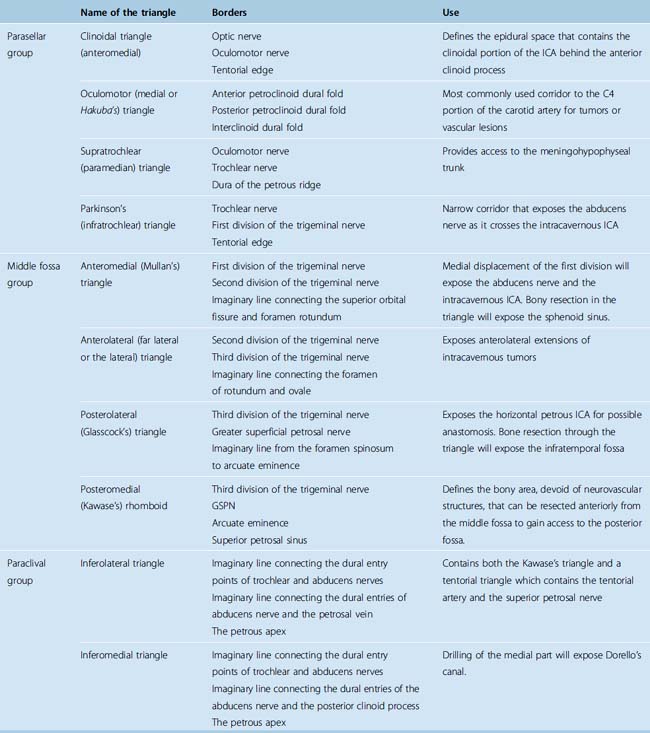
The entry to the CS is accomplished through multiple surgical corridors. Various such corridors have been described by pioneering surgeons. Four of these are the most commonly used and versatile; however, a much more extensive, systematic description has been provided by Fukushima.13 A description of the triangles is provided in Table 34-1; however, several of the triangles deserve special consideration. Two of the four most commonly used triangles are on the roof of the CS, which are the clinoidal and occulomotor triangles. The lateral wall bears the supratrochlear and the infratrochlear (Parkinson’s) triangles. The supratrochlear or the superior triangle is formed by the oculomotor and the trochlear nerves and the posterior margin is formed by dura along the petrous ridge. The supratrochlear triangle provides an exposure of the meningohypohyseal trunk. The infratrochlear or the lateral triangle was first described by Parkinson in 1965. It is bound by the trochlear and the first division of the trigeminal nerves. The dura of the petrous ridge forms the posterior margin. The medial triangle is used to access the cavernous ICA for a direct approach to intracarotid aneurysms and most CS neoplasms. It is delimited by the intradural carotid artery, the posterior clinoid process, the porus oculomotorus, and the siphon angle of the carotid artery. Two additional triangles describe important entry routes through the petrous bone. The posterolateral triangle was first described by Glasscock in 1968 to define the horizontal intrapetrous ICA. The exposure at this triangle is used to gain proximal control of the ICA in an intracavernous procedure. The posteromedial triangle was described by Kawase to represent the anterior petrous projection of the bone that has to be drilled from the petrous apex to gain access to the posterior fossa without encountering neurovascular structures within the bone.
PATHOLOGY
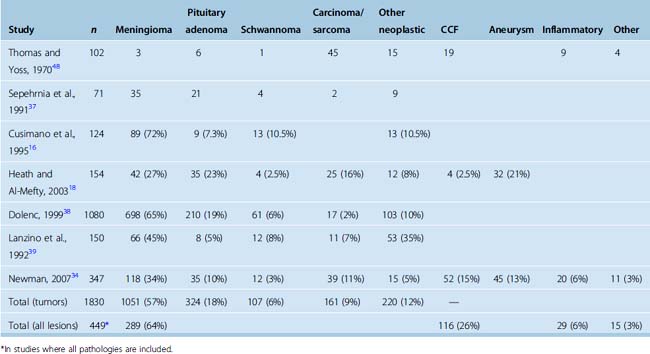
The reported relative incidences of different CS pathologies have varied considerably in the literature, mostly due differences in study methodology. Earlier studies focused on symptomatology and reported high incidences of CS syndrome due to trauma and iatrogenic injuries. With advances in diagnostic radiology, the spectrum of lesions became more clearly defined. The three most common space-occupying lesions in the CS are tumors, vascular lesions, and infectious/inflammatory lesions. Again, most clinical series are biased by their institutional referral patterns. A summary of large studies is provided in Table 34-2, which shows that roughly two thirds of CS masses are tumors and roughly one fourth are vascular pathologies. Among neoplastic lesions, meningioma is the most commonly reported pathology in most modern series and makes up roughly two thirds of all tumors. Invasive pituitary adenomas and schwannomas (of the trigeminal nerve and other cranial and noncranial nerves) are also commonly encountered. A list of reported pathologies is provided in Table 34-3. The surgical results in malignant pathologies are not satisfactory, and surgery is seldom indicated in such cases, mostly to establish a tissue diagnosis where radiologic studies are indecisive. The role of surgery and its extent is the source of another debate and is discussed below. Surgical results in invasive pituitary adenomas are poor.14 In fact, several authors have reported that the total resection rate for invasive pituitary adenomas is the lowest among all benign nonmeningeal tumors of the CS. Conversely, the surgical results for schwannomas and neurinomas are better than in meningiomas. Cavernous hemangiomas of the CS can also be resected with good surgical results, if an intracapsular resection can be achieved. Surgery remains the best treatment option for chordomas and chondrosarcomas; however, the CS portion is difficult to resect totally, more so in chordomas.
| Tumors |
INCIDENCE AND CLINICAL MANIFESTATIONS
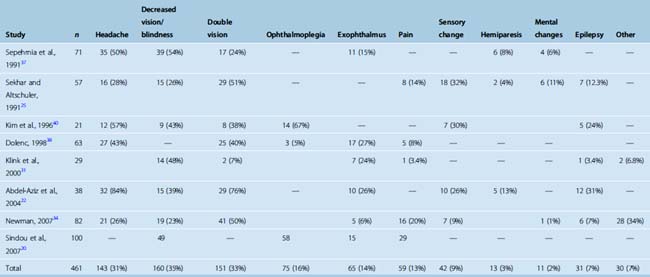
The most common presenting symptom of a CS meningioma is decreased vision in the ipsilateral eye. The incidence of this symptom varies between 23% and 54% among studies (Table 34-4). Headaches are present in roughly one third of patients. A classical finding in CS meningiomas is disturbance of ocular movements. Proptosis and varying degrees of ocular movement limitations ranging from complete ophthalmoplegia to slight functional disturbances can be observed. Trigeminal involvement resulting in facial pain syndromes or sensory changes is observed in only a minority of patients. Other findings such as hemiparesis, mental changes, or epilepsy are observed in even a smaller minority. Incidental CS meningiomas are commonly found during cranial radiologic studies. Almost none of the clinical studies on treatment of CS meningiomas report the incidence of asymptomatic cases. Nakamura and colleagues15 reported that CS meningiomas made up 17% of 41 incidentally found meningiomas. A quick glance at the published series indicates that the symptomatology differs slightly between meningiomas and nonmeningeal tumors of the CS. The incidences of headache and double vision are higher in nonmeningial tumors of the CS.
DIAGNOSTIC STUDIES
As CT and MRI provide a significant amount of information both on the pathoanatomy of the lesion and its effects on surrounding neurovascular structures, angiography is not universally indicated. However, if there is a significant risk of internal carotid involvement, digital subtraction angiography (DSA) can provide very valuable functional information. First, DSA can provide supplementary diagnostic information on the carotid involvement by demonstrating occlusion, narrowing, luminal changes, and pseudoaneurysm formation. Second, using cross compression, the DSA will provide information on the vascular reserve of the contralateral ICA and the posterior circulation. Rupture of the internal carotid artery may occur during CS exploration, which may necessitate a carotid takedown. A postoperative major disabling stroke is reported in roughly 5% of cases with CS meningiomas.16–18 In patients with a significant risk of internal carotid involvement, a clearer and more objective picture on the vascular reserve can be provided with a balloon occlusion test. The test consists of angiographic catheterization of the internal carotid artery and occlusion of the internal carotid artery with an intraluminal balloon with constant neurologic monitoring. The development of neurologic symptomatology on closure of the ICA indicates that the contralateral ICA and the posterior circulation cannot provide adequate perfusion compensation, which will significantly affect the surgical strategy. The balloon occlusion test can also be combined with single-photon emission computed tomography (SPECT) imaging or xenon blood flow studies to increase its reliability. Patients are ultimately stratified to groups of low-intermediate and high risk of neurologic complications after a possible carotid takedown.
NATURAL COURSE OF THE DISEASE
Very little is known on the natural course of CS meningiomas. A large proportion of cases present to clinical attention with significant symptomatology such as decreased vision, proptosis, and disturbance in conjugated gaze. A significant proportion of cases are nevertheless incidentally found on radiologic studies. Nakamura and colleagues15 analyzed 41 meningioma patients with incidentally found meningiomas, and this cohort included 7 CS meningiomas. Patients were observed without interventions for a fairly short mean period of 43 months, and from serial imaging studies the authors calculated a mean absolute growth rate of 1.34 cm3/yr and a mean tumor doubling time of 13.6 years (1.73–49.6 years). Similarly, to analyze the incidence of growth in incidentally found skull base meningiomas, Bindal and colleagues19 observed 40 patients clinically for a mean of 83 months and radiologically for a mean of 76 months. During this follow-up, 7 patients (17%) experienced tumor growth and 11 patients (27.5%) experienced neurologic progression. These studies indicate that a significant proportion of CS meningiomas do progress and therefore some form of treatment is indicated in these cases.
TREATMENT
Choosing the Optimal Management Strategy
The rate of total resection in CS meningiomas is very low. This is dictated by the invasive nature of meningiomas, their vascularity, and the commonly hard consistency. Attempts at resection of such a tumor in a very tight space that contains highly fragile and extremely important neurovascular structures is naturally associated with significant surgical morbidity. Safe resection can certainly be performed in a subset of meningiomas that have a soft consistency and poor vascularity; however, these cases constitute only a minority and the information on these tumor characteristics can be obtained only during surgery. In a recent article, Sindou and collleagues20 showed that attempts at resection of the intracavernous component are associated with a significantly higher rate of visual impairment and cranial nerve morbidity than resection of only the extracavernous portion. These facts led several authors to plan alternative treatment strategies such as maximal safe resection of the tumor outside the CS with or without adjuvant radiosurgery. The aim of such treatment combinations is to debulk the tumor to a size that can safely be treated with radiosurgery, which is used to obtain long-term tumor growth control. Several publications have indicated that this is an acceptable strategy with good short-term results, most certainly avoiding the very high complication rate of aggressive surgical treatment strategies. The literature contains one study that compares the results of aggressive surgical excision with maximal safe resection combined with Gamma-Knife radiosurgery.21 The study concluded that extracavernous resection followed by gamma-knife radiosurgery is as effective as radical surgery and that the third-year tumor volume growth control and cranial nerve outcome are better in the combination treatment group.21 At our institution, gamma-knife radiosurgery became available in 1997 and we have changed our treatment paradigm to resection of the extracavernous part followed by immediate adjuvant radiosurgery. The study was a retrospective comparison of these two periods when two different management strategies were used in the same clinic. Other centers have also reported similar experiences.22–24 There are varying opinions on the timing of radiosurgery. While some advocate immediate postoperative use, others choose to use it in case of radiologically documented volumetric growth or symptomatic recurrence. However, as combination of maximal safe surgery with adjuvant radiosurgery is a fairly recent concept, long-term results are still not available and there are no prospective, randomized studies to compare the results to maximal surgery alone to evaluate the benefits of radiosurgery. Small but symptomatic CS meningiomas can be successfully treated with radiosurgery alone with adequate short-term tumor growth control and minimal morbidity. Secondary CS involvement may be seen in meningiomas of the tuberculum sella and in clinoidal, medial sphenoid wing, and sphenopetroclival meningiomas. CS involvement significantly decreases the chance of good surgical results in such cases unless the invasion is confined to the lateral wall of the CS. Less radical strategies are being reported in the recent literature and these do not significantly differ from those of primary CS meningiomas.
Surgical Treatment
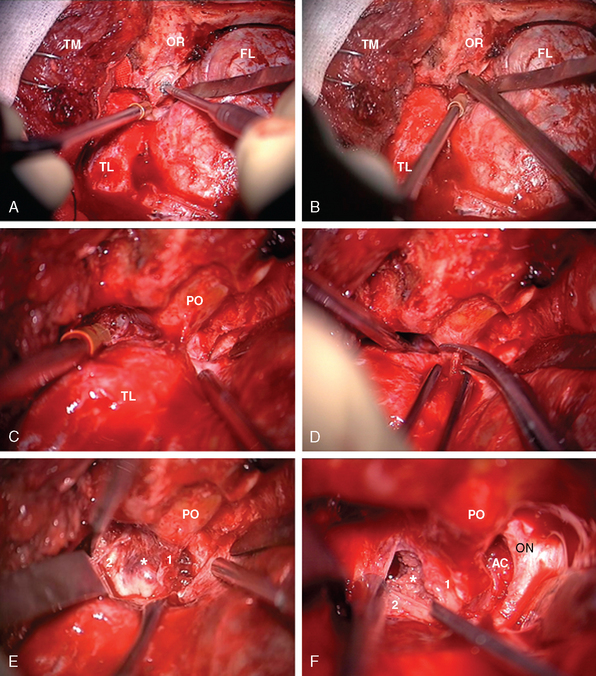
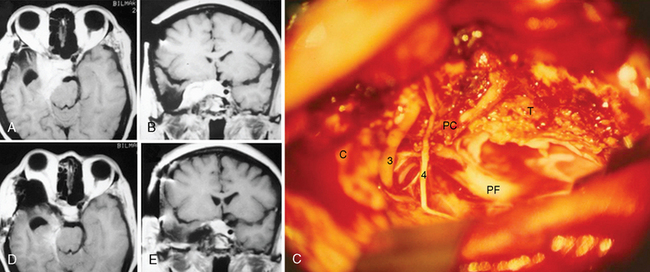
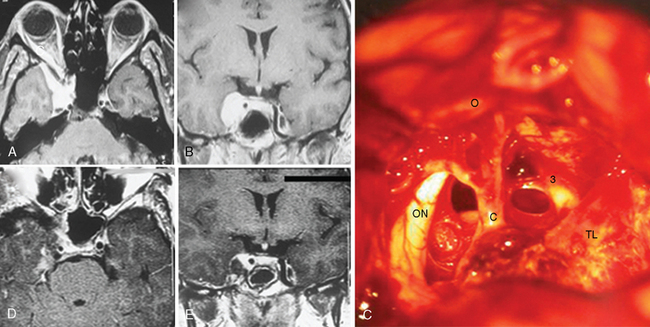
The frontotemporal epidural–subdural approach of Dolenc forms the basis of all anterolateral approaches to the CS. The techniques described by Hakuba, Dolenc, and Kawase are the most widely used approaches.7,8 The Dolenc approach is basically a frontotemporal craniotomy, which also combines exposure and splitting of the dura at the superior orbital fissure to gain an epidural access to the lateral wall of the CS (Fig. 34-1). The similar Hakuba approach exposes the CS purely extradurally. The Kawase approach involves a frontotemporal–orbitozygomatic craniotomy (or, alternatively, a simple temporal craniotomy). The basic approach can easily be modified in a modular fashion. Most of these modifications are based on use of different skin flaps, different scalp flap dissections, and different osteotomies. Certainly, the basic operation can be modified by combination with a transpetrosal approach. For all approaches, the patient is placed in the supine position and the head is fixed using a pin head rest. When using three-pin fixation the single pin is placed at the inion and the double pins are placed on the contralateral frontal convexity, a safe distance away from the temporal squama, the frontal sinuses, and the planned incision trajectory. The head is rotated approximately 30 degrees and slightly flexed. The basic approach uses a pterional or frontotemporal skin flap. However, for exposure of the orbital rim a longer skin incision is used, which starts anterior to the ipsilateral tragus and extends in a curvilinear fashion, crossing the midline to the contralateral midpupillary line behind the frontal hairline. The basic pterional or frontotemporal craniotomy is routinely performed with pneumatic or electric osteotomes to decrease operative time. Such a frontotemporal craniotomy can be extended using zygomatic and orbital osteotomies. A zygomatic osteotomy enables a low lateral retraction of the temporal muscle to gain additional frontobasal to superior viewing angle. This can be further improved by removal of the orbital rim. The orbitozygomatic osteotomy provides a substantial amount of basal to superior viewing angle and has the most extensive access to the CS. In the basic frontotemporal epidural approach, the craniotomy is followed by drilling of the orbital roof and exposure of the periorbit. Special care must be taken not to damage the periorbit. The dorsal surface of the superior orbital fissure is then exposed in its entirety. The dural fold extending from the temporal dura through the superior orbital fissure to the periorbit is then incised from its lateral aspect to obtain a plane of dissection between the two layers of the lateral wall of CS, and the dural reflection is peeled posteriorly from CN V(1). The next step in CS exploration is the opening of the optic canal by removal of the anterior clinoidal process. This can be performed extradurally or after opening of the dura and cisternal cerebrospinal fluid (CSF) drainage. Although there is no systematically collected evidence to support either method, anecdotal reports have indicated that injury to the optic nerve can occur in extradural clinoidectomy but not with an intradural approach. In either approach, the anterior clinoidal process is removed using a diamond drill. Drilling starts at the center of the anterior clinoidal process and performed under constant irrigation, for short periods to minimize the mechanical and thermal injury to the optic nerve. Variations on the bony anatomy of the anterior clinoid process are encountered where the internal carotid process is encircled by bone; therefore the anterior clinoidal process should be skeletonized and gently broken but never extracted in one piece. Exposure of the optic canal simultaneously exposes the clinoidal and the ophthalmic segments of the ICA and the distal dural ring. The extradural exposure advances with drilling of the temporal bone and further peeling of the outer leaf of the lateral wall of the CS toward the Gasserian ganglion. CN V(3) is exposed at the foramen rotundum. The middle meningeal artery is sectioned and coagulated at the foramen spinosum, which is then plugged with Surgicel and bone-waxed. The greater petrosal nerve is exposed and protected to avoid the risk of xeropthalmia. The majority of meningiomas are not confined to the interdural layer at the lateral wall of the CS. Therefore, the pure epidural approach is seldom sufficient for meningioma resection. Extension into surrounding structures such as optic canal, orbit, sella, Meckel’s cave, and the posterior fossa is addressed with slight modifications of the basic approach. The hard consistency of meningiomas and their rich vascular supply further complicate their surgery. Further, meningiomas can encircle, encase, and even invade the adventitia of the ICA. The ICA can be encased and even invaded within the CS, in the C4 segment, and attempts at resection of the intracavernous part are associated with a significant risk of ICA rupture. To predict this risk preoperatively, Sekhar and Altschuler classified intracavernous neoplasms into five grades, depending on the number of areas involved and the degree of encasement of the ICA25 (Table 34-5). This was later supported by a systematic radiologic description and its clinical correlation and used as the Hirsh grade.26 The risk of postoperative major disabling stroke in meningiomas that invade the CS is in the range of 5%.16–18 Several authors have reported that such invasion can be addressed with a high-flow bypass procedure, the risks and gains of which should be carefully considered and planned. If such an intracavernous resection is planned, proximal and distal vascular control is necessary. Proximal control can be obtained by an exposure of the petrous segment of the ICA by unroofing of the petrous carotid canal posterior to foramen ovale and medial to foramen spinosum. Distal control can be obtained after sylvian dissection proximal to the posterior communicating artery in the ophthalmic segment.
TABLE 34-5 Sekhar classification of intracavernous neoplasms
| Grade | Cavernous sinus involvement | Intracavernous ICA |
|---|---|---|
| I | One area* | Not involved |
| II | More than one area | Displaced but not totally encased |
| III | Entire cavernous sinus | Totally encased |
| IV | Entire cavernous sinus | Encased with narrowing, pseudoaneurysm or occlusion |
| V | Bilateral cavernous sinuses | Encased |
OUTCOME OF SURGERY
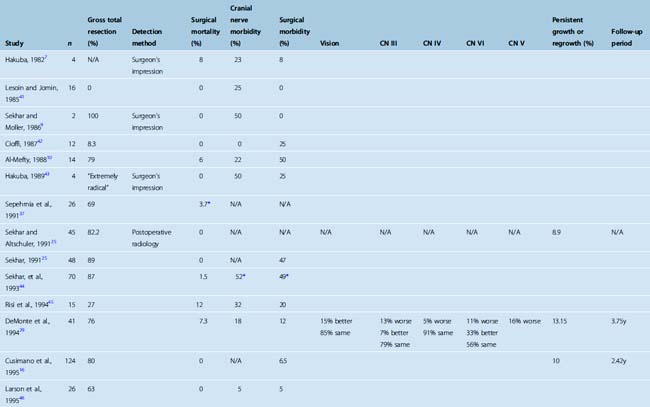
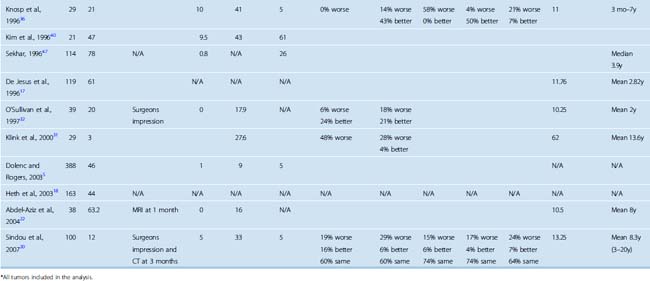
Total resection of a meningioma within the CS is one of the greatest surgical challenges. Despite the optimistic initial reports, which indicated total resection rates approaching as high as 100%, more recent studies after the MRI era have established that gross total resection is possible but is very rarely achieved in meningiomas that involve the CS proper. A significant proportion of studies published before 1994 reported total resection rates in the range of 81% to 100% (Table 34-6). In contrast, the resection rate in more recent studies is far lower and the most recent study has reported a 12% total resection rate.20 This reported rate is based on CT follow-up at 3 months, which may indicate that results with MRI follow-up may be even worse.20 Failure to perform total resections is due to the invasive nature of meningiomas, their intricate relationship to the neurovascular structures within the CS, and invasion of cranial nerves and the internal carotid artery by the tumor. It must also be mentioned that even in the case of a gross total resection, the result is far from an oncologic resection and is at best a Simpson grade 3 resection. However; it must also be emphasized that the total resection rates are very much dependent on the methodology. The extent of resection can be determined by the surgeon’s impression, which can be misleading in such intricate anatomy with such an invasive tumor. CT and MRI provide objective information on the extent of resection, but the sensitivity of MRI is far superior to CT in detection of tumor residuals within the CS (Figs. 34-2, 34-3, and 34-4). Most of the earlier studies on CS meningiomas have suffered from this methodological bias, and the surgical results were most likely not as perfect. Contrast-enhanced MRI studies within the first 24 hours after surgery are a very reliable means to evaluate surgical results, as demonstrated for most intracranial tumors.27 Such a change of methodology may be one of the reasons for the dramatic decline in reported total resection rates.
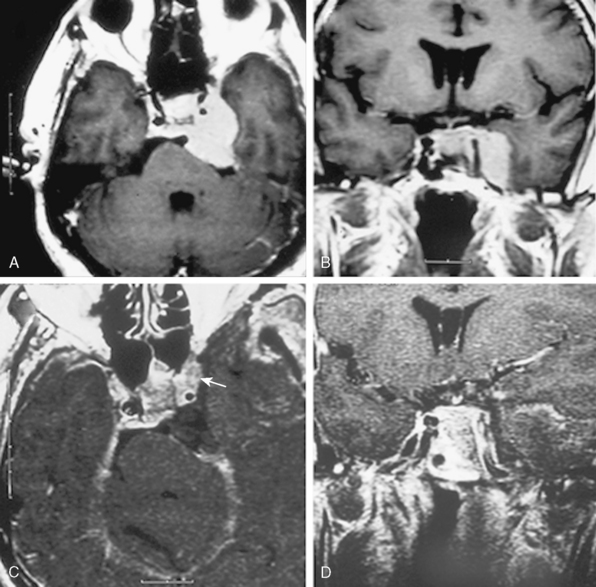
FIGURE 34-2 Preoperative axial (A) and coronal (B) as well as postoperative axial (C) and coronal (D) contrast enhanced T1-weighted MRI images of the same patient presented in Figure 34-1 with a left-sided CS meningioma. Postoperative contrast-enhanced MRI images obtained within the first 24 hours after surgery clearly demonstrate the residual tumor (arrow on axial image).
With the advancements in the last decades, the mortality associated with CS surgery has decreased significantly. Today, CS explorations can be performed with mortality rates comparable to those associated with other common neurosurgical procedures (see Table 34-6). The risk of surgical morbidity in the recent literature is in the range of 5%. However, much higher rates, as high as 61%, have also been reported. Invasive skull-base approaches are associated with significant morbidity. The most feared ones are vascular complications. Major disabling strokes due to internal carotid artery closure are observed in roughly 5% of patients. Venous complications are less predictable, and in most cases catastrophic. Especially in subtemporal approaches, temporal lobe edema or venous infarctions can be life threatening. Hematomas in the surgical cavity or postoperative hydrocephalus are also reported. Diabetes insipidus can be observed after CS surgery but is transient in most cases. CSF leaks are a very significant complication of skull-base surgery, and when not detected and promptly treated may be complicated by meningitis. The importance of packing of the air sinuses during surgery with muscle tissue cannot be overemphasized. CSF leaks can also result after drilling of pneumatized anterior clinoid processes.
In addition to general surgical complications, CS explorations are associated with a significant risk of transient or permanent worsening of cranial nerve function. Preoperative visual deficits are common in CS meningiomas. Compromised vision in the ipsilateral eye has been reported in 24% to 80% of patients with CS meningiomas.28–33 The deficit is attributed to physical compression by the large tumor, the impingement of the optic nerve at the falciform ligament, and to vascular compromise. Decompression of the optic nerve may improve vision. Newman34 reported that 25% of their 20 patients with preoperative visual deficits had immediate improvement after surgery, while 30% had decreased vision postoperatively. Among all cranial nerve functions, optic nerve function is the only one that had a significant chance of improvement after CS surgery. However, decreased vision can also be encountered with or without preexisting, preoperative visual impairment. The exact cause of this iatrogenic injury to the optic nerve has not been clearly demonstrated. Injury to microvasculature around the optic nerve, vasospasm, and direct mechanical injury to the optic nerve during anterior clinoid removal have been suspected as the cause. Several studies have drawn attention to the heat damage related to the use of a diamond drill.5 In large tumors, attempts at extradural removal of the anterior clinoid process may put extra tension on the already impinged optic nerve. In a retrospective analysis, Yonekawa and colleagues35 found a 10% risk of postoperative visual dysfunction in cases, where the anterior clinoid was removed extradurally, while no dysfunction was noted in intradurally resected cases. A comparable rate of postoperative optic nerve damage (7%) was reported in a large series that utilized extradural anterior clinoid removal.34 Many studies have not reported such dysfunction, as it can be easily missed clinically without dedicated assessment. The most common complication after CS explorations is a VIth cranial nerve palsy. Reports indicate that preoperative deficits in abducens nerve function did not improve after surgery. Worsening of CN VI function, in contrast, is common and was reported in 76% of patients who did not have preoperative dysfunction. These deficits were temporary in some patients and improved to some degree in 50%. In the long term, however, a majority of these patients will have some degree of impairment. Ophthalmologic correction of such a dysconjugate gaze is not simple. Impairment in trochlear nerve function is more difficult to evaluate, especially in the setting of incomplete occulomotor nerve palsy. Oculomotor nerve palsy also commonly complicates CS explorations. As in abducens nerve function, preoperative deficits rarely improve after surgery. A new IIIrd nerve palsy is induced in the majority of patients after CS explorations. Previous reports have concluded that the severity of this deficit is also predictive of its prognosis. The majority of cases with incomplete IIIrd nerve palsies improve in time. Complete deficits are far less likely to improve.36 Trigeminal nerve dysfunction is also observed after CS surgery, and xerophthalmia is the most significant complication, which rarely improves over time and must be treated aggressively to prevent corneal ulcerations. The surgical cranial nerve complication rate is not the same in all CS pathologies. The risk of cranial nerve morbidity is higher in meningiomas due to their invasive nature, their frequently hard consistency, and their highly vascular nature.
RECURRENCE
When compared to other meningiomas, the recurrence rate in CS meningiomas is low. Although the follow-up period varies among studies, the reported recurrence rate ranges from 10% to 13.3% after surgery alone (2–8.3 years mean follow-up period) and 6% to 13.6% after radiosurgery (1.6–4.12 years mean follow-up period). Sindou and colleagues20 compared the recurrence rates after surgery alone or surgery with adjuvant radiotherapy or radiosurgery and found no significant difference. There was no statistically significant difference in recurrence between the patients who underwent resection of only the extracavernous portion and those who underwent a subtotal resection of the intracavernous tumor. With a mean follow-up of 8.3 years, Sindou and colleagues20 have reported that most recurrences occurred between 3 and 9 years. Similarly Klink and colleagues31 reported the mean time to progression to be 8.2 years.
[1] Parkinson D. Lateral sellar compartment O.T. (cavernous sinus): history, anatomy, terminology. Anat Rec. 1998;251:486-490.
[2] Browder J. Treatment of carotid artery-cavernous sinus fistula. Report of a case. Arch Ophthalmol. 1937;18:95-102.
[3] Parkinson D. A surgical approach to the cavernous portion of the carotid artery. Anatomical studies and case report. J Neurosurg. 1965;23:474-483.
[4] Taptas J.N. The so-called cavernous sinus: a review of the controversy and its implications for neurosurgeons. Neurosurgery. 1982;11:712-717.
[5] Dolenc V., Rogers L. Microsurgical Anatomy and Surgery of the Central Skull Base. New York: Springer-Verlag, 2003.
[6] Umansky F., Nathan H. The lateral wall of the cavernous sinus. With special reference to the nerves related to it. J Neurosurg. 1982;56:228-234.
[7] Hakuba A., Nishimura S., Shirakata S., Tsukamoto M. [Surgical approaches to the cavernous sinus. Report of 19 cases (author’s transl)]. Neurol Med Chir (Tokyo). 1982;22:295-308.
[8] Dolenc V. Direct microsurgical repair of intracavernous vascular lesions. J Neurosurg. 1983;58:824-831.
[9] Sekhar L.N., Moller A.R. Operative management of tumors involving the cavernous sinus. J Neurosurg. 1986;64:879-889.
[10] Al-Mefty O., Smith R.R. Surgery of tumors invading the cavernous sinus. Surg Neurol. 1988;30:370-381.
[11] Bouthillier A., van Loveren H.R., Keller J.T. Segments of the internal carotid artery: a new classification. Neurosurgery. 1996;38:425-432. discussion 432–3
[12] Knosp E., Muller G., Perneczky A. The paraclinoid carotid artery: anatomical aspects of a microneurosurgical approach. Neurosurgery. 1988;22:896-901.
[13] Fukushima T. Direct operative approach to the vascular lesions in the cavernous sinus: summary of 27 cases. Mt Fuji Workshop on Cerebrovascular Diseases. 1988;6:169-189.
[14] Pamir M.N., Kilic T., Ozek M.M., et al. Non-meningeal tumours of the cavernous sinus: a surgical analysis. J Clin Neurosci. 2006;13:626-635.
[15] Nakamura M., Roser F., Michel J., et al. The natural history of incidental meningiomas. Neurosurgery. 2003;53:62-70. discussion 70–1
[16] Cusimano M.D., Sekhar L.N., Sen C.N., et al. The results of surgery for benign tumors of the cavernous sinus. Neurosurgery. 1995;37:1-9. discussion 9–10
[17] De Jesus O., Sekhar L.N., Parikh H.K., et al. Long-term follow-up of patients with meningiomas involving the cavernous sinus: recurrence, progression, and quality of life. Neurosurgery. 1996;39:915-919. discussion 919–20
[18] Heth J.A., Al-Mefty O. Cavernous sinus meningiomas. Neurosurg Focus. 2003;14:e3.
[19] Bindal R., Goodman J.M., Kawasaki A., et al. The natural history of untreated skull base meningiomas. Surg Neurol. 2003;59:87-92. discussion 92
[20] Sindou M., Wydh E., Jouanneau E., et al. Long-term follow-up of meningiomas of the cavernous sinus after surgical treatment alone. J Neurosurg. 2007;107:937-944.
[21] Pamir M.N., Kilic T., Bayrakli F., Peker S. Changing treatment strategy of cavernous sinus meningiomas: experience of a single institution. Surg Neurol. 2005;64(Suppl. 2):S58-66.
[22] Abdel-Aziz K.M., Froelich S.C., Dagnew E., et al. Large sphenoid wing meningiomas involving the cavernous sinus: conservative surgical strategies for better functional outcomes. Neurosurgery. 2004;54:1375-1383. discussion 1383–4
[23] Duma C.M., Lunsford L.D., Kondziolka D., et al. Stereotactic radiosurgery of cavernous sinus meningiomas as an addition or alternative to microsurgery. Neurosurgery. 1993;32:699-704. discussion 704–5
[24] Friedlander R.M., Ojemann R.G., Thornton A.F. Management of meningiomas of the cavernous sinus: conservative surgery and adjuvant therapy. Clin Neurosurg. 1999;45:279-282.
[25] Sekhar L.N., Altschuler E.M. Meningiomas of the cavernous sinus. In: Al-Mefty O., editor. Meningiomas. New York: Raven Press; 1991:445-460.
[26] Hirsch W.L., Sekhar L.N., Lanzino G., et al. Meningiomas involving the cavernous sinus: value of imaging for predicting surgical complications. AJR Am J Roentgenol. 1993;160:1083-1088.
[27] Ekinci G., Akpinar I.N., Baltacioglu F., et al. Early-postoperative magnetic resonance imaging in glial tumors: prediction of tumor regrowth and recurrence. Eur J Radiol. 2003;45:99-107.
[28] Jacob M., Wydh E., Vighetto A., Sindou M. Visual outcome after surgery for cavernous sinus meningioma. Acta Neurochir (Wien). 2008;150:421-429. discussion 429
[29] DeMonte F., Smith H.K., al-Mefty O. Outcome of aggressive removal of cavernous sinus meningiomas. J Neurosurg. 1994;81:245-251.
[30] Maruyama K., Shin M., Kurita H., et al. Proposed treatment strategy for cavernous sinus meningiomas: a prospective study. Neurosurgery. 2004;55:1068-1075.
[31] Klink D.F., Sampath P., Miller N.R., et al. Long-term visual outcome after nonradical microsurgery patients with parasellar and cavernous sinus meningiomas. Neurosurgery. 2000;47:24-31. discussion 31–2
[32] O’Sullivan M.G., van Loveren H.R., Tew J.M.J. The surgical resectability of meningiomas of the cavernous sinus. Neurosurgery. 1997;40:238-244. discussion 245–7
[33] George B., Ferrario C.A., Blanquet A., Kolb F. Cavernous sinus exenteration for invasive cranial base tumors. Neurosurgery. 2003;52:772-780. discussion 780–2
[34] Newman S. A prospective study of cavernous sinus surgery for meningiomas and resultant common ophthalmic complications (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2007;105:392-447.
[35] Yonekawa Y., Ogata N., Imhof H.G., et al. Selective extradural anterior clinoidectomy for supra- and parasellar processes. Technical note. J Neurosurg. 1997;87:636-642.
[36] Knosp E., Perneczky A., Koos W.T., et al. Meningiomas of the space of the cavernous sinus. Neurosurgery. 1996;38:434-442. discussion 442–4
[37] Sepehrnia A., Samii M., Tatagiba M. Management of intracavernous tumours: an 11–year experience. Acta Neurochir Suppl (Wien). 1991;53:122-126.
[38] Dolenc V. Tumors involving the cavernous sinus. In: Kaye A., Black P., editors. Operative Neurosurgery. Philadelphia: Churchill-Livingstone; 1999:657-670.
[39] Lanzino G., Hirsch W.L., Pomonis S., et al. Cavernous sinus tumors: neuroradiologic and neurosurgical considerations on 150 operated cases. J Neurosurg Sci. 1992;36:183-196.
[40] Kim D.K., Grieve J., Archer D.J., Uttley D. Meningiomas in the region of the cavernous sinus: a review of 21 patients. Br J Neurosurg. 1996;10:439-444.
[41] Lesoin F., Jomin M., Bouchez B., et al. Management of cavernous sinus meningiomas. Neurochirurgia (Stuttg). 1985;28:195-198.
[42] Cioffi F.A., Bernini F.P., Punzo A., et al. Cavernous sinus meningiomas. Neurochirurgia (Stuttg). 1987;30:40-47.
[43] Hakuba A., Tanaka K., Suzuki T., Nishimura S. A combined orbitozygomatic infratemporal epidural and subdural approach for lesions involving the entire cavernous sinus. J Neurosurg. 1989;71:699-704.
[44] Sekhar L.N., Ross D.A., Sen C. Cavernous sinus and sphenocavernous neoplasms. In: Sekhar L.N., Pi J., editors. Surgery of Cranial Base Tumors. New York: Raven Press; 1993:521-604.
[45] Risi P., Uske A., de Tribolet N. Meningiomas involving the anterior clinoid process. Br J Neurosurg. 1994;8:295-305.
[46] Larson J.J., van Loveren H.R., Balko M.G., Tew J.M.J. Evidence of meningioma infiltration into cranial nerves: clinical implications for cavernous sinus meningiomas. J Neurosurg. 1995;83:596-599.
[47] Sekhar L.N., Patel S., Cusimano M., et al. Surgical treatment of meningiomas involving the cavernous sinus: evolving ideas based on a ten year experience. Acta Neurochir Suppl. 1996;65:58-62.
[48] Thomas J.E., Yoss R.E. The parasellar syndrome: Problems in determining etiology. Mayo Clin Proc. 1970;45:617-623.