26 Spinal Cord Tumors
Primary Tumors Involving the Spinal Cord
Epidemiology and Genetics
Primary tumors involving the spinal cord are uncommon1 and tend to occur in young patients. In adults, they are outnumbered by primary brain tumors at a ratio of roughly 20 : 1; for children, a ratio of 5 : 1 is reported by most centers. Tumors arising from the spinal canal and vertebrae show a more heterogeneous age distribution. The rarity of primary spinal tumors makes it difficult to establish a clear-cut association with specific cytogenetic abnormalities. Some investigators have found an association between spinal and brain ependymomas and the loss of sequences in chromosome arm 22q, suggesting the presence of one or more ependymoma tumor suppressor genes in this location.2–4 Pediatric ependymomas and astrocytomas have been associated with loss of chromosome arm 17p.3,4 Some myxopapillary and papillary ependymomas have been associated with bcl-2 oncoprotein expression.5 Other ependymomas have been associated with mutations in the MEN1 gene and loss of heterozygosity in the chromosome arm 11q region.6
Prior exposure to therapeutic irradiation probably increases the risk for developing spinal canal meningiomas, soft tissue sarcomas, vertebral body sarcomas, and possibly spinal cord gliomas, although the magnitude of this risk is unclear. Neurofibromatosis is strongly associated with an increased incidence of spinal canal neurofibrosarcoma and has been associated with both intracranial and spinal cord ependymomas as well.2 This latter association may be indirect, with the actual cause being the loss of a separate ependymoma tumor suppressor gene located near the NF2 gene.3
Anatomy
Approximately 10% of all primary tumors involving the cord arise from the vertebrae, 65% from the spinal canal, and 25% from the cord proper. For purposes of treatment, it is useful to group primary tumors based on whether they arise from the cord, spinal canal, or vertebrae (Table 26-1). This system helps to predict whether radical surgery is feasible. It also suggests whether the normal spinal cord must be included within the high-dose irradiation volume or could instead be partially excluded from treatment with the use of sophisticated irradiation techniques.
Table 26-1 Common Primary Tumor Types Involving the Spinal Cord
| Location | Histologic Types |
|---|---|
| Vertebral body (excluding myeloma/plasmacytoma) | Osteogenic sarcoma |
| Chondrosarcoma | |
| Chordoma | |
| Spinal canal | Neurofibrosarcoma |
| Malignant schwannoma | |
| Meningioma | |
| Spinal cord/cauda equina | Astrocytoma |
| Ependymoma | |
| Oligodendroglioma |
Pathology
Grade is an important consideration for several tumor types. Low-grade spinal canal sarcomas appear to be at low risk for both local and distant recurrence if completely resected. High-grade, malignant meningiomas are probably at higher risk for recurrence than the more typical benign meningiomas. The importance of grade is uncertain for ependymomas, as some series have identified grade as a significant prognostic factor,7–9 and others have not.10 The overall risk of subsequent intracranial failure for low-grade spinal ependymomas is low, on the order of 7%.11 However, this value is probably higher for histologically malignant ependymomas.12,13 Myxopapillary ependymomas reportedly have a particularly favorable prognosis.9
Grade is clearly the most important factor influencing outcome for spinal astrocytomas.14,15 Although prolonged survival is generally the rule for patients with low-grade astrocytomas, malignant astrocytomas such as glioblastoma multiforme (GBM) and highly anaplastic (Grade 3) astrocytomas (HAAs) are aggressive tumors characterized by rapid local recurrence following treatment, craniospinal axis dissemination, and short survival.13–17 The pilocytic astrocytoma subtype reportedly has a better prognosis than the diffuse fibrillary astrocytoma subtype.18
Diagnostic and Staging Studies
The workup for primary cord tumors is chiefly radiographic. Magnetic resonance imaging (MRI) with gadolinium for contrast is the imaging study of choice.19 Standard or metrizamide-enhanced computed tomographic (CT) myelography is indicated for patients unable to undergo MRI (e.g., pacemaker-dependent patients). Intramedullary tumors usually extend for multiple segments of cord, and sagittal MRI is particularly useful for delineating the rostral and caudal extent of tumor. Imaging of the entire craniospinal axis is required for all ependymomas, because spinal axis metastases develop in 5% to 15% of intracranial ependymomas.20 These spinal “drop metastases” can produce the initial symptoms of a disseminated, primary intracranial ependymoma.
The entire neuraxis should also be imaged for cord HAAs and GBMs. Low-grade spinal astrocytomas and oligodendrogliomas do not require imaging of the entire craniospinal axis. CT scanning of the chest to rule out pulmonary metastases should be performed for vertebral osteogenic sarcomas and chondrosarcomas, as well as for spinal canal neurofibrosarcomas. Other tests such as angiography may be of limited use for AVMs or meningiomas. CSF cytology may be informative, but its use in determining treatment or influencing outcome is controversial. No chemical markers appear to be clinically useful in the diagnosis or follow-up of spinal tumors. The role of the bromodeoxyuridine (BrdU) and Ki-67 labeling indices as prognostic indicators for spinal ependymomas is under investigation.21,22 An elevated cyclin D1 labeling index, high MIB-1 proliferation index, and p53 immunolabeling might be indicative of high grade in ependymomas, although a correlation with clinical outcome is unclear.5,23
Staging System
Presently there is no commonly accepted TNM-type staging system for primary cord tumors. Primary vertebral body tumors and spinal canal tumors should be staged using the American Joint Committee on Cancer (AJCC) staging systems for bone and soft tissues, respectively.24
Standard Therapeutic Approaches
Surgical exploration combining maximal resection (preferably en bloc) with minimal risk of iatrogenic injury is the preferred initial treatment procedure. In addition to decompressing the cord, essential histologic information is obtained. There is no role for preoperative irradiation or irradiation without a prior attempt at histologic diagnosis. As many as 4% of radiographically diagnosed “cord gliomas” have proven to be benign upon pathologic examination, with infarcts, demyelinating processes, amyloidosis, and sarcoidosis comprising some of the disease processes which mimic gliomas.25
The extent of resection is variable for primary cord gliomas. Using modern microsurgical techniques with intraoperative monitoring, complete resection of low-grade gliomas has become more common, and outcome has been successful without the use of adjuvant radiotherapy.26,27,97 Postoperative megavoltage irradiation provides favorable long-term results following subtotal resection. Craniospinal irradiation is employed in the management of malignant as well as benign, multifocal ependymomas.
The favorable location of ependymomas of the cauda equina usually permits complete resection. Intramedullary ependymomas frequently have tissue planes separating the tumor and cord that facilitate complete resection, as well. Low-grade astrocytomas and oligodendrogliomas are infiltrative and generally lack tissue planes separating the tumor from normal cord. Aggressive resection in this situation can result in substantial neurologic injury, so subtotal resection or biopsy is often performed. However, aggressive resection has been performed successfully in some expert hands.28 If frozen sections suggest HAA or GBM, resection is usually abandoned after biopsy is performed.
Role of Radiation Therapy
The radiotherapeutic approach is different for each situation (Table 26-2). Postoperative irradiation is not indicated for completely resected low-grade gliomas.26,29,30 Incompletely resected low-grade astrocytomas and ependymomas are generally treated with postoperative focal irradiation. High-grade (malignant) astrocytomas are palliatively irradiated with focal fields. Malignant ependymoma and multifocal, benign ependymomas are treated with curative-intent, craniospinal irradiation.
Table 26-2 Radiotherapeutic Management of Primary Spinal Tumors
| Type | Treatment and Total Radiation Dose, Gy* |
|---|---|
| Low-grade glioma,† complete resection | Observation |
| Low-grade glioma,† subtotal resection | Focal field XRT,‡ 50.4 |
| High-grade (malignant) glioma (HAA, GBM) | Focal field XRT, 54 |
| Malignant ependymoma and benign multifocal ependymoma | Craniospinal XRT to 45, then 9 focal boost to gross tumor sites (54 total) |
| Meningioma, completely resected | Observation |
| Meningioma, subtotally resected | Observation vs. focal field XRT, 50.4-54, or charged particle beams,§ 52-54 Eq |
| Spinal canal sarcomas and vertebral body chondrosarcomas, chordomas, osteogenic sarcomas | Charged particle beams,§ 60-72 Eq |
HAA, Highly anaplastic (Grade 3) astrocytoma; GBM, glioblastoma multiforme; XRT, x-ray therapy.
* Total dose prescribed using standard (1.8-Gy) once-daily fractions with megavoltage photons unless otherwise specified.
† Includes astrocytoma, ependymoma, and oligodendroglioma.
‡ Megavoltage photon irradiation.
Completely resected spinal canal meningiomas require no additional therapy, since the risk of recurrence is only on the order of 6%.31 However, subtotally resected meningiomas have a higher risk of local recurrence, making postoperative irradiation a reasonable consideration.
Nerve sheath sarcomas are approached in a similar manner: postoperative irradiation is generally withheld if the tumor has been completely resected. Particle beam irradiation is given if macroscopic tumor remains. Incompletely removed vertebral chondrosarcomas and chordomas are likewise irradiated with charged particles (see Table 26-2). Osteogenic sarcomas are treated with a combination of chemotherapy, surgery, and postoperative particle beam irradiation if complete resection is not achieved.
Simulation
Low-grade cord gliomas are treated with megavoltage photon fields encompassing the radiographically apparent lesion (gross target volume [GTV]) with a 3- to 5-cm margin of normal spinal cord (or brainstem for high cervical lesions) both rostrally and caudally (clinical target volume [CTV]). The preoperative sagittal MRI is the most useful study for determining the size and location of the GTV. Field width for non-IMRT cases rarely needs to exceed 7 to 8 cm, since only the cord requires irradiation. Sophisticated immobilization devices are useful when treating with IMRT, and thermoplastic face masks are useful for conventionally-treated tumors located in the cervical spine. A typical clinical, non-IMRT setup for a patient with a thoracic cord glioma is shown in Fig. 26-1.
FIGURE 26-1 • Clinical setup showing cephalad and caudal field borders for a localized low-grade thoracic spinal cord glioma.
Whether to include an associated syrinx (a dilated, fluid-filled intramedullary cavity) in the treatment volume is controversial. At times, the syrinx results from local mass effect causing obstruction and dilation of the central canal of the spinal cord; in this situation, the syrinx is not actually part of the neoplastic process, but instead represents a reaction of normal tissue. At other times, the tumor itself may be from a cystic cavity or syrinx as part of the neoplastic process, and in this situation the syrinx must be included in the GTV treatment volume. Clinically distinguishing these situations from one another is often difficult; consultation with the neurosurgeon regarding his or her impression at the time of surgery is often beneficial. In general, a small syrinx can easily be included in the treatment volume. An extensive syrinx extending for virtually the entire length of the cord can be technically difficult to encompass with conventional treatment fields. IMRT can be particularly beneficial in this situation (Fig. 26-2).
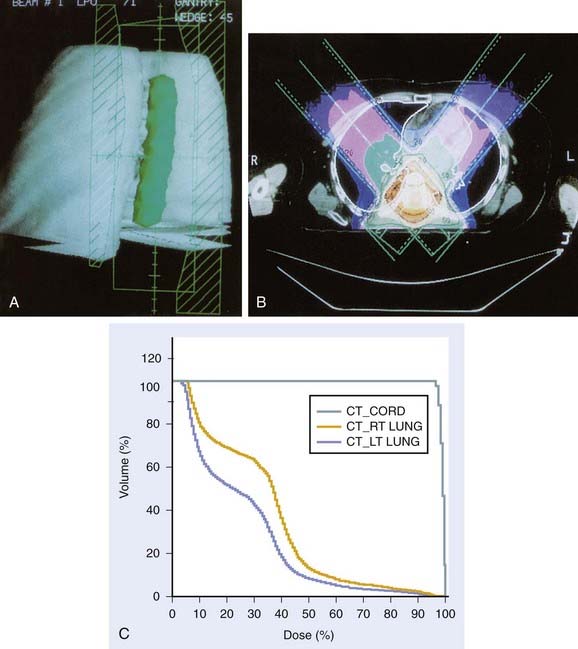
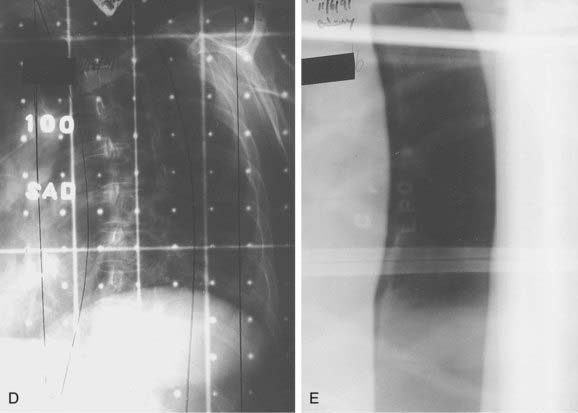
FIGURE 26-2 • A, Beam’s eye view achieved using three-dimensional conformal reconstruction of a thoracic cord glioma target volume and adjacent lungs with a posterior oblique wedged-pair irradiation technique. Custom low melting point alloy blocking or multileaf collimation is designed using the beam’s eye view of the target volume. B, Transverse section showing dosimetry from the treatment plan developed using the target volume derived from A. Note the substantial portion of lung included in the beam’s exit path at this level. C, Dose-volume histogram derived from the treatment plan shown in B using dosimetry information for the entire treatment volume. Virtually the entire target volume receives 100% of the total dose of 50.4 Gy at 1.8 Gy per fraction. Despite the impressive amount of lung included in the exit beam shown in B, the histogram shows that only 10% to 15% of each lung actually receives more than 25 Gy (50% of the total dose) under this plan. Nonetheless, 40% of the left lung and 60% of the right lung receives a dose greater than 15 Gy (30% of the total dose). See also Fig. 26-2. D, Verification simulator film taken with the beam’s eye view geometry based on the treatment plan developed in A, B, and C. E, Port film taken during treatment on the linear accelerator using the treatment field shown in D confirms the accuracy of the daily patient setup.
When IMRT is not utilized, tumors in the upper cervical spine are generally treated with opposed lateral fields to avoid unnecessary irradiation of the aerodigestive mucosa. Tumors located more caudally are often treated with differentially weighted anterior-posterior–posterior-anterior (AP-PA) fields using compensators. Use of beam-split abutting opposed lateral and AP-PA fields to treat tumors at the cervicothoracic junction is discouraged; the risk of overdosing the normal cord and underdosing the tumor owing to setup variation or error far outweighs any advantage in reducing acute morbidity. Again, IMRT is of particular benefit for tumors located in this region, eliminating the problems of possible overlap/underdosing inherent in the use of abutting fields (Fig. 26-3). When the use of abutting fields is unavoidable, a matching beam-split technique using independent jaws is required. The match line should be shifted 1 cm after every 10 Gy to minimize the risk of inadvertent overlap. A medulloblastoma-type “gap” technique should only be used when treating the craniospinal axis, and the gap should never be located over a site of macroscopic disease.
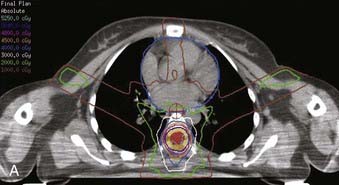
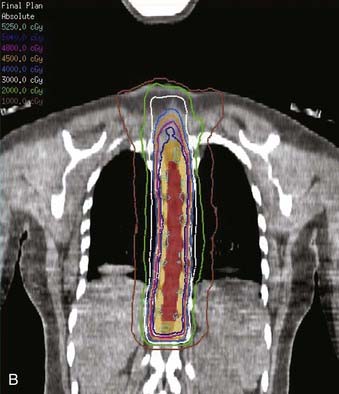
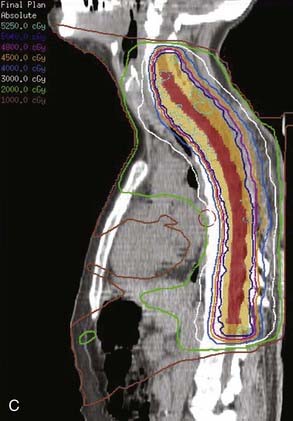
FIGURE 26-3 • The advantages of intensity modulated radiation therapy (IMRT) are highlighted by this difficult low-grade astrocytoma involving the thoracic cord, associated with a neoplastic syrinx extending cephalad through the cervicothoracic junction into the cervical cord. These three images demonstrate the superior dose-homogeneity IMRT provides within the high-dose treatment volume and the substantially greater sparing of adjacent normal structures throughout the treatment volume (compare the isodose curves involving the lung with the discussion in Fig. 26-2). Conventional treatment techniques are not able to provide this level of precise dose-localization, although, theoretically, stereotaxic body radiosurgery would provide similar advantages. A, Transverse image. B, Coronal image. C, Sagittal image. The 5040 cGy isodose line is shown in dark blue.
Wedged pair, posterior, oblique fields offer the theoretical advantage of decreased morbidity by minimizing exit dose in any given location; however, the planning and setup are technically more difficult, and scrupulous verification is necessary to ensure adequate coverage during treatment. Care must also be taken with this technique to ensure that the kidneys, liver, or substantial portions of the lungs are not irradiated beyond tolerance. The use of three-dimensional (3D) conformal treatment planning with dose-volume histograms has greatly improved the reliability and safety of this technique (see Fig. 26-2).
It is worth noting that a series from Iowa with small numbers of cauda ependymomas found that adjuvant thecal sac irradiation was beneficial when piecemeal rather than en bloc resection was performed.32 However, several other series found no advantage to the use of traditional extended fields over focal fields.8,33 Following an uncomplicated resection, the use of fields extended inferiorly and laterally to encompass the entire sacrum appear unnecessary from both a clinical and theoretical standpoint.
Spinal canal sarcomas as well as sarcomas of bone generally require extremely high doses of radiation, far exceeding the tolerance of the normal cord, for local control to be achieved. Charged particle beams (protons and helium where available) are generally considered to be the standard of care in this setting, as they have the physical ability to minimize the dose delivered to the adjacent normal spinal cord while adequately encompassing the tumor, which is generally located only a few millimeters away. A technical description of such treatment techniques is available in Chapters 68 and 69. The development of image-guided/intensity modulated photon radiation therapy (IGRT/IMRT) offers a new, promising alternative to charged particle beams in this situation.101
Radiation Treatment Plan
Malignant ependymomas and benign multifocal ependymomas are treated with 1.8-Gy fractions. The entire craniospinal axis is electively treated to a dose of 45 Gy, followed by a boost to macroscopic disease sites to 50.4 to 54 Gy total. Treating the craniospinal axis with lower doses (on the order of 20 Gy) provides no benefit compared to using localized radiation fields alone.90
Unless observation is chosen, subtotally resected spinal canal meningiomas may be irradiated in one of two ways: either 1.8-Gy daily fractions to a total dose of 50.4 to 54 Gy, or with twice-a-day fractions of 1 Gy (minimum 5- to 6-hour interfraction interval) to 54 Gy. (The hyperfractionated approach has the theoretical advantage of minimizing the risk of radiation injury to the normal spinal cord and has been used safely when incidentally irradiating the cervical cord during treatment for brainstem gliomas.34) Because the risk of long-term local recurrence for subtotally resected intracranial meningiomas is less than 10% when the minimum tumor dose is 52 Gy or greater,35 a reasonable approach is to use a plan in which the maximum dose in the treatment volume is 54 Gy, with a minimum dose to the meningioma of 52 Gy.
Brachytherapy
There is no established role for brachytherapy in the management of primary cord gliomas. Temporary 192Ir and permanent 125I implants have been used rarely to manage paraspinal sarcomas, occasionally with concurrent placement of a gold foil shield separating the seeds from the cord. Outcome has been poor in situations in which resection of the tumor has required opening of the dura.36 Currently, brachytherapy plays little role in the management of most primary tumors involving the spinal cord.
Critical Normal Tissues (Radiation Myelitis)
The spinal cord and cauda equina are the critical dose-limiting structures in the treatment of primary tumors involving the spinal cord. The cauda equina, consisting of peripheral nerve, is probably more resistant to radiation injury than the cord. Myelopathy was observed to be much more frequent when intrathecal 198Au was used alone or in addition to external beam treatment20,37; this form of therapy is now obsolete.
It has been postulated that the risk of cord injury depends on the location and length of cord irradiated, but no contemporary data exist to support this widely held notion. Older reports in the literature suggested that the thoracic cord was more sensitive to radiation injury than the cervical cord.38 During the period when this information was gathered, it was common to treat with only one field per day. Since most fields involving the cervical cord were opposed laterals, the dose delivered daily to the cervical cord was the prescribed mid-plane dose. The thoracic cord, on the other hand, was usually irradiated with AP-PA opposed fields, and the radiation dose it received on “PA days” was substantially higher than the mid-plane dose. Since fraction size dominates as a risk factor for radiation cord injury, the radiobiologic dose received by the cord under the “one-field-a-day” approach was much greater than the prescribed mid-plane dose. Thus, the reputed anatomic variability in cord sensitivity appears to be a radiobiologic artifact created by an obsolete treatment technique.39 Unlike the brain, where a relationship apparently exists between the volume irradiated and the subsequent risk of radiation injury, the length of cord irradiated appears to have little impact on the risk of radiation injury.40,41
The radiation tolerance of the normal spinal cord using once-daily fractions of 1.8 to 2 Gy has been traditionally listed as 4542 to 50 Gy.38,43 The 45-Gy value reportedly entails a 5% risk of myelitis at 5 years (TD5/5), which seems a gross overestimate. A University of Florida review of head and neck cancer patients whose cervical cords were incidentally irradiated found a 0.4% incidence of radiation myelitis with total doses between 45.01 and 50 Gy (2 of 471), compared with a 0% incidence with 40.01 to 45 Gy (0 of 514 patients), and 0% incidence (0 of 75 patients) with doses of more than 50 Gy.44 A 6% incidence of cervical myelitis was reported in 72 head and neck cancer patients whose cords were treated with at least 55 Gy.45 In that series, the minimum follow-up was 2 years, radiation fraction sizes ranged from 1.5 to 2 Gy, and 26 patients received total doses in excess of 60 Gy. Based partly on these data, contemporary experts have suggested that the TD5/5 for human spinal cord is actually on the order of 57 to 61 Gy, and the TD5/50 is in the range of 68 to 73 Gy.40,41,46,47 The spinal cord is particularly vulnerable to injury when larger fraction sizes are used; the Medical Research Council Lung Cancer Working Party reported on radiation myelopathy developing in 1048 lung cancer patients undergoing thoracic irradiation with palliative intent.48 No radiation myelitis developed using 3 Gy in 10 fractions (30 Gy total); but myelitis developed in 2.5% of patients receiving 3 Gy in 13 fractions (39 Gy total).
Although it seems logical to assume that cord injured by growing tumor and surgical trauma would be more sensitive to radiation injury than incidentally irradiated normal spinal cord, many series suggest that cord involved by tumor may be safely irradiated to standard tolerance levels. In the Massachusetts General Hospital (MGH) series, 26 patients with various histologic conditions were postoperatively irradiated to the equivalent of 40 to 50 Gy with standard fractionation and none developed radiation myelopathy.49 Likewise, none of 29 Medical College of Virginia spinal cord ependymoma and astrocytoma patients treated with doses between 45 and 55 Gy developed radiation-related neurologic deficits.50 In the series from the University of California at San Francisco (UCSF), 39 patients with primary cord and cauda tumors received total doses ranging between 45 and 54.7 Gy and only one case of radiation myelitis developed.13 Higher total radiation doses have been well tolerated; four ependymoma patients were treated at the Mayo Clinic to doses of 55 Gy and greater without complications.12
Outcome
Treating primary spinal tumors with postoperative irradiation is generally successful in terms of survival, less so in terms of local control, and difficult to evaluate in terms of long-term neurologic outcome. Survival of patients with low-grade spinal gliomas is substantially better than that achieved with histologically similar intracranial gliomas, probably because salvage of locally recurrent tumors with additional surgery is often effective. The exact degree of neurologic impairment caused by irradiation is difficult to determine because injury due to direct tumor growth as well as surgical trauma must be taken into account. As a general rule, it seems that a majority of patients either improve neurologically or stabilize with irradiation.51
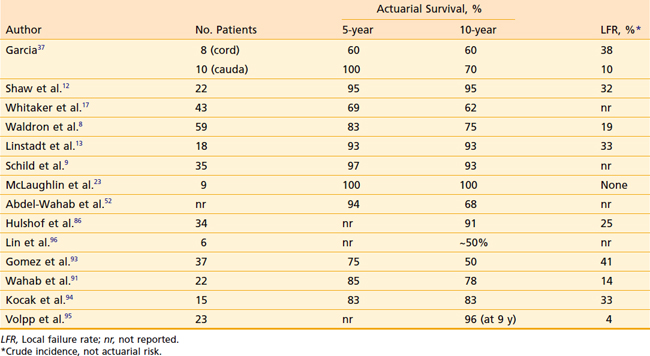
Spinal ependymoma patients do well, with 5- and 10-year survival rates in the 70% to 100% range (Table 26-3). Unfortunately, local recurrence ultimately develops in roughly one third of irradiated patients. Delayed local failures are far from rare; most series include several late recurrences developing more than 5 or 10 years after initial treatment, indicating the need for long-term follow-up. The majority of recurrences develop within the irradiated volume.
A Mallinckrodt series of 37 patients with primary cord tumors found a statistically significant dose–response relationship: Survival was 23% for those receiving less than 40 Gy total dose, compared with 83% for those with total doses of 40 Gy or greater.37 This analysis combined ependymomas, astrocytomas, unbiopsied tumors, and lymphoma together.
When ependymomas have been analyzed exclusively, no series has found a statistically significant dose-response relationship in the 40 to 54 Gy range.* In the Mayo Clinic series, four patients were irradiated to total doses of 55 Gy or greater, with one in-field failure still noted at this dose level.12 The UCSF series included nine ependymoma patients treated with doses between 45 and 50.4 Gy and another nine with doses between 50.4 and 52.9 Gy. The local recurrence rate was 33% in both dose ranges.13 Presently, there are no convincing data that demonstrate improved outcome with total doses above 50.4 Gy.
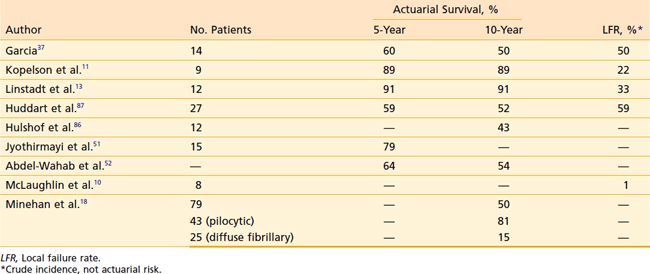
Results for low-grade spinal astrocytomas are similar to those achieved with ependymomas, with long-term survival in the 60% to 90% range but a relatively high incidence of local recurrence (Table 26-4). Patients with pilocytic astrocytomas probably derive little survival benefit from postoperative irradiaton.100 Most failures develop within 2 to 3 years of treatment. Low-grade astrocytomas tend to fail locally, although craniospinal axis metastases are occasionally encountered.11,13 Like ependymomas, there is no evidence suggesting improved local control or survival with total doses exceeding 50.4 Gy.11,13,92
Postoperative irradiation for malignant astrocytomas provides palliation, but long-term results are dismal. Extremely rapid local recurrence, CSF dissemination, and short survival times are the rule, regardless of the total dose of radiation used (Table 26-5).
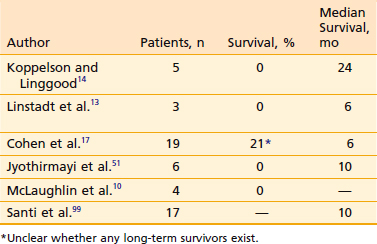
Table 26-5 High-Grade Spinal Astrocytomas (Including Highly Anaplastic [Grade 3] Astrocytomas and Glioblastoma Multiforme): Reported Results With Postoperative Irradiation
Spinal canal sarcomas respond favorably to charged particle irradiation. Castro and coworkers53,54 at Lawrence Berkeley Laboratory (LBL) treated 14 patients with neurofibrosarcoma and other soft tissue sarcoma histologies whose tumors abutted or encircled the spinal cord. All had macroscopic residual tumor when irradiated. With a mix of photons and helium and neon ions, a median dose of 65 GyEq was delivered to the tumor, but the cord dose was generally limited to less than 45 GyEq. The 4-year actuarial local control rate was 56%, and the 4-year actuarial survival rate was 77%. There was one case of radiation myelitis in a patient whose cord dose reached 55 GyEq.
The experience with primary tumors arising from the vertebral bodies is similar. Twenty-four LBL patients were irradiated with particles for spinal chordoma and chondrosarcoma.54 Mean doses were 72 GyEq (chondrosarcoma) and 65 GyEq (chordoma); gross tumor was present at the time of irradiation in 92% of cases. The 3-year actuarial local control rate for chondrosarcoma was 83%; the 3-year survival rate was 69%. Corresponding values for chordoma were 33% local control and 48% survival. There were no neurologic complications. Fourteen patients with sacral chordomas did particularly well, with a 5-year actuarial survival rate of 85% and a 55% local control rate.55 Similar results have been reported with the use of protons at MGH.56 The 5-year survival rate for proton treatment of cervical chondrosarcomas and chordomas using doses of 56.8 to 80 GyEq was 58%; as with the LBL series, better outcome was seen with chondrosarcomas than with chordomas.57 Investigators in Hannover, Germany also reported a benefit from high-dose (60 to 70 Gy) postoperative radiotherapy after subtotal resection of spinal chordoma, although results were best when en bloc resection could be performed.58
Experience with vertebral osteogenic sarcomas is limited. Five such patients were treated at LBL; local control was achieved in two without treatment-related complications.54 Fifteen osteogenic sarcoma patients treated at MGH with mixed protons and photons achieved a 5-year local control rate of 59%, and a 5-year overall survival rate of 44%.59
Treatment of Recurrent Spinal Cord Gliomas
Surgery is the mainstay of salvage treatment. Low-grade tumors can be treated with reexcision including cordectomy, if necessary. These tumors appear to be particularly appropriate candidates for radical resection. In the experience at New York University, 29 patients (26 of whom had recurrent intramedullary tumors, with 18 previously irradiated) underwent attempted radical resection.17 Complete resection was obtained in 14, with “99% removal” in another seven. Although all three patients with malignant astrocytomas died from neuraxis dissemination, only one local recurrence was reported for low-grade tumors. Follow-up was short (range: 6 to 36 months), so long-term local control and survival rates are unknown. Lower extremity neurologic function appeared satisfactory, with stabilization or improvement in 72% of patients. However, patients unable to stand or walk preoperatively did not improve. Long-term disease-free survival following recurrence has also been reported following total removal or cordectomy in both the Mayo Clinic and UCSF series.12,13 Salvage cordectomy has been reported to result in protracted (6-year) survival following local failure in one patient with a spinal cord GBM, although ultimately the patient died from CSF-borne intracranial metastasis.98
Reirradiation has been attempted sporadically. Both experimental and clinical data suggest that the spinal cord and lung, unlike the heart and kidney, partially recover from subclinical radiation injury, suggesting that reirradiation of the cord might therefore be safely attempted.60 In the UCSF series, 16 patients with spinal cord tumors suffered local failure. Four underwent repeat subtotal resection and reirradiation. No patients with multifocal or high-grade disease benefitted from retreatment. Two others with low-grade recurrences survived several years with high Karnofsky Performance Status values (≥80) following reirradiation. Both had initially undergone subtotal resections (one localized low-grade astrocytoma, one localized low-grade ependymoma) followed by 50.4 Gy to focal fields. After developing in-field recurrence and undergoing repeat subtotal resection, both patients were reirradiated with focal fields with 1-Gy fractions given twice daily to doses of 30 and 27 Gy. The cumulative doses were 77 Gy to the cauda equina for the ependymoma and 80 Gy to the cervical cord for the astrocytoma. No long-term radiation injuries developed.
Spinal Cord Compression from Metastatic Disease
Natural History
Epidural spinal metastases develop in 5% of patients with systemic cancer at some time during their illness. The incidence of spinal cord compression has decreased over the past 15 years, likely due to earlier detection and treatment of vertebral metastases as MRI has become more widely available. In 1992, it was estimated that there were 20,000 cases of cord and cauda compression annually in the United States,61 that number decreased to 12,700 by 2007.88 Primary carcinomas originating from the breast, lung, and prostate account for about half of all cases of cord compression. Other histologic types (including lymphoma, renal cell carcinoma, myeloma, melanoma, gastrointestinal carcinomas, gynecologic carcinomas, and sarcomas) account for the remainder.
Work-Up
History, physical, and a high index of suspicion are essential for making the diagnosis of cord compression. All patients with back pain and a history of cancer should be evaluated for possible spinal cord compression. One prospective series of cancer patients suggested that the likelihood of cord compression was approximately 30% when any one of the following risk factors was present: back pain, abnormal neurologic examination, or evidence of vertebral metastases on plain x-rays.62 When two factors were present, the probability of cord compression was 60% to 70%; when all three features were present, the likelihood of cord compression was more than 90%.
Imaging
MRI with gadolinium is the radiographic study of choice to evaluate possible malignant spinal cord compression. Patients unable to undergo MRI should be evaluated by metrizamide-enhanced CT myelography. In addition to the clinical area of interest, the study should evaluate the entire spine to determine the complete extent of the lesion and to detect possible synchronous lesions located elsewhere along the spinal axis. One retrospective series reported that multiple levels of cord compression existed in 39% of patients with apparently clinically localized spinal cord compression, and that more than two thirds of patients with multiple levels of spinal cord compression had more than one spinal region involved.63
Diagnostic Approach
When the sagittal screening MRI detects epidural disease, a full MRI of the affected area is performed. This approach results in more rapid performance of the definitive study compared with the old approach of first performing plain films with or without bone scans, followed by MRI when radiographic or scintigraphic abnormalities were noted.64 It also results in a less expensive MRI examination if no epidural disease is detected and has proven to be cost-effective compared with the traditional approach.64 This approach might be criticized as excessively “high-tech,” and it probably detects far more cases of painful bony metastases than actual cord compression. Nonetheless, the patient’s long term functional status is clearly superior when a painful bony metastasis is irradiated early on, rather than later when neurologic deficits from cord compression have developed.
A widespread belief held among radiation oncologists is that referral for radiation treatment of spinal cord compression occurs most frequently on Fridays, generally late in the afternoon. The validity of this belief is supported by a retrospective study of 443 spinal cord compression patients from the Netherlands, which showed that 30% of such referrals came on Friday, 12% on Monday, 17% on Tuesday, 15% on Wednesday, 20% on Thursday, 5% on Saturday, and 1% on Sunday (P < .002).65
Treatment
Management options vary among irradiation, neurosurgical decompression with or without postoperative irradiation, and chemotherapy. In the past, irradiation and corticosteroids were the mainstay of treatment,61 but improvements in neurosurgical technique during the past decade have now made initial surgical debulking and decompression followed by postoperative radiotherapy the current gold standard of care where available.89
Steroids
Glucocorticoid therapy (dexamethasone) is generally started immediately upon diagnosis; the initial dose is controversial. Extremely high dexamethasone doses (100 mg intravenous bolus upon diagnosis followed by 24 mg orally qid for 3 days, then tapered) did not influence neurologic outcome in two prospective studies, but appeared to hasten pain relief.66,67 At UCSF, 10 mg of dexamethasone is given intravenously, followed by 4 to 6 mg orally or intravenously every 6 hours. The dexamethasone dose is gradually tapered once the initial high-dose radiation fractions are completed.
Some investigators have suggested that steroids may be safely omitted for favorable patients with spinal cord compression undergoing irradiation (those presenting without neurologic deficits or radiculopathy symptoms only, and no massive vertebral involvement). One prospective phase II trial from Italy contained 20 such patients; all were ambulatory at the end of treatment, and median survival was 14 months.68 Conversely, a randomized trial of high-dose dexamethasone (96 mg over 4 days, then tapered) versus no steroid was performed in 57 irradiated spinal cord compression patients in Denmark. The corticosteroid treatment resulted in superior outcome with respect to ambulation rates 6 months after treatment (59% for the steroid group versus 33% for the group not receiving steroids).69 However, there was no difference between groups with respect to median survival.
Surgery
The traditional approach, decompressive laminectomy, offers poor exposure for the majority of tumors that arise from anterior bony structures. A successful outcome (defined as ambulation after treatment) is achieved in about 30% of patients. Historically, complications have been high, with 10% surgical mortality, 10% significant morbidity, and 10% of patients experiencing further neurologic deterioration.70 More recently, selective surgery with vertebral body resection and stabilization has been performed for anterior tumors.71 This approach appears to have substantially improved results with approximately 80% to 90% of patients ambulatory postoperation, although mortality and serious morbidity rates remain in the 5% to 15% range.
A randomized prospective study comparing the combination of modern selective surgery plus postoperative irradiation with palliative radiotherapy verified the benefit of combined treatment.89 In that study, 101 patients with newly diagnosed spinal cord compression were given 100 mg dexamethasone immediately, followed by 24 mg q6hr until treated with either radiation or surgery. Patients were randomized to either radiotherapy (30 Gy in 10 fractions) within 24 hours of diagnosis or surgical debulking/decompression within the same time period. The surgical patients all received postoperative radiotherapy (30 Gy in 10 fractions) beginning within 14 days of surgery. The combined modality patients demonstrated superior post-treatment ambulation rates (84% vs 57%); this was true both for patients who were ambulatory at the time of treatment (94% vs 74%) and those unable to walk at study entry (62% of the combined modality group regained the ability to walk, compared to only 19% in the radiation arm). Additionally, the median duration of ambulatory status was significantly longer in the surgery plus x-ray therapy (XRT) group (153 days) than in the radiotherapy alone group (54 days). Median survival was also significantly better in the combined treatment group (126 days vs 100 days for XRT alone). Putting this palliative benefit of combined treatment into perspective, it appears that, on average, the combined treatment patients preserved their ambulatory status for the rest of their lifespans, while the average radiation-alone patient preserved the ability to walk for only 50% to 60% of his or her remaining lifespan before becoming bedridden.
Nonetheless, there are several situations where irradiation is contraindicated:
Irradiation
Treatment of cord compression with either irradiation alone or postoperative irradiation appears to achieve better results than decompressive laminectomy alone. A large retrospective review from Memorial Sloan-Kettering Cancer Center (MSKCC) examined results with radiation alone (170 patients) and surgery plus postoperative XRT (65 patients).73 There was no significant difference in outcome between the two treatment groups, with 46% of patients ambulatory following treatment in the surgery plus XRT group versus 49% in the XRT-alone group. Conversely, a retrospective study from Australia found the combination of surgery followed by irradiation (38 patients) to be superior to either surgery alone (19 patients) or radiotherapy alone (37 patients).74 A prospective, randomized trial from the University of California at Los Angeles compared irradiation alone with decompressive laminectomy and postoperative XRT. The study was small (16 patients received surgery plus XRT; 13 received XRT alone), but found no difference between treatment arms with respect to pain relief, improved ambulation, or improved sphincter function.75 Another small, randomized, prospective trial from the MD Anderson Center compared radiotherapy alone versus surgical decompression plus irradiation and found no difference in outcome.76 It is important to note that there are no data comparing irradiation alone with selective anterolateral-approach surgery, and this new procedure (perhaps combined with postoperative XRT) might prove to be superior to XRT alone.
Radiation doses are usually given once daily with initially large fractions (3 to 5 Gy) and then decreased in size after 3 to 4 doses and taken to total doses in the 20 to 40 Gy range. The UCSF treatment policy has been to deliver 4 Gy daily in three fractions, followed by 2 Gy in 12 fractions (total dose 36 Gy in 15 fractions given over 3 weeks). This regimen appears to be well tolerated and achieves satisfactory outcome. Alternative schemes such as 4 Gy in five, 3 Gy in 10 or (for selected patients with highly favorable long-term prognosis) 2 Gy in 20 are reasonable. An Italian series reported that a short course scheme of a single 8-Gy fraction followed by a second 8-Gy fraction given 1 week later was comparable in outcome to a more protracted regimen using daily fractions of 5 Gy over 3 days, 4 days of rest, and then 3 Gy over 5 days (30 Gy total).77
Chemotherapy
Prognostic Factors
The most important factor influencing functional outcome is the extent of neurologic impairment when treatment begins.78,79 In the MSKCC series of patients treated with XRT alone, 79% of patients who were ambulatory at the start of irradiation remained so at the end of treatment.73 In contrast, only 45% of paraparetic patients became ambulatory, and only 3% of paralyzed patients later walked. Results were similar for the patients treated with both surgery and postoperative XRT (64%, 45%, and 10% post-treatment ambulation rates, respectively).
The Rigshospitalet in Copenhagen reported similar post-treatment ambulation rates in 345 patients treated with laminectomy, radiation, or both.80 Of initially ambulatory patients, 79% remained ambulatory, compared with 21% of paraparetic patients. There did appear to be a better outcome for paralyzed patients treated with laminectomy plus XRT (13% post-treatment ambulation rate) versus XRT alone (4%).
One retrospective review has suggested a better outcome with laminectomy plus XRT than with XRT alone for paraparetic patients.81 In that series, post-treatment ambulation rates were 64% for XRT alone, but 82% with combined therapy. No series has suggested a better outcome for ambulatory patients treated with combined surgery plus XRT compared with XRT alone.
Somewhat counterintuitively (given the usual rush to initiate XRT as soon as possible), a longer time interval between the onset of motor deficits and the initiation of radiotherapy appears to result in better functional outcome. A retrospective review of 96 spinal cord compression patients from Hannover, Germany found that 89% of patients showed improved motor function with irradiation when their motor symptoms had been present for 14 days or longer before the start of radiotherapy; only 2% deteriorated.82 In contrast, only 12% of patients improved when their motor symptoms had been present for less than 14 days before the start of radiation, and 49% deteriorated despite treatment. The effect was particularly pronounced when motor symptoms had been present for less than 48 hours before the start of irradiation: Only 6% of such patients experienced improved motor function from treatment while 65% deteriorated. A follow-up prospective, observational study of 98 patients from the same investigators confirmed these findings.83 In that series the posttreatment ambulation rates were 86% for patients whose symptoms developed more than 14 days before the start of irradiation, 55% for symptoms developing 8 to 14 days before treatment, and 35% for those whose symptoms developed 1 to 7 days before treatment.
These findings strongly indicate that the intrinsic biology of the tumor causing the spinal cord compression is the major factor influencing functional outcome,83 and the slower the development of motor deficits, the better the functional prognosis. Consequently, while the perceived need to initiate radiotherapy emergently may provide psychological benefit to patients and the treatment team, it may not provide any meaningful actual improvement in the patient’s long-term functional outcome.
Survival
Survival correlates best with ambulatory status at the end of treatment.84 In a series of 56 breast cancer patients irradiated for cord compression, the 1-year actuarial survival rate was 66% for patients who were ambulatory at the end of irradiation versus 10% for nonambulatory patients.85 For irradiated patients with spinal cord compression caused by prostate cancer, median survival for post-treatment walking patients was 10 months, compared with 2 months for nonwalking patients.77
1 Michalski J, Garcia D. Spinal Canal. In: Perez C, Brady L, editors. Principles and practice of radiation oncology. ed 3. Philadelphia: Lippincott-Raven; 1998:851.
2 von Haken MS, White EC, Daneshvar-Shyesther L, et al. Molecular genetic analysis of chromosome arm 17p and chromosome arm 22q DNA sequences in sporadic pediatric ependymomas. Genes Chromosomes Cancer. 1996;17:37.
3 Hulsebos TJ, Oskam NT, Bijleveld EH, et al. Evidence for an ependymoma tumour suppressor gene in chromosome region 22pter-22q11.2. Br J Cancer. 1999;81:1150.
4 Huang B, Starostik P, Kuhl J, et al. Loss of heterozygosity on chromosome 22 in human ependymomas. Acta Neuropathol (Berl). 2002;103:415.
5 Rushing EJ, Brown DF, Hladik CL, et al. Correlation of bcl-2, p53, and MIB-1 expression with ependymoma grade and subtype. Mod Pathol. 1998;11:464.
6 Lamszus K, Lachenmayer L, Heinemann U, et al. Molecular genetic alterations on chromosomes 11 and 22 in ependymomas. Int J Cancer. 2001;91:803.
7 Whitaker SJ, Bessell EM, Ashley SE, et al. Postoperative radiotherapy in the management of spinal cord ependymoma. J Neurosurg. 1991;74:720.
8 Waldron JN, Laperriere NJ, Jaakkimainen L, et al. Spinal cord ependymomas: a retrospective analysis of 59 cases. Int J Radiat Oncol Biol Phys. 1993;27:223.
9 Schild SE, Nisi K, Scheithauer BW, et al. The results of radiotherapy for ependymomas: The Mayo Clinic experience. Int J Radiat Oncol Biol Phys. 1998;42:953.
10 McLaughlin MP, Buatti JM, Marcus RBJr, et al. Outcome after radiotherapy of primary spinal cord glial tumors. Radiat Oncol Investig. 1998;6:276.
11 Kopelson G, Linggood RM, Kleinman GM, et al. Management of intramedullary spinal cord tumors. Radiology. 1980;135:473.
12 Shaw EG, Evans RG, Scheithauer BW, et al. Radiotherapeutic management of adult intraspinal ependymomas. Int J Radiat Oncol Biol Phys. 1986;12:323.
13 Linstadt DE, Wara WM, Leibel SA, et al. Postoperative radiotherapy of primary spinal cord tumors. Int J Radiat Oncol Biol Phys. 1989;16:1397.
14 Kopelson G, Linggood RM. Intramedullary spinal cord astrocytoma versus glioblastoma: The prognostic importance of histologic grade. Cancer. 1982;50:732.
15 Rodrigues GB, Waldron JN, Wong CS, et al. A retrospective analysis of 52 cases of spinal cord glioma managed with radiation therapy. Int J Radiat Oncol Biol Phys. 2000;48:837.
16 Cooper P, Epstein F. Radical resection of intramedullary spinal cord tumors in adults. Recent experience in 29 patients. J Neurosurg. 1985;63:492.
17 Cohen A, Wisoff J, Allen J, et al. Malignant astrocytomas of the spinal cord. J Neurosurg. 1989;70:50.
18 Minehan KJ, Shaw EG, Scheithauer BW, et al. Spinal cord astro cytoma: pathological and treatment considerations. J Neurosurg. 1995;83:590.
19 Wara W, Linstadt D, Larson D. Management of primary brain stem gliomas and spinal cord gliomas. Semin Radiat Oncol. 1991;1:50.
20 Marks JE, Adler SJ. A comparative study of ependymomas by site of origin. Int J Radiat Oncol Biol Phys. 1982;8:37.
21 Asai A, Hoshino T, Edwards M, et al. Predicting the recurrence of ependymomas from the bromodeoxyuridine labeling index. Childs Nerv Syst. 1992;8:273.
22 Schroder R, Ploner C, Ernestus RI. The growth potential of ependymomas with varying grades of malignancy measured by the Ki-67 labeling index and mitotic index. Neurosurg Rev. 1993;16:145.
23 McLaughlin MP, Marcus RBJr, Buatti JM, et al. Ependymoma: results, prognostic factors and treatment recommendations. Int J Radiat Oncol Biol Phys. 1998;40:845.
24 AJCC. Bone and soft tissue sarcoma. In: Greene FL, Page DL, Fleming I, et al, editors. AJCC Cancer Staging Manual. ed 6. New York: Springer; 2002:185.
25 Lee M, Epstein FJ, Rezai AR, et al. Nonneoplastic intra-medullary spinal cord lesions mimicking tumors. Neurosurgery. 1998;43:788.
26 Lee TT, Gromelski EB, Green BA. Surgical treatment of spinal ependymoma and post-operative radiotherapy. Acta Neurochir (Wien). 1998;140:309.
27 Chang C, McCormick P, Stein B, et al. Radical Treatment of Primary Intramedullary Spinal Cord Tumors. Kyoto, Japan: International Congress of Radiation Oncology; 1993. p 95.
28 Epstein F, Epstein N. Surgical treatment of spinal cord astrocy-tomas of childhood. A series of 19 patients. J Neurosurg. 1982;57:685.
29 Constantini S, Miller D, Allen J, et al. Radical excision of intramedullary spinal cord tumors: surgical morbidity and long-term follow-up evaluation in 164 children and young adults. J Neurosurg. 2000;93:183.
30 Goh K, Velasquez L, Epstein F. Pediatric intramedullary spinal cord tumors: is surgery alone enough? Pediatr Neurosurg. 1997;27:34.
31 Solero CL, Fornari M, Giombini S, et al. Spinal meningiomas: review of 174 operated cases. Neurosurgery. 1989;25:153.
32 Wen BC, Hussey DH, Hitchon PW, et al. The role of radiation therapy in the management of ependymomas of the spinal cord. Int J Radiat Oncol Biol Phys. 1991;20:781.
33 Lodin K, Dattoli M, Milcu M: Radiation Therapy for Adult Spinal Cord Ependymomas: The Case for Limited Volume Treatment, Scottsdale, Ariz.: Proceedings from the 72nd Annual American Radium Society Meeting, April 21–25, 1990; 1990:5.
34 Linstadt DE, Edwards MS, Prados M, et al. Hyperfractionated irradiation for adults with brainstem gliomas. Int J Radiat Oncol Biol Phys. 1991;20:757.
35 Goldsmith B, Wara W, Wilson C, et al. Post-operative external beam irradiation for sub-totally resected meningioma. Int J Radiat Oncol Biol Phys. 1992;24:126.
36 Armstrong J, Fass D, Bains M, et al. Paraspinal tumors: techniques and results of brachytherapy. Int J Radiat Oncol Biol Phys. 1991;20:787.
37 Garcia D. Primary spinal cord tumors treated with surgery and postoperative irradiation. Int J Radiat Oncol Biol Phys. 1985;11:1933.
38 Wara WM, Phillips TL, Sheline GE, et al. Radiation tolerance of the spinal cord. Cancer. 1975;35:1558.
39 Schultheiss TE. Spinal cord radiation “tolerance”: doctrine versus data. Int J Radiat Oncol Biol Phys. 1990;19:219.
40 Levin V, Leibel S, Gutin P. Neoplasms of the central nervous system. In: Devita V, Hellman S, Rosenberg S, editors. Cancer Principles and Practice of Oncology. ed 6. Philadelphia: Lippincott Williams & Wilkins; 2001:2117.
41 Schultheiss TE, Kun LE, Ang KK, et al. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys. 1995;31:1093.
42 Rubin P, Cooper R, Phillips T. Radiation Biology and Radiation Pathology Syllabus. In: Rubin P, Cooper R, Phillips T, editors. Radiation Biology and Radiation Pathology Syllabus. Chicago: American College of Radiology, 1975.
43 Phillips TL, Buschke F. Radiation tolerance of the thoracic spinal cord. Am J Roentgenol Radium Ther Nucl Med. 1969;105:659.
44 Marcus R, Million R. The incidence of myelitis after irradiation of the cervical spinal cord. Int J Radiat Oncol Biol Phys. 1990;19:3.
45 Jeremic B, Djuric L, Mijatovic L. Incidence of radiation myelitis of the cervical spinal cord at doses of 5500 cGy or greater. Cancer. 1991;68:2138.
46 Kim YH, Fayos JV. Radiation tolerance of the cervical spinal cord. Radiology. 1981;139:473.
47 van der Kogel AJ. Retreatment tolerance of the spinal cord. Int J Radiat Oncol Biol Phys. 1993;26:715.
48 Macbeth FR, Wheldon TE, Girling DJ, et al. Radiation myelopathy: estimates of risk in 1048 patients in three randomized trials of palliative radiotherapy for non–small cell lung cancer. The Medical Research Council Lung Cancer Working Party. Clin Oncol (R Coll Radiol). 1996;8:176.
49 Kopelson G. Radiation tolerance of the spinal cord previously damaged by tumor and operation: long term neurological improvement and time-dose-volume relationships after irradiation of intraspinal gliomas. Int J Radiat Oncol Biol Phys. 1982;8:925.
50 Chun H, Schmidt-Ullrich R, Wolfson A, et al. External beam radiotherapy for primary spinal cord tumors. J Neurooncol. 1990;9:211.
51 Jyothirmayi R, Madhavan J, Nair MK, et al. Conservative surgery and radiotherapy in the treatment of spinal cord astrocytoma. J Neurooncol. 1997;33:205.
52 Abdel-Wahab M, Corn B, Wolfson A, et al. Prognostic factors and survival in patients with spinal cord gliomas after radiation therapy. Am J Clin Oncol. 1999;22:344.
53 Castro J, Collier J, Petti P, et al. Charged particle radiotherapy for lesions encircling the brain stem or spinal cord. Int J Radiat Oncol Biol Phys. 1989;17:477.
54 Nowakowski VA, Castro JR, Petti PL, et al. Charged particle radiotherapy of paraspinal tumors. Int J Radiat Oncol Biol Phys. 1992;22:295.
55 Schoenthaler R, Castro JR, Petti PL, et al. Charged particle irradiation of sacral chordomas. Int J Radiat Oncol Biol Phys. 1993;26:291.
56 Austin-Seymour M, Munzenrider J, Goitein M, et al. Progress in low-LET heavy particle therapy: intracranial and paracranial tumors and uveal melanomas. Radiat Res Suppl. 1985;8:S219.
57 Munzenrider J. Proton therapy with the Harvard cyclotron. Int Cong Ser. 1994;1077:83.
58 Klekamp J, Samii M. Spinal chordomas—results of treatment over a 17-year period. Acta Neurochir (Wien). 1996;138:514.
59 Hug EB, Fitzek MM, Liebsch NJ, et al. Locally challenging osteo- and chondrogenic tumors of the axial skeleton: results of combined proton and photon radiation therapy using three-dimensional treatment planning. Int J Radiat Oncol Biol Phys. 1995;31:467.
60 Neider C, Milas L, Ang K. Tissue tolerance to reirradiation. Semin Radiat Oncol. 2000;10:200.
61 Byrne T. Spinal cord compression from epidural metastases. N Engl J Med. 1992;327:614.
62 Rodichok LD, Harper GR, Ruckdeschel JC, et al. Early diagnosis of spinal epidural metastases. Am J Med. 1981;70:1181.
63 Cook A, Lau T, Tomlinson M, et al. Magnetic resonance imaging of the whole spine in suspected malignant spinal cord compression: impact on management. Clin Oncol (R Coll Radiol). 1998;10:39.
64 Ruckdeschel J, Patterson S, Cuthbertson D, et al. Rapid, cost-effective diagnosis of spinal cord compression due to cancer. Cancer Control. 1995;2:320.
65 Poortmans P, Vulto A, Raaijmakers E. Always on a Friday? Time pattern of referral for spinal cord compression. Acta Oncol. 2001;40:88.
66 Greenberg H, Kim J, Posner J. Epidural spinal cord compression from metastatic tumor: results with a new treatment protocol. Ann Neurol. 1980;8:361.
67 Vecht CJ, Haaxma-Reiche H, van Putten WL, et al. Initial bolus of conventional versus high-dose dexamethasone in metastatic spinal cord compression. Neurology. 1989;39:1255.
68 Maranzano E, Latini P, Beneventi S, et al. Radiotherapy without steroids in selected metastatic spinal cord compression patients. A phase II trial. Am J Clin Oncol. 1996;19:179.
69 Sorensen S, Helweg-Larsen S, Mouridsen H, et al. Effect of high-dose dexamethasone in carcinomatous metastatic spinal cord compression treated with radiotherapy: a randomised trial. Eur J Cancer. 1994;30A:22.
70 Black P. Spinal metastasis: current status and recommended guidelines for management. Neurosurgery. 1979;5:726.
71 Sundaresan N, Digiacinto GV, Hughes JE, et al. Treatment of neoplastic spinal cord compression: results of a prospective study. Neurosurgery. 1991;29:645.
72 Ghogawala Z, Mansfield F, Borges L. Spinal radiation before surgical decompression adversely affects outcomes of surgery for symptomatic metastatic spinal cord compression. Spine. 2001;26:818.
73 Gilbert R, Kim J, Posner J. Epidural spinal cord compression from metastatic tumor: diagnosis and treatment. Ann Neurol. 1978;3:40.
74 Milcross C, Davies M, Fisher R, et al. The efficacy of treatment for malignant epidural spinal cord compression. Australas Radiol. 1997;41:137.
75 Young RF, Post EM, King GA. Treatment of spinal epidural metastases. Randomized prospective comparison of laminectomy and radiotherapy. J Neurosurg. 1980;53:741.
76 Payne R, Gaughan E, Chou C, et al. A randomized trial of radiation therapy alone versus best decompressive surgery plus radiaton for single site spinal cord compression: interim analysis. Proc Annu Meet Am Soc Clin Oncol. 1997;16:A275. (Abstract)
77 Maranzano E, Latini P, Beneventi S, et al. Comparison of two different radiotherapy schedules for spinal cord compression in prostate cancer. Tumori. 1998;84:472.
78 Kim RY, Spencer SA, Meredith RF, et al. Extradural spinal cord compression: analysis of factors determining functional prognosis—prospective study. Radiology. 1990;176:279.
79 Turner S, Marosszeky B, Timms I, et al. Malignant spinal cord compression: a prospective evaluation. Int J Radiat Oncol Biol Phys. 1993;26:141.
80 Sorensen S, Borgesen SE, Rohde K, et al. Metastatic epidural spinal cord compression. Results of treatment and survival. Cancer. 1990;65:1502.
81 Landmann C, Hunig R, Gratzl O. The role of laminectomy in the combined treatment of metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 1992;24:627.
82 Rades D, Blach M, Nerreter V, et al. Metastatic spinal cord compression. Influence of time between onset of motoric deficits and start of irradiation on therapeutic effect. Strahlenther Onkol. 1999;175:378.
83 Rades D, Heidenreich F, Karstens J. Final results of a prospective study of the prognostic value of the time to develop motor deficits before irradiation in metastatic spinal cord compression. Int J Radiat Oncol Biol Phys. 2002;53:975.
84 Kovner F, Spigel S, Rider I, et al. Radiation therapy of metastatic spinal cord compression. Multidisciplinary team diagnosis and treatment. J Neurooncol. 1999;42:85.
85 Maranzano E, Latini P, Checcaglini F, et al. Radiation therapy of spinal cord compression caused by breast cancer: report of a prospective trial. Int J Radiat Oncol Biol Phys. 1992;24:301.
86 Hulshof MC, Menten J, Dito JJ, et al. Treatment results in primary intraspinal gliomas. Radiother Oncol. 1993;29:294.
87 Huddart R, Traish D, Ashley S, et al. Management of spinal astrocytoma with conservative surgery and radiotherapy. Br J Neurosurg. 1993;7:473.
88 Abraham JL, Banffy MB, Harris MB. Spinal cord compression in patients with advanced metastatic cancer. JAMA. 2007;299:937-946.
89 Patchell RA, Tibbs PA, Regine WF. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: a randomized trial. Lancet. 2005;366:643-648.
90 Combs SE, Kelter V, Weizel T, et al. Influence of radiotherapy concept on the outcome of patients with localized ependymomas. Int J Radiat Oncol Biol Phys. 2008;71:972-978.
91 Wahab SH, Simpson, Michalski JM, et al. Long term outcome with post-operative radiation therapy for spinal canal ependymoma. J Neurooncol. 2007;83:85-89. Epub 2007 Jan 6
92 Abdel-Wahab M, Etuk B, Palermo J, et al. Spinal cord gliomas: a multi-institutional analysis. Int J Radiat Oncol Biol Phys. 2006;64:1060-1071.
93 Gomez DR, Missett BT, Wara WM, et al. High failure rate I spinal ependymomas with long-term follow-up. Neuro Oncol. 2005;7:254-259.
94 Kocak Z, Garipagaoglu M, Adli M, et al. Spinal cord ependymomas in adults: analysis of 15 cases. J Exp Clin Cancer Res. 2004;23:201-206.
95 Volpp PB, Han K, Kagan AR, et al. Outcome in treatment for intradural spinal cord ependymomas. Int J Radiat Oncol Biol Phys. 2007;69:1199-1204.
96 Lin YH, Huang CI, Wong TT, et al. Treatment of spinal cord ependymomas by surgery with or without postoperative radiotherapy. J Neurooncol. 2005;71:205-210.
97 Nakamura M, Ishii K, Watanabe K, et al. Surgical treatment of intramedullary spinal cord tumors: prognosis and complications. Spinal Cord. 2008;46:282-286.
98 Marchan EM, Sekula RF, Jannetta PJ, et al. Long-term survival enhanced by cordectomy in a patient with spinal glioblastoma multiforme and paraplegia. Case report. J Neurosurg Spine. 2007;7:656-659.
99 Santi M, Mena H, Wong K, et al. Spinal cord malignant astrocytomas. Clinicopathologic features in 36 cases. Cancer. 2003;98:554-561.
100 Minehan KJ, Brown PD, Scheithauer BW, et al. Prognosis and treatment of spinal cord astrocytoma. Int J Radiat Oncol Biol Phys. 2007;69:1199-1204.
101 Terezakis SA, Lovelock DM, Bilsky MH, et al. Image-guided intensity-modulated photon radiotherapy using multifractionated regimen to paraspinal chordomas and rare sarcomas. Int J Radiat Oncol Biol Phys. 2007;69:1502-1508.